Abstract
Development of a novel group of antiviral agents, acyclic nucleoside phosphonates, has provided a new perspective for treating human immunodeficiency virus (HIV) infection. One of the compounds, 9-(R)-[2-(phosphonomethoxy)propyl]adenine (PMPA) (tenofovir), has been shown to confer complete protection against AIDS in a simian model of the infection. The aim of our study was to investigate whether the antiviral efficacy of PMPA, which depends mainly on inhibition of virus-induced DNA polymerase or of reverse transcriptase, could be contributed by immunomodulatory potential of this drug. We screened for its ability to activate production of cytokines and chemokines that are known to interfere with the replication and/or the entry of HIV in cells. Using the in vitro test system of mouse macrophages and lymphocytes, it has been found that PMPA stimulates macrophage secretion of interleukin-1β (IL-1β), IL-10, and tumor necrosis factor alpha. Production of the chemokines RANTES and macrophage inflammatory protein 1α was activated in both macrophages and lymphocytes, and also in human cell line U937. Other cytokines—i.e., IL-2, IL-12, IL-13, and gamma interferon—remained uninfluenced by PMPA. The cytokines were stimulated in a dose-dependent fashion, with rapid onset, and peak concentrations were achieved within 5 to 24 h. The findings contribute to a more complex understanding of mechanisms of antiviral effectiveness of PMPA and support the view that this drug could become a promising candidate for therapeutic exploitation in anti-HIV preventive medicine.
Acyclic phosphonate analogs are antiviral agents effective against replication of both DNA and retroviruses (32), including human immunodeficiency virus (HIV) (12, 16, 56), simian immunodeficiency virus (SIV) (52), and feline immunodeficiency virus (29). One of the compounds endowed with prominent antiretroviral potential is 9-(R)-[2-(phosphonomethoxy)propyl]adenine (PMPA) (tenofovir). It has been found to inhibit replication of HIV in vitro (3) and to significantly reduce plasma RNA levels of HIV (17) and SIV (50). Obviously, the major mechanism of antiviral action of acyclic nucleoside phosphonates is the inhibition of virus-induced DNA polymerase or of reverse transcriptase (40, 55).
It is well recognized that various immune defense mechanisms are activated during virus infections to combat the disease progression. A number of cytokines have been shown to more or less effectively interfere with the virus replication. The most anti-HIV efficient among them seem to be interleukin-13 (IL-13) (42) and IL-16 (24). IL-2 (1) and IL-12 (10) can restore some cellular immune functions impaired by immunosuppressive retroviral peptides in infected patients (28). Presumably, the mechanism of antireplication action of cytokines is their ability to modify expression of transcriptional factors, such as NF-κB (27) and cyclic AMP response element-binding protein (47), required for transcription of HIV gene.
Another tactic of activated anti-HIV immune defense apparatus is restriction of the virus penetration in cells. The entry of HIV-1 in the cells of the immune system is primarily mediated by the CD4 receptor; however, the G-protein-coupled 7-transmembrane chemokine receptors, called coreceptors, are needed to ensure a productive infection (7). Macrophage-tropic isolates (R5) of HIV type 1 (HIV-1) mainly use CCR5 (36); lymphocytotropic viruses (R5X4) use CXCR4 receptor (25). The native ligands for CCR5, which is used by both R5 and R5X4 (dualtropic) HIV-1 strains, are chemokines such as RANTES, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and MCP-2 (5, 7). Blocking the appropriate chemokine receptors through binding to RANTES or MIP-1α or -1β has been shown to prevent the HIV membrane fusion (22). The HIV entry also can be indirectly influenced by certain cytokines through regulation of chemokine receptors expression. Thus, IL-4 up-regulates CXCR4 (26) and favors entry of X4-tropic HIV-1 strains in CD4+ T cells (43). Conceivably, down-regulation of CD4 expression by some cytokines, e.g., by IL-16 (30) or gamma interferon (IFN-γ) (20), contributes to the inhibition of HIV fusion.
Several data show that the early treatment with PMPA confers protection against AIDS in a simian model of the infection (6, 51, 53). This prompted us to investigate whether this effect could be at least partially contributed by immunomodulatory interventions. The aim of the study was therefore to analyze, in an in vitro system of mouse macrophages and lymphocytes, the potential of PMPA to activate the secretion of cytokines (IL-1, IL-2, IL-10, IL-12, IL-13, tumor necrosis factor alpha [TNF-α], and IFN-γ) and chemokines (RANTES and MIP-1α) that interfere with replication and/or cell entry of HIV. Preliminary data on immunostimulatory activity of several related acyclic nucleoside phosphonates have been published recently (64, 65).
MATERIALS AND METHODS
Test compound.
PMPA, i.e., 9-(R)-[2-(phosphonomethoxy)propyl]adenine (Fig. 1), was synthesized according to a previously described procedure (31). Stock 10 mM solution of its sodium salt (obtained by neutralization of the suspension of free acid form with NaOH) was prepared in incomplete phenol red-free RPMI 1640 medium containing NaHCO3 (Sigma, St. Louis, Mo.). It was sterile filtered using nonpyrogenic 0.22-μm-pore-size filters (Costar, Cambridge, Mass.). Working concentrations were prepared by diluting the stock solution in complete RPMI 1640 (described below) and used fresh. The possible presence of endotoxin was determined using a chromogenic Limulus amoebocyte lysate assay (Kinetic-QCL; BioWhittaker, Walkersville, Md.); the samples contained <0.1 endotoxin units/ml at a PMPA concentration of 100 μM.
FIG. 1.
Chemical structure of PMPA
Animals, cells, and cell cultures.
Female C57BL/6 mice, purchased from Charles River, Sulzfeld, Germany, were used as donors of peritoneal macrophages and splenocytes. Animals, killed by cervical dislocation, were intraperitoneally injected with sterile saline. Collected and pooled lavage cells from 5 to 12 mice were washed, resuspended in culture medium, and seeded into 96-well flat-bottom microplates (catalog no. 3595; Costar) in 100-μl volumes containing 2 × 105 cells. Adherent peritoneal cells (macrophages) were isolated by incubating the cells for 2 h at 37°C with 5% CO2 and three times vigorously shaking the plate and washing to remove nonadherent cells. They were further cultured for indicated time intervals in complete RPMI 1640 medium. Lymphocytes were prepared from pooled spleens. After removal of erythrocytes using red blood cell lysing buffer and subsequent thorough washing, the cells in complete RPMI 1640 medium were seeded into 96-well round-bottom microplates (catalog no. 3799; Costar) at 5 × 105/well in final volume of 100 μl. The human promonocytic cell line U937 was obtained from the Institute of Microbiology, Academy of Sciences, Prague, Czech Republic. The complete culture medium contained 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, gentamicin (50 μg/ml), and 2 × 10−5 M 2-mercaptoethanol (all from Sigma). PMPA was added immediately after setting the cells into microplates. All experimental variants were run in triplicate. All protocols used in the experiments were approved by the Commission for the Laboratory Animal Welfare of the Czech Academy of Sciences.
Antibodies and cytokine assays.
When indicated, antibodies against murine TNF-α or IL-10 (R&D Systems; Abingdon, United Kingdom) were added at a concentration of 1 μg/ml, 30 min before the application of PMPA. The cytokines in supernatants of macrophages and lymphocytes, stored at −70°C until used, were determined using enzyme-linked immunosorbent assay detection kits purchased from R&D Systems.
In vivo administration of PMPA.
A fresh solution of PMPA was prepared in sterile saline. It was applied subcutaneously, at a concentration of 5 mg/kg of body weight, either as a single injection or repeatedly for consecutive 5 days (n = 4, each group). Mice (C57BL/6) were sacrificed 5 h after the last injection, and the obtained serum was analyzed for concentrations of RANTES, TNF-α, and IL-10.
Statistical analysis.
The data were evaluated by analysis of variance and Dunnet's test using the PRISM program (GraphPad Software, San Diego, Calif.).
RESULTS AND DISCUSSION
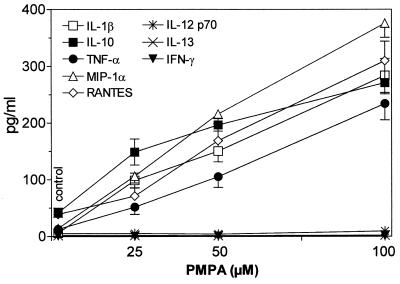
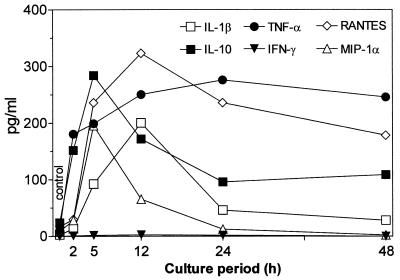
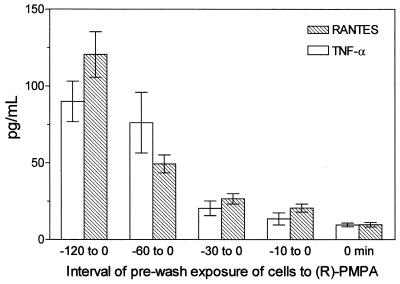
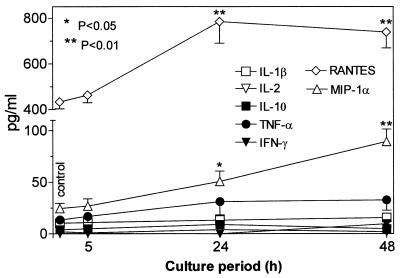
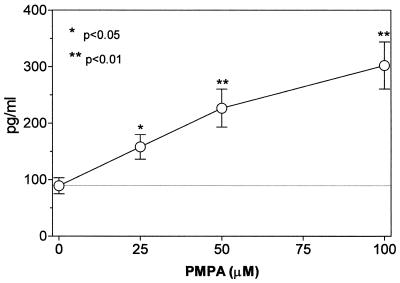
The present results document the potential of the antiviral agent PMPA to activate production of several cytokines. The concentrations of IL-1β, IL-10, and TNF-α and the chemokines RANTES and MIP-1α have been found to be substantially increased in supernatants of mouse macrophages cultured 5 h in the presence of PMPA (Fig. 2). Their secretion was enhanced in a dose-dependent manner, the stimulatory effects being apparent starting with 25 μM PMPA. This concentration equals 7.2 μg/ml, which is very close to the maximum concentration of drug in serum determined in HIV-infected humans after the infusion of 3 mg/kg/day (17). Incidentally, similar maximum concentrations of drug in serum have been observed after intravenous dosing of other acyclic nucleotide analogues such as cidofovir and adefovir applied in doses of 3 mg/kg (15). It may be presumed that even higher in vitro concentrations of PMPA (up to 100 μM) are relevant to expected peak serum levels that can be generated by substantially enhanced doses (20 to 30 mg/kg) given to SIV-infected macaques (50, 51, 54). Production of IL-12 p70, IL-13, and IFN-γ remained uninfluenced. The stimulatory effects PMPA are characterized by a dose-dependent mode of action and rapid onset. Enhanced levels of IL-10 and TNF-α are detectable as early as 2 h following the 50 μM PMPA exposure (Fig. 3). Other cytokines, stimulated by PMPA, occurred at the 5-h interval. The peak concentrations differed greatly among cytokines, however. They were reached at 5 h (IL-10 and MIP-1α), 12 h (IL-1β and RANTES), or 24 h (TNF-α). While IL-1β, IL-10, and MIP-1α declined rapidly thereafter, elevated concentration of TNF-α and RANTES persisted for the whole observation period of 48 h. Rapid appearance of PMPA-induced IL-10 is in contrast with its delayed secretion with respect to secretion of many other cytokines, including TNF-α, that are induced by various immune stimuli like lipopolysaccharide (39). It has been found that 30- to 60-min pulsing of macrophages with PMPA is sufficient to trigger the production of cytokines (TNF-α and RANTES). The longer the prewash (i.e., pulsing) cultivation period was, the higher the secretion of cytokines achieved during the consecutive postwash 5 h later was (Fig. 4). This pattern of the action might reflect the ability of PMPA to cross the cell membrane and reach a critical intracellular concentration. Whether or not PMPA has to be phosphorylated to acquire immunomodulatory properties remains unclear, however.
FIG. 2.
In vitro production of cytokines by mouse peritoneal macrophages (2 × 106/ml) cultured for 5 h in the presence of various concentrations of PMPA. The drug stimulates production of IL-1β, IL-10, and TNF-α and the chemokines RANTES and MIP-1-α. Secretion of IL-12, IL-13, and IFN-γ is uninfluenced. The individual points represent means (± standard errors of the means [error bars]) of data pooled from two independent experiments.
FIG. 3.
Dynamics of in vitro cytokine secretion by mouse peritoneal macrophages (2 × 106/ml) stimulated with 100 μM PMPA. The supernatant concentrations of cytokines and chemokines were assayed at the indicated times after addition of the compound. The earliest onset of the stimulatory response was recorded for TNF-α and IL-10 (2 h), followed by those for IL-1β, RANTES, and MIP-1α (5 h). The peak concentrations were attained within the interval of 5 h (MIP-1α) to 24 h (TNF-α). IFN-γ remained nonstimulated irrespective of the length of cultivation. Enhanced concentrations of several cytokines (from most to least: TNF-α, RANTES, and IL-10) could be detected as late as the 48-h interval, while others (IL-1β and MIP-1α) declined rapidly toward the interval of 24 h. All experimental variants were run in triplicate. The data are representative of one of two identical experiments.
FIG. 4.
In vitro secretion of TNF-α and RANTES by mouse peritoneal macrophages (2 × 106/ml) cultured for indicated the time intervals in the presence of 50 μM PMPA. PMPA was then thoroughly washed out (at time zero), and the cells were kept for the consecutive 5 h in PMPA-free medium. Error bars, standard errors of the means.
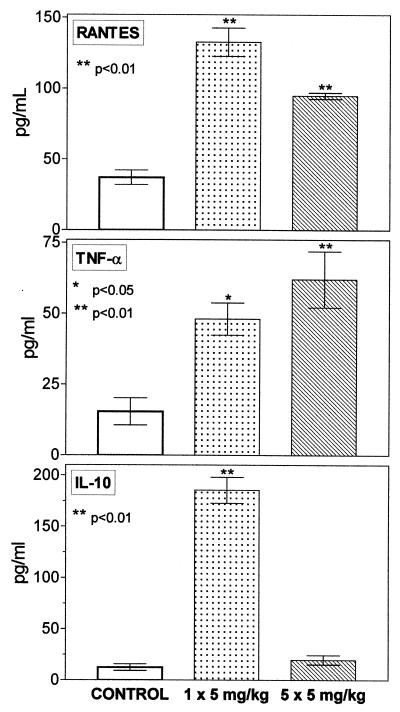
In response to in vitro PMPA exposure, splenocytes could be activated as well, as shown by increased production of chemokines RANTES and MIP-1 α (but not of other test cytokines, including IL-2) at intervals of 24 and 48 h (Fig. 5). Importantly, RANTES also was augmented in the human promonocyte cell line U937 (Fig. 6). Furthermore, PMPA was found to be effective under in vivo conditions in mice. Applied as a single dose or repeatedly on 5 consecutive days (5 mg/kg, subcutaneously), it increased concentration of RANTES and TNF-α in serum. Also, the levels of IL-10 were greatly enhanced, but only after the single dose of PMPA (Fig. 7).
FIG. 5.
In vitro production of chemokines RANTES and MIP-1α is enhanced in mouse splenocytes upon stimulation with PMPA (100μM). No changes in secretion of other cytokines—including IL-1β, IL-2, IL-10, TNF-α, and IFN-γ—were observed. The cells were cultured at a density of 5 × 106/ml, in triplicate for each experimental variant. Error bars, standard errors of the means.
FIG. 6.
PMPA stimulates secretion of chemokine RANTES by human monocyte leukemia cells U937. The concentration of RANTES was determined in supernatants of cells (2 × 106/ml) cultured in triplicate for 5 h in the presence of various concentrations of PMPA. The data are means ± standard errors of the means (error bars). of two identical experiments.
FIG. 7.
PMPA administered in vivo stimulates production of RANTES, TNF-α, and IL-10. The drug was administered subcutaneously to C57BL/6 mice as a single injection or repeatedly on five consecutive days, always at a dose of 5 mg/kg (n = 4, each group). The animals were sacrificed 5 h subsequent to the last injection, at which time serum concentrations of the cytokines were determined. Error bars, standard errors of the means.
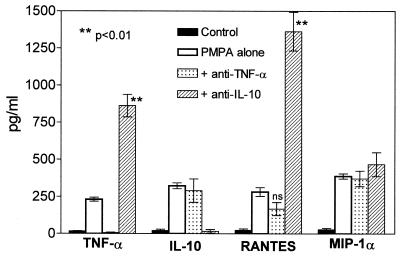
Since TNF-α and IL-10 have been found to precede the production of all other cytokines, their presumptive subsidiary effects on the overall cytokine production in the test system were analyzed. Removal of PMPA-induced IL-10 by means of its neutralization with anti-IL-10 antibody greatly enhanced expression of concomitantly produced TNF-α (by >300%) and RANTES (by >500%), but this treatment had no effect on MIP-1α secretion. Administration of anti-TNF-α antibody only marginally decreased PMPA-induced RANTES and remained without any influence on MIP-1α and IL-10 production (Fig. 8). It follows, therefore, that production of IL-10 and RANTES is virtually independent on PMPA-induced TNF-α, whereas PMPA-induced IL-10 significantly diminishes both TNF-α and RANTES. Completely independent on both TNF-α and IL-10 is the PMPA-activated secretion of MIP-1α. The data thus prove that all these cytokines are directly stimulated by PMPA. In accordance with generally accepted view on the down-regulatory immune activity of IL-10, this cytokine has a negative feedback function in the system. On the other hand, our findings are at variance with some data claiming TNF-α to be an up-regulator of chemokine secretion (61).
FIG. 8.
Antibodies against TNF-α or IL-10 change expression of PMPA-activated cytokines. Both anti-TNF-α and anti-IL-10 antibodies, applied at a final concentration of 1 μg/ml, completely inhibited the production of TNF-α and IL-10, respectively, induced by 50 μM PMPA. Anti-TNF-α antibody had no effect on the secretion of PMPA-induced IL-10 and MIP-1α and only marginally suppressed RANTES production. Neutralization of IL-10 with anti-IL-10 antibody greatly enhanced the expression of TNF-α and RANTES. Similar to anti-TNF-α, anti-IL-10 did not affect the secretion of MIP-1α. The data document that PMPA-induced IL-10 substantially attenuates the secretion of other PMPA-activated cytokines, such as TNF-α and RANTES, but not MIP-1α. On the other hand, PMPA-induced TNF-α virtually does not interfere with the production of other concomitantly secreted cytokines (IL-10, RANTES, and MIP-1α). The experiments were performed using mouse (C57BL/6) resident peritoneal macrophages cultured 5 h in the presence of 50 μM PMPA. The data are mean values (± standard errors of the means (error bars) for three independent experiments.
The transduction pathways and transcriptional mechanisms underlying the immunostimulatory effects of PMPA are presently under investigation. Herein, we have focused on discussing the possible relevance of the cytokine- and especially chemokine-enhancing potential to the established antiviral effectiveness of PMPA. It should be stressed that all cytokines that have been found to be stimulated by PMPA are known to influence viral immunity, including defense mechanisms against retroviruses, notably HIV.
The major role in therapeutic approaches against HIV is generally considered to be played by β-chemokines. The data accumulated so far suggest that RANTES and MIP-1α and -1β are prominent inhibitors of HIV infection of both T cells (11, 22, 62) and macrophages (8, 18, 63). Another antiviral agent, very efficient in many viral infections, is TNF-α. However, it has been found either to stimulate (23, 45) or inhibit (38, 60) HIV infection. Anti-HIV activity of TNF-α may be related to the chemokine system, because TNF-α can delay and down-modulate expression of CCR5 receptor in human monocytic cells (38) and in peripheral blood lymphocytes, particularly in synergism with IL-13 (2). This action may be at least partially mediated by RANTES and MIP-1α and -1β themselves (33). Most reports support the view on the inhibitory effects of IL-10 upon HIV infection (35, 48, 49, 59), though it occasionally exerts the opposite effect. Namely, IL-10 was found to reduce the ability of CD8+ from HIV-infected patients to suppress HIV replication (4) and to enhance cytokine-induced HIV-1 expression in monocytes (58) and in T cells (46). The data on the effects of IL-1 suggest that it rather up-regulates HIV infection (13, 44). With respect to the ability of IL-1β to stimulate secretion of RANTES and MIP-1α and -1β (41), the possibility that under some conditions this cytokine also could suppress entry of HIV in cells cannot be excluded.
Obviously, the interference of certain cytokines with replication of viruses, notably with HIV, is ambiguous. The ultimate direction of the action may be governed by multiple functional interactions among them. Noteworthy, anti-inflammatory IL-10 and pro-inflammatory TNF-α are frequently produced concomitantly upon various immune stimuli, and they can reciprocally influence secretion of each other, as well as secretion of many other cytokines. Thus, TNF-α enhances expression of IL-10 (57) and IL-1β (21), while IL-10 in turn inhibits production of both TNF-α and IL-1β (19). TNF-α also is a potent activator of secretion of chemokines RANTES and MIP-1α/β (61). Synthesis of them has been found either uninfluenced (61) or inhibited by IL-10 (34). Biological actions of TNF-α are influenced by the capability of IL-10 to modify both negatively and positively expression of TNF-α receptor (37). Furthermore, IL-10 enhances expression of a natural antagonist of IL-1, i.e., IL-1Ra (9), which has been suggested to have a perspective in treatment of HIV infection (13).
Altogether, the ability of cytokines to regulate virus infectivity and/or disease progression depends primarily on the mode of their intrinsic immune potential. Cytokines such as TNF-α and IL-10 reduce HIV infection at the stage of virus replication. Chemokines, e.g., RANTES and MIP-1α mainly function as inhibitors of the virus entry, though some of them, namely macrophage-derived chemokine may suppress HIV replication as well (14). The ultimate effect results undoubtedly from an integration of synergistic and antagonistic actions of individual cytokines.
Herein we document that PMPA (tenofovir) stimulates production of several cytokines that interfere with HIV replication (TNF-α, IL-10, and IL-1β) and chemokines (RANTES and MIP-1α) that inhibit entry of HIV in cells. The data provide new insights contributing to a more complex understanding of mechanisms of PMPA protective effects against immunodeficiency virus infection. Ancillary to the antimetabolic activity, which is the main mode of antiviral effectiveness of various acyclic nucleoside phosphonates, immunomodulatory potential, especially chemokine-stimulatory potential, of the drug could be of therapeutic importance. The present findings favor the view that PMPA could become a promising candidate for therapeutic exploitation in anti-HIV preventive medicine.
ACKNOWLEDGMENTS
This work was supported by grant 305/00/0048 and 203/96/K001 from the Grant Agency of the Czech Republic, and by Gilead Sciences (Foster City, Calif.).
REFERENCES
- 1.Arnó A, Ruiz L, Juan M, Jou A, Balagué M, Zayat M K, Marfil S, Martínez-Picado J, Martínez M A, Romeu J, Pujol-Borell R, Lane C, Clotet B. Efficacy of low-dose subcutaneous interleukin-2 to treat advanced human immunodeficiency virus type 1 in persons with <250/μL CD4 T cells and undetectable plasma virus load. J Infect Dis. 1999;180:56–60. doi: 10.1086/314831. [DOI] [PubMed] [Google Scholar]
- 2.Bailer R T, Lee B, Montaner L J. IL-13 and TNF-α inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur J Immunol. 2000;30:1340–1349. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J, Aquaro S, Perno C F, Witvrouw M, Holý A, De Clercq E. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem Biophys Res Commun. 1996;219:337–341. doi: 10.1006/bbrc.1996.0234. [DOI] [PubMed] [Google Scholar]
- 4.Barker E, Mackewicz C E, Levy J A. Effects of TH1 and TH2 cytokines on CD8+ cell response against human immunodeficiency virus: implications for long-term survival. Proc Natl Acad Sci USA. 1995;92:11135–11139. doi: 10.1073/pnas.92.24.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 6.Bischofberger N, Tsai C-C, Sabo A, Follis K E, Grant R F, Beck T W, Dailey P J, Black R. Antiviral efficacy of PMPA in macaques chronically infected with SIV. Antivir Res. 1996;30:A42. . (Abstract.) [Google Scholar]
- 7.Cammack N. Human immunodeficiency virus type 1 entry and chemokine receptors: a new therapeutic target. Antivir Chem Chemother. 1999;10:53–62. doi: 10.1177/095632029901000201. [DOI] [PubMed] [Google Scholar]
- 8.Capobianchi M R, Abbate I, Antonelli G, Turriziani O, Dolei A, Dianzani F. Inhibition of HIV type 1 BaL replication by MIP-1α, MIP-1β, and RANTES in macrophages. AIDS Res Hum Retrovir. 1998;14:233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- 9.Cassatella M A, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix C W, Wolf S F, Shearer G M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Collier A C, Coombs R W, Nienow J, Paradise M, Yang H H, Troxel S, Boggs J, Ebeling D, Jaffe H S, Corey L. Phase I/II study of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in advanced HIV infection. 1993. The First National Conference on Human Retroviruses and Related Infections. Bethesda, Md. [Google Scholar]
- 13.Corley P A. Interleukin-1 receptor antagonist as a treatment of HIV infection. Med Hypotheses. 2000;54:513–518. doi: 10.1054/mehy.1998.0817. [DOI] [PubMed] [Google Scholar]
- 14.Cota M, Mengozzi M, Vicenzi E, Panina-Bordignon P, Sinigaglia F, Transidico P, Sozzani S, Mantovani A, Poli G. Selective inhibition of HIV replication in primary macrophages but not T lymphocytes by macrophage-derived chemokine. Proc Natl Acad Sci USA. 2000;97:9162–9167. doi: 10.1073/pnas.160359197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cundy K C. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cundy K C, Barditch-Crovo P A, Walker A C, Collier D, Ebeling D, Toole J, Jaffe H S. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1995;39:2401–2405. doi: 10.1128/aac.39.11.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks S G, Barditch-Crovo P, Lietman P S, Hwang F, Cundy K C, Rooney J F, Hellmann N S, Safrin S, Kahn J O. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10-produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhawan S, Heredia A, Wahl L M, Epstein J S, Meltzer M S, Hewlett I K. Interferon-gamma-induced downregulation of CD4 inhibits the entry of human immunodeficiency virus type-1 in primary monocytes. Pathobiology. 1995;63:93–99. doi: 10.1159/000163939. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello C A, Cannon J G, Wolff S M. Tumor necrosis factor/cachectin is an endogenous pyrogen and induces production of interleukin-1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 23.Duh E J, Maury W J, Folks T M, Fauci A S, Rabson A B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauci A S. An elusive soluble suppressor. Nature. 1995;378:561. doi: 10.1038/378561a0. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 26.Galli G, Annunziato F, Mavilia C, Romagnani P, Cosmi L, Manetti R, Pupilli C, Maggi E, Romagnani S. Enhanced HIV expression during Th2-oriented responses explained by the opposite regulatory effect of IL-4 and IFN-γ on fusin/CXCR4. Eur J Immunol. 1998;28:3280–3290. doi: 10.1002/(SICI)1521-4141(199810)28:10<3280::AID-IMMU3280>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Ghassemi M, Asadi F K, Andersen B R, Novak R M. Mycobacterium avium induces HIV upregulation through mechanisms independent of cytokine induction. AIDS Res Hum Retrovir. 2000;16:435–440. doi: 10.1089/088922200309098. [DOI] [PubMed] [Google Scholar]
- 28.Haraguchi S, Good R A, Cianciolo G J, Engelman R W, Day N K. Immunosuppressive retroviral peptides: Immunopathological implications for immunosuppressive influences of retroviral infections. J Leukoc Biol. 1997;61:654–666. doi: 10.1002/jlb.61.6.654. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann K A, Donath A, Beer B, Egberink H F, Horzinek M C, Lutz H, Hoffmann-Fezer G, Thum I, Thefeld S. Use of two virostatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol. 1992;35:167–175. doi: 10.1016/0165-2427(92)90129-e. [DOI] [PubMed] [Google Scholar]
- 30.Hermann E, Darcissac E, Idziorek T, Capron A, Bahr G M. Recombinant interleukin-16 selectively modulates surface receptor expression and cytokine release in macrophages and dendritic cells. Immunobiology. 1999;97:241–248. doi: 10.1046/j.1365-2567.1999.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holý A, Masojídková M. Synthesis of enantiomeric N-(2-phosphonomethoxypropyl) derivatives of purine and pyrimidine bases. 1. The stepwise approach. Collect Czech Chem Commun. 1995;60:1196–1212. [Google Scholar]
- 32.Holý A, Votruba I, Merta A, Èerný J, Veselý J, Vlach J, Šedivá K, Rosenberg I, Otmar M, Høebabecký H, Trávníèek M, Vonka V, Snoeck R, De Clercq E. Acyclic nucleotide analogues: synthesis, antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. Antivir Res. 1990;13:295–311. doi: 10.1016/0166-3542(90)90014-x. [DOI] [PubMed] [Google Scholar]
- 33.Hornung F, Scala G, Lenardo M J. TNF-α-induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–6187. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- 34.Hu S X, Chao C C, Ehrich L C, Sheng W S, Sutton R L, Rockswold G L, Peterson P K. Inhibition of microglial cell RANTES production by IL-10 and TGF-β. J Leukoc Biol. 1999;65:815–821. doi: 10.1002/jlb.65.6.815. [DOI] [PubMed] [Google Scholar]
- 35.Chang J, Naif H M, Li S, Jozwiak R, HoShon M, Cunningham A L. The inhibition of HIV replication in monocytes by interleukin 10 is linked to inhibition of cell differentiation. AIDS Res Hum Retrovir. 1996;12:1227–1236. doi: 10.1089/aid.1996.12.1227. [DOI] [PubMed] [Google Scholar]
- 36.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodorski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 37.Joyce D A, Gibbons D P, Green P, Steer J H, Feldmann M, Brennan F M. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol. 1994;24:2699–2705. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 38.Lane B R, Markovitz D M, Woodford N L, Rochford R, Strieter R M, Coffey M J. TNF-α inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- 39.Lefebvre D‘Hellencourt C, Diaw L, Cornillet P, Guenounou M. Differential regulation of TNFα, IL-1β, IL-6, IL-8, TNFβ, and IL-10 by pentoxifylline. Int J Immunopharmacol. 1996;18:739–748. doi: 10.1016/s0192-0561(97)85556-7. [DOI] [PubMed] [Google Scholar]
- 40.Merta A, Votruba I, Rosenberg I, Otmar M, Høebabeck. ı H, Bernaerts R, Holý A. Inhibition of herpes simplex virus DNA polymerase by diphosphates of acyclic phosphonylmethoxyalkyl nucleotide analogues. Antiviral Res. 1990;13:209–218. doi: 10.1016/0166-3542(90)90066-g. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto N G, Medberry P S, Hesselgesser J, Boehlk S, Nelson P J, Krensky A M, Perez H D. Interleukin-1β induction of the chemokine RANTES promoter in the human astrocytoma line CH235 requires both constitutive and inducible transcription factors. J Neuroimmunol. 2000;105:78–90. doi: 10.1016/s0165-5728(00)00195-8. [DOI] [PubMed] [Google Scholar]
- 42.Montaner L J, Dozle A G, Collin M, Herbein G, Illei P, James W, Minty A, Caput D, Ferrara P. Interleukin 13 inhibits human immunodeficiency virus type I production in primary blood-derived human macrophages in vitro. J Exp Med. 1993;178:743–747. doi: 10.1084/jem.178.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penn M L, Grivel J-C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poli G, Kinter A L, Fauci A S. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonists. Proc Natl Acad Sci USA. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poli G, Kinter A L, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbi M F, Finnegan A, Al-Harthi L, Song S, Roebuck K A. Interleukin-10 enhances tumor necrosis factor-α activation of HIV-1 transcription in latently infected cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:321–331. doi: 10.1097/00042560-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 47.Rohr O, Schwartz C, Aunis D, Schaeffer E. CREB and COUP-TF mediate transcriptional activation of the human immunodeficiency virus type 1 genome in Jurkat T cells in response to cyclic AMP and dopamine. J Cell Biochem. 1999;75:404–413. [PubMed] [Google Scholar]
- 48.Saville M W, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–3599. [PubMed] [Google Scholar]
- 49.Schuitemaker H. IL4 and IL10 as potent inhibitors of HIV1 replication in macrophages in vitro: a role for cytokines in the in vivo virus host range? Res Immunol. 1994;145:588–592. doi: 10.1016/s0923-2494(05)80038-0. [DOI] [PubMed] [Google Scholar]
- 50.Silvera P, Racz P, Racz K, Bischofberger N, Crabbs C, Yalley-Ogunro J, Greenhouse J, Jiang J B, Lewis M G. Effect of PMPA and PMEA on the kinetics of viral load in simian immunodeficiency virus-infected macaques. AIDS Res Hum Retrovir. 2000;16:791–800. doi: 10.1089/088922200308783. [DOI] [PubMed] [Google Scholar]
- 51.Tsai C-C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 52.Tsai C-C, Follis K E, Sabo A, Grant R F, Bartz C, Notte R E, Benveniste R E, Bischofberger N. Preexposure prophylaxis with of 9-(2-phosphonylmethoxyethyl)adenine against simian immunodeficiency virus infection in macaques. J Infect Dis. 1994;169:260–266. doi: 10.1093/infdis/169.2.260. [DOI] [PubMed] [Google Scholar]
- 53.Van Rompay K K A, Berardi C J, Aguirre N L, Bischofberger N, Lietman P S, Pedersen N C, Marthas M L. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998;12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Van Rompay K K A, Miller M D, Marthas M L, Margot N A, Dailey P J, Canfield D R, Tarara R P, Cherrington J M, Aguirre N L, Bischofberger N, Pedersen N C. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J Virol. 2000;74:1767–1774. doi: 10.1128/jvi.74.4.1767-1774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Votruba I, Trávníèek M, Rosenberg I, Otmar M, Merta A, Høebabecký H, Holý A. Inhibition of avian myeloblastosis virus reverse transcriptase by diphosphates of acyclic phosphonylmethyl nucleotide analogues. Antivir Res. 1990;13:287–293. doi: 10.1016/0166-3542(90)90013-w. [DOI] [PubMed] [Google Scholar]
- 56.Walker R E, Vogel S E, Jaffe H S, Polis M A, Kovacs J A F J, Davey R T, Ebeling D, Cundy K C, Paar D, Markowitz N, Masur H, Lane H C. A phase I/II study of PMEA in HIV infected patients. 1993. The First National Conference on Human Retroviruses and Related Infections. Bethesda, Md. [Google Scholar]
- 57.Wanidworanum C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- 58.Weissman D, Poli G, Fauci A S. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:442–449. [PubMed] [Google Scholar]
- 59.Weissman D, Poli G, Fauci A S. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retrovir. 1994;10:1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 60.Wong G H W, Kamb A, Goeddel D V. Antiviral properties of TNF. In: Beutler B, editor. Tumor necrosis factors: the molecules and their emerging role in medicine. New York, N.Y: Raven Press, Ltd; 1992. pp. 371–381. [Google Scholar]
- 61.Xiao B-G, Mousa A, Kivisäkk P, Seiger A, Bakhiet M, Link H. Induction of β-family chemokines mRNA in human embryonic astrocytes by inflammatory cytokines and measles virus protein. J Neurocytol. 1998;27:575–580. doi: 10.1023/a:1006918110952. [DOI] [PubMed] [Google Scholar]
- 62.Yang A G, Zhang X, Torti F, Chen S Y. Anti-HIV type 1 activity of wild-type and functional defective RANTES intrakine in primary human lymphocytes. Hum Gene Ther. 1998;9:2005–2018. doi: 10.1089/hum.1998.9.14-2005. [DOI] [PubMed] [Google Scholar]
- 63.Ylisastigui L, Vizzavona J, Drakopoulou E, Paindavoine P, Calvo C F, Parmentier M, Gluckman J C, Vita C, Benjouad A. Synthetic full-length and truncated RANTES inhibit human immunodeficiency virus type 1 infection of primary macrophages. AIDS. 1998;12:977–984. [PubMed] [Google Scholar]
- 64.Zídek Z, Franková D, Holý A. Activation of cytokine production by antiviral acyclic nucleoside phosphonates. Eur Cytokine Netw. 2000;11(Special issue):189. [Google Scholar]
- 65.Zídek Z, Holý A, Franková D. Immunomodulatory properties of antiviral acyclic nucleotide analogues: cytokine stimulatory and nitric oxide costimulatory effects. Int J Immunopharmacol. 1997;19:587–597. doi: 10.1016/s0192-0561(97)00047-7. [DOI] [PubMed] [Google Scholar]