Abstract
Analysis of the transport functions of individual Candida albicans plasma membrane drug efflux pumps is hampered by the multitude of endogenous transporters. We have stably expressed C. albicans Cdr1p, the major pump implicated in multiple-drug-resistance phenotypes, from the genomic PDR5 locus in a Saccharomyces cerevisiae mutant (AD1-8u−) from which seven major transporters of the ATP-binding cassette (ABC) family have been deleted. High-level expression of Cdr1p, under the control of the S. cerevisiae PDR5 promoter and driven by S. cerevisiae Pdr1p transcriptional regulator mutation pdr1-3, was demonstrated by increased levels of mRNA transcription, increased levels of nucleoside triphosphatase activity, and immunodetection in plasma membrane fractions. S. cerevisiae AD1-8u− was hypersensitive to azole antifungals (the MICs at which 80% of cells were inhibited [MIC80s] were 0.625 μg/ml for fluconazole, <0.016 μg/ml for ketoconazole, and <0.016 μg/ml for itraconazole), whereas the strain (AD1002) that overexpressed C. albicans Cdr1p was resistant to azoles (MIC80s of fluconazole, ketoconazole, and itraconazole, 30, 0.5, and 4 μg/ml, respectively). Drug resistance correlated with energy-dependent drug efflux. AD1002 demonstrated resistance to a variety of structurally unrelated chemicals which are potential drug pump substrates. The controlled overexpression of C. albicans Cdr1p in an S. cerevisiae background deficient in other pumps allows the functional analysis of pumping specificity and mechanisms of a major ABC transporter involved in drug efflux from an important human pathogen.
Candida albicans is an asexual diploid fungus that causes opportunistic infections commonly seen in immunocompromised and debilitated patients (9, 30). An estimated 33 to 55% of patients with human immunodeficiency virus infection and AIDS contract oropharyngeal candidosis (34), and the synthetic triazole fluconazole has been the mainstay of their treatment. The widespread use of prolonged fluconazole therapy has increased the incidence of treatment failure due to fluconazole-resistant C. albicans (3, 14, 21, 34, 42). A number of studies have identified the major azole resistance mechanisms (1, 20, 38, 41, 42, 44–46). These include overexpression of, or mutations in, the drug target, 14α-sterol demethylase; mutations in other parts of the sterol biosynthesis pathway; and, most commonly, overexpression of drug efflux proteins.
C. albicans possesses transporters such as Cdr1p and Cdr2p with homology to proteins of the ATP-binding cassette (ABC) family (10, 16, 18, 19, 31), as well as Benrp, which has homology to the major facilitator superfamily (MFS) class of drug-proton antiport efflux pumps (1, 5, 36, 46). The BENr gene encodes a transporter associated with resistance to benomyl and methotrexate when it is expressed in Saccharomyces cerevisiae. The C. albicans CDR1 gene is a homologue of S. cerevisiae PDR5, which encodes a multidrug efflux pump, and CDR1 is the gene most often associated with energy-dependent drug efflux in fluconazole-resistant clinical isolates (37, 38, 44).
We have developed a yeast secretory vesicle drug pump assay to investigate drug translocation mechanisms for specific transporters heterologously expressed in S. cerevisiae (5). A limitation of this assay is that the vesicles contain other endogenous membrane transporters. S. cerevisiae possesses 29 genes with homology to ABC transporters (10), although only a subset of these is expressed in membrane vesicles. Recently, several S. cerevisiae mutants have been developed from which genes encoding major ABC transporters have been deleted (11). In addition, the S. cerevisiae pdr1-3 mutation has been shown to hyperinduce the PDR5 gene promoter and cause high-level functional overexpression of the Pdr5p protein in yeast plasma membranes (2, 7, 12). In the present study our objective was to investigate C. albicans Cdr1p by stably expressing functional Cdr1p in S. cerevisiae. Overexpression sufficient to demonstrate a phenotype was achieved by replacing the chromosomal copy of PDR5 with C. albicans CDR1 in a pdr1-3 mutant depleted of endogenous membrane transporters. Such a heterologous expression system would be valuable in studies to determine pump specificities and to screen for pump antagonists.
MATERIALS AND METHODS
Bacterial and yeast strains and growth media.
Plasmids were maintained in Escherichia coli DH5α. The CDR1 gene was obtained from C. albicans ATCC 10261. The S. cerevisiae strains used in the study were AD1-8u− (MATα pdr1-3, his1 ura3 Δyor1::hisG Δsnq2::hisG Δpdr5::hisG Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG; AD1-8u− was derived from AD12345678 [11]) and AD124567 (MATα pdr1-3 his1 Δyor1::hisG Δsnq2::hisG Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG [11]). E. coli was cultured in Luria-Bertani medium (35). C. albicans was maintained on YEPD (yeast extract, 10 g/liter; Bacto Peptone, 20 g/liter; glucose, 20 g/liter), and S. cerevisiae was maintained on YEPD, complete synthetic medium (CSM; Bio 101, Vista, Calif.), or CSM without uracil (CSM − URA; Bio 101), as required.
Plasmid construction and yeast transformation.
Expand DNA polymerase (Roche Diagnostics N.Z. Ltd., Auckland, New Zealand) was used to amplify by PCR the CDR1 open reading frame (ORF) and transcriptional termination region (4.8 kb) from C. albicans ATCC 10261 genomic DNA with primers containing SpeI restriction sites, primers 5′-CTTTAAAAGGTCAACTAGTAAAAAATTATG-3′ and 5′-CAATAATACACTAGTTTGCAACGGAAG-3′. The PCR product was digested with SpeI and was cloned into plasmid pSK-PDR5PPUS (see Fig. 1) that had previously been cut with SpeI and dephosphorylated with alkaline phosphatase (New England Biolabs, Beverly, Mass.). The orientation of the CDR1 ORF was confirmed by sequencing to be the same as that of PDR5p, and the plasmid was designated pKEN1002. Plasmid pKEN1002 was linearized with XbaI and was used to transform S. cerevisiae AD1-8u− to for uracil prototrophy (Ura+) by the lithium acetate transformation protocol (Alkali-Cation Yeast kit; Bio-101). The entire CDR1 ORF DNA in pKEN1002 was sequenced, and the CDR1 ORFs from C. albicans ATCC 10261 and S. cerevisiae AD1-8u−/pKEN1002 transformant AD1002 were amplified from genomic DNA by PCR with Pfx DNA polymerase (Gibco BRL, Life Technologies, Rockville, Md.) and sequenced.
FIG. 1.
Construction of plasmid pKEN1002 (A) and integration of C. albicans CDR1 at the chromosomal PDR5 locus of S. cerevisiae AD1-8u− (B).
Northern analysis of RNA extracted from S. cerevisiae.
Total RNA was extracted from S. cerevisiae as described previously (1). RNA (20 μg) was electrophoresed in agarose gels, vacuum blotted onto a Hybond+ nylon membrane (Amersham Pharmacia Biotech New Zealand, Auckland, New Zealand), and fixed by UV irradiation. Membranes were hybridized with [α-32P]dCTP-labeled probes under high-stringency conditions as described by Cannon et al. (6). A C. albicans CDR1-specific probe (ORF nucleotides [nt] 1 to 497) was generated by PCR amplification, and the S. cerevisiae PMA1-specific probe (ORF nt −835 to 1598) was obtained as a 2.4-kb BamHI fragment from plasmid pDP100 (40).
Immunodetection of Cdr1p.
Crude protein extracts were prepared from S. cerevisiae cells grown in YEPD broth to the mid-exponential phase. Plasma membrane fractions of these cells were obtained by sucrose gradient centrifugation as described by Monk et al. (29). Protein samples (40 μg) were separated by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels (8% [wt/vol] acrylamide) and either stained with Coomassie blue or electroblotted (100 V, 1 h, 4°C) onto nitrocellulose membranes (Highbond-C; Amersham). Western blots were incubated with a 1:200 dilution of anti-Cdr1p antibodies (provided by D. Sanglard, Institute of Microbiology, University Hospital, Lausanne, Switzerland). Immunoreactivity was detected with horseradish peroxidase-labeled swine anti-rabbit immunoglobulin G antibodies (Dako Corp., Carpinteria, Calif.) at a 1:500 dilution.
Genomic DNA extraction and Southern analysis of CDR1 gene integrated into S. cerevisiae genome.
Genomic DNA was prepared from S. cerevisiae cells as described previously (39). Genomic DNA (5 μg) was digested with a restriction endonuclease (EcoRV, SpeI, BamHI, PstI, or EcoRI; New England Biolabs), separated in a 0.75% agarose gel, and transferred to a Hybond+ nylon membrane (Amersham). Membranes were hybridized with a [α-32P]dCTP-labeled, C. albicans CDR1-specific probe under high-stringency conditions (6).
MIC determination.
The MICs of antifungal agents for S. cerevisiae cells were determined by a microdilution test based on the macrodilution reference method of the National Committee for Clinical Laboratory Standards (29a). Cells (10-μl cell suspension, 2 × 105 cells/ml) were inoculated into 90 μl of CSM − URA buffered with 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 18 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES, pH 7.0) and containing 0.67% (wt/vol) yeast nitrogen base (YNB) in microtiter plate wells. For uracil-requiring strain AD1-8u−, the medium was supplemented with 0.02% (wt/vol) uridine. The wells contained doubling dilutions of antifungal agents (final concentrations were as follows: fluconazole, 30 to 0.058 μg/ml; itraconazole and ketoconazole, 8 to 0.016 μg/ml). The microtiter plates were incubated at 30°C for 48 h with shaking, and then the growth of cells in individual wells (the optical density at 590 nm [OD590]) was measured with a microplate reader (EL 340; Bio-Tek, Winooski, Vt.). The MIC at which 80% of cells were inhibited (MIC80) was the lowest concentration of drug that inhibited the growth yield by at least 80% compared to the growth found for a no-drug control.
NTPase assays.
Yeasts were grown in YEPD (pH 5.5) at 30°C until they reached the late exponential phase of growth (OD600, 7), washed twice in ice-cold distilled water, and incubated on ice for 2 h to minimize glucose-stimulated Pma1p activity. The cells were resuspended in homogenizing medium (50 mM Tris [pH 7.5], 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) and disrupted with a Braun homogenizer. Cell debris and unbroken cells were removed by centrifugation at 3,000 × g at 4°C for 10 min. A crude membrane fraction was isolated from the cell-free supernatant by centrifugation at 30,000 × g at 4°C for 45 min. Plasma membranes were prepared by centrifugation of the supernatant obtained after selective precipitation of mitochondria at pH 5.2, as described by Goffeau and Dufour (15). The plasma membranes were resuspended in 10 mM Tris (pH 7.0)–0.5 mM EDTA–20% (vol/vol) glycerol and were stored at −80°C. The protein concentration was determined by a micro-Bradford (Bio-Rad Laboratories, Hercules, Calif. [4]) assay with bovine gamma globulin as the standard. Nucleoside triphosphatase (NTPase) activity was measured by incubating the plasma membrane fractions (10 μg) at 30°C in a final volume of 120 μl containing 6 mM nucleoside triphosphate (NTP) and 7 mM MgSO4 in 59 mM MES-Tris buffer (pH 6.0 to 8.0). To eliminate possible contributions from nonspecific phosphatases and vacuolar or mitochondrial ATPases, 0.2 mM ammonium molybdate, 50 mM KNO3, and 10 mM NaN3 respectively, were included in the assay mixtures (29). Other ATPase inhibitors (20 μM oligomycin, 20 μM aurovertin B, or 100 μM vanadate) were added to the reaction mixture where indicated. After 30 min the reaction was stopped by the addition of 130 μl of a solution containing 1% (wt/vol) SDS, 0.6 M H2SO4, 1.2% (wt/vol) ammonium molybdate, and 1.6% (wt/vol) ascorbic acid. The amount of inorganic phosphate released from NTPs was measured at 750 nm after 10 min of incubation at room temperature.
Fluconazole accumulation by S. cerevisiae cells.
The net rate of fluconazole accumulation by early-exponential-phase S. cerevisiae cells was measured as described previously (1). To examine the energy dependence of fluconazole accumulation, the assay mixtures contained 20 mM sodium azide.
Rhodamine 6G efflux by S. cerevisiae cells.
The efflux of rhodamine 6G (Sigma) from intact S. cerevisiae cells was determined by adapting the method described by Kolaczkowski et al. (22). Yeast cells from YEPD cultures in the exponential growth phase (OD600, 0.5) were collected by centrifugation (3,000 × g, 5 min, 20°C) and washed three times with water. The cells were resuspended at a concentration of 0.5 × 106 to 1.0 × 107 cells per ml in HEPES-NaOH (50 mM; pH 7.0) containing 5 mM 2-deoxyglucose and 10 μM rhodamine 6G. In some experiments fluconazole (10 μM) was also added. Cell suspensions were incubated at 30°C with shaking (200 rpm) for 90 min to allow rhodamine accumulation under glucose starvation conditions. The starved cells were washed twice in HEPES-NaOH, and portions (400 μl) were incubated at 30°C for 5 min before the addition of glucose (final concentration, 2 mM) to initiate rhodamine efflux. At specified intervals after the addition of glucose, the cells were removed by centrifugation, and triplicate 100-μl volumes of the cell supernatants were transferred to the wells of 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark). The rhodamine fluorescence of the samples was measured with a Cary Eclipse spectrofluorimeter (Varian Inc., Mulgrave, Victoria, Australia). The excitation wavelength was 529 nm (slit 5), and the emission wavelength was 553 nm (slit 10).
Drug susceptibility disk assays.
Drug susceptibility was measured by disk assays on CSM − URA plates (containing 1.5% [wt/vol] agar). The plates were seeded with yeast cells suspended in top agar (5 ml, 105 cells/ml). For the uracil-dependent parental strain, agar was supplemented with 0.02% uridine. Five microliters of drug solution or solvent control was spotted onto sterile Whatman paper disks on the top agar. The indicated amounts of the following drugs were applied to individual disks: fluconazole (Pfizer Ltd., Sandwich, Kent, United Kingdom), 6.5 nmol; ketoconazole (Janssen Research Foundation, Beerse, Belgium), 0.094 nmol; itraconazole (Janssen), 0.35 nmol; miconazole (Janssen), 0.084 nmol; amphotericin B (E. R. Squibb & Sons, Princeton, N.J.), 54 nmol; rhodamine 6G (Sigma, Penorse, Auckland, New Zealand), 10 nmol; rhodamine 123 (Sigma), 50 nmol; trifluoperazine (Sigma), 100 nmol; benomyl (Nippon Roche), 10 nmol; cycloheximide (Sigma), 5 nmol; carbonyl cyanide p-chlorophenylhydrazone (CCCP; Sigma), 490 nmol; oligomycin (Sigma), 10 nmol; nigericin (Sigma), 100 nmol; tamoxifen (Sigma), 25 nmol; naftifine (Novartis), 50 nmol; quinidine (Sigma), 500 nmol; valinomycin (Sigma), 20 nmol; verapamil (Sigma), 1,000 nmol. The agar plates were incubated at 30°C for 48 h or until clear growth inhibition zones were visible.
RESULTS
Integration of C. albicans CDR1 gene at the PDR5 locus in S. cerevisiae AD1-8u−.
The function of C. albicans Cdr1p was studied with diminished-background endogenous ABC transporter interference by expressing CDR1 in the S. cerevisiae pdr1-3 mutant AD1-8u−, from which seven major ABC transporters have been deleted. This was achieved by using a new protein overexpression system (11) that uses the multidrug resistance regulatory mutation pdr1-3 to up-regulate the PDR5 promoter and that results in overexpression of the Pdr5p protein primarily located in plasma membranes (2, 12). Hyperinduction of Cdr1p was achieved by integrating the CDR1 ORF at the S. cerevisiae AD1-8u− PDR5 locus downstream from the PDR5 promoter. First, the CDR1 ORF and its transcription terminator region was amplified from C. albicans ATCC 10261 genomic DNA by PCR and cloned into the SpeI site in plasmid pSK-PDR5PPUS, which is located between the PDR5 promoter and the PDR5 stop codon (Fig. 1). The resulting plasmid, pKEN1002, was linearized with XbaI and transformed into S. cerevisiae AD1-8u− (ΔPDR5; from which nt −360 to 1163 were deleted) with selection for Ura+ transformants. This selection protocol was made possible by the presence of the S. cerevisiae URA3 gene in the PDR5 terminator region of pKEN1002.
The Ura+ S. cerevisiae transformants demonstrated lower levels of sensitivity to azoles than the parental strain, and one transformant (AD1002) was selected for further analysis. The doubling time of AD1002 in YEPD- and CSM-based media was the same as that for the parental strain. To confirm the integration of CDR1 at the PDR5 locus in AD1002, uncut total DNA and restricted genomic DNAs were hybridized with a C. albicans CDR1-specific probe. The probe hybridized with uncut genomic DNA, and there was no evidence of episomal plasmid. The hybridization of the probe with single bands of genomic DNA of the expected sizes after digestion with five separate restriction endonucleases (EcoRV, 5,467 bp; SpeI, 4,776 bp; BamHI, 4,272 bp; PstI, 2,236 bp; EcoRI, 1,042 bp) indicated that a single integration event had occurred at the PDR5 locus. The sequence of the CDR1 gene from donor strain C. albicans ATCC 10261 was determined and compared with the sequence of a C. albicans strain (strain 1001 [32]) in the GenBank database (accession number X77589). There were 45 nucleotide differences (over the ORF of 4,503 nt) between the two DNA sequences but only two amino acid changes: F427Y and V916I substitutions in ATCC 10261. The paucity and conservative nature of the amino acid substitutions indicate that the CDR1 gene is functionally highly conserved between strains. The CDR1 gene from plasmid pKEN1002, which had been passaged through E. coli, had differences of 21 nt from the template ATCC 10261 sequence but only 5 amino acid differences: E214Q, S842T, S1021L, E1177K, and A1416E substitutions in pKEN1002. By contrast, there were no nucleotide differences between the sequence of CDR1 amplified from the S. cerevisiae AD1002 transformant and the sequence of CDR1 in plasmid pKEN1002 used in the transformation. The latter result confirms the high fidelity of the DNA polymerase used to amplify the gene from C. albicans genomic DNA and suggests that the differences between the pKEN1002 and C. albicans ATCC 10261 CDR1 sequences were selected in E. coli. The mutations in pKEN1002 suggest that Cdr1p expression in E. coli is not favored, an hypothesis supported by the observation that only one E. coli strain that had been transformed with pKEN1002 was obtained, which precluded testing of other clones for similar mutations. None of the CDR1 sequences (C. albicans 1002, C. albicans ATCC 10261, pKEN1002, or S. cerevisiae AD1002) contained the CTG codon, which is decoded by S. cerevisiae as leucine but which is decoded by C. albicans as serine. Substitution of leucine for serine in Cdr1p heterologously expressed in S. cerevisiae AD1002, with consequential effects on protein function, is therefore not a problem.
Expression of C. albicans CDR1 in S. cerevisiae AD1002.
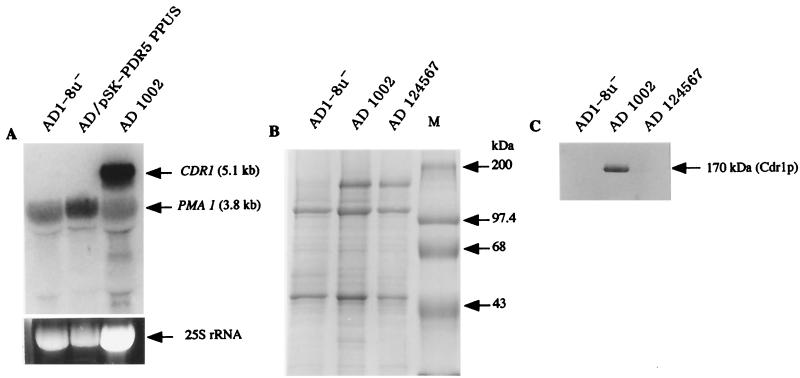
The expression of C. albicans CDR1 in AD1002 was investigated by a Northern analysis of total RNA and by immunodetection in plasma membrane-enriched subcellular fractions. The levels of expression of PMA1 and CDR1 mRNAs by S. cerevisiae AD1-8u− and by this strain transformed with pSK-PDR5PPUS or pKEN1002 (AD1002) were measured. PMA1 mRNA, which encodes the constitutively expressed plasma membrane H+-ATPase, was expressed in all strains (Fig. 2A). CDR1 mRNA was expressed only in cells transformed with pKEN1002. Expression of Cdr1p (Fig. 2B) was examined by SDS-polyacrylamide gel electrophoresis (PAGE) analysis of plasma membrane proteins from these strains and S. cerevisiae AD124567, which overexpresses Pdr5p (12). No major plasma membrane protein bands of the size expected for ABC transporters (170 kDa [10, 24, 32]) were detected by Coomassie blue staining in samples from parental strain AD1-8u−. This confirmed the depletion of endogenous pumps in this strain with multiple deletions. In contrast, samples from both AD1002 and AD124567 contained a major protein band at 170 kDa which accounted for 10 to 20% of the Coomassie blue-stained plasma membrane protein. Only the 170-kDa protein from AD1002 reacted with anti-Cdr1p antibodies (Fig. 2C).
FIG. 2.
Expression of C. albicans CDR1 mRNA and Cdr1p in S. cerevisiae AD1002. (A) Total RNA (20 μg) from parental AD1-8u− cells, AD1-8u− cells transformed with shuttle plasmid pSK-PDR5PPUS, or AD1002 cells was hybridized with a mixture of [α-32P] dCTP-labeled C. albicans CDR1 and S. cerevisiae PMA1 (control) probes. The lower part of panel A shows a portion of the ethidium bromide-stained agarose gel before vacuum blotting. (B) Plasma membrane proteins separated through an 8% polyacrylamide gel and stained with Coomassie blue. Lane M, molecular mass markers. (C) Plasma membrane proteins separated through an 8% polyacrylamide gel, electroblotted onto nitrocellulose, and incubated with anti-C. albicans Cdr1p antibodies. Antibodies were detected with horseradish peroxidase-labeled anti-immunoglobulin G.
Antifungal sensitivities of S. cerevisiae cells expressing Cdr1p.
The phenotypic effects of Cdr1p expression in S. cerevisiae strains with a depleted ABC transporter background on antifungal sensitivity was measured. Parental strain AD1-8u− was exquisitely sensitive to fluconazole, ketoconazole, and itraconazole (Table 1). Transformant AD1002 was markedly less sensitive to fluconazole, ketoconazole, and itraconazole, for which there were 48-, >31-, and >250-fold increases in the MICs, respectively (Table 1). Thus, expression of Cdr1p in this transformant conferred cross-resistance to different azole antifungal drugs, as has been shown in other S. cerevisiae strains and in C. albicans (1, 32). These results, together with those of SDS-PAGE, Western, and Northern analyses, indicated that the C. albicans drug resistance gene, CDR1, is functionally overexpressed in S. cerevisiae AD1002.
TABLE 1.
Antifungal sensitivities of S. cerevisiae cells expressing Cdr1p
| S. cerevisiae strain | MIC80 (μg/ml)
|
||
|---|---|---|---|
| Fluconazole | Ketoconazole | Itraconazole | |
| AD1-8u−a | 0.625 | <0.016 | <0.016 |
| AD1002b | 30 | 0.5 | 4 |
Parental strain.
Strain expressing Cdr1p (the MICs were unaffected by supplementing the medium with uridine [0.02%; wt/vol]).
NTPase activity of AD1002.
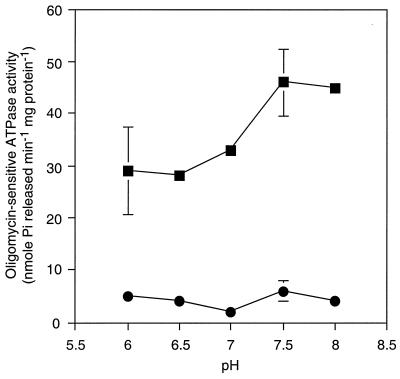
Plasma membrane fractions from S. cerevisiae AD1002 possessed at least an order of magnitude higher oligomycin-sensitive ATPase activity than parental strain AD1-8u− over the pH range 6.0 to 8.0 (Fig. 3). This activity had a pH optimum of about 7.5, and thus, the ATPase activity of AD1002 was readily distinguished from the Pma1p ATPase activity of S. cerevisiae, which has a pH optimum of 6.0 (12). Furthermore, the activity of Pma1p is insensitive to oligomycin (29) and is specific for ATP (12). C. albicans Cdr1p expressed in S. cerevisiae AD1002 also showed oligomycin-sensitive UTPase, CTPase, and GTPase activities similar to the ATPase activity, and all these NTPase activities had alkaline pH optima (Table 2). The activity of each NTPase of AD1002 was sensitive to 100 μM vanadate but was insensitive to 20 μM aurovertin B. Mitochondrial ATPase activity therefore made a negligible contribution to the ATPase activities of these membrane preparations. The ATPase activities of AD1002 were unaffected by the addition of fluconazole (up to 80 μM) to the assay mixture, indicating that this substrate does not stimulate ATP hydrolysis.
FIG. 3.
Oligomycin-sensitive C. albicans Cdr1p ATPase activity in plasma membrane fractions. Membrane fractions were isolated from from S. cerevisiae AD1002 (■) or parental AD1-8u− cells (●). ATPase assays were carried out at 30°C for 30 min as described in Materials and Methods. The oligomycin-sensitive activity was determined as the difference in ATPase activity in the presence and absence of 20 μM oligomycin. The ATPase activity was completely sensitive to vanadate (100 μM) and was insensitive to aurovertin B (20 μM). The results are the means ± standard deviations of four determinations carried out with two membrane preparations.
TABLE 2.
Oligomycin-sensitive NTPase activities in plasma membrane fractions of parental strain S. cerevisiae AD1-8u− and S. cerevisiae AD1002 expressing Cdr1p
| S. cerevisiae strain | Oligomycin-sensitive NTPase activity (nmol of Pi min−1 mg of protein−1)a
|
|||||
|---|---|---|---|---|---|---|
| UTPase
|
CTPase
|
GTPase
|
||||
| pH 6.5 | pH 7.5 | pH 6.5 | pH 7.5 | pH 6.5 | pH 7.5 | |
| AD1-8u− | 1 | 0 | 1 | 0 | 0 | 0 |
| AD1002 | 16 | 30 | 24 | 40 | 27 | 46 |
NTPase activities were determined in assays similar to those that provided the data presented in Fig. 3. Values represent the differences in NTPase activities measured in the presence and absence of 20 μM oligomycin. The results are the means of two experiments, which did not vary more than 10%.
Fluconazole accumulation by AD1002.
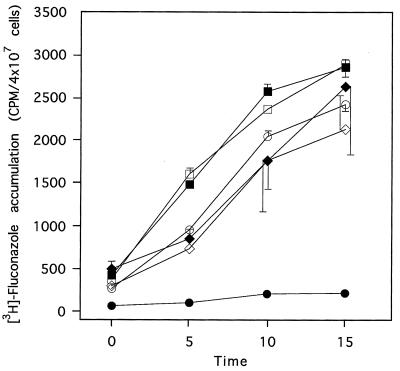
The levels of accumulation of [3H] fluconazole by S. cerevisiae AD1-8u− and transformants AD1002 and AD/pSK-PDR5PPUS were measured (Fig. 4). Energized AD1-8u− or AD/pSK-PDR5PPUS cells accumulated fluconazole over a 15-min time course, whereas AD1002 cells did not. Addition of the respiratory chain inhibitor sodium azide to the assay mixture had no effect on the level of accumulation of fluconazole by AD1-8u− or AD/pSK-PDR5PPUS cells but greatly increased the level of accumulation by AD1002 cells. These results were consistent with the drug resistance of AD1002 cells being due to energy-dependent drug efflux and indicated that the overexpressed ABC-type transporter Cdr1p functioned as expected.
FIG. 4.
Azide-dependent fluconazole accumulation by S. cerevisiae AD1002. [3H]fluconazole accumulation was measured in the presence (open symbols) or absence (closed symbols) of sodium azide (20 mM). The strains used were AD1-8u− (■, □), AD/pSK-PDR5PPUS (⧫, ◊), and AD1002 (●, ○). Results are the means ± standard deviations of six separate determinations with two batches of cells.
Cdr1p-mediated rhodamine efflux.
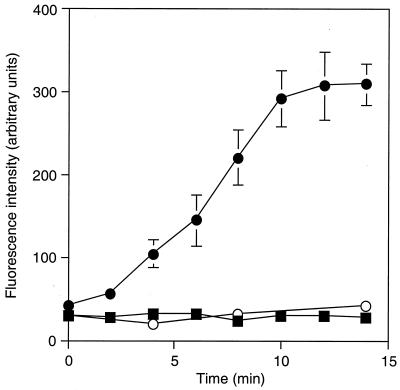
The glucose-dependent efflux of rhodamine 6G from S. cerevisiae AD1002 was demonstrated (Fig. 5). Efflux from deenergized (by incubation with 2-deoxyglucose), rhodamine 6G-preloaded cells of AD1002 required the presence of glucose; by 10 min following glucose addition the extracellular rhodamine 6G concentration had increased more than sixfold. In contrast, efflux of rhodamine 6G from parental AD1-8u− cells was undetectable in the presence of glucose (or the absence of glucose [data not shown]). Both strains showed similar survival rates following rhodamine pretreatment and accumulated equivalent amounts of rhodamine during pretreatment in the presence of 2-deoxyglucose, as demonstrated by measurement of the amount of rhodamine 6G released following cell lysis. Fluconazole inhibited rhodamine efflux from AD1002 cells. The addition of fluconazole (10 μM) during preincubation of AD1002 cells with rhodamine in the presence of 2-deoxyglucose resulted in a 32% reduction in the concentration of released fluorescence 10 min following the addition of glucose.
FIG. 5.
Energy-dependent Cdr1p-mediated efflux of rhodamine 6G from yeast cells. Deenergized cells were preloaded with rhodamine 6G as described in Materials and Methods. The efflux of rhodamine was followed by direct measurement of the fluorescence in cell supernatants following the addition of glucose (2 mM) to suspensions of S. cerevisiae AD1002 (●) or AD1-8u− (■) cells. The fluorescence in the supernatants of AD1002 cells incubated in the absence of added glucose is also shown (○). The results are the means ± standard deviations of triplicate determinations from a representative experiment which was undertaken three times.
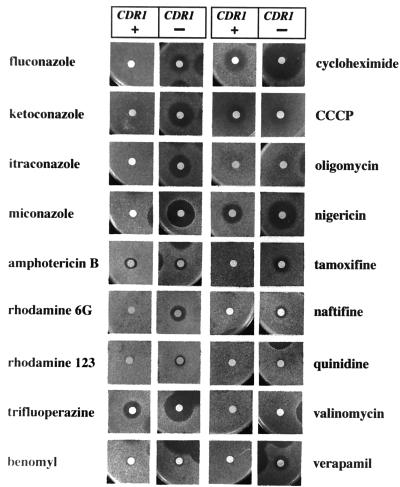
Ability of Cdr1p to mediate resistance to a variety of drugs.
We compared the sensitivities of parental strain S. cerevisiae AD1-8u− and transformant AD1002 to a variety of drugs in order to assess the function of Cdr1p (Fig. 6). The differential sensitivities of these two strains are likely to be due to the drug efflux driven by Cdr1p. AD1002 showed cross-resistance to all azoles tested and to the sterol biosynthesis inhibitor naftifine, but not to the antifungal amphotericin B.
FIG. 6.
Sensitivity of S. cerevisiae AD1002 expressing Cdr1p to various drugs and chemicals. S. cerevisiae AD1-8u− (CDR1-negative [CDR1 −]) or AD1002 (CDR1-positive [CDR1 +]) cells (5 × 105) seeded in top agar on CSM agar plates (containing uracil for AD1-8u−) were exposed to drugs or chemicals applied to filter disks and were incubated at 30°C for 48 h. The sensitivity of AD1002 to drugs or chemicals was unaffected by supplementation of the medium with uridine (0.02%, wt/vol). The amounts of the individual drugs applied to the disks are given in the Materials and Methods section.
Transformant AD1002 showed clear resistance to the fluorescent dyes rhodamine 6G and rhodamine 123, with rhodamine 6G showing greater cytotoxicity for the parental S. cerevisiae strain. The reduced susceptibility of AD1002 to rhodamine 6G is consistent with our demonstration that rhodamine 6G is actively effluxed from these cells.
We further discovered that Cdr1p conferred resistance to growth inhibition by the following drugs: multidrug resistance modifier trifluoperazine, protein synthesis inhibitor cycloheximide, ionophoric peptide nigericin, anticancer drug tamoxifen, and calcium channel blocker verapamil. The structures and targets of these drugs are diverse, and our results indicate that Cdr1p has a wide pumping specificity. The drug resistance phenotypes conferred by Cdr1p were similar to those conferred by the overexpression of Pdr5p (22). Parental strain AD1-8u− was, however, resistant to tubulin synthesis inhibitor benomyl, mitochondrial ATPase inhibitor oligomycin, potassium channel blocker quinidine, and K+-selective ionophoric cyclodepsipeptide valinomycin at the concentrations used in the present study; and so these may or may not be substrates of Cdr1p.
DISCUSSION
Energy-dependent drug efflux is a major mechanism of resistance in clinical C. albicans isolates refractory to fluconazole treatment. A successful clinical outcome may be obtained by using a higher dosage of fluconazole or by applying one of the recently developed triazoles such as voriconazole, which are more potent. Another potential therapeutic strategy is to obtain and utilise antifungal adjuvants that inhibit the efflux pumps, thereby counteracting the pumps' ability to lower intracellular antifungal concentrations to subinhibitory levels. C. albicans, like S. cerevisiae, contains many ORFs with homology to either ABC or MFS drug pumps. This does not mean, however, that they are all involved in antifungal drug export; they may either be insufficiently expressed or have other physiological functions. An analysis of gene expression in fluconazole-resistant isolates has indicated that resistance most often correlates with overexpression of ABC-like gene products Cdr1p and Cdr2p (38, 44). To determine whether pump antagonists can sensitize cells that overexpress these pumps to conventional drugs, it is necessary to either determine pump specificity or screen for inhibitory compounds by using yeasts that overexpress functional pumps. The application of both of these strategies in vivo is complicated by the presence of multiple endogenous pumps with various pumping specificities. We have addressed these problems by the stable overexpression of Cdr1p in a yeast depleted of the major drug efflux pumps: Pdr5p, Yor1p, Snq2p, Ycf1p, Pdr10p, Pdr11p, and Pdr15p. Although none of these pumps is essential, they confer on cells overlapping capacities to tolerate xenobiotics (11, 22).
Integration of the CDR1 ORF into genomic DNA resulted in stable inheritance of a single copy of the gene at the locus for the S. cerevisiae homologue PDR5. Fusion of the CDR1 ORF to the PDR5 promoter in a strain that contains the transcriptional regulator mutation pdr1-3 ensured overexpression of Cdr1p. This overexpression was demonstrated in terms of increased levels of CDR1 mRNA and in the appearance of a new 170-kDa protein band that accounted for 10 to 20% of the plasma membrane protein which reacted with anti-C. albicans Cdr1p antibodies. This protein was functional. Its expression conferred on S. cerevisiae drug resistance, increased levels of plasma membrane NTPase activity, an energy-dependent reduction in the intracellular levels of fluconazole accumulation, and energy-dependent rhodamine 6G efflux. The drug resistance phenotype was due to the overexpression of Cdr1p and not simply the pdr1-3 mutation, as this mutation was also present in the hypersensitive parental strain, AD1-8u−.
Plasma membranes from the Cdr1p-overexpressing strain displayed oligomycin-sensitive NTPase activity with biochemical properties, including pH activity profiles, similar to those of Pdr5p, the S. cerevisiae multidrug efflux pump homologous to C. albicans Cdr1p (12). The pH optimum for UTPase activity (pH 7.0 to 8.0) was significantly higher than that (pH 6.5) reported by Krishnamurthy et al. with a plasmid-based expression system (25). Interestingly, the specific activity of Cdr1p ATPase was four to five times lower than the Pdr5p ATPase activity of Pdr5p-overexpressing strain AD124567U+ measured under the same conditions (unpublished data). Although we cannot exclude possible effects due to mutational changes that occurred during cloning, the reduced activity of Cdr1p may explain why clinical fluconazole resistance can be overcome by increased dosage. The reduced ATPase activity also validates the search for pump antagonists, such as the immunosuppressive agent cyclosporine, which may interact directly with multidrug efflux transporters, and potentiates the effect of fluconazole in vitro and in vivo (27, 28).
Expression of Cdr1p in AD1002 conferred the ability to efflux rhodamine 6G. Rhodamine 6G efflux is associated with S. cerevisiae Pdr5p and Yor1p (11, 22), but both genes that encode these proteins are deleted from AD1002. Rhodamine efflux has also been associated with azole resistance in Candida (8) and, in particular, with Cdr1p expression (26). Our results confirm, therefore, that the Cdr1p in AD1002 functions normally and that rhodamine 6G and fluconazole are Cdr1p substrates.
Expression of Cdr1p reduced the sensitivity of AD1-8u− to a variety of structurally unrelated compounds which could be pump substrates. The spectrum of compounds to which Cdr1p conferred resistance was similar to that to which Pdr5p confers resistance (22). Recent experimental evidence suggests that Cdr1p may be involved in the distribution of phosphatidylethanolamine across the plasma membrane lipid bilayer (13), analogous to the “flippase” activity ascribed to human Cdr1p homologues Mdr2p and Mdr3p (17, 33, 43). Other studies suggest that Pdr5p and Cdr1p might also transport membrane sterols (22, 23, 25). These observations seem to indicate a broad Cdr1p substrate specificity that includes amphipathic molecules that contain both hydrophobic and hydrophilic domains. An alternative interpretation is that Cdr1p solely affects membrane lipid and/or sterol composition and that this has a secondary effect on other mechanisms by which membranes transport a variety of compounds. Our results demonstrate an effect of Cdr1p expression on drug sensitivity in the absence of seven other major S. cerevisiae transporters. If the resistance phenotype is mediated by secondary effects on other transporters, it cannot involve these seven ABC pumps.
Heterologous hyperexpression of functional membrane proteins in S. cerevisiae is hard to achieve, possibly due to protein trafficking control within the cell. Our success may be due to the use of the pdr1-3 and PDR5 promoter system, which involves a pleiotropic activator of a membrane protein expression pathway. The ability to express high levels of specific membrane transporters in an S. cerevisiae strain depleted of endogenous pumps opens the possibility of studying pumping mechanisms in vivo and in vitro. This heterologous expression system will prove useful in screening for pump substrates and antagonists by both in vitro membrane pump NTPase activity assays and whole-cell sensitization assays with substrates such as fluconazole and rhodamine 6G. It may also allow the hyperexpression of a wide range of biologically, pharmaceutically, and agrochemically relevant plasma membrane proteins.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the Health Research Council of New Zealand, the New Zealand Lotteries Board, and the Sumitomo Foundation, Japan.
We also thank D. Sanglard for the kind gift of anti-C. albicans Cdr1p antibodies. We are grateful to S. Aoki (Nippon Dental University) for valuable advice.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 3.Boschman C R, Bodnar U R, Tornatore M A, Obias A A, Noskin G A, Englund K, Postelnick M A, Suriano T, Peterson L R. Thirteen-year evolution of azole resistance in yeast isolates and prevalence of resistant strains carried by cancer patients at a large medical center. Antimicrob Agents Chemother. 1998;42:734–738. doi: 10.1128/aac.42.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Cannon R D, Fischer F J, Niimi K, Niimi M, Arisawa M. Drug pumping mechanisms in Candida albicans. Jpn J Med Mycol. 1998;39:73–78. doi: 10.3314/jjmm.39.73. [DOI] [PubMed] [Google Scholar]
- 6.Cannon R D, Niimi K, Jenkinson H F, Shepherd M G. Molecular cloning and expression of the Candida albicans β-N-acetylglucosaminidase (HEX1) gene. J Bacteriol. 1994;176:2640–2647. doi: 10.1128/jb.176.9.2640-2647.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvajal E, van-den-Hazel H B, Cybularz-Kolaczkowska A, Balzi E, Goffeau A. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol Gen Genet. 1997;256:406–415. doi: 10.1007/s004380050584. [DOI] [PubMed] [Google Scholar]
- 8.Clark F S, Parkinson T, Hitchcock C A, Gow N A R. Correlation between rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in drug resistance. Antimicrob Agents Chemother. 1996;40:419–425. doi: 10.1128/aac.40.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. doi: 10.3109/10408419309113523. [DOI] [PubMed] [Google Scholar]
- 10.Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins. Nat Genet. 1997;15:137–145. doi: 10.1038/ng0297-137. [DOI] [PubMed] [Google Scholar]
- 11.Decottignies A, Grant A M, Nichols J W, de Wet H, McIntosh D B, Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- 12.Decottignies A, Kolaczkowski M, Balzi E, Goffeau A. Solubilization and characterization of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast. J Biol Chem. 1994;269:12797–12803. [PubMed] [Google Scholar]
- 13.Dogra S, Krishnamurthy S, Gupta V, Dixit B L, Gupta C M, Sanglard D, Prasad R. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast. 1999;15:111–121. doi: 10.1002/(SICI)1097-0061(19990130)15:2<111::AID-YEA350>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Fox R, Neal K R, Leen C L S, Ellis M E, Mandal B K. Fluconazole resistant candida in AIDS. J Infect. 1991;22:201–204. doi: 10.1016/0163-4453(91)91767-r. [DOI] [PubMed] [Google Scholar]
- 15.Goffeau A, Dufour J-P. Plasma membrane ATPase from the yeast Saccharomyces cerevisiae. Methods Enzymol. 1988;157:528–533. doi: 10.1016/0076-6879(88)57101-x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 17.Higgins C F. Flip-flop: the transmembrane translocation of lipids. Cell. 1994;79:393–395. doi: 10.1016/0092-8674(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 18.Higgins C F. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson H F. Ins and outs of antimicrobial resistance: era of the drug pumps. J Dent Res. 1996;75:736–742. doi: 10.1177/00220345960750020201. [DOI] [PubMed] [Google Scholar]
- 20.Joseph-Horne T, Hollomon D W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149:141–149. doi: 10.1111/j.1574-6968.1997.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 21.Kitchen V S, Savage M, Harris J R W. Candida albicans resistance in AIDS. J Infect. 1991;22:204–205. doi: 10.1016/0163-4453(91)91789-z. [DOI] [PubMed] [Google Scholar]
- 22.Kolaczkowski M, van der Rest M, Cybularz-Kolaczkowska A, Soumillion J P, Konings W N, Goffeau A. Anticancer drugs, ionophoric peptides, and steroids as substrates of yeast multidrug transporter Pdr5p. J Biol Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis D P. Efflux-mediated resistance to fluconazole could be modulated by sterol homeostasis in Saccharomyces cerevisiae. J Antimicrob Chemother. 2000;46:199–203. doi: 10.1093/jac/46.2.199. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy S, Chatterjee U, Gupta V, Prasad R, Das P, Snehlata P, Hasnain S E, Prasad R. Deletion of transmembrane domain 12 of CDR1, a multidrug transporter from Candida albicans, leads to altered drug specificity: expression of a yeast multidrug transporter in baculovirus expression system. Yeast. 1998;14:535–550. doi: 10.1002/(SICI)1097-0061(19980430)14:6<535::AID-YEA254>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy S, Gupta V, Snehlata P, Prasad R. Characterisation of human steroid hormone transport mediated by Cdr1p, a multidrug transporter of Candida albicans, belonging to the ATP binding cassette super family. FEMS Microbiol Lett. 1998;158:69–74. doi: 10.1111/j.1574-6968.1998.tb12802.x. [DOI] [PubMed] [Google Scholar]
- 26.Maesaki S, Marichal P, Vanden Bossche H, Sanglard D, Kohno S. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J Antimicrob Chemother. 1999;44:27–31. doi: 10.1093/jac/44.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti O, Entenza J M, Sanglard D, Bille J, Glauser M P, Moreillon P. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother. 2000;44:2932–2938. doi: 10.1128/aac.44.11.2932-2938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetti O, Moreillon P, Glauser M P, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000;44:2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monk B C, Kurtz M B, Marrinan J A, Perlin D S. Cloning and characterization of the plasma membrane H+-ATPase from Candida albicans. J Bacteriol. 1991;173:6826–6836. doi: 10.1128/jb.173.21.6826-6836.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 30.Odds F C. Candida and candidosis. 2nd ed. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 31.Ouellette M, Legare D, Papadopoulou B. Microbial multidrug-resistance ABC transporters. Trends Microbiol. 1994;2:407–411. doi: 10.1016/0966-842x(94)90620-3. [DOI] [PubMed] [Google Scholar]
- 32.Prasad R, De Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 33.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mfr2 gene. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 34.Ruhnke M, Eigler A, Tennagen I, Geiseler B, Engelmann E, Trautmann M. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol. 1994;32:2092–2098. doi: 10.1128/jcm.32.9.2092-2098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 38.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherer S, Stevens D A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987;25:675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seto-Young D, Na S, Monk B C, Haber J E, Perlin D S. Mutational analysis of the first extracellular loop region of the H+-ATPase from Saccharomyces cerevisiae. J Biol Chem. 1994;269:23988–23995. [PubMed] [Google Scholar]
- 41.Vanden Bossche H, Marichal P, Odds F C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 1994;2:393–400. doi: 10.1016/0966-842x(94)90618-1. [DOI] [PubMed] [Google Scholar]
- 42.Vanden Bossche H, Warnock D W, Dupont B, Kerridge D, Gupta S S, Improvisi L, Marichal P, Odds F C, Provost F, Ronin O. Mechanisms and clinical impact of antifungal drug resistance. J Med Vet Mycol. 1994;32(Suppl. 1):189–202. doi: 10.1080/02681219480000821. [DOI] [PubMed] [Google Scholar]
- 43.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specifity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 44.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White T C. Antifungal drug resistance in Candida albicans. ASM News. 1998;63:427–433. [Google Scholar]
- 46.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]