Abstract
Susceptibilities to 13 antimicrobial agents were determined by measurement of MICs for 60 isolates of Streptococcus bovis from blood cultures. Thirty-eight isolates (63.3%) had high-level resistance to erythromycin (MICs, ≥128 μg/ml). Among the 38 erythromycin-resistant strains, 21 isolates (55%) had inducible resistance to macrolides-lincosamides-streptogramin B (iMLS isolates) and 17 (45%) had constitutive resistance to macrolides-lincosamides-streptogramin B (cMLS isolates). Tetracycline resistance was also found among all of the erythromycin-resistant strains. None of the strains displayed resistance to penicillin, chloramphenicol, or vancomycin. Detection of erythromycin resistance genes by PCR and sequencing indicated that all 17 cMLS isolates were positive for the ermB gene and that 7 of 21 iMLS isolates carried the ermB gene and the remaining 14 iMLS isolates carried the ermT gene. Sequence analysis of amplified partial ermB fragments (594 bp) from S. bovis isolates revealed a 99.8% nucleotide identity and a 100% amino acid homology compared with the sequences from gene banks. The sequences of amplified fragments with primers targeted for ermC were shown to be very similar to that of ermGT (ermT) from Lactobacillus reuteri (98.5% nucleotide identity). This is the first report to describe the detection of the ermT class of erythromycin resistance determinants in S. bovis. The high rate of inducible erythromycin resistance among S. bovis isolates in Taiwan was not reported before. The iMLS S. bovis isolates were shown to be heterogeneous by randomly amplified polymorphic DNA analysis. These results indicate that the prevalence of inducible erythromycin resistance in S. bovis in Taiwan is very high and that most of the resistant strains carry the ermT or the ermB gene.
Streptococcus bovis is a group D streptococcus frequently found as part of the commensal bowel flora in humans and animals. This organism is recognized as a cause of endocarditis in elderly people and an uncommon cause of septicemia and meningitis in newborn infants (2, 10, 19). Bacteremia due to S. bovis has been reported to be associated mostly with underlying colonic neoplasms and to a lesser extent with gastrointestinal tract and oropharyngeal carcinoma (2). Clinical isolates of S. bovis are usually susceptible to penicillin. However, macrolides and related drugs have been suggested as alternatives for treatment of streptococcal infections when the patient is allergic to penicillin.
High rates of erythromycin resistance have been recognized among streptococci in Taiwan since the mid-1990s (3, 12, 34, 35). Recent studies found erythromycin resistance in 23.5 to 81.3% of streptococci in Taiwan, depending on the species (12, 34, 35). Two major mechanisms account for erythromycin resistance in many gram-positive bacteria: target site modification and active efflux (16, 26, 30). Target site modification, generally known as macrolide-lincosamide-streptogramin B (MLS) resistance, is mediated by Erm methylases, which methylate 23S rRNA and induce ribosome modification. Expression of MLS resistance in streptococci can be either constitutive or inducible (11, 16, 27). Macrolide efflux, which is effected by a membrane protein encoded by the mefA or the mefE gene, has been reported in group A streptococci, Streptococcus pneumoniae, and other streptococci (1, 4, 22, 32).
The objectives of the present study were to determine the incidence and patterns of antimicrobial resistance among S. bovis isolates that cause significant infections and to classify the phenotypes and genotypes of erythromycin-resistant strains.
MATERIALS AND METHODS
Bacterial strains.
A total of 60 nonredundant isolates of S. bovis isolated from cultures of blood from distinct patients between 1996 and 2000 were collected from the Bacteriology Laboratory, National Taiwan University Hospital, a 2,000-bed teaching hospital in northern Taiwan. The S. bovis isolates were identified to the species level by conventional methods as well as with a commercial identification system, the API 20 Strep system (bioMérieux Vitek, Inc., Hazelwood, Mo.). The S. bovis isolates were differentiated from enterococci and other viridans group streptococci by growth on bile esculin medium and at 45°C but not in 6.5% NaCl or at 10°C and by urease negativity (6). With the API system, the S. bovis isolates were further identified as biotype I, II/1, or II/2 (5, 6).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing of the isolates was performed by a standard agar dilution method according to the guidelines established by the National Committee for Clinical Laboratory Standards (20). The isolates were grown on Trypticase soy agar plates supplemented with 5% sheep blood at 37°C. Bacterial inocula were prepared by suspending the grown bacteria in normal saline and adjusting the suspension to a 0.5 McFarland standard. By using a Steers replicator, an organism density of 104 CFU/spot was inoculated onto Mueller-Hinton agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.) supplemented with 5% sheep blood with various concentrations of antimicrobial agents, and the plate was then incubated aerobically at 35°C for 24 h.
The following antimicrobial agents were obtained as standard reference powders of known potency for laboratory use: penicillin, erythromycin, clindamycin, tetracycline, chloramphenicol, gentamicin, and vancomycin, from Sigma Chemical Co. (St. Louis, Mo.); cefotaxime, from Hoechst AG (Frankfurt, Germany); imipenem, from Merck Sharp & Dohme (West Point, Pa.); clarithromycin, from Abbott Laboratories (North Chicago, Ill.); quinupristin-dalfopristin, from Rhone-Poulenc Rorer (Amstelveen, The Netherlands); ciprofloxacin, from Bayer Corporation (West Haven, Conn.); and teicoplanin, from Marion Merrill Dow (Kansas City, Mo.). Staphylococcus aureus ATCC 29213 and S. pneumoniae ATCC 49619 were used as control organisms in each batch.
Double-disk test.
The conventional double-disk induction test was performed to test the erythromycin resistance phenotypes (28). Erythromycin resistance was classified on the basis of the double-disk test with erythromycin and clindamycin. The disks were placed 15 to 20 mm apart on Mueller-Hinton agar (Becton Dickinson Microbiology Systems) supplemented with 5% sheep blood, and the plates were incubated aerobically at 35°C for 18 h. Blunting of the clindamycin inhibition zone proximal to the erythromycin disk indicated an inducible type of MLS resistance (iMLS), and resistance to both erythromycin and clindamycin indicated a constitutive type of resistance (cMLS). Susceptibility to clindamycin with no blunting indicated the M phenotype (30).
Detection of erythromycin resistance genes.
DNA samples from S. bovis isolates were prepared with a DNA isolation kit (Puregene; Gentra Systems, Inc., Minneapolis, Minn.), according to the manufacturer's instructions. The DNAs of the erythromycin-resistant isolates were amplified with primers specific for the ermA, ermB, ermC, ermTR, and mef genes (29, 31). Amplification reactions were performed in volumes of 50 μl containing 1× PCR buffer, each deoxynucleoside triphosphate at a concentration of 0.2 μM, 2 mM MgCl2, 1 pmol of each primer, and 1 U of Taq polymerase. PCR products were resolved by electrophoresis on 1.5% agarose gels. The expected amplicons were 640 bp for ermA, ermB, and ermC; 530 bp for ermTR; and 348 bp for mef. Another pair of primers that targeted ermC was also used (17). The tetracycline resistance genes of tet(M) and the int gene were also examined by PCR (9, 21).
RAPD analysis.
The preparation of the isolates for randomly amplified polymorphic DNA (RAPD) analysis, the DNA for which was generated by arbitrarily primed PCR, was performed as described previously (13). The following two arbitrary oligonucleotide primers were used: primer OP-H2 (5′-TCGGACGTGA-3′) and primer OP-H7 (5′-CTGCATCGTG-3′) (Operon Technologies, Inc., Alameda, Calif.). The amplification products were electrophoresed in a 1.2% agarose gel. Isolates which differed by two or more major bands were considered to have different patterns.
RESULTS
Biotype, antimicrobial susceptibility patterns, and prevalence of erythromycin resistance.
With the API 20 Strep system, the S. bovis isolates were divided into three subtypes. Among the 60 isolates tested, 4 biotype I, 3 biotype II/1, and 53 biotype II/2 isolates were identified. The four biotype I isolates and the three biotype II/1 isolates were all susceptible to erythromycin. The results of testing of the susceptibilities to 13 antibiotics obtained by the agar dilution method are shown in Table 1. The S. bovis isolates studied were all susceptible to penicillin, cefotaxime, imipenem, chloramphenicol, vancomycin, and teicoplanin. However, of the 60 S. bovis isolates tested, 38 (63%) isolates were highly resistant to two macrolides tested, erythromycin and clarithromycin. The MIC ranges, the MICs at which 50% of isolates are inhibited (MIC50s), and the MIC90s of these two macrolides were similar. The MIC50s of erythromycin and clarithromycin were >512 and 256 μg/ml, respectively, and the MIC90s of erythromycin and clarithromycin were ≥512 μg/ml. The MIC50 of clindamycin was 0.06 μg/ml, which was much lower than those of erythromycin and clarithromycin. The activity of quinupristin-dalfopristin was not high, with an MIC50 of 4 μg/ml and an MIC90 of 8 μg/ml. The rate of tetracycline resistance was 75%.
TABLE 1.
Susceptibilities of 60 clinical isolates of S. bovis to various antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)
|
% Resistant | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Penicillin | ≤0.03–0.12 | 0.06 | 0.12 | 0 |
| Imipenem | ≤0.03–≤0.03 | ≤0.03 | ≤0.03 | 0 |
| Cefotaxime | ≤0.03–0.5 | ≤0.03 | 0.25 | 0 |
| Erythromycin | 0.03–>512 | >512 | >512 | 63.3 |
| Clarithromycin | ≤0.03–>512 | 256 | 512 | 63.3 |
| Clindamycin | 0.03–256 | 0.06 | 256 | 28.3,a 35b |
| Quinupristin-dalfopristin | 1–16 | 4 | 8 | NAc |
| Tetracycline | 0.5–64 | 64 | 64 | 75 |
| Chloramphenicol | 1–2 | 2 | 2 | 0 |
| Gentamicin | 1–32 | 2 | 4 | NA |
| Ciprofloxacin | 1–4 | 2 | 4 | NA |
| Vancomycin | 0.25 | 0.25 | 0.25 | 0 |
| Teicoplanin | 0.06–0.5 | 0.25 | 0.5 | 0 |
For cMLS isolates.
For iMLS isolates.
NA, no interpretive NCCLS breakpoints are available.
Resistance phenotypes.
Since the incidence of erythromycin resistance was high in the S. bovis isolates tested, the double-disk test was performed to determine the resistance phenotypes. Two phenotypes were observed among the 38 erythromycin-resistant isolates. Twenty-one strains (55%) presented an inducible phenotype (iMLS isolates) with typical blunting of the clindamycin zone of inhibition, and 17 (45%) exhibited the constitutive phenotype (cMLS isolates). None of the strains displayed the M phenotype.
Erythromycin resistance determinants.
To understand the mechanism of erythromycin resistance, macrolide resistance genes were detected by PCR and sequencing. Amplification with the primers specific for ermB revealed that 17 cMLS isolates and 7 iMLS isolates contained this gene. Sequence analysis of amplified partial ermB fragments (594 bp, not including the primers) from four randomly selected resistant isolates revealed 99.8% identity (at the DNA level) and 100% amino acid homology with published sequences of ErmB from Streptococcus or Enterococcus. None of the isolates contained the ermA, ermC, ermTR, or mef gene. However, amplification with one pair of primers that initially targeted ermC (17) was positive for 14 of 21 iMLS isolates. These amplification products were subsequently sequenced but were found to be more similar to ermGT from Lactobacillus reuteri (98.5% of nucleotides identical) than to ermC (77.5% identity).
Since all the erythromycin-resistant isolates were also resistant to tetracycline, tests for the detection of tet(M) and int genes were also performed. The results revealed that all the erythromycin-resistant isolates contained tet(M) and int genes.
RAPD patterns.
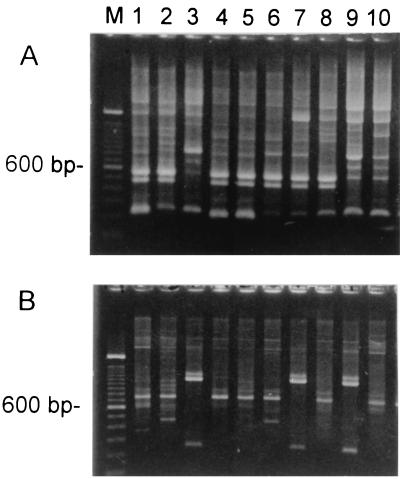
To understand whether the high prevalence of inducible erythromycin resistance of S. bovis was due to clonal spread, RAPD analysis was performed with 21 iMLS isolates. The results are partially shown in Fig. 1. The isolates in lanes 4 and 5 were considered to have the same RAPD patterns. The isolates in the other lanes were considered different. Eighteen RAPD patterns were identified with two different primers. These results indicate that these isolates correspond to a heterogeneous population rather than the expansion of a single clone.
FIG. 1.
RAPD patterns of S. bovis isolates obtained with two primers. Lane M, molecular size marker (100-bp ladder; Gibco BRL, Gaithersburg, Md.). (A) Primer OP-H2; (B) primer OP-H7. The isolates in lanes 4 and 5 were considered to have the same RAPD patterns. The isolates in the other lanes were considered different.
DISCUSSION
Introduction of erythromycin into clinical practice was rapidly followed by resistance to MLS antibiotics. A high incidence of erythromycin-resistant streptococci in Taiwan has been recognized since the mid-1990s. In the present study, the incidence of resistance to erythromycin among the S. bovis isolates tested was also very high. Only 36.7% of isolates were fully erythromycin susceptible, and the remaining isolates (63.3%) had high-level resistance to erythromycin. The rate of resistance to erythromycin among S. bovis isolates in Taiwan was similar to those for other streptococci. Therefore, it is of concern that S. bovis, like other streptococci, exhibits a high level of resistance to macrolides, making them of limited use for empirical therapy. S. bovis isolates were found to be more resistant to erythromycin and clindamycin than other streptococcal species in Denmark (25). The susceptibility to quinupristin-dalfopristin was also tested in the present study. For clinical use, quinupristin-dalfopristin may be an alternative in patients with endocarditis due to streptococci. However, the MICs of quinupristin-dalfopristin for the S. bovis isolates were shown to be high. A study of antimicrobial susceptibility among viridans group streptococci in The Netherlands in 1997 revealed that the activity of quinupristin-dalfopristin is somewhat dependent on the species, with S. bovis being the least susceptible (18). Although vancomycin-resistant S. bovis isolates have been reported (23), our results indicated that all isolates remained susceptible to vancomycin and teicoplanin.
In the present study, the prevalence of the iMLS phenotype among S. bovis isolates was much higher than that among other streptococci in Taiwan. The iMLS phenotype was not common in other streptococcal species in Taiwan. For example, the incidence of the iMLS phenotype was only 3.1% among Streptococcus pyogenes isolates (36). The incidence of iMLS phenotypes in other streptococci was also very low (22). Our S. bovis isolates were predominantly biotype II/2. This is in agreement with a recent report from Clarridge et al. (5). Among our 60 isolates, only 4 biotype I isolates were recovered, and these 4 biotype I isolates were all susceptible to erythromycin. Since all 38 erythromycin-resistant isolates were biotype II/2, more data are needed to obtain a final conclusion as to whether isolates of biotype II/2 are more resistant. The RAPD patterns of isolates with the iMLS phenotype suggest that these isolates correspond to a heterogeneous population rather than the expansion of a single clone. Since the RAPD analysis results were preliminary, pulsed-field gel electrophoresis might be further performed for epidemiological study.
This is the first report characterizing the ermT class of erythromycin resistance determinants in S. bovis. The ermA, ermC, ermTR, and mef genes were not detected in any of our S. bovis isolates. Detection of erythromycin resistance genes showed that target site modification mediated by the ermB or ermT gene was the most common mechanism responsible for erythromycin resistance in S. bovis isolates in Taiwan. Although the PCR result obtained with one pair of ermC-specific primers was positive, the sequence data revealed that the sequence of the amplification product was more similar to that of ermT. The nucleotides of the amplified fragment from S. bovis obtained with primers based on the ermC sequence were shown to be 98.5% identical to those of ermGT from L. reuteri (33). Recently, Roberts et al. (26) suggested a new nomenclature for naming of the MLS genes and proposed the use of a single letter rather than the two-letter designations presently used in the literature. The ermT includes ermGT, and ermA includes ermTR. Differences in the prevalence of erm gene classes in streptococci have been reported. The ermTR (ermA) gene has been found to be predominant among iMLS group A streptococcus isolates in Finland (100%) (14) and Canada (100%) (8). The ermTR gene has also been found in group A streptococcus isolates in Taiwan, although there were only four iMLS isolates (36). This finding indicates that the distribution of resistance genes varies among species.
Since the erythromycin-resistant isolates were also revealed to be resistant to tetracycline, the isolates were tested for the presence of the tet(M) and int genes by PCR. The association of tet(M) with the integrase gene was found in all but one of the tetracycline-resistant S. bovis strains. Many of the erm genes are associated with conjugative or nonconjugative transposons. Several tet(M)-containing conjugative transposons have been described in streptococci (including S. bovis), enterococci, and staphylococci (7, 21). It has been reported that Tn916 and Tn1545 are associated with resistance to tetracycline as well as resistance to other drugs (9). In the present study, tet(M) and the integrase gene were detected in most ermB- or ermT-containing strains. However, more work will be necessary to determine the nature of the resistance element, which possibly contains a transposon, on which the integrase gene is located.
High rates of erythromycin resistance among streptococci and other organisms in Taiwan have been reported previously (3, 34, 35). Because S. bovis isolates are part of the normal flora of humans, they are challenged by every antibiotic treatment, and selection of highly resistant strains may occur. Although S. bovis displayed some phenotypic resemblance to other viridans group streptococci (6), the phylogeny determined by analysis of several genes showed that S. bovis has a unique trait among streptococcal species (15, 24). The presence of ermB and ermT in S. bovis suggests that interactions between streptococci and other organisms might exist. Although erythromycin is not widely used for the treatment of S. bovis infections, the significance of the high prevalence of macrolide resistance in S. bovis isolates cannot be ignored.
ACKNOWLEDGMENT
This work was supported by grant NSC 88-2314-B-002-240 from the National Science Council of Taiwan.
REFERENCES
- 1.Arpin C, Daube H, Tessier F, Quentin C. Presence of mefA and mefE genes in Streptococcus agalactiae. Antimicrob Agents Chemother. 1999;43:944–946. doi: 10.1128/aac.43.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballet M, Gevigney G, Gare J P, Delahaye F, Etienne J, Delahaye J P. Infective endocarditis due to Streptococcus bovis, a report of 53 cases. Eur Heart J. 1995;16:1975–1980. doi: 10.1093/oxfordjournals.eurheartj.a060856. [DOI] [PubMed] [Google Scholar]
- 3.Chang S C, Chen Y C, Luh K T, Hsieh W C. Macrolides resistance of common bacteria isolated from Taiwan. Diagn Microbiol Infect Dis. 1995;23:147–154. doi: 10.1016/0732-8893(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 4.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Clarridge J E, III, Attorri S M, Zhang Q, Bartell J. 16S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J Clin Microbiol. 2001;39:1549–1552. doi: 10.1128/JCM.39.4.1549-1552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coykendall A. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989;2:315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David F, de Cespedes G, Delbos F, Horaud T. Diversity of chromosomal genetic elements and gene identification in antibiotic-resistant strains of Streptococcus pneumoniae and Streptococcus bovis. Plasmid. 1993;29:147–153. doi: 10.1006/plas.1993.1017. [DOI] [PubMed] [Google Scholar]
- 8.De Azavedo J C S, Yeung R H, Bast D J, Duncan C L, Norgia S B, Low D E. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario. Antimicrob Agents Chemother. 1999;43:2144–2147. doi: 10.1128/aac.43.9.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Barbeyrac B, Dupon M, Rodriguez P, Renaudin H, Bebear C. A Tn1545-like transposon carries the tet(M) gene in tetracycline resistant strains of Bacteroides ureolyticus as well as Ureaplasma urealyticum but not Neisseria gonorrhoeae. J Antimicrob Chemother. 1996;37:223–232. doi: 10.1093/jac/37.2.223. [DOI] [PubMed] [Google Scholar]
- 10.Grant R J, Whitehead T R, Orr J E. Streptococcus bovis meningitis in an infant. J Clin Microbiol. 2000;38:462–463. doi: 10.1128/jcm.38.1.462-463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horinouchi S, Byeon W H, Weisblum B. A complex attenuator regulates inducible resistance to macrolides, lincosamides, and streptogramin type B antibiotics in Streptococcus sanguis. J Bacteriol. 1983;154:1252–1262. doi: 10.1128/jb.154.3.1252-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh P R, Chen H M, Huang A H, Wu J J. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239–2242. doi: 10.1128/aac.39.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsueh P R, Teng L J, Lee L N, Yang P C, Ho S W, Luh K T. Dissemination of high-level penicillin-, extended-spectrum cephalosporin-, and erythromycin-resistant Streptococcus pneumoniae clones in Taiwan. J Clin Microbiol. 1999;37:221–224. doi: 10.1128/jcm.37.1.221-224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataja J, Huovinen P, Skurnik M, Seppala H The Finnish Study Group for Antimicrobial Resistance. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura Y, Hou X, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lina G, Quaglia A, Reverdy M E, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouton J W, Endtz H P, den Hollander J G, van den Braak N, Verbrugh H A. In-vitro activity of quinupristin/dalfopristin compared with other widely used antibiotics against strains isolated from patients with endocarditis. J Antimicrob Chemother. 1997;39(Suppl. A):75–80. doi: 10.1093/jac/39.suppl_1.75. [DOI] [PubMed] [Google Scholar]
- 19.Muhlemann K, Graf S, Tauber M G. Streptococcus bovis clone causing two episodes of endocarditis 8 years apart. J Clin Microbiol. 1999;37:862–863. doi: 10.1128/jcm.37.3.862-863.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: tenth informational supplement, M100–S10 (M7). Wayne, Pa: National Committee for Laboratory Standards; 2000. [Google Scholar]
- 21.Olsvik B, Olsen I, Tenover F C. Detection of tet(M) and tet(O) using the polymerase chain reaction in bacteria isolated from patients with periodontal disease. Oral Microbiol Immunol. 1995;10:87–92. doi: 10.1111/j.1399-302x.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 22.Ono T, Shiota S, Hirota K, Nemoto K, Tsuchiya T, Miyake Y. Susceptibilities of oral and nasal isolates of Streptococcus mitis and Streptococcus oralis to macrolides and PCR detection of resistance genes. Antimicrob Agents Chemother. 2000;44:1078–1080. doi: 10.1128/aac.44.4.1078-1080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob Agents Chemother. 1997;41:24–29. doi: 10.1128/aac.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol. 1998;36:41–47. doi: 10.1128/jcm.36.1.41-47.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renneberg J, Niemann L L, Gutschik E. Antimicrobial susceptibility of 278 streptococcal blood isolates to seven antimicrobial agents. J Antimicrob Chemother. 1997;39:135–140. doi: 10.1093/oxfordjournals.jac.a020858. [DOI] [PubMed] [Google Scholar]
- 26.Roberts M C, Sutcliff J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolides and macrolides-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosato A, Vicarini H, Leclercq R. Inducible or constitutive expression of resistance in clinical isolates of streptococci and enterococci cross-resistant to erythromycin and lincomycin. J Antimicrob Chemother. 1999;43:559–562. doi: 10.1093/jac/43.4.559. [DOI] [PubMed] [Google Scholar]
- 28.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 29.Seppälä H, Skuruik M, Soini H, Roberts M C, Huovinen P. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob Agents Chemother. 1998;42:257–262. doi: 10.1128/aac.42.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannock G W, Luchansky J B, Miller L, Connell H, Thode-Andersen S, Mercer A A, Klaenhammer T R. Molecular characterization of a plasmid-borne (pGT633) erythromycin resistance determinant (ermGT) from Lactabacillus reuteri 100-63. Plasmid. 1994;31:60–71. doi: 10.1006/plas.1994.1007. [DOI] [PubMed] [Google Scholar]
- 34.Teng L J, Hsueh P R, Chen Y C, Ho S W, Luh K T. Antimicrobial susceptibility of viridans group streptococci in Taiwan with an emphasis on the high rates of resistance to penicillin and macrolides in Streptococcus oralis. J Antimicrob Chemother. 1998;41:621–627. doi: 10.1093/jac/41.6.621. [DOI] [PubMed] [Google Scholar]
- 35.Wu J J, Lin K Y, Hsueh P R, Liu J W, Pan H I, Sheu S M. High incidence of erythromycin-resistant streptococci in Taiwan. Antimicrob Agents Chemother. 1997;41:844–846. doi: 10.1128/aac.41.4.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J J, Wu H M, Huang A H, Fu H M, Lee C T, Wu J J. Prevalence of polyclonal mefA-containing isolates among erythromycin-resistant group A streptococci in southern Taiwan. J Clin Microbiol. 2000;38:2475–2479. doi: 10.1128/jcm.38.7.2475-2479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]