Abstract
Low-income countries (LICs) and lower-middle-income countries (LMICs) are still deprived of the optimum doses of coronavirus disease 2019 (COVID-19) vaccines for their population, equal access and distribution, as well as mass immunization roadmaps to be implemented for achieving herd immunity and protection from the ongoing pandemic. In this short report, we are interacting with the world public health experts, as well as national and global leaders for warranting the mass vaccination drive to be more progressive against COVID-19 with equitable access of vaccines to LICs or LMICs to save the lives of the poorest country people and refugees. From several scientific databases, such as Google Scholar, PubMed, as well as national and international news websites, the data were collected data by utilizing appropriate keywords regarding the topic. Bangladesh might be exemplified in this brief communication as the representative of LMIC. As of October 14, 2021, 48% of the world's people have received at least one dose of the COVID-19 vaccine. In contrast, only 2.5% of people from LICs have come in under COVID-19 vaccination for at least a single shot. Both LICs and LMICs need far more vision and ambition, including political, administrative, and diplomatic progress along with enhancing the vaccination drive for their population to be immunized through simultaneous mass vaccination progress of other countries with implementing public health safety measures against the COVID-19 pandemic.
Keywords: Bangladesh, COVID-19 outbreak, Lower-middle-income country (LMIC), Successful mass vaccination
1. Introduction
Since March 8, 2020, Bangladesh, the representative of a lower-middle-income country (LMIC), has been experiencing the adverse effects of severe acute respiratory coronavirus 2 (SARS-CoV-2) infection that caused the catastrophic coronavirus disease 2019 (COVID-19) pandemic worldwide ( 1 , 2 ). As of October 15, 2021, the country witnessed 1.56 million cumulative infected cases with 27,737 deaths due to this deadly viral outbreak, while nearly 240 million confirmed COVID-19 cases and 4.9 million deaths had been reported worldwide ( 3 ). Utilizing several vaccine platforms and high advancements in science led to the current availability of few of the COVID-19 vaccines with varied efficacies and protection levels in vaccinated people, while other vaccines are under clinical trials, and presently vaccine drive is progressively moving ahead across many countries to vaccinate more significant proportions of their population at the earliest feasible time ( 4 - 7 ). Several drugs, therapies, immunomodulatory agents, and supportive therapies are also being employed in emergency purposes to alleviate the clinical severity and lessening death rates in COVID-19 patients; however, any effective drugs and treatments are still awaited, though continuous research and trials are being performed to find out the choice of the therapeutic module ( 8 - 13 ).
Seeing the continued ongoing threats of the COVID-19 pandemic waves, some challenges are needed to be handled appropriately, including the development of more efficacious vaccines, addressing concerning safety issues of post-vaccination adverse effects, breaking vaccine hesitancy, diplomacy and equitable access to all the countries, ramping up vaccine production, affordability and deployment, designing strategies to tackle the threats being posed by emerging SARS-CoV-2 variants and mutants on vaccine efficacy, checking vaccine breakthrough events with vaccinated people getting infected with the virus or its variants, and enhancing global vaccination drive to the optimum desired levels of achieving herd immunity and protecting the global population against COVID-19 ( 14 - 23 ). This study has briefly pointed out several potential factors, including hesitancy towards the COVID-19 vaccine, which might be the barrier to mass vaccination during the current COVID-19 pandemic situation in many low-income (LICs) and middle-income countries or lower-middle countries (LMICs), such as Bangladesh. In addition, a tentative roadmap and several practical recommendations were reported to properly achieve the mass vaccination drive by subduing its potential challenges in LICs and LMICs.
2. Materials and Methods
Several electronic scholarly databases, such as Google Scholar, Semantic Scholar, PubMed, Science Direct, Springer Link, Scopus, and Europe PMC, have been searched utilizing several keywords, alone or in combination, including “COVID-19 pandemic”, “COVID-19 vaccine”, “low-income countries (LICs)”, “lower-middle-income countries (LMICs)”, “mass vaccination”, “mass immunization”, “herd immunity”, “vaccine hesitancy”, “vaccination challenges”, “vaccine affordability”, and “Bangladesh” to find out the relevant articles regarding the topic. Several national and international news websites were also utilized to collect the data or information. The data published from the last end of 2020 to September 2021 were scrutinized and finalized for writing this scientific report.
3. Results
3.1. Vaccine Hesitancy and Herd Immunity Status in LMICs
Amid the ongoing global COVID-19 vaccination drive efforts, safe and effective vaccines must be required broadly for all age groups to substantially reduce the mortality and morbidity from this deadly SARS-CoV-2 infection. In addition, there should be equitable access to the vaccine to all the nations and for all classes of people, including health workers, to ascertain mass immunological protection levels for a majority of the population to restrain the further spread of this pandemic virus and also protect non-vaccinated people via achieving herd immunity ( 24 - 26 ).
In this regard, vaccine hesitancy was a prime concern to inoculate mass people at the community level for achieving indirect immunity, which is required to save the most vulnerable people ( 15 ). The most common attributing factor for this unwillingness to vaccination is the adverse effects of vaccines. However, this hesitancy is overcome through a vaccination campaign with a clear message towards the general population highlighting the safety and efficacy of the vaccines, aiding to achieve a successful global COVID-19 vaccination campaign to counter the ongoing pandemic ( 15 ).
The vaccine acceptance rate is significantly higher in LICs and middle-income countries (80.3%), compared to the developed countries (64.6% and 30.4% for the United States of America and Russia, respectively) ( 27 ). In the meantime, the vaccine shortage and distribution inequality have crucially risen to concern levels for LMICs. Based on several parameters, such as varying environmental, biological, social, and behavioral factors, the exact threshold level of herd immunity against COVID-19 is less understood for many LMICs, including Bangladesh. At the same time, the global data demonstrate the ceiling values between 55% and 82% of the total population ( 28 , 29 ). Recently, one forecasting study on fully vaccinated people keeping in view future vaccination rates, pointed out that many countries are still far away from achieving desired levels of herd immunity that are needed to render complete protection and slow down or check the ongoing spread of COVID-19. Accordingly, worldwide vaccination campaigns must be progressively enhanced to high levels along with tackling various challenges posed and adopting the recommended prevention and control strategies to fight against COVID-19 ( 30 - 32 ).
Since the emerging SARS-CoV-2 variants and mutants are leading to a rapid surge of COVID-19 cases while posing threats on the vaccine efficacy of the available SARS-CoV-2 vaccines with providing varying degrees of protection levels, including lower efficacy being reported, modifications in such vaccines or designing newer vaccines may be possibly required under the events of some emerging variants possessing higher virulence and lethality, as well as potential to spread more rapidly ( 16 , 18 , 21 , 33 - 35 ).
3.2. COVID-19 Vaccination and Early Experiences
To battle against the COVID-19 pandemic, recently, several vaccine candidates got emergency use authorization and are being used across the globe. Bangladesh commenced a priority-based COVID-19 vaccination program on February 7, 2021 ( 36 ). Initially, though the country launched the mass vaccination program impressively, the government failed to continue due to the export prohibition of the COVISHIELD vaccine manufactured by the Serum Institute of India (SII) in collaboration with Oxford-AstraZeneca. Till October 13, 2021, the country has administered near 56 million doses (33.51 per 100 people) of the COVID-19 vaccine ( 37 ). However, the infection rate of this virus is not still negligible rather than resurging with tremendous spreading capacity in so many LMICs, and limited triumph was observed in impeding the community transmission chain in the achievement of herd immunity against the delta variant of the virus ( 1 , 38 ). The delta variant of concern has already deduced 10%-13% and 16% efficacy of BNT162b2 and ChAdOx1 vaccines, respectively ( 39 ).
There were numerous projected obstacles and challenges for LMICs to get inoculated against COVID-19 before 2023. In such circumstances, LMICs necessitate some exceptional and realistic strategies to subdue these hindrances and hurdles to execute a successful mass vaccination against COVID-19. A long-term solution to the current pandemic and several clinical and socio-economic benefits will appear through rational mass immunization.
The plan of actions or policies of the two early successful vaccination programs in Bangladesh might be helpful for authorities to comprehend the gravity and emergency preparedness for quick and efficient rollout attributed to a mass inoculation program against COVID-19. One was an impressive tetanus toxoid immunization, which augmented around 11 times higher by only 10 years (1984 to 1994: 6% vs. 67%), and complete protection among children of 12-23 months old increased from an insignificant percentage to almost 60% ( 40 ). On the other hand, the country's most prominent public health milestone was to sustain the nationwide expanded program on immunization, and a polio-free nation's declaration by the regional certification committee of the World Health Organization (WHO) South Asian region in 2014 ( 41 ). These preceding accomplishments and experiences might be helpful appliances in the ahead of the mass vaccination programs.
3. 3. Affordability and Challenges
Undoubtedly, the vital and most prominent challenges for a resource-constrained country are to ascertain enough doses of safe and effective vaccines and abundance immunization at the community level. Apart from these, having approved vaccines is not capacious to attain the minimum threshold of herd immunity against the COVID-19 pandemic globally due to several drawbacks of development and large-scale production, pricing affordability, appropriate allocation, and deployment with public confidence and trust ( 22 , 28 ).
In addition, the inadequate and fragile healthcare infrastructure and information management may jeopardize the fast-track vaccination for LMICs, including Bangladesh, one of the most vulnerable countries in this case ( 42 , 43 ). However, the government of Bangladesh has the promised distribution of COVID-19 vaccines to all citizens free of cost after procurement from several vaccine-producing companies. Furthermore, under the COVAX (COVID-19 vaccine and global access) facility, a task force for equitable distribution of COVID-19 vaccines worldwide initiatives by Global Alliance for Vaccines and Immunization, Coalition for Epidemic Preparedness Innovations, and WHO, Bangladesh will get 20% immunization for high-risk group people ( 22 ). In the meantime, apart from the Oxford-AstraZeneca, Bangladesh has approved several more vaccines, such as China's Sinopharm and Sinovac, Russia's Sputnik V, US's Pfizer-bioNTech, as well as Johnson and Johnson (single dose) vaccines for emergency use against COVID-19. The country has also already received some Pfizer-BioNtech and Sinopharm vaccines shipments following an influential diplomatic relationship and administrative process with the vaccine producer countries ( 44 ).
Vaccine unwillingness was obvious in several countries during the inception phase of the current mass vaccination program against COVID-19. Little knowledge of immunization and the lack of mass campaigns might be prominent reasons behind the skepticism. In this regard, the dominant groups are female and geriatric patients ( 27 ). Additionally, propaganda and infodemics about the adverse effects of COVID-19 vaccines had worsened the situation of hesitation ( 45 ). Although this reluctance behavior has lessened, and people are now enthused to accept vaccines, it is still one of the major concerning factors due to the vaccine coverage inequality. The city area has the privileged availability of vaccines; in contrast, most of the rural areas are still deprived of this facility. The economical minority is also a huge problem as they are not much concerned about the health and precautionary measures against COVID-19. Many LMICs stand last in vaccine coverage as the number of people having both doses is not very poor so far. The younger generation is still deprived of even the first dose as citizens below age 25 years are not allowed to register for vaccines to date in many LMICs ( 45 ).
Moreover, most of the preeminent COVID-19 vaccines, such as Pfizer (-70°C), Moderna (-20°C), and Gamaleya (-18°C) demand more fastidious maintenance though luckily, Sinopharm, Johnson, and AstraZeneca vaccines require cheaper governance that can efficiently be stored in fridges at 2-8°C ( 22 ). In order to tackle the potential cold chain error and shipping hassle that is one of the biggest challenges, Bangladeshi experts in partnership with the United Kingdom Research and Innovation are struggling to develop a blueprint and model for effective nationwide vaccine freight ( 46 ).
4. Recommendations
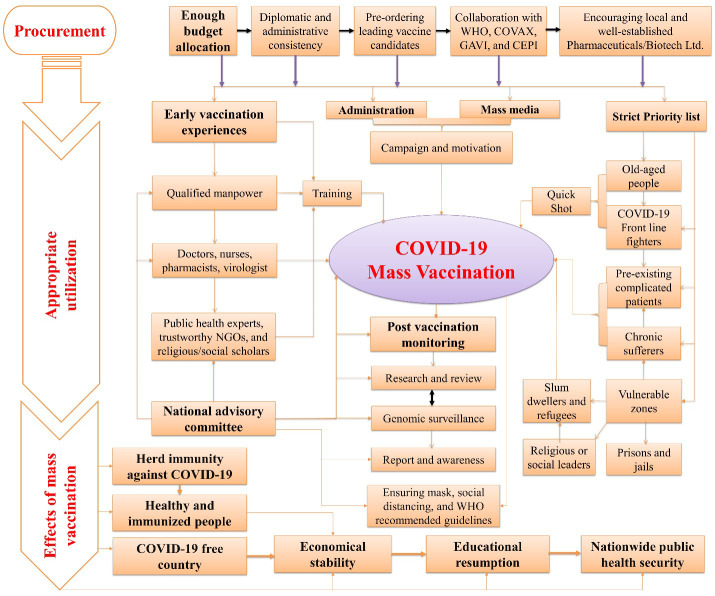
Although the rollout of quick vaccination induces a beacon of hope, the infection rate keeps escalating steadily in so many LMICs and refugees ( 47 ). Unforeseen adverse reactions and new virulent variants as of SARS-CoV-2 genome mutation could bring a terrifying consequence by thwarting vaccines' efficacy. However, several instructions and effective guidelines were illustrated in figure 1, which might be recommended at this current stage for resource-constraint countries. First, safe and effective vaccines should rapidly be procured through a smooth and sustainable diplomatic and administrative process. Second, cold chain management must be ensured for appropriate storage to maintain the stability and efficacy of the vaccines. Concurrently, equitable distribution and deployment of countrywide vaccination after securing the availability of the vaccines need to be expedited based on several unanimous implications, such as a strict priority list to quickly inoculate the highest risky population. In contrast, previous immunization experiences and proactive public awareness might mitigate vaccine hesitancy among general people and the health community, accelerating the proper vaccination program countrywide. Cultural, social, or political leaders might be enlisted with the medical and health workers in formulating a national messaging community to generate and spread urgent public health campaigns with accessible vibes and venues. Finally, the WHO recommended post-vaccination routine surveillance, citing potential adverse effects, particularly in older-aged recipients with comorbidities, which require be carefully realizing and executing countrywide in a part of successful immunization against COVID-19 pandemic ( 48 ).
Figure 1.
Mass vaccination against COVID-19 pandemic in low-income and middle-income countries (LMICs): challenges, recommendations, and impacts.
COVID-19 = Coronavirus Disease 2019, WHO = World Health Organization, GAVI = Global Alliance for Vaccines and Immunization, CEPI = Coalition for Epidemic Preparedness Innovations, NGOs = Non-Governmental Organizations.
5. Conclusion
Most of the LICs and LMICs, including Bangladesh, need far more vision and ambition to be immunized through nationwide vaccination. Highly holistic, political, administrative, and diplomatic progress must be required to triumph over the current challenges and implement the stated recommendations for achieving urgent mass vaccination drive successfully for the entire population to terminate the current COVID-19 pandemic soon by achieving desired levels of herd immunity and protection at the earliest time.
Authors' Contribution
Study concept and design: M. J. H.
Acquisition of data: S. M. A. R.
Analysis and interpretation of data: T. B. E.
Drafting of the manuscript: S. M.
Critical revision of the manuscript for important intellectual content: M. J. H. and M. R. I.
Statistical analysis: K. D.
Administrative, technical, and material support: M. J. H.
Conflict of Interest
The authors declare that they have no conflict of interest.
Grant Support
The authors received no financial support for this article.
References
- 1.Bari MS, Hossain MJ, Akhter S, Emran TB. Delta variant and black fungal invasion: A bidirectional assault might worsen the massive second/third stream of COVID-19 outbreak in South-Asia. Ethics Med Public Health. 2021; 19: 100722. doi: 10.1016/j.jemep.2021.100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain MJ, Kuddus MR, Rahman SMA. Knowledge, attitudes, and behavioral responses toward COVID-19 during early phase in Bangladesh: a questionnaire-based study. Asia Pac J Public Health. 2021;33(1):141–144. doi: 10.1177/1010539520977328. [DOI] [PubMed] [Google Scholar]
- 3.WHO Coronavirus (COVID-19) Dashboard. Accessed: October 15, 2021. Available from: https://covid19.who.int/table .
- 4.García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, et al. An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times. Vaccines. 2021; 9(5):433. doi: 10.3390/vaccines9050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal Yatoo M, Hamid Z, Parray OR, Wani AH, Ul Haq A, Saxena A, et al. COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccin Immunother. 2020; 16(12):2891–2904. doi: 10.1080/21645515.2020.1788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawat K, Kumari P, Saha L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021; 892 doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. DRAFT landscape of COVID-19 candidate vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- 8.Hossain MJ, Rahman SMA. Repurposing therapeutic agents against SARS-CoV-2 infection: most promising and neoteric progress. Expert Rev Anti Infect Ther. 2021;19(8):1009–1027. doi: 10.1080/14787210.2021.1864327. [DOI] [PubMed] [Google Scholar]
- 9.Haider T, Gour V, Pandey V, Kanwar IL, Tiwari R, Vishwakarma M, et al. COVID-19 Infection: Targeting Possibilities for Treatment. Crit Rev Ther Drug Carrier Syst. 2021;38(3):75–115. doi: 10.1615/CritRevTherDrugCarrierSyst.2021035392. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal Yatoo M, Hamid Z, Rather I, Nazir QUA, Bhat RA, Ul Haq A, et al. Immunotherapies and immunomodulatory approaches in clinical trials - a mini review. Hum Vaccin Immunother. 2021;17(7):1897–1909. doi: 10.1080/21645515.2020.1871295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabaan AA, Al-Ahmed SH, Sah R, Tiwari R, Yatoo MI, Patel SK, et al. SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter this emerging pandemic. Ann Clin Microbiol Antimicrob. 2020;19(1):40. doi: 10.1186/s12941-020-00384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA, Tirupathi R, et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines. 2021;9(5):436. doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hossain MJ, Jannat T, Brishty SR, Roy U, Mitra S, Rafi MO, et al. Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far. Biologics. 2021; 1(2):252–284. [Google Scholar]
- 14.Blasi F, Gramegna A, Sotgiu G, Saderi L, Voza A, Aliberti S, Amati F. SARS-CoV-2 vaccines: A critical perspective through efficacy data and barriers to herd immunity. Respir Med. 2021;180: 106355. doi: 10.1016/j.rmed.2021.106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhama K, Sharun K, Tiwari R, Dhawan M, Emran TB, Rabaan AA, Alhumaid S. COVID-19 vaccine hesitancy - reasons and solutions to achieve a successful global vaccination campaign to tackle the ongoing pandemic. Hum Vaccin Immunother. 2021;17(10):3495–3499. doi: 10.1080/21645515.2021.1926183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqi T, Malik JA, Mulla AH, Al Hagbani T, Almansour K, Ubaid MA, Alghamdi S, Anwar S. An overview of SARS-COV-2 epidemiology, mutant variants, vaccines, and management strategies. J Infect Public Health. 2021;14(10):1299–1312. doi: 10.1016/j.jiph.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai CC, Chen IT, Chao CM, Lee PI, Ko WC, Hsueh PR. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20(8):1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 18.Raman R, Patel KJ, Ranjan K. COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules. 2021;11(7):993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharun K, Dhama K. COVID-19 Vaccine Diplomacy and Equitable Access to Vaccines Amid Ongoing Pandemic. Arch Med Res. 2021;52(7):761–3. doi: 10.1016/j.arcmed.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snehota M, Vlckova J, Cizkova K, Vachutka J, Kolarova H, Klaskova E, Kollarova H. Acceptance of a vaccine against COVID-19 - a systematic review of surveys conducted worldwide. Bratisl Lek Listy. 2021;122(8):538–547. doi: 10.4149/BLL_2021_086. [DOI] [PubMed] [Google Scholar]
- 21.Tareq AM, Emran TB, Dhama K, Dhawan M, Tallei TE. Impact of SARS-CoV-2 delta variant (B.1.617.2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccin Immunother. 2021;2:1–2. doi: 10.1080/21645515.2021.1963601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan ZP, Yang M, Lai CL. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals. 2021;14(5):406. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain MJ, Kuddus MR, Rashid MA, Sultan MZ. Understanding and Dealing the SARS-CoV-2 Infection: An Updated Concise Review. Bangladesh Pharm J. 2021; 24(1): 61–75. [Google Scholar]
- 25.Garg S, Singh MM, Deshmukh CP, Bhatnagar N, Borle AL, Kumar R. Critical interpretative synthesis of herd immunity for COVID-19 pandemic. J Family Med Prim Care. 2021;10(3):1117–1123. doi: 10.4103/jfmpc.jfmpc_1127_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redwan EM. COVID-19 pandemic and vaccination build herd immunity. Eur Rev Med Pharmacol Sci. 2021;25(2):577–579. doi: 10.26355/eurrev_202101_24613. [DOI] [PubMed] [Google Scholar]
- 27.Solís Arce JS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossain MJ. Is Bangladesh moving toward herd Immunity? current COVID-19 perspective. Bangladesh J Infect Dis. 2020;7(2):63–6. [Google Scholar]
- 29.Schaffer DeRoo S, Pudalov NJ, Fu LY. Planning for a COVID-19 Vaccination Program. J Am Med Assoc. 2020;323(24):2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 30.Cihan P. Forecasting fully vaccinated people against COVID-19 and examining future vaccination rate for herd immunity in the US, Asia, Europe, Africa, South America, and the World. Appl Soft Comput. 2021;111:107708. doi: 10.1016/j.asoc.2021.107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadkhoda Herd Immunity to COVID-19. Am J Clin Pathol. 2021;155(4):471–472. doi: 10.1093/ajcp/aqaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma N, Vyas S, Mohapatra A, Khanduri R, Roy P, Kumar R. Combating COVID-19 pandemic in India: Demystifying the concept of herd immunity. J Family Med Prim Care. 2021;10(4):1515–1519. doi: 10.4103/jfmpc.jfmpc_1971_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm E, Kronig I, Neher RA, Eckerle I, Vetter P, Kaiser L. Geneva Centre for Emerging Viral Diseases. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27(8):1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldwell JM, Le X, McIntosh L, Meehan MT, Ogunlade S, Ragonnet R, O'Neill GK, Trauer JM, McBryde ES. Vaccines and variants: Modelling insights into emerging issues in COVID-19 epidemiology. Paediatr Respir Rev. 2021;39:32–39. doi: 10.1016/j.prrv.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharun K, Tiwari R, Dhama K, Emran TB, Rabaan AA, Al Mutair A. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccin Immunother. 2021;17(10):3491–3494. doi: 10.1080/21645515.2021.1923350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan AM. COVID-19 vaccination: How’s Bangladesh doing compared to the rest of the world? Daily Star.Accessed October15,2021. Available from: https://www.thedailystar.net/coronavirus-deadly-new-threat/news/covid-19-vaccination-hows-bangladesh-doing-compared-the-rest-the-world-2049289 .
- 37.Our World in Data. Coronavirus vaccinations. Accessed October 14, 2021. Available from: https://ourworldindata.org/covid-cases .
- 38.Hossain MJ, Soma MA, Islam MR, Emran TB. Urgent call for actionable measures to fight the current co-epidemic of dengue burden during the SARS-CoV-2 delta variant era in South-Asia. Ethics Med Public Health. 2021;19:100726. doi: 10.1016/j.jemep.2021.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021 doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamil K, Bhuiya A, Streatfield K, Chakrabarty N. The immunization programme in Bangladesh: impressive gains in coverage, but gaps remain. Health Policy Plan. 1999;14(1):49–58. doi: 10.1093/heapol/14.1.49. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. World polio day: Bangladesh continues efforts to sustain the polio-free status. Accessed October 15, 2021. Available from: https://www.who.int/bangladesh/news/detail/28-10-2019-world-polio-day-bangladesh-continues-efforts-to-sustain-the-polio-free-status .
- 42.Hossain MJ. Impact of COVID-19 Pandemic among health care providers in Bangladesh: a systematic review. Bangladesh J Infect Dis. 2020;7(2): 8–15. [Google Scholar]
- 43.Hossain MJ. Social Organizations and Mass Media in COVID-19 Battle: A Bidirectional Approach in Bangladesh. Asia Pac J Public Health. 2021;33(4):467–468. doi: 10.1177/10105395211002601. [DOI] [PubMed] [Google Scholar]
- 44.Tribune report. Bangladesh clears Johnson & Johnson’s single-dose Covid-19 vaccine for use. Accessed October 15, 2021. Available from: https://www.dhakatribune.com/health/coronavirus/2021/06/15/johnson-johnson-s-covid-19-vaccine-approved-for-emergency-use .
- 45.Machingaidze S, Wiysonge C.S. Understanding COVID-19 vaccine hesitancy. SNat Med. 2021; 27:1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 46.The logistics of mass vaccination in Bangladesh and beyond. UK Research and Innovation. Accessed October 15, 2021. Available from: https://www.birmingham.ac.uk/news/latest/2020/09/blueprint-to-vaccinate-in-Bangladesh.aspx .
- 47.Kondilis E, Papamichail D, McCann S, et al. The impact of the COVID-19 pandemic on refugees and asylum seekers in Greece: A retrospective analysis of national surveillance data from 2020. EClinicalMedicine. 2021; 37: 100958. doi: 10.1016/j.eclinm.2021.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molla MMA, Disha JA, Yeasmin M, Ghosh AK, Nafisa T. Decreasing transmission and initiation of countrywide vaccination: Key challenges for future management of COVID-19 pandemic in Bangladesh. Int J Health Plann Manage. 2021 doi: 10.1002/hpm.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]