Abstract
Purpose:
Level III inferior vena cava tumor thrombectomy for renal cancer is one of the most challenging open urologic oncology surgeries. We present the initial series of completely intracorporeal robotic level III inferior vena cava tumor thrombectomy.
Materials and Methods:
Nine patients underwent robotic level III inferior vena cava thrombectomy and 7 patients underwent level II thrombectomy. The entire operation (high intrahepatic inferior vena cava control, caval exclusion, tumor thrombectomy, inferior vena cava repair, radical nephrectomy, retroperitoneal lymphadenectomy) was performed exclusively robotically. To minimize the chances of intraoperative inferior vena cava thrombus embolization, an “inferior vena cava-first, kidney-last” robotic technique was developed. Data were accrued prospectively.
Results:
All 16 robotic procedures were successful, without open conversion or mortality. For level III cases (9), median primary kidney (right 6, left 3) cancer size was 8.5 cm (range 5.3 to 10.8) and inferior vena cava thrombus length was 5.7 cm (range 4 to 7). Median operative time was 4.9 hours (range 4.5 to 6.3), estimated blood loss was 375 cc (range 200 to 7,000) and hospital stay was 4.5 days. All surgical margins were negative. There were no intraoperative complications and 1 postoperative complication (Clavien 3b). At a median 7 months of followup (range 1 to 18) all patients are alive. Compared to level II thrombi the level III cohort trended toward greater inferior vena cava thrombus length (3.3 vs 5.7 cm), operative time (4.5 vs 4.9 hours) and blood loss (290 vs 375 cc).
Conclusions:
With appropriate patient selection, surgical planning and robotic experience, completely intracorporeal robotic level III inferior vena cava thrombectomy is feasible and can be performed efficiently. Larger experience, longer followup and comparison with open surgery are needed to confirm these initial outcomes.
Keywords: vena cava, inferior, robotics, thrombectomy
Locally advanced renal cancer with inferior vena cava tumor thrombus is infrequent, occurring in 4% to 10% of patients.1 Absent systemic metastases, prognosis is typically good, dictated largely by pathological TNM stage, grade and subtype, but not thrombus extent.1 Complete surgical excision is the only curative option. Radical nephrectomy with caval thrombectomy, with or without (neo)adjuvant therapy, confers an encouraging 5-year CSS of 40% to 65%.2 The Mayo classification subdivides caval thrombi into 4 categories based on their cephalad extent,3 which has implications on surgical complexity, blood loss, transfusion rates and perioperative complications but not CSS (table 1, fig. 1).1 Level III denotes a thrombus whose proximal extent is intrahepatic yet infradiaphragmatic. Open surgical IVC tumor thrombectomy is a major undertaking, associated with prolonged recovery, significant morbidity, a 25% to 40% complication rate and a 5% to 10% perioperative mortality rate.1,2
Table 1.
Mayo classification of IVC tumor thrombi3
| Thrombus Level | Proximal Extent of Thrombus | No. Robotic Cases Reported | Surgical Maneuvers Necessary | Mean Blood Loss (L)* | Mean Units Packed Red Blood Cells Transfused* | Periop Complications (%)* | 5-Yr CSS (%)* |
|---|---|---|---|---|---|---|---|
| 0 | Renal vein | Many | Milk back thrombus, staple at renal vein-IVC junction | 0.6 | 3 | 8.6 | - |
| I | Up to 2 cm into IVC | 5 | Partial circumference Satinsky clamping or limited cross-clamping of IVC | 1.0 | 4 | 15.2 | 31.7 |
| II | More than 2 cm into IVC, yet infrahepatic | 5 | IVC exclusion + cross-clamping: control of lumbar veins, infrarenal IVC, contralat renal vein + suprarenal infrahepatic IVC | 1.3 | 7 | 14.1 | 26.3 |
| III | Intrahepatic, yet infradiaphragmatic†,* | 9 (current study) | All maneuvers for level II thrombi, + control of short hepatic veins, intrahepatic IVC, occasionally porta hepatis (Pringle), suprahepatic IVC or intrapericardial IVC | 2.7 | 16 | 17.9 | 39.4 |
| IV | Supradiaphragmatic | 0 | Cardiopulmonary bypass | 2.5 | 18 | 30 | 37 |
| p Value | Not applicable | Not applicable | Not applicable | <0.001 | <0.001 | <0.001 | 0.87 |
These data are obtained for the Mayo Clinic open surgical series of IVC thrombectomy.1
Spiros et al subcategorized level III thrombi into 4 subgroups depending on the need for dissection of the hepatic veins and degree of IVC control required to extract the thrombus, as IIIa (intrahepatic) thrombus extending into the retrohepatic IVC but below the main hepatic veins, IIIb (hepatic) thrombus reaching/extending into ostia of major hepatic veins, IIIc (infradiaphragmatic) thrombus extending above major hepatic veins but infradiaphragmatic, and IIId (suprahepatic, supradiaphragmatic) thrombus extending into the intrapericardial IVC but not into the right heart.17
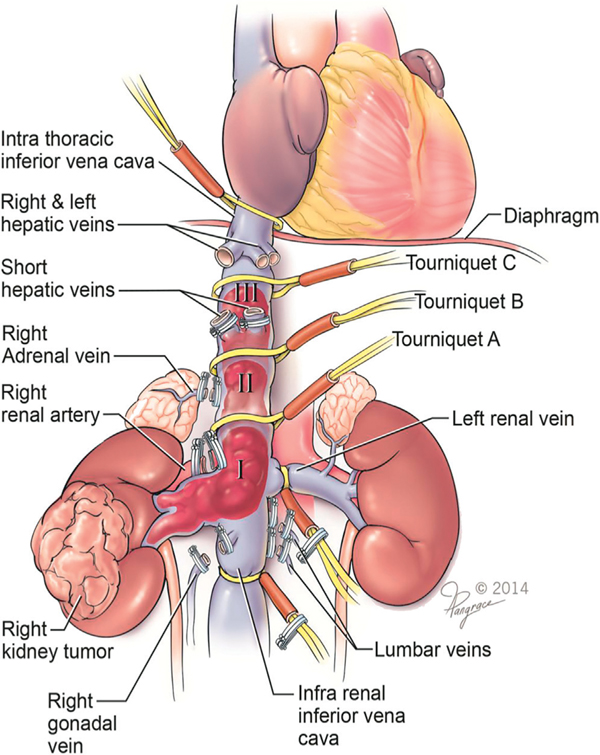
Figure 1.
Inferior vena cava control for which individualized surgical planning is necessary. Note various locations of Rummel tourniquet placement for achieving proximal control of IVC as dictated by thrombus level.
Minimally invasive IVC thrombectomy has evolved during the last 15 years. In the laboratory, laparoscopic level II and level III/IV caval thrombectomy techniques were first developed by our team in the early 2000s.4,5 Pure laparoscopic renal vein thrombectomy6 was followed by robotic level I and II caval thrombectomy.7 Recently the technique and initial clinical case reports of robotic level III thrombectomy were described.8–10 We present the initial series of robotic level III tumor thrombectomy in 9 patients. Furthermore, we add 7 cases of robotic Mayo level II thrombectomy to the literature.
MATERIALS AND METHODS
Sixteen patients underwent completely intracorporeal robotic tumor thrombectomy for level III (9) and level II (7) IVC thrombus by a single surgeon (June 2013 to February 2015). Exclusion criteria comprised patients with Mayo level 0-I (less than 2 cm into IVC), suprahepatic thrombus, metastatic disease (more than 1 site), unacceptable anesthetic risk or those undergoing venacavectomy. After informed consent, data were collected prospectively in our institutional review board approved databases. Complications were graded according to the Clavien-Dindo system.11
Patient Evaluation
Abdominopelvic imaging delineates thrombus anatomy (length/diameter, intrahepatic extent, distance from main hepatic veins, arterialization, bland thrombus extent), IVC anatomy (diameter, presence of flow, wall invasion, bilateral renal vein locations), hepatic anatomy (number/location of short/main hepatic veins, liver size/involvement), renal anatomy (number of renal arteries/veins, venous flow/collaterals, renal tumor size/stage) and retroperitoneal anatomy (adenopathy, venous collaterals) (fig. 2). Additional evaluation included renal/hepatic function testing, metastatic evaluation (CT chest, bone scan, occasionally positron emission tomography-CT) and evaluating for leg deep vein thrombosis, with anesthesia, cardiopulmonary, medical oncology and surgical (cardiovascular, hepatobiliary) consultations obtained as indicated.
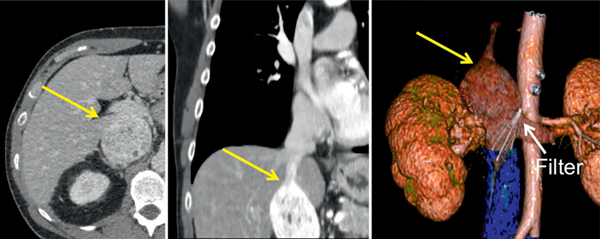
Figure 2.
Right side level III caval tumor thrombus (7 cm in length, yellow arrows). Before referral to our center transjugular IVC filter had been inserted straight through tumor thrombus, apparently in attempt to secure distal bland thrombus (blue). Obliquely malpositioned IVC filter (white arrow) at level of left renal vein ostium was also removed during robotic IVC thrombectomy.
Preoperative Preparation
Angioembolization of the tumor bearing kidney is performed, especially for patients with a left side or large renal tumor, significant perirenal collaterals or arterialized thrombus. Intraoperative monitoring (arterial, central venous, Swan Ganz) also included real-time, transesophageal echocardiography to assess cardiac hemodynamics, thrombus extent/tip stability during manipulation and caval flow cessation upon tourniquet occlusion. Followup included biochemical tests, chest x-ray and abdominal-pelvic scanning at 3 to 6 months and per surgeon discretion thereafter.
Robotic Technique
Right Side Thrombus.
Complete caval exclusion with cross-clamping is performed routinely. The patient is secured in a right side up, 60-degree lateral position. A 7-port approach (4 robotic, 2 assistant, 1 liver retraction) with the Si or Xi da Vinci® robot is used. Vascular dissection begins in the inter-aorto-caval region and infrarenal IVC is controlled caudal to any bland thrombus, as confirmed by laparoscopic ultrasound. Lumbar veins are secured. Infrarenal IVC is controlled with intra-abdominally controlled vessel loop Rummel tourniquets.
Inter-aorto-caval dissection proceeds cephalad to achieve the 3 goals of 1) Rummel tourniquet control of left renal vein precisely at its caval junction and to the right of any lumbar vein that may be draining directly into the proximal left renal vein, 2) transection of right renal artery and 3) mobilization of left edge of suprarenal IVC, occasionally requiring control of a nonpaired lumbar vein.
High proximal control of intrahepatic IVC requires transection of SH veins (fig. 3). Right adrenalectomy is performed. Intrahepatic IVC is circumferentially mobilized and a double-fenestrated robotic grasper is passed left-to-right posterior to the IVC to position a Rummel tourniquet. The right renal vein is dissected.
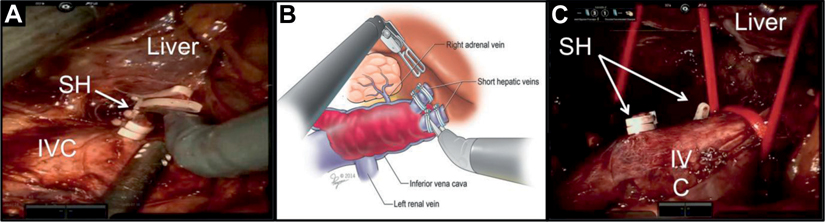
Figure 3.
Ligating SH veins. A, level III thrombectomy requires control of intrahepatic IVC and for this SH veins need to be transected. SH veins are short, wide, thin walled and 1 to 5 in number. B, anterior retraction of caudate lobe places SH veins on stretch. Each SH is individually controlled, thereby exposing intrahepatic IVC. C, after right adrenalectomy, mobilization and Rummel control of high intrahepatic IVC are achieved.
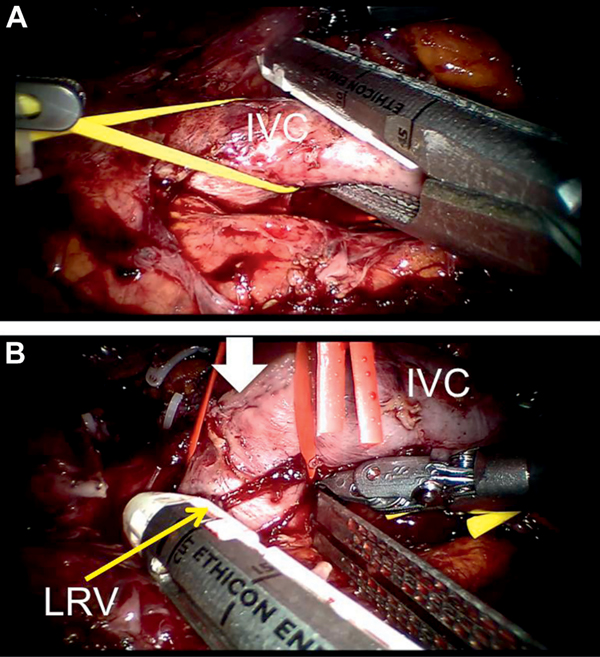
After alerting the anesthesiologist the 3 Rummel tourniquets are tightly cinched sequentially (infrarenal IVC, left renal vein, intrahepatic IVC). The thrombus bearing right renal vein is transected with Endo GIA™ stapler (vascular load 45 mm). Blood flow cessation within the excluded caval segment is confirmed by laparoscopic Doppler or by needle aspiration. A longitudinal cavotomy is made to excise the intraluminal thrombus en bloc with stapled right renal vein stump/ostium and any involved caval wall. The excised thrombus is immediately entrapped in an Endo Catch™ bag. The cavotomy is suture repaired with single layer running stitch (4-zero Prolene® or 5-zero Gore-Tex®). Care is taken not to narrow the IVC, with at least 50% of the lumen maintained (fig. 4). The IVC is irrigated with heparinized water to eliminate any intracaval clots and air bubbles. Rummel tourniquets are released to restore caval flow. Radical nephrectomy with ipsilateral retroperitoneal lymph node dissection is completed. The entrapped specimens are extracted intact.
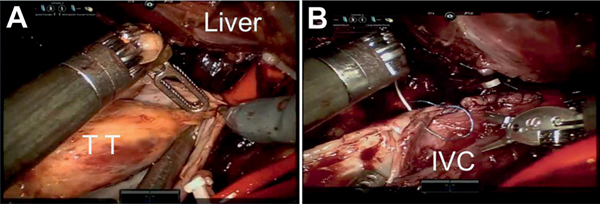
Figure 4.
Level III tumor thrombectomy. Rescue stitch is preplaced on IVC for safety in case emergent control is needed. Rummel tourniquets are secured from distal to proximal. A, cephalad tip of tumor thrombus (TT) being freed. Note: intrahepatic TT extends to within 1 cm of proximal IVC Rummel. B, IVC reconstruction using 4-zero Prolene or 5-zero Gore-Tex suture.
Left Side Thrombus.
Our strategy is caval thrombectomy first and left radical nephrectomy last (fig. 5). Reliable preoperative angioembolization of the left main and any secondary renal arteries is critical. This is important since intraoperatively the left renal vein will be disconnected well before the left renal artery can be robotically secured. During surgery the patient is secured in the right side up position to perform caval thrombectomy first. Important surgical aspects include preservation of right adrenal gland, transient bull-dog clamping of the right renal artery and Rummel control of the right renal vein. After thrombectomy and caval reconstruction the right kidney is revascularized. The patient is then repositioned left side up and the robot re-docked to complete left radical nephrectomy and lymphadenectomy.
Figure 5.
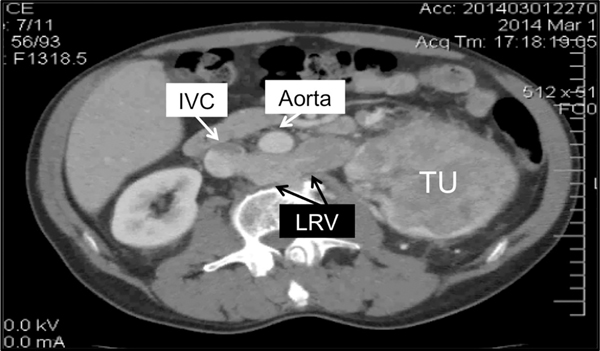
Left side IVC tumor thrombus. CT showing 4.1 cm level III thrombus emanating from large left renal tumor (TU) via retroaortic left renal vein (LRV).
RESULTS
Among 9 level III thrombi 3 were left side (table 2). Mean IVC tumor thrombus length was 5.7 cm (range 4 to 7). In all 9 patients proximal caval control was secured intrahepatically, transecting a median of 2 SH veins (1 to 5) per patient. Median operative time was 4.9 hours (range 4.5 to 6.3), estimated blood loss was 375 cc (range 200 to 7,000) and transfusions were necessary in 3 patients (33%). Pathology revealed negative surgical/vascular margins in all cases. Mean number of lymph nodes retrieved was 8 (range 0 to 15) with a single positive node in 1 patient. There were no intraoperative complications and 1 postoperative complication, a subphrenic abscess requiring percutaneous drainage (Clavien 3b). In 2 patients with completely occlusive thrombus and extensive distal bland thrombus extending into iliac veins we staple transected the cava without reconstruction or hemodynamic sequelae (fig. 6). During a median of 7 months of followup (range 1 to 18) all patients are alive, 8 without evidence of disease. One patient with preexisting lumbar metastasis underwent spinal surgery after IVC thrombectomy.
Table 2.
Level III IVC thrombi
| Median/Mean/No. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Age (yrs) | 59 | 37 | 72 | 63 | 52 | 72 | 67 | 55 | 51 | 61/59.6 |
| Gender | M | M | M | M | M | F | M | M | M | |
| BMI (kg/m2) | 26.4 | 40 | 27.1 | 22 | 35 | 38 | 28 | 22.5 | 31.5 | 27.5/29.8 |
| ASA® class | III | II | III | III | II | III | III | II | II | |
| Renal tumor: | ||||||||||
| Size (cm) | 8.0 | 9.0 | 8.0 | 10.8 | 5.3 | 6.1 | 10.2 | 9.0 | 7.0 | 8.5/8.3 |
| Side | Rt | Lt | Lt | Lt | Rt | Rt | Rt | Rt | Rt | |
| IVC thrombus length (cm) | 5.0 | 6.0 | 4.1 | 5.4 | 7.0 | 7.0 | 4.0 | 7.0 | 7.0 | 5.7/5.8 |
| Preexisting metastasis | No | No | No | No | No | No | Yes | No | No | |
| Neoadjuvant therapy | Tyrosine kinase inhibitor, 3 mos | No | No | No | No | Prior partial nephrectomy | No | No | No | |
| Preop renal embolization | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Proximal IVC control level | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | Intrahepatic | |
| No. hepatic veins taken | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 2 | 5 | 2/2.6 |
| O.R. time (hrs) | 4.7 | 5.7 | 4.5 | 5.6 | 5.0 | 6.3 | 4.7 | 4.8 | 4.5 | 4.9/5.1 |
| Blood loss (cc) | 200 | 500 | 200 | 800 | 7,000* | 1,300 | 200 | 350 | 200 | 375/493 |
| Blood transfusion | No | No | No | Yes (1 u) | Yes (10 u) | Yes (8 u) | No | No | No | |
| Hospital stay (days) | 22 | 5 | 3 | 3 | 4 | 10 | 5 | 3 | 2 | 4.5/6.8 |
| Intraop complication | No | No | No | No | No* | No | No | No | No | |
| Postop complication | Clavien IIIb† | No | No | No | No | No | No | No | No | |
| Histology | RCC | RCC | RCC | RCC | RCC | RCC | RCC | RCC | RCC | RCC (9) |
| Tumor grade | 3 | 3 | 4 | 3 | 2 | 2 | 3 | 3 | ||
| pTNM stage | T3bNxMx | T3bNxMx | T3bN0M0 | T3bNxMx | T3cN0M0 | T3bN0M0 | T3bN0M1 | T3bN0Mx | T3bNoMx | – |
| Mos followup | 18 | 11 | 8 | 8 | 6 | 2 | 1 | 1 | 1 | 7/7.6 |
| Current status | Alive/NED | Alive/NED | Alive/NED | Alive/NED | Alive/NED | Alive/NED | Alive‡ | Alive/NED | Alive/NED |
Patient had a 400 cc blood loss during IVC thrombectomy and radical nephrectomy part of the procedure. Immediately before concluding the procedure, during suctioning of the operative field, a clip on a posterior lumbar vein got dislodged, which was controlled robotically without open conversion, with a 7,000 cc total blood loss.
Subphrenic collection developed requiring percutaneous drainage.
Patient underwent excision of large lumbar spinal metastasis 2 weeks after IVC thrombectomy.
Figure 6.
Robotic IVC transection. Completely occlusive left side level III thrombus. A, patients with completely obstructive thrombi have nonfunctional, no-flow IVC. Since robust collaterals have long been recruited, complete robotic transection of IVC was performed with Endo GIA vascular stapler to prevent embolism of any distal bland thrombus after first cinching intrahepatic IVC and right renal vein tourniquets. B, left renal vein (LRV) transected with vascular stapler. Arrow denotes staple line of transected IVC.
Table 3 presents data for level II thrombi. On comparing level II and III cases (table 4), the latter trended toward somewhat greater thrombus length (median 3.4 vs 5.7 cm), operative time (4.5 vs 4.9 hours), blood loss (280 vs 375 cc), transfusion rate (14% vs 33%) and hospital stay (4.0 vs 4.5 days), with shorter followup (21 vs 7 months). Mean followup for the entire cohort was 15.3 months (range 1 to 39). One patient with pN1 disease is being treated on a clinical trial. Two patients, 1 with a single stable lung metastasis and the other with prior spinal metastasis, are undergoing targeted therapy.
Table 3.
IVC level II thrombi
| Median/Mean | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pt No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Median/Mean |
| Age (yrs) | 66 | 72 | 74 | 65 | 82 | 69 | 36 | 69/66.2 |
| Gender | M | M | M | M | M | M | F | |
| BMI (kg/m2) | 40.3 | 26.7 | 41.9 | 32.2 | 24 | 31 | 28 | 31.6/32.6 |
| ASA class | III | II | III | III | II | II | III | |
| Renal tumor: | ||||||||
| Size (cm) | 11 | 6.5 | 19.5 | 8.7 | 7.2 | 8.5 | 6.7 | 8.5/9.7 |
| Side | Rt | Rt | Lt | Rt | Rt | Lt | Rt | |
| IVC thrombus length (cm) | 2.5 | 3.5 | 2 | 5.3 | 3 | 4.5 | 3.1 | 3.4/3.1 |
| Preexisting metastasis | 2.2 cm Node pericaval | No | No | No | No | No | No | |
| Neoadjuvant therapy | No | No | No | No | No | No | No | |
| Preop renal embolization | Yes | No | No | Yes | Yes | Yes | No | |
| Proximal IVC control level | Suprarenal IVC | Suprarenal IVC | Suprarenal IVC | Suprarenal IVC | Suprarenal IVC | Suprarenal IVC | Suprarenal IVC | |
| No. hepatic veins taken | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0/0.3 |
| O.R. time (hrs) | 4.0 | 4.5 | 6.1 | 5 | 4.5 | 3 | 3.5 | 4.5/4.5 |
| Blood loss (cc) | 400 | 300 | 200 | 280 | 700 | 100 | 100 | 280/297 |
| Blood transfusion | No | Yes | No | No | No | No | No | |
| Hospital stay (days) | 7 | 4 | 8 | 5 | 3 | 4 | 1 | 4/4.6 |
| Tumor histology | RCC | RCC | RCC | RCC | RCC | RCC | RCC | |
| Tumor grade | 2 | 3 | 4 | 3 | 4 | 3 | 2 | |
| TNM stage | T3aN1Mx | T3aN0Mx | T4aNxMx | T3bNxMx | T3bNxMx | T3aN0Mx | T3bNxMx | |
| Mos followup | 39 | 29 | 24 | 18 | 8 | 3 | 1 | 21/20.2 |
| Current status | Alive with Mets | Alive/NED | Alive/NED | Alive/NED | Alive with Mets | Alive/NED | Alive/NED |
Table 4.
IVC level II vs III
| Level II | Level III | |
|---|---|---|
| Median/mean pt age | 69/66.2 | 61/59.6 |
| No. gender: | ||
| M | 6 | 7 |
| F | 1 | 1 |
| Median/mean kg/m2 BMI | 31.6/32.6 | 27.5/29.8 |
| No. ASA class: | ||
| II | 3 | 4 |
| III | 4 | 5 |
| Renal tumor: | ||
| Median/mean cm | 8.5/9.7 | 8.5/8.3 |
| No. rt side | 5 | 6 |
| No. lt side | 2 | 3 |
| Median/mean cm IVC thrombus length | 3.4/3.1 | 5.7/5.8 |
| No. preexisting metastasis: | ||
| No | 6 | 8 |
| Yes | 1 Enlarged node | 1 |
| No. neoadjuvant therapy: | ||
| No | 6 | 7 |
| Yes | 2 (tyrosine kinase inhibitor) | |
| No. preop renal embolization: | ||
| Yes | 4 | 8 |
| No | 3 | 1 |
| No. level of proximal IVC control | 7 (suprarenal IVC) | 9 (retro/intrahepatic IVC) |
| Median/mean hepatic veins taken | 0/0.3 | 2/2.6 |
| Median/mean hrs O.R. time | 4.5/4.5 | 4.9/5.1 |
| Median/mean cc blood loss | 280/297 | 375/493 |
| No. transfusion: | ||
| No | 6 | 6 |
| Yes | 1 | 3 |
| No. no open conversions | 7 | 9 |
| Median/mean days hospital stay | 4/4.6 | 4.5/6.8 |
| No. no intraop complications | 7 | 9 |
| No. postop complications | None | 1 (subphrenic abscess, drained percutaneously) |
| No. tumor histology RCC | 6 | 9 |
| No. tumor grade 2/3/4 | 2/3/2 | 2/5/1 |
| No. TNM stage | T3a N0Mx (2) | T3bN0Mx (7) |
| T3aN1Mx (1) | T3cN0Mx (1) | |
| T3bN0Mx (3) T4aN0Mx (1) |
T3bNoM1 (1) | |
| No. neg surgical margins | 7 | 9 |
| Median/mean mos followup | 21/20.2 | 7/7.6 |
| No. current status: | ||
| Alive/NED | 4 | 8 |
| Alive with Mets | 2 |
DISCUSSION
We have reported the initial clinical series of robotic level III IVC tumor thrombectomy, performed completely intracorporeally. Also included are some of the initial reported cases of robotic left side IVC thrombectomy and robotic IVC transection, procedures with their own unique robotic surgical considerations. No patient was associated with open conversion or mortality.
For level III thrombectomy the surgical approach centers on the IVC, with technical complexity dictated by cranial extent of tumor thrombus. Successful robotic IVC thrombectomy surgery requires thorough knowledge of surgical anatomy, detailed preoperative preparation and meticulous robotic technique. Caval tumor thrombi can grow rapidly. Therefore, imaging (magnetic resonance imaging/CT) should ideally be repeated within 2 weeks of planned surgery. Precise preoperative knowledge of the exact locations, distances and diameters of important, pertinent vascular structures is immensely helpful when working in the magnified robotic intraoperative field. Preoperative renal angioinfarction, albeit controversial due to its potential for inducing perirenal inflammatory reaction,12 was used in selected patients to advantage.
During open surgical IVC thrombectomy, major pulmonary embolism can occur due to intraoperative tumor thrombus dislodgment, an event associated with a 75% mortality rate.13 To minimize the chances of this occurring during robotic surgery, we developed an “IVC-first, kidney-last” technique. The kidney is mobilized only at the end of the case, after the thrombus has been extracted and the IVC repaired. Caval mobilization is performed with a strict “minimal-touch” technique, thus minimizing the chances of inadvertent thrombus manipulation and embolization. When mobilizing the IVC, pericaval tissues are retracted principally, rather than the IVC itself. Based on our own prior open surgical experience, it is our subjective impression that open surgery entails earlier renal, and more extensive caval, manipulation in order to expose the retrocaval space for controlling the lumbar veins. Conversely, during robotic surgery, use of the 30-degree up or down robotic lens and the miniaturized, wristed instrumentation facilitates lumbar vein control with minimal retraction or manipulation of the IVC.
IVC thrombectomy can result in considerable blood loss. Inadvertent hemorrhage can occur during dissection of the renal hilum, a region typically containing multiple newly developed aberrant, large caliber, thin walled, high flow venous collaterals. Also, malignant infiltration of hilar and/or pericaval tissues can lead to considerable oozing, requiring meticulous robotic technique. To minimize the chances of major hemorrhage, we evolved toward a “midline-first, lateral-last” robotic operative strategy, in which the inter-aorto-caval region is dissected first, and dissection of the laterally located renal hilar region is deferred toward the end of the case. Deeply located paraspinal venous collaterals, invisible to the surgical field, also have considerable potential for hemorrhage, as occurred in patient 5, which was nevertheless controlled robotically. Overall we recommend substantial IVC control be secured before any renal hilar manipulation.
Releasing 1 to 5 short hepatic veins, as necessary, allowed us to expose the critical 2 to 3 cm of intrahepatic IVC. Thus, high proximal IVC control could be secured without the need for suprahepatic dissection. After the thrombus bearing IVC segment has been excluded by cinching down the various Rummel tourniquets, the ipsilateral, thrombus bearing renal vein is transected with an Endo GIA vascular stapler. Such transection of the thrombus bearing renal vein is an important maneuver of this robotic operation. It affords 3 specific benefits. 1) It eliminates back-bleeding from the tumor bearing kidney. Since all caval tourniquets are already cinched down, there is no chance of thrombus embolization. 2) Renal vein transection frees the excluded IVC segment, which can be rotated medial-to-lateral to confirm that all posterior venous branches into the IVC segment have been controlled. 3) Immediately upon completion of thrombectomy, the en bloc thrombus and stapled renal vein ostium are entrapped in an impermeable Endo Catch bag, eliminating local spillage.
Occasionally the IVC wall may be directly infiltrated by tumor thrombus, requiring its excision, as occurred in 2 of our cases. We were readily able to locally excise the caval wall and primarily suture repair with minimal luminal narrowing. Larger areas of caval wall involvement would require excision with vein or synthetic patch or interposition grafting. Although we were fully prepared to do this, it was not necessary in any case. In another case the thrombus was friable, yet we could remove it en bloc with delicate sharp dissection off the IVC endothelial surface and immediately entrap in an Endo Catch bag without local spillage. Finally, we developed the robotic technique for left side tumor thrombectomy as described.
Open surgical IVC thrombectomy is one of the most challenging procedures in urological oncology, with a 5% to 10% perioperative mortality rate.1,2 Simultaneous multiorgan access to the kidneys, liver, vena cava and thorax has required a variety of open surgical muscle cutting approaches, such as thoracoabdominal, bilateral subcostal (chevron) with concomitant median sternotomy or extended flank incisions. Major perioperative complications occur in up to 38% of cases, including hemodynamic instability, visceral injury, coagulopathy, hepatic/renal dysfunction, reoperation, sepsis and death.14,15 The Mayo Clinic reported their 30-year experience (1970 to 2000) with open surgical IVC thrombectomy in 540 patients, 77% of whom had level 0-I thrombi and only 5% (28) had level III thrombi.1,13,16 Specifically in patients with level III thrombi, mean O.R. time was 4.6 hours (3–8.3), blood loss was 2.7 liters (0.6–15) and the majority of patients required blood transfusions (mean 9 units per patient, range 0 to 46). There were 30-day complications in 18% of the patients, including intraoperative/perioperative death in 14%.1 Our departmental experience at the University of Southern California revealed 43 patients who underwent open surgical level III thrombectomy during a 32-year period (1978 to 2012). Mean O.R. time was 6.1 hours (3.8–14), mean 14 units of blood were transfused per patient (0–37) and perioperative mortality occurred in 1 patient (2.3%), none since 1990.17 Advances in anesthetic monitoring and use of refined liver transplant, vascular bypass and retroperitoneal surgical techniques have improved outcomes considerably.18 Comparing the 1970 to 1989 era vs the 1990 to 2000 era, early open surgical complications decreased from 13.4% to 8.1% (p=0.06) and operative mortality decreased from 8.1% to 3.8% (p=0.28).1
Our initial experience with robotic IVC level III thrombectomy is encouraging. Median O.R. time, blood loss and hospital stay was 4.9 hours, 375 cc and 4.5 days, respectively. Blood transfusions were required in only 33% of patients (mean 4 units per patient). None of the 16 patients undergoing level II-III thrombectomy had open conversion or mortality. Perioperative efficiency and outcomes in patients with level III thrombi were only marginally different from those in patients with level II thrombi (table 4).
To date, 78 cases of minimally invasive IVC thrombectomy have been reported during the last 15 years.8 Of these, most (67%) have been level I thrombi and most (91%) were performed with hand-assisted or straight laparoscopic surgery, occasionally involving open surgical control of the IVC. Robotic level II thrombectomy was recently reported in 5 cases7 and level III thrombectomy was presented in abstract form.9,10
The potential exists for robotic IVC surgery to be extended further. We recently reported the initial case of total thoracoscopic control of the intrapericardial supradiaphragmatic vena cava for a high level III thrombus.19 Vascular control (supradiaphragmatic IVC, porta hepatis, infrarenal IVC, left renal vein) was achieved minimally invasively (fig. 7). Given the large size of the intrahepatic thrombus, inferior vena cavectomy, juxtahepatic suture closure of the IVC and right radical nephrectomy were completed in open surgery.19 More recently in the perfused cadaveric model we developed an exclusively transabdominal robotic technique for infradiaphragmatic control of the suprahepatic IVC, thus providing a possible alternative to transthoracic IVC control (unpublished data).
Figure 7.
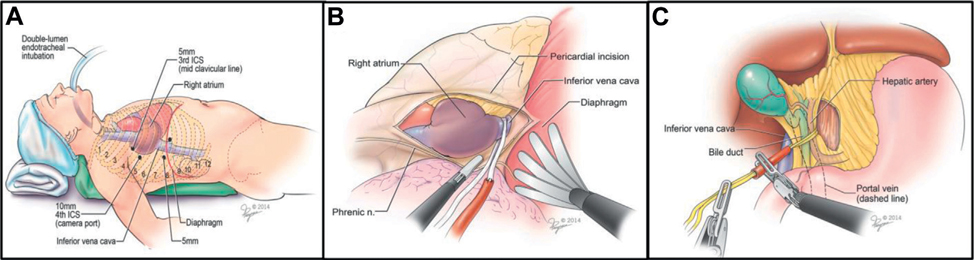
Control of suprahepatic IVC and porta hepatis. A, port placement for transthoracic control of IVC. After selective endotracheal intubation 4 transthoracic ports are placed into right hemithorax. To optimize operative exposure low pressure CO2 is insufflated. B, completely thoracoscopic control of intrapericardial IVC. Pericardium is incised anterior to phrenic nerve. Diaphragm is retracted caudally with fan retractor to enable blunt retrocaval dissection. Umbilical tape is positioned around the intrapericardial IVC. C, Pringle maneuver. Using transabdominal ports, retroperitoneum is incised medial to IVC and lateral to hepatoduodenal ligament. Window is created posterior to porta hepatis into lesser omental sac and Rummel tourniquet is positioned. Note: patient had large, infiltrating, completely occluding, high intrahepatic tumor thrombus in nonfunctional IVC. Using techniques depicted in parts A to C, vascular control was exclusively achieved minimally invasively. Subsequently, inferior venacavectomy, juxtahepatic suture closure of IVC and radical nephrectomy were completed with open conversion.19
Shortcomings of this study include the small number of patients and limited followup. Careful comparison with open surgical IVC thrombectomy is lacking and warranted to determine the proper place of robotic surgery in this arena. It is emphasized that robotic vena cava surgery is an advanced undertaking that requires considerable robotic experience.
CONCLUSIONS
We have reported the initial series of robotic level III inferior vena cava thrombectomy for kidney cancer. All necessary surgical maneuvers could be performed completely robotically without open conversion or mortality. This demonstration of efficient robotic performance of the challenging vascular, oncologic and reconstructive procedures inherent herein opens the door for major renal, caval and hepatic robotic surgeries in the future. Although our experience is only initial, we believe that robotic IVC thrombus surgery has considerable potential for the future.
Abbreviations and Acronyms
- BMI
body mass index
- CSS
cancer specific survival
- CT
computerized tomography
- IVC
inferior vena cava
- Mets
metastasis
- NED
no evidence of disease
- O.R.
operating room
- RCC
renal cell carcinoma
- SH
short hepatic
REFERENCES
- 1.Blute ML, Leibovich BC, Lohse CM et al. : The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004; 94: 33. [DOI] [PubMed] [Google Scholar]
- 2.Al Otaibi M, Abou Youssif T, Alkhaldi A et al. : Renal cell carcinoma with inferior vena caval extension: impact of tumour extent on surgical outcome. BJU Int 2009; 104: 1467. [DOI] [PubMed] [Google Scholar]
- 3.Neves RJ and Zincke H: Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987; 59: 390. [DOI] [PubMed] [Google Scholar]
- 4.Fergany AF, Gill IS, Kaouk JH et al. : Laparoscopic radical nephrectomy with level II vena caval thrombectomy: survival porcine study. J Urol 2002; 168: 2629. [DOI] [PubMed] [Google Scholar]
- 5.Meraney AM, Gill IS, Desai M et al. : Laparoscopic inferior vena cava and right atrial thrombectomy utilizing deep hypothermic circulatory arrest. J Endourol 2003; 17: 275. [DOI] [PubMed] [Google Scholar]
- 6.Desai MM, Gill IS, Ramani AP et al. : Laparoscopic radical nephrectomy for cancer with level I renal vein involvement. J Urol 2003; 169: 487. [DOI] [PubMed] [Google Scholar]
- 7.Abaza R: Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol 2011; 59: 652. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, de Castro Abreu AL and Gill IS: Robotic inferior vena cava thrombus surgery: novel strategies. Curr Opin Urol 2014; 24: 140. [DOI] [PubMed] [Google Scholar]
- 9.Abreu AL, Azhar R, Chopra S et al. : Robotic level 3 cava thrombectomy. J Urol, suppl, 2014; 191: e735, abstract V7–02. [Google Scholar]
- 10.Cheng J and Bratslavsky G: The first report of robotic assisted radical nephrectomy with retrohepatic vena caval tumor thrombectomy and extended retroperitoneal lymph node dissection. J Urol, suppl, 2014; 191: e618. [DOI] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N and Clavien PA: Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian VS, Stephenson AJ, Goldfarb DA et al. : Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology 2009; 74: 154. [DOI] [PubMed] [Google Scholar]
- 13.Shuch B, Crispen PL, Leibovich BC et al. : Cardiopulmonary bypass and renal cell carcinoma with level IV tumour thrombus: can deep hypothermic circulatory arrest limit perioperative mortality? BJU Int 2011; 107: 724. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney P, Wood CG, Pisters LL et al. : Surgical management of renal cell carcinoma associated with complex inferior vena caval thrombi. Urol Oncol 2003; 21: 327. [DOI] [PubMed] [Google Scholar]
- 15.Kaag MG, Toyen C, Russo P et al. : Radical nephrectomy with vena caval thrombectomy: a contemporary experience. BJU Int 2011; 107: 1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearnes RJ and Blute ML: Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol 2008; 5: 329. [DOI] [PubMed] [Google Scholar]
- 17.Patil MB, Montez J, Loh-Doyle J et al. : Level III-IV inferior vena caval thrombectomy without cardiopulmonary bypass: long-term experience with intrapericardial control. J Urol 2014; 192: 682. [DOI] [PubMed] [Google Scholar]
- 18.Spiros D, Dervenis C, Lytras D et al. : Liver transplantation techniques with preservation of the natural venovenous bypass: effect on surgical resection of renal cell carcinoma invading the inferior vena cava. World J Surg 2004; 28: 614. [DOI] [PubMed] [Google Scholar]
- 19.Hui DS, Gill IS and Cunningham MJ: Minimally invasive approach to the supradiaphragmatic inferior vena cava: total thoracoscopic caval isolation. Innovations 2014; 9: 145. [DOI] [PubMed] [Google Scholar]