Abstract
The current experiment aimed to assess the effect of the synthetic antioxidants ethoxyquin (EQ) and/or butylated hydroxytoluene (BHT) on the liver function tests, hematological parameters, and liver histoarchitecture in rats. A total of 50 male Sprague-Dawley rats were divided into five groups of 10 animals per group. The first group served as the control and did not receive any treatments, and the second group served as the vehicle control and was orally administrated 1 ml of corn oil day after day for consecutive 45 and 90 days. The third group (EQ) was orally administered 1 ml of EQ dissolved in corn oil day after day for consecutive 45 and 90 days in a dose of 1/5 LD50, and the fourth group (BHT) was orally received 1 ml of BHT dissolved in corn oil day after day for consecutive 45 and 90 days in a dose of 1/5 LD50. The fifth group (combination group) was orally administered both EQ and BHT at the same doses and durations described above. The present results showed that the final body weight was significantly decreased in the EQ- or BHT-treated group particularly at 90 days of exposure to both compounds. Furthermore, the liver weight was significantly elevated in EQ, BHT, and co-exposed groups at 45 and 90 days of exposure, compared to the control group. Moreover, EQ, BHT, and their co-exposure caused a significant elevation in the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes, as well as total bilirubin at 45 and 90 days of exposure. On the other hand, there was no significant change in the total albumin. Hemoglobin value, red blood cells, white blood cells, platelets, and differential leucocyte count at 45 and 90 days of exposure were significantly decreased. Histopathological significant findings in the liver were observed as vascular congestions, vacuolations, hydropic degenerations, lipidosis, and swelling, particularly in the co-exposed group for 90 days. These findings confirmed the hepatotoxic potential of EQ and BHT; therefore, it is recommended to control and limit the utilization of such chemicals.
Keywords: EQ, BHT, Feed additives, Antioxidants, ALT, AST, Total bilirubin
1. Introduction
Feed additives are substances intentionally added to animal feeds or water in small quantities to improve feed and food quality, as well as animals' health, promote performance, and enhance digestibility. They have been added to commercial feeds to stop lipid peroxidation and oxidative rancidity, adjust the pH during processing, prevent microbial spoilage of feed, and enhance the physical or chemical properties of the diet ( 1 - 4 ). Antioxidants are preservative additives that are included especially in pets’ food, to enhance the quality of the food and prevent harmful spoilage. Most pet foods contain fats and oils essential for the pet’s health, which are required to be stabilized. The antioxidant preservatives prevent the fats and oils in the food from going rancid, leading to the accumulation of possibly harmful degradation substances and unpleasant odors ( 5 ). Ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline, [EQ]) is a synthetic antioxidant commonly used for pets, livestock, and aquaculture feeds in order to stop lipid peroxidation. It cannot be used for human consumption as food additives, except for spices; however, it can reach human beings from poultry, farmed fish, and eggs ( 6 ). Joint Food and Agricultural Organization and World Health Organization Meeting on Pesticide Residues (JMPR) recognized an acceptable daily intake for EQ as 0.005 mg/kg body weight according to clinical signs observed in dogs exposed to 2.5 mg EQ/kg body weight in feed ( 7 ). It was recently shown to cause many unhealthy side effects in animals fed on feed containing this compound ( 8 ). Błaszczyk and Skolimowski ( 9 ) have reported that EQ is carcinogenic and toxic to animals.
Butylated hydroxytoluene (2, 6-di-tert-butyl-p-kresol or [BHT]; 2, 6-di-tert-butyl-4-methyl phenol), a synthetic phenolic antioxidant, is used in processed foods, cosmetics, pharmaceutical, and petrochemical products ( 10 ). It is commonly used to preserve nutritive value, flavor, freshness, and color of foods and animal feed ( 11 ). It is used either singly or in combination with BHA and/or EQ at a maximum concentration of 150 mg/kg ( 12 ) as a feed additive for all animal species, except for dogs. The assessment of BHT toxicity showed that it had teratogenic, carcinogenic, and mutagenic effects since BHT led to the development of hepatocellular tumors in rats and mice, DNA development inhibitions in monkeys, and endocrine disturbance in Zebrafish ( 13 ). Accordingly, this study aimed to estimate the outcomes of the single or co-exposure of EQ and BHT at 45 and 90 days. For this purpose, body and liver weight, liver function tests, hematological examination, and histopathological examination were assessed in this study.
2. Materials and Methods
2.1. Tested Compounds
EQ (C14H19NO) was bought from Sigma-Aldrich Chemical Company, Saint Louis, MO, USA (≥75% purity), and BHT (C15H24O) was obtained from Alpha Chemica, India (99% pure). The doses of EQ and BHT were selected to be 1/5 LD 50=235 mg/kg b.w.t ( 14 ) and 1/5 LD 50=132 mg/kg b.w.t ( 15 ), respectively. Both substances were dissolved in corn oil.
2.1.1. Animals and Experimental Design
A total of 50 male Sprague-Dawley rats weighing 240-300g were obtained from Laboratory Animal Farm, Faculty of Veterinary Medicine, Zagazig University, Egypt. The rats were kept with water and feed ad libitum throughout the acclimatization period (2 weeks).
The rats were divided into five groups of 10 animals per group. The first group was placed as the control and did not receive any treatments, and the second group was considered a vehicle control and orally given 1 ml of corn oil day after day for consecutive 45 and 90 days. The third group (EQ) was orally administered EQ dissolved in 1 ml corn oil day after day for consecutive 45 and 90 days in a dose of 235 mg / gm b.w.t to each rat ( 14 ). Moreover, the fourth group (BHT) was orally received BHT dissolved in 1 ml corn oil day after day for consecutive 45 and 90 days in a dose of 132 mg/ gm b.w.t to each rat ( 15 ). The fifth group (combination group) was orally administered both EQ and BHT in doses of 500mg/kg b.w.t and durations described above. It should be noted that the rats were orally administrated all treatments using gastric gavages.
2.2. Body Weight and Liver Weights
The body weight was observed weekly till the end of the experiment. The body and liver weights of the treated and control rats were taken at 45 and 90 days.
2.3. Blood and Tissue Sampling
At 45 and 90 days of the experiment, from the medial canthus of the eye of all rats (orbital vessels), the blood samples were collected after anesthesia of rats by Ketamine-Xylazine (0.05-0.10 ml/100 gm) intraperitoneally. The blood samples were then divided into two portions. The first part of the blood was put in a clean test tube and centrifuged at 3000 rpm for 15 min to separate sera for the completion of liver function tests. The second part was collected for hematological analysis into sterilized Wasserman tubes containing dipotassium salts of ethylene diamine tetraacetic acid. Following that, the rats were euthanized by cervical dislocation. The liver was directly excised and weighed. Furthermore, the liver tissue specimens were divided and washed with sterile physiological saline. For histopathological studies, the liver tissues were fixed in 10% neutral buffered formalin.
2.4. Evaluation of Some Liver Function Tests in Serum
Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were estimated by the method of Babson, Shapiro ( 16 ). Albumin evaluation depends on dye-binding methods which have been accepted since the 1950s ( 17 ). Total bilirubin was estimated by the method described by Dangerfield and Finlayson ( 18 ).
2.5 Hematological Examination
Hematological analysis was evaluated by an automated blood cell analyzer (Sysmex XT-2000iV, Kobe, Japan) ( 19 ).
2.6. Histopathological Examination
Liver specimens were dehydrated in graded ethanol (70%-100%), cleared in xylene, and embedded in paraffin. Thereafter, 5-µm thick sections were prepared and then stained with hematoxylin and eosin ( 20 ).
2.7. Statistical Analysis
In order to assess the influence of EQ, BHT, and their combination, the results were expressed as mean±SE. Statistical comparisons were conducted using a two way-ANOVA in SPSS software (version 22). Furthermore, Duncan’s multiple comparisons post hoc test was performed to compare the mean values among the treated groups and corresponding control. A P-value less than 0.05 was considered statistically significant ( 21 ).
3. Results
3.1. Effect of Ethoxyquin and/or Butylated Hydroxytoluene on Body and Liver Weights
Data in table 1 showed the EQ and/or BHT effect on the final body weight (FBW), bodyweight change (BWC), and liver weight (LW) of Sprague-Dawley male rats. The results showed that the differences in the exposure duration did not induce significant changes on FBW; however, they significantly influence the BWC, where it was significantly higher at day 45. The duration of exposure also significantly affects the LW, and it was higher at 90 days of exposure.
Table 1.
Changes of the body and liver weights in male Sprague-Dawley rats treated with EQ and/or BHT for 45 and 90 days
| IBW (g) | FBW (g) | BWC (g) | LW (g) | ||
|---|---|---|---|---|---|
| Main effects | |||||
| Duration | 45 days | 277.00±7.00 | 337.90±9.5 | 65.10a 0.09 | 13.00b±0.50 |
| 90 days | 272.00±10.00 | 325.0±11.00 | 51.50b±0.10 | 14.00a±0.60 | |
| Sig. | NS | NS | * | * | |
| Treatments | Control | 283.0b ±12.00 | 352.0 ab ±5.00 | 67.1 a±0.33 | 9.40 d ±0.300 |
| VH | 318.0 ab±12.00 | 382.0 a±12.00 | 64.5 a±0.29 | 13.00 cd±0.30 | |
| EQ | 272.0 b±6.00 | 331.0b±13.00 | 57.5 b±0.23 | 14.00 b±0.30 | |
| BHT | 259.5 bc±11.00 | 327.0 b±9.00 | 67.4 a±0.31 | 14.80 b±0.40 | |
| EQ+BHT | 239.0 c±2.00 | 264.0 c±4.00 | 25.12c±0.11 | 16.00 a ±0.30 | |
| Sig. | *** | *** | *** | *** | |
| Interaction effect | |||||
| 45 days | Control | 271.0± 18.00 | 347.0 b±4.00 | 76.02 a±0.15 | 9.40 de ±0.80 |
| VH | 311.0±13.00 | 374.0 b±18.00 | 73.21a±0.18 | 12.80 d ±0.70 | |
| EQ | 282.0±7.00 | 354.0 b±16.00 | 73.17 a±0.19 | 14.50 c±0.50 | |
| BHT | 281.0±8.00 | 344.0 b±7.00 | 63.15 ab±0.13 | 14.0 cd ±0.40 | |
| EQ+ BHT | 241.0±5.00 | 269.0 d ±6.00 | 28.33 d±0.02 | 15.8 b±0.30 | |
| 90 days | Control | 296.0±16.00 | 357.0 abᵇ±9.00 | 61.25 b±0.11 | 9.40 e± 0.40 |
| VH | 326.0±22.00 | 391.0 a ±16.00 | 65.23 ab±0.10 | 14.20 cd±0.30 | |
| EQ | 263.0±8.00 | 307.0 c±13.00 | 44.47 c±0.09 | 15.20 bc±0.30 | |
| BHT | 237.6±12.00 | 310.0 c±10.00 | 73.26 a±0.21 | 15.70 b±0.40 | |
| EQ+ BHT | 238.0±1.00 | 258.0 d ±4.00 | 20.17 d±0.03 | 17.00 a ±0.40 | |
| Sig. | NS | * | *** | * | |
Data are expressed as mean±SD. Means within the same column carrying different superscripts are significant differences (P<0.05) (n=5/group). NS= not significant,*=P<0.05, ***=P<0.001.
As shown in table 1, separate exposure to EQ or BHT did not significantly alter the FBW, while their combined exposure significantly decreases the FBW of rats, compared to the control group. Exposure of rats to EQ resulted in a significant reduction of BWC, compared to the control, while BHT did not significantly alter the BWC. On the other hand, a more prominent reduction of BWC was recorded in the EQ+BHT co-exposed group, compared to the other groups. The results also revealed that the highest LW was observed in the co-exposed group (EQ+BHT), followed by the separately exposed groups (EQ or BHT), compared to the control group. The effect of interaction (duration*treatment) on FBW caused significant decreases in the EQ- or BHT-treated group particularly at 90 days of exposure. On the other hand, a more prominent reduction of FBW was noted in the co-exposed group, compared to the other groups.
The BWC was also affected by the interaction effect (duration*treatment), where EQ significantly decreased the BWC at 90 days but not at 45 days; however, BHT did not show any difference, compared to the control group, on both durations. Additionally, the lowest gain was obtained by the co-exposed group at both durations. EQ and BHT exhibited a significant increase in the LW, compared to the control group, and this increase was more noticeable by increasing the duration of exposure. The combined exposure to EQ and BHT significantly increased the LW, compared to the separate exposure at 90 days.
3.2. Effects of Ethoxyquin and/or Butylated Hydroxytoluene on Some Liver Function Tests in the Serum
Changes in the liver function markers in the serum of Sprague-Dawley male rats exposed to EQ and/or BHT for 45 and 90 days are summarized in table 2. Our data showed that the ALT activity was significantly increased with increasing the exposure duration. However, the opposite occurred for the level of albumin content. On the other hand, the exposure duration did not significantly influence the levels of AST or the total bilirubin. Regarding the effect of treatment, the results showed a significant (P<0.001) elevation in ALT, AST, and total bilirubin in all treated groups, compared to the control group. Moreover, the EQ+BHT groups showed significantly higher levels of such parameters, compared to the EQ group. On the other hand, there was no significant change in the total albumin as influenced by the different treatments. The results of the interaction effect between exposure time and treatments showed that the highest values of ALT, AST, and total bilirubin were observed in the BHT and EQ+BHT groups at 90 days, followed by the same groups at 45 days. EQ also showed similar higher levels of these parameters at both durations, compared to the control group. On the other hand, there were no significant changes in the total albumin due to the interaction effect of different treatments and the exposure duration.
Table 2.
Changes in the liver function tests in the serum of male Sprague-Dawley rats treated with EQ and/or BHT for 45 and 90 days
| ALT(u/L) | AST (U/L) | Total bilrubin (mg/dL) | Albumin (g/dL) | ||
|---|---|---|---|---|---|
| The main effect | |||||
| Duration | 45 days | 111.0 b±9.11 | 128.95±4.50 | 0.94 a ±0.05 | 3.50±0.10 |
| 90 days | 159.0 a±15.5 | 131.6±8.40 | 0.85 b±0.10 | 3.40±0.10 | |
| Sig. | *** | NS | * | NS | |
| Treatments | Control | 69.70 c±1.8 | 89.00 c ±6.00 | 0.69 c±0.02 | 3.59±0.16 |
| VH | 70.0 c ±8.40 | 86.6 c±4.00 | 0.70 c±0.10 | 3.24±0.16 | |
| EQ | 128.0 b±3.30 | 122.8 b±5.00 | 0.80 b±0.10 | 3.69±0.14 | |
| BHT | 180.8 a±32.1 | 160.0 a ±6.00 | 1.02 a±0.04 | 3.57±0.04 | |
| EQ+ BHT | 187.0 a ±3.40 | 143.6 a±2.00 | 1.20 a ±0.10 | 3.54±0.10 | |
| Sig. | *** | *** | *** | NS | |
| The interaction effect | |||||
| 45 days | Control | 68 d ±2.72 | 99.00 d±2.00 | 0.87 d±0.10 | 3.65±0.10 |
| VH | 68.66 d ±1.25 | 96.0 d ±1.50 | 0.91 d±0.10 | 3.22±0.32 | |
| EQ | 129.87 c±0 .68 | 166 .0 c ±0.60 | 1.09 c±0.03 | 4.10±0.02 | |
| BHT | 180.0 b±1.50 | 152.6 b±1.70 | 1.20 b±0.02 | 3.48±0.03 | |
| EQ+ BHT | 182.6 b ±5.70 | 140.6 b±4.00 | 1.26 b±0.02 | 3.40 ±1.00 | |
| 90 days | Control | 74.00 d ±2.70 | 99.0 d±10.00 | 0.86 d ±0.10 | 3.54±0.28 |
| VH | 72.0 d±2.80 | 97.0 d±2.00 | 0.90 d±0.02 | 3.27±0.10 | |
| EQ | 137.0 c±1.5 | 169.0 c±1.00 | 1.07 c±0.030 | 3.33±0.05 | |
| BHT | 195 a±1.80 | 185.0 a±2.00 | 1.40 a±0.10 | 3.65±0.02 | |
| EQ+BHT | 191.0 a±3.00 | 176.6 a±1.00 | 1.44 a±0.03 | 3.66±0.17 | |
| Sig. | *** | *** | *** | NS | |
Data are expressed as mean±SD. Means within the same column carrying different superscripts are significant differences (P<0.05) (n=5/group). NS=not significant,*=P<0.05, ***=P<0.001.
3.3. Effects of Ethoxyquin and Butylated Hydroxytoluene on Some Hematological Parameters
Table 3 tabulates the changes of hematological parameters of Sprague-Dawley male rats treated with EQ and/or BHT for 45 and 90 days. The hemoglobin (Hb) value, white blood cells (WBCs), red blood cells (RBCs), and differential leucocytes count were significantly influenced by the duration of exposure to EQ and/or BHT, where their values were significantly reduced by increasing the duration of exposure to 90 days, compared to their values at 45 days. On the other hand, opposite results were recorded for the platelets count. The effect of treatments showed a similar reducing impact of EQ and BHT on RBCs and WBCs count, compared to the control group, while the reducing effect of EQ was more obvious than that of BHT on Hb, blood platelets, and differential leucocytes count. Moreover, the lowest values of all hematological parameters were observed in the EQ+BHT group. The obtained results related to the interaction between time and treatment showed significant effects on Hb value. The Hb level in the EQ-, BHT-, and EQ+BHT-treated groups were lower than that in the control group at 45 days, and their values showed a more significant decrease at 90 days. The interaction effect on the RBCs and platelets count showed a significant (P<0.001) decrease in all treated groups, compared to the control group, at both durations. Similar results were obtained for the WBC count; however, the lowest value was in the EQ+BHT group, compared to the other treated groups.
Table 3.
Changes in the hematological parameters in the blood of male Sprague-Dawley rats treated with EQ and/or BHT for 45 and 90 days
| HB(g/dl) | RBCS (1086/cmm) | WBCs (1083/cmm) | Platelet (1083/cmm) | D. Leucocytic count% | ||
|---|---|---|---|---|---|---|
| The main effect | ||||||
| Duration | 45 days | 11.70 a ±0.50 | 4.00 a± 0.22 | 13.00 a±1.00 | 884.0 b±44.00 | 10.90 a±0.70 |
| 90 days | 10.60 b±0.50 | 3.59 b±0.20 | 10.60 b±0.65 | 963.0 a ±36.00 | 10.00 b±0.70 | |
| Sig. | *** | *** | *** | *** | * | |
| Treatments | Control | 14.00 a±0.40 | 4.90a±0.10 | 16.20 a±0.30 | 1192.0a±8.00 | 10.00 a±0.20 |
| VH | 14.00 a±0.10 | 4.90 a±0.06 | 15.20 a±1.30 | 1200.0 a±48.00 | 10.00 a±0.40 | |
| EQ | 10.85bc±1.10 | 3.80bc±0.50 | 12.90b±0.30 | 824.00c±8.00 | 7.50b±1.30 | |
| BHT | 11.50 b±0.40 | 4.00 b±0.22 | 13.70 b±0.90 | 931.00 b±46.00 | 9.00 ab±0.40 | |
| EQ+ BHT | 10.00 c±0.30 | 3.10 c±0.17 | 11.30 c±0.40 | 944.00 b±30.00 | 9.40 ab±0.30 | |
| Sig. | *** | *** | *** | *** | *** | |
| The interaction effect | ||||||
| 45 days | Control | 14.60 a±0.30 | 5.00 a±0.05 | 16.70 a±0.10 | 1172 a±5.00 | 10.00 a±0.40 |
| VH | 14.40 a±0.10 | 5.20 a±0.08 | 16.00 a ±0.80 | 1177 a±15.50 | 10.50 a±0.20 | |
| EQ | 10.70 b±0.30 | 3.30 cd±0.21 | 13.00 b±0.60 | 832.0 b±14.00 | 8.10 c±0.10 | |
| BHT | 10.60 b±0.10 | 3.50 b±0.12 | 12.0 b±0.40 | 812.0 b±0.04 | 9.00 b±0.30 | |
| EQ+ BHT | 10.20 b±0.10 | 2.90 de±0.04 | 11.48 c±0.40 | 815.0 b±10.00 | 9.80 b±0.40 | |
| 90 days | Control | 13.60 a ±0.80 | 4.80 a±0.18 | 15.60 a± 0.30 | 1212 a±3.00 | 10.50 a±0.20 |
| VH | 13.60 a ±0.80 | 4.6 a±0.13 | 16.40 a±1.20 | 1200 a±32.00 | 10.50 a±0.60 | |
| EQ | 9.00 c ±0.10 | 2.56 e±0.05 | 12.60 b±0.20 | 816.0 d±6.00 | 6.80 d±0.40 | |
| BHT | 7.90 de±0.30 | 2.95 deᵉ±0.12 | 12.40 b±0.50 | 819.0 d±26.00 | 9.00 b±0.20 | |
| EQ+ BHT | 7.00 d±0.10 | 2.96 deᵉ±0.26 | 11.00 c±0.70 | 811.0 d±48.00 | 9.00 b± 0.40 | |
| Sig. | *** | *** | *** | *** | *** | |
Data are expressed as mean±SD. Means within the same column carrying different superscripts are significant differences (P<0.05) (n=5/group). NS= not significant,*=P<0.05,***=P<0.001.
Concerning the interaction effect (duration*treatments) on differential leucocytes count, the results showed that the lowest values were obtained by the EQ treatments at both durations, and it was more clear at 90 days, compared to all other groups. The BHT and EQ+BHT groups also significantly decreased the differential leucocytes count, compared to the control group; however, their effect was similar at both durations of exposure.
3.4. Histopathological Findings
3.4.1. Effect of Ethoxyquin on Liver Histoarchitecture
3.4.1.1. After 45 Days of Exposure
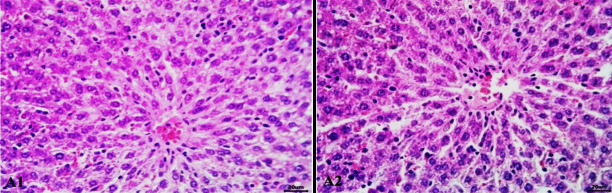
Histopathological findings of the liver tissue of rats (control) showed normal histological pictures; at 45 days (Figure 1; A1) and at 90 days (Figure 1; A2).
Figure 1.
Histopathological findings of the liver tissue of rats (control) showed normal histological pictures; at 45 days (A1) and at 90 days (A2). (H&E staining scale bars: 20 μm).
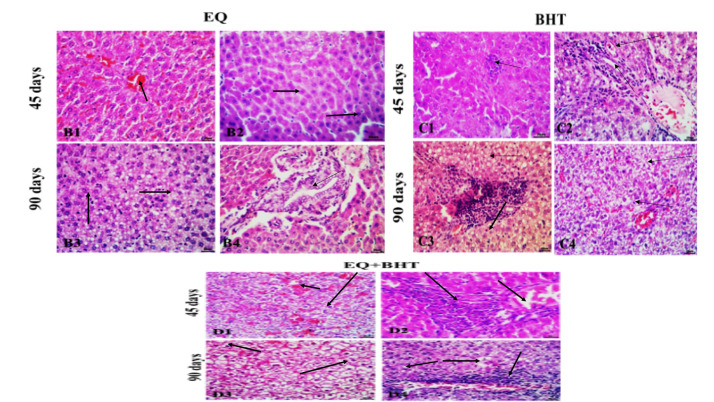
The microscopic examination of hepatic cells of male Sprague-Dawley rats treated with EQ for 45 days showed mild degenerative and vascular changes (congestion of the central veins) and sinusoidal dilatations (Figure 2; B1); however, few hepatocytes exhibited vacuolations (Figure 2; B2).
Figure 2.
Histopathological findings of the liver tissue of rats that administrated EQ for 45 days showing mild degenerative and vascular changes (B1) and hepatocytes vacuolations (B2). While at 90 days showed vascular congestions, and hepatocytes vacuolations (B3), mild hyperplastic proliferations of biliary system (B4). (H&E staining scale bars: 20 μm). The liver tissue of rats that administrated BHT for 45 days showed focal mononuclear aggregates (C1), vacuolar, and hydropic degenerations (C2). (H&E staining scale bars: 30 μm). While, at 90 days showed cellular swelling, necrosis (C3), vacuolar and hydropic degenerations (C4). (H&E staining scale bars: 20 μm). the liver tissue of rats administrated EQ +BHT for 45 days showed marked cellular swelling, vacuolar and hydropic degenerations (D1) , lipidosis, focal leukocytic aggregations and congestions (D2). While at 90 days showed marked cellular swellings, steatosis (D3), intralobular and portal leukocytic aggregations (D4) (H&E staining scale bars: 20 μm).
3.4. 1.2. After 90 Days of Exposure
The hepatic cells of rats that administrated EQ for 90 days were similar to those at 45 days; however, the lesions were more pronounced. Most specimens revealed vascular congestions, and hepatocytes vacuolations particularly (Figure 2; B3). The biliary system showed very mild hyperplastic proliferation (Figure 2; B4). This was accompanied in most cases by portal congestions and mononuclear cell infiltration.
3.4.2. Effect of Butylated Hydroxytoluene on the Liver Histoarchitecture
3.4.2.1. After 45 Days of Exposure
The effect of oral administration of BHT to male Sprague-Dawley rats for 45 days on hepatic cells showed more pronounced vascular, degenerative, and inflammatory alterations. Focal mononuclear aggregates (Figure 2; C1), vacuolar and hydropic degenerations, lipidosis, portal congestions, and mononuclear cellular infiltrations were the common findings (Figure 2; C2).
3.4.2.2. After 90 Days of Exposure
The deleterious effects of BHT were very clear in all specimens taken from the liver tissue of the rats administrated BHT for 90 days. The hepatic cells had marked cellular swelling, single-cell necrosis, vacuolar and hydropic degenerations, lipidosis, focal leukocytic aggregations, and vascular congestions (Figure 2; C3 and C4)
3.4.3. Effect of Ethoxyquin+Butylated Hydroxytoluene on the Liver Histoarchitecture
3.4.3.1. After 45 Days of Exposure
The combination of EQ and BHT exaggerated the histopathological alterations, compared to either of them. The hepatic cells suffered from marked cellular swelling, single-cell necrosis, vacuolar and hydropic degenerations, lipidosis, focal leukocytic aggregations, and vascular congestions (Figure 2; D1 and D2).
3.4.3.2. After 90 Days of Exposure
The worst lesions were reported in the liver tissue of the rats that administrated EQ and BHT in combination for 90 days, where most specimens exhibited marked cellular swellings, steatosis, single-cell necrosis, vacuolar and hydropic degenerations, focal coagulative necrosis, intralobular and portal leukocytic aggregations, and noticeable vascular congestions (Figure 2; D3 and D4).
4. Discussion
Although feed additives are now considered to be without potential adverse effects, and as generally recognized as safe, there have been problems concerning the safety of some of these chemicals. The results of the present study revealed that the FBW of the male Sprague-Dawley rats exposed to EQ and/or BHT for 90 days was significantly reduced, compared to that in the control group. These results are compatible with those obtained by Kim ( 22 ) who reported that EQ lowered body weight in female mice, Ohshima, Layug ( 23 ) who observed that EQ reduced body weight gain in chicks, Wang, Ai ( 24 ) who demonstrated that EQ decreased FBW in fish, as well as Saheb and Saheb ( 25 ) who found that BHT reduced the bodyweight of both male and female rats.
Powell, Connelly ( 26 ) also observed slightly lower body weights in rats administrated BHT, compared to the control group. Similarly, Kisley, Barrett ( 27 ) reported that the administration of BHT caused weight loss in rats. Faine, Rodrigues ( 28 ) also found that BHT led to decreased body weight gain in rats. Ornsrud, Arukwe ( 29 ) attributed the loss of body weight to the sensory properties (taste and odor) of EQ. It decreased nutrient adjustment efficiency and accumulated in muscles when dietary concentration elevated. The accumulated EQ was related to physiological stress, particularly inhibiting energy metabolism and adenosine triphosphate production ( 6 , 8 , 30 ). These changes may be related to the increased activity of the thyroid gland as a consequence of the enzyme induction in the liver by BHT and the increased mobilization of thyroxin (T4) in the liver. Consequently, this effect on thyroid gland increases the basal metabolic rate ( 31 ). Indeed, liver weight was significantly increased in the EQ and/or BHT groups, compared to the control group, after 45 and 90 days of exposure. These results are consistent with those obtained by Saheb and Saheb ( 25 ) who observed that relative liver weights increased in males given BHT, Bailey, Srinivasan ( 32 ) who found that relative liver weights were significantly increased in cockerels fed on EQ, Safer and al-Nughamish ( 33 ) who found that rats fed on BHT showed a significant increase in liver weights, and Al-Harbi, Al-Hasawi ( 34 ) who reported that liver weight of rats was significantly elevated following the administration of BHT. These effects might be related to the ability of EQ to react with the site I of the mitochondrial respiratory chain and caused the inhibition of oxygen consumption in the mitochondria of liver cells with mitochondrial fatty acid β-oxidation disturbance ( 35 ).
This result could be also associated with the ability of BHT to induce cellular hypertrophy and cellular proliferation ( 36 ). These results are reinforced by the histopathological findings in the liver tissue, where most specimens revealed vascular congestions and hepatocytes vacuolations, particularly in the EQ-treated rats. Moreover, the hepatic cells have marked cellular swelling and lipidosis in the BHT-treated group. Correspondingly, the present study showed that ALT and AST activity was significantly elevated in the EQ- and/or BHT-treated groups at 45 and 90 days, compared to the control group. Our results are in line with the findings obtained by Bernhard, Rasinger ( 7 ) who found a significant increase in plasma ALT levels in mice exposed to EQDM, Mean, DeäŸEr ( 15 ) who observed a significant increase in the ALT activity when BHT orally administered to female Wistar albino rats, Altuntaş and Değer ( 37 ) who showed that the level of ALT and AST were significantly increased in female rats fed on BHT, and Panicker, George ( 38 ) who reported that the level of ALT and AST were significantly increased in the serum of male rats administrated BHT. Interestingly, EQDM caused an increase in frequency and severity of necrosis in livers that was accompanied by elevated ALT and AST levels in serum ( 7 ). The presented effects may be induced by the electrophilic metabolites of BHT which can attack macromolecules of hepatocytes ( 39 ). Furthermore, during liver damage, the transport functions of hepatocytes are impaired and serum enzyme activity increased due to leakage from the plasma membrane ( 40 , 41 ). The injury of hepatic cells is often related to alterations in the serum and liver levels of some enzymes, such as ALT and AST ( 42 ). Both are present in high concentrations in the liver. These enzymes present in hepatocytes and contribute to the number of metabolic reactions. When there is any hepatocytic injury or hepatocellular reactions to toxic agents, serum levels of ALT and AST will increase. Obviously, these results are nourished by histological findings in the liver tissue, where the liver showed marked cellular swellings, steatosis, single-cell necrosis, vacuolar and hydropic degenerations, focal coagulative necrosis, intralobular and portal leukocytic aggregations, and noticeable vascular congestions.
The present study exhibited that the serum level of total bilirubin was significantly increased in the EQ- and/or BHT-treated group after 45 and 90 days. These results agree with the findings inferred by Gupta and Boobis ( 43 ), as well as Bernhard, Rasinger ( 44 ) who found an increased level of total bilirubin in the plasma of fish that exposed to the highest doses of EQ. Bernhard, Rasinger ( 44 ) observed a highly significant increase in total bilirubin in male rats fed on BHT in the diet. These may be assigned to hepatocellular damages and necrosis accompanied by elevated ALT/AST enzyme levels and decreased RBCs count. Harmonically, the histopathological findings of the liver tissue clearly explain this damage and the alteration in the liver function. Concerning serum albumin level, the results of our study demonstrated no significant effect at 45 and 90 days of exposure to EQ and/or BHT. Regarding the effect of EQ and/or BHT exposure on different hematological parameters, the present study cleared that Hb value was significantly decreased in the EQ- and/or BHT-treated groups after 45 and 90 days of exposure. These results are in agreement with EFSA ( 45 ), where the level of Hb was decreased in female rats administrated EQ, Cottrell, Andrews ( 46 ) and Nechat and kc ( 47 ) who mentioned a significant decrease in the Hb content after EQ administration. The rapid oxidation of Hb to methemoglobin or oxidative stress induced by EQ and the heavy production of reactive oxygen species are the predictable causes of Hb level changes ( 47 ). The significant changes in the Hb levels may be caused by the early decrease in food and water intake when BHT-containing diets were started. Such diet-related effects have been observed by Maejima and Nagase ( 48 ), as well as Zaporowska and Wasilewski ( 49 ). Therefore, it seems likely that the RBCs count was significantly declined in the EQ- and/or BHT-treated group after 45 and 90 days of exposure, compared to the control group. Our results agree with the findings of studies by Nechat and kc ( 47 ) who found that the count of RBCs was significantly decreased after 72 and 96 hours of exposure to EQ in fish. Jayalakshmi and Sharma ( 50 ) also studied the effect of BHT on RBCs in vitro, and the results showed excessive damage to the cell membrane of RBCs. The authors declared that BHT may cause hemolysis through modification of membrane integrity by their lipid peroxidation clear effect and reduce the concentrations of acetylcholine esterase and ATPase, both are membrane-bound enzymes ( 51 ) and related to severe anemic state or hemolyzing capacity of EQ ( 47 ). Taken together, the present study revealed that EQ- and/or BHT-treated groups had a significant decrease in WBCs count after 45 and 90 days of exposure, compared to the control group. These results agree with the findings obtained by EFSA ( 45 ). Our study stated that the platelets count was significantly decreased in the EQ- and/or BHT-treated groups, compared to the control group at 45 and 90 days. These results are comparable with the findings obtained by Cottrell, Andrews ( 46 ). The authors emphasized that a significant change in platelets was likely to be an adaptive response to further BHT-related suppression of normal platelet function ( 46 ).
Regarding the histopathological findings of the liver tissue, the results of the present study were time-dependent, where oral administration of EQ for 45 days showed few vacuolations of hepatocytes, mild degenerative and vascular changes, vacuolar and hydropic degenerations, and lipidosis. However, after 90 days, the histopathological findings were more pronounced and more severe. Moreover, the biliary system showed hyperplastic proliferation and necrosis. These results are in line with the findings stated by Bernhard, Rasinger ( 44 ) and Additives and Feed ( 45 ). The histopathological drawbacks in the liver tissue of EQ-treated rats may be attributed to the pro-oxidant effect and attack on macro and micro-molecules of EQ to the hepatic cells ( 6 ). Concerning BHT exposure, after 45 days, vacuolar and hydropic degenerations, as well as portal congestions were evident. This result agreed with that obtained by ( 52 ). Focal mononuclear aggregates, mononuclear cellular infiltrations, and lipidosis were common findings. These results are comparable to those obtained by Mean, DeäŸEr ( 15 ). However, after 90 days, histopathological findings showed single cell necrosis in the liver of male rats. This result agreed with the findings obtained by Elgazar ( 53 ) and Olsen, Meyer ( 54 ). The histopathological changes in the BHT-treated rats may be due to hepatotoxicity as a result of the formation of BHT reactive metabolite, such as the BHT-quinone method, which attacks the macromolecules of hepatocytes ( 39 ).
5. Conclusion
The current study found that BHT and EQ could induce hematological alterations and disturb the liver function and histoarchitecture in exposed rats suggesting their hepatotoxic potentials. Therefore, the utilization of such compounds should be controlled and limited to avoid the extensive alterations applied upon the vital body organs, such as the liver.
Authors' Contribution
A. H. A.: Conceptualization, investigation, supervision, writing-review and editing.
A. T. M.: Conceptualization, investigation, methodology, validation, project administration, writing-original draft, writing-review and editing.
D. Y. H.: Conceptualization, investigation, methodology, validation, formal analysis, writing-original draft.
M. H. G.: Conceptualization, formal analysis, funding acquisition, supervision, writing-review and editing. All authors read and approved the final version of this manuscript.
Ethics
According to the general rules of the National Institutes of Health for the Care and Use of Laboratory Animals in scientific investigations, the experiment was handled and affirmed by the Ethics of Animal Use in Research Committee (EAURC), Zagazig University, Egypt.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors extend their appreciation to their own University for the support and scientific encouragement.
References
- 1.Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos M, Bories G, et al. Safety and efficacy of sodium saccharin when used as a feed flavour for piglets, pigs for fattening, calves for rearing and calves for fattening. EFSA J. 2018;16(3):e05208. doi: 10.2903/j.efsa.2018.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dada AA. Improvement of tilapia (Oreochromis niloticus Linnaeus, 1758) growth performance fed three commercial feed additives in diets. J Aquac Res Development. 2015;6:1–3. [Google Scholar]
- 3.Erickson MC, Doyle MP. The Challenges of Eliminating or Substituting Antimicrobial Preservatives in Foods. Annu Rev Food Sci Technol. 2017;8:371–90. doi: 10.1146/annurev-food-030216-025952. [DOI] [PubMed] [Google Scholar]
- 4.Murbach Teles Andrade BF, Nunes Barbosa L, da Silva Probst I, Fernandes Júnior A. Antimicrobial activity of essential oils. J Essent Oil Res. 2014;26(1):34–40. [Google Scholar]
- 5.Tortosa V, Pietropaolo V, Brandi V, Macari G, Pasquadibisceglie A, Polticelli F. Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study. Molecules. 2020;25(9) doi: 10.3390/molecules25092229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaszczyk A, Augustyniak A, Skolimowski J. Ethoxyquin: An Antioxidant Used in Animal Feed. Int J Food Sci. 2013;2013:585931. doi: 10.1155/2013/585931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhard A, Rasinger JD, Wisloff H, Kolbjornsen O, Secher Myrmel L, Berntssen MHG, et al. Subchronic dietary exposure to ethoxyquin dimer induces microvesicular steatosis in male BALB/c mice. Food Chem Toxicol. 2018;118:608–25. doi: 10.1016/j.fct.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Błaszczyk A, Skolimowski J. Apoptosis and cytotoxicity caused by ethoxyquin and two of its salts. Cell Mol Biol Lett. 2005;10(1):15–21. [PubMed] [Google Scholar]
- 9.Błaszczyk A, Skolimowski J. Cytotoxicity and Genotoxicity of Ethoxyquin Used As an Antioxidant. Food Rev Int. 2015;31(3):222–35. [Google Scholar]
- 10.Chen C, Shaw YS. Cyclic metabolic pathway of a butylated hydroxytoluene by rat liver microsomal fractions. Biochem J. 1974;144(3):497–501. doi: 10.1042/bj1440497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu LL, Yu HH, Liang XF, Li N, Wang X, Li FH, et al. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides) Fish Shellfish Immunol. 2018;72:220–9. doi: 10.1016/j.fsi.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Berntssen MHG, Hoogenveen R, Bernhard A, Lundebye AK, Ornsrud R, Zeilmaker MJ. Modelling of the feed-to-fillet transfer of ethoxyquin and one of its main metabolites, ethoxyquin dimer, to the fillet of farmed Atlantic salmon (Salmon salar L.) Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019;36(7):1042–54. doi: 10.1080/19440049.2019.1605208. [DOI] [PubMed] [Google Scholar]
- 13.Achar JC, Nam G, Jung J, Klammler H, Mohamed MM. Microbubble ozonation of the antioxidant butylated hydroxytoluene: Degradation kinetics and toxicity reduction. Environ Res. 2020;186:109496. doi: 10.1016/j.envres.2020.109496. [DOI] [PubMed] [Google Scholar]
- 14.Dewhurst I. Ethoxyquin. JMPR Evaluations. 1998 [Google Scholar]
- 15.Mean S, DeäŸEr Y, Y1ld1s1m S. Effects of butylated hydroxytoluene on blood liver enzymes and liver glutathione and glutathione-dependent enzymes in rats. Bulg J Vet Med. 2018;21:461–9. [Google Scholar]
- 16.Babson AL, Shapiro PO, Williams PAR, Phillips GE. The use of a diazonium salt for the determination of glutamic-oxalacetic transaminase in serum. Clinica Chimica Acta. 1962;7(2):199–205. doi: 10.1016/0009-8981(62)90010-4. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Banerjee D. Methods of albumin estimation in clinical biochemistry: Past, present, and future. Clin Chim Acta. 2017;469:150–60. doi: 10.1016/j.cca.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Dangerfield WG, Finlayson R. Estimation of bilirubin in serum. J Clin Pathol. 1953;6(3):173–7. doi: 10.1136/jcp.6.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buttarello M, Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol. 2008;130(1):104–16. doi: 10.1309/EK3C7CTDKNVPXVTN. [DOI] [PubMed] [Google Scholar]
- 20.Suvarna KS, Layton C, Bancroft JD. Bancroft's theory and practice of histological techniques E-Book: Elsevier Health Sciences. 2018. [Google Scholar]
- 21.Burton JL. The external examination: An often-neglected autopsy component. Current Diagnostic Pathology. 2007;13(5):357–65. [Google Scholar]
- 22.Kim HL. Preparation and dietary effects of ethoxyquin hydrochloride. J Toxicol Environ Health. 1985;15(5):663–71. doi: 10.1080/15287398509530694. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima M, Layug DV, Yokota H-o, Ostrowski-Meissner HT. Effect of graded levels of ethoxyquin in alfalfa leaf extracts on carotenoid and cholesterol concentrations in chicks. Anim Feed Sci Technol. 1996;62(2):141–50. [Google Scholar]
- 24.Wang J, Ai Q, Mai K, Xu W, Xu H, Zhang W, et al. Effects of dietary ethoxyquin on growth performance and body composition of large yellow croaker Pseudosciaena crocea. Aquaculture. 2010;306(1):80–4. [Google Scholar]
- 25.Saheb JL, Saheb SA. Toxic effects of butylated hydroxytoluene on rats. Can J Comp Med. 1977;41(2):195–201. [PMC free article] [PubMed] [Google Scholar]
- 26.Powell CJ, Connelly JC, Jones SM, Grasso P, Bridges JW. Hepatic responses to the administration of high doses of BHT to the rat: Their relevance to hepatocarcinogenicity. Food Chem Toxicol. 1986;24(10):1131–43. doi: 10.1016/0278-6915(86)90299-1. [DOI] [PubMed] [Google Scholar]
- 27.Kisley LR, Barrett BS, Dwyer-Nield LD, Bauer AK, Thompson DC, Malkinson AM. Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis. 2002;23(10):1653–60. doi: 10.1093/carcin/23.10.1653. [DOI] [PubMed] [Google Scholar]
- 28.Faine LA, Rodrigues HG, Galhardi CM, Ebaid GM, Diniz YS, Fernandes AA, et al. Butyl hydroxytoluene (BHT)-induced oxidative stress: effects on serum lipids and cardiac energy metabolism in rats. Exp Toxicol Pathol. 2006;57(3):221–6. doi: 10.1016/j.etp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Ornsrud R, Arukwe A, Bohne V, Pavlikova N, Lundebye AK. Investigations on the metabolism and potentially adverse effects of ethoxyquin dimer, a major metabolite of the synthetic antioxidant ethoxyquin in salmon muscle. J Food Prot. 2011;74(9):1574–80. doi: 10.4315/0362-028X.JFP-10-547. [DOI] [PubMed] [Google Scholar]
- 30.EUN J-B, Hearnsberger JO, Kim JM. Antioxidants, Activators, and Inhibitors Affect the Enzymic Lipid Peroxidation System of Catfish Muscle Microsomes. J Food Sci. 1993;58(1):71–4. [Google Scholar]
- 31.Søndergaard D, Olsen P. The effect of butylated hydroxytoluene (BHT) on the rat thyroid. Toxicol Lett. 1982;10(2):239–44. doi: 10.1016/0378-4274(82)90081-9. [DOI] [PubMed] [Google Scholar]
- 32.Bailey CA, Srinivasan LJ, McGeachin RB. The effect of ethoxyquin on tissue peroxidation and immune status of single comb White Leghorn cockerels. Poult Sci. 1996;75(9):1109–12. doi: 10.3382/ps.0751109. [DOI] [PubMed] [Google Scholar]
- 33.Safer AM, al-Nughamish AJ. Hepatotoxicity induced by the anti-oxidant food additive, butylated hydroxytoluene (BHT), in rats: an electron microscopical study. Histol Histopathol. 1999;14(2):391–406. doi: 10.14670/HH-14.391. [DOI] [PubMed] [Google Scholar]
- 34.Al-Harbi H, Al-Hasawi Z, Binnaser Y, Al-Hasawi R, Al-Solami F, Al-Ghamdi A. Effect of Polyethylene and Butylated Hydroxytoluene on the histological structure and some enzymes of rat liver. J Biosci Appl Res. 2020;6(2):68–82. [Google Scholar]
- 35.Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44(1):34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 36.Briggs D, Lok E, Nera EA, Karpinski K, Clayson DB. Short-term effects of butylated hydroxytoluene on the Wistar rat liver, urinary bladder and thyroid gland. Cancer Lett. 1989;46(1):31–6. doi: 10.1016/0304-3835(89)90211-5. [DOI] [PubMed] [Google Scholar]
- 37.Altuntaş G, Değer Y. The effects of butylated hydroxyl toluene on the total antioxidant status/total oxidant stress and biochemical parameters in rats. World J Pharm Pharm Sci. 2017;6:199–210. [Google Scholar]
- 38.Panicker V, George S, Krishna D. Toxicity study of butylated hydroxyl toluene (BHT) in rats. Int J Pharm Sci. 2014;3:758–63. [Google Scholar]
- 39.Nakagawa Y, Tayama K. Nephrotoxicity of butylated hydroxytoluene in phenobarbital-pretreated male rats. Arch Toxicol. 1988;61(5):359–65. doi: 10.1007/BF00334616. [DOI] [PubMed] [Google Scholar]
- 40.Jang IS, Chae KR, Kang TS, Kim YK, Kim CK, Hwang JH, et al. Effects of Long-Term Vitamin E and Butylated Hydroxytoluene Supplemented Diets on Murine Intestinal and Hepatic Antioxidant Enzyme Activities. Asian-Australas J Anim Sci. 1999;12(6):932–8. [Google Scholar]
- 41.Lin HM, Yen FL, Ng LT, Lin CC. Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J Ethnopharmacol. 2007;111(1):129–36. doi: 10.1016/j.jep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa Y, Tayama K, Nakao T, Hiraga K. On the mechanism of butylated hydroxytoluene-induced hepatic toxicity in rats. Biochem Pharmacol. 1984;33(16):2669–74. doi: 10.1016/0006-2952(84)90643-9. [DOI] [PubMed] [Google Scholar]
- 43.Gupta PK, Boobis A. ETHOXYQUIN (addendum). Joint FAO/WHO Meeting on Pesticide Residues: Pesticide Residues in Food. 2005. pp. 20–9. [Google Scholar]
- 44.Bernhard A, Rasinger JD, Betancor MB, Caballero MJ, Berntssen MHG, Lundebye AK, et al. Tolerance and dose-response assessment of subchronic dietary ethoxyquin exposure in Atlantic salmon (Salmo salar L.) PLoS One. 2019;14(1):e0211128. doi: 10.1371/journal.pone.0211128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Additives EPo, Feed PoSuiA. Safety and efficacy of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species. 2015;13(11):4272. doi: 10.2903/j.efsa.2022.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottrell S, Andrews CM, Clayton D, Wild BJ, Powell CJ. Haematological and platelet effects of butylated hydroxytoluene (BHT, E321) Comp Haematol Int. 1994;4(2):102–7. [Google Scholar]
- 47.Nechat S, kc C. Ethoxyquin on Haematological and Biochemical Parameters in the Fish, Anabas testudineus (Bloch, 1792) Int J Zool Invest. 2017;3:1–10. [Google Scholar]
- 48.Maejima K, Nagase S. Effect of starvation and refeeding on the circadian rhythms of hematological and clinico-biochemical values, and water intake of rats. Jikken Dobutsu. 1991;40(3):389–93. doi: 10.1538/expanim1978.40.3_389. [DOI] [PubMed] [Google Scholar]
- 49.Zaporowska H, Wasilewski W. Significance of reduced food and water consumption in rats intoxicated with vanadium. Comp Biochem Physiol C Toxicol. 1991;99(3):349–52. doi: 10.1016/0742-8413(91)90254-q. [DOI] [PubMed] [Google Scholar]
- 50.Jayalakshmi CP, Sharma JD. Effect of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) on rat erythrocytes. Environ Res. 1986;41(1):235–8. doi: 10.1016/s0013-9351(86)80185-2. [DOI] [PubMed] [Google Scholar]
- 51.Kumar PS, George T, Bai NJ, Krishnamurthy S. Effect of lipid antioxidants on rat erythrocyte hemolysis. Int J Vitam Nutr Res. 1979;49(4):352–8. [PubMed] [Google Scholar]
- 52.Ibrahim IA, Hassan AGA, Abdel-Hamid A, Shalaby , Dessouki AA, Habib D. Biochemical Studies on The Effect of Sodium Nitrite and Butylated Hydroxytoluene in Rats. CVMJ. 2014;14(2):265–278. [Google Scholar]
- 53.Elgazar AF, editor. Effects of Butylated Hydroxytoluene and Butylated Hydroxyanisole against Hepatotoxicity Induced by Carbon Tetrachloride in Rats. 2013. [Google Scholar]
- 54.Olsen P, Meyer O, Bille N, Würtzen G. Carcinogenicity study on butylated hydroxytoluene (BHT) in Wistar rats exposed in utero. Food Chem Toxicol. 1986;24(1):1–12. doi: 10.1016/0278-6915(86)90256-5. [DOI] [PubMed] [Google Scholar]