Abstract
Background
Serum total bilirubin has been reported to have antioxidant properties against chronic respiratory diseases. The objective of our study is to evaluate the association of total bilirubin (TB) with annual lung function decline in COPD patients with different GOLD stages.
Methods
This study used pooled data from two observational and prospective cohorts of 612 COPD patients whose TB levels were measured at baseline. The associations between TB and postbronchodilator FEV1, FEV1pred, FVC, FVCpred, FEV1/FVC, and the rate of their decline were all determined using linear regression models in the total population and strata of GOLD stages.
Results
Serum TB was positively related to FEV1 and FVC in the total group (β 0.02, 95% CI 0.001~0.02, P = 0.025 and β 0.02, 95% CI 0.002~0.03, P = 0.022, respectively). Additionally, TB was inversely associated with the annual decline in FEV1 and FEV1pred (β 4.91, 95% CI 1.68~8.14, P = 0.025 and β 0.21, 95% CI 0.06~0.36, P = 0.022, respectively) when adjusted for multivariables. After stratification, the significant associations merely persisted in COPD patients with GOLD 2 and GOLD 3–4.
Conclusion
Increased TB level was related to less annual decline in FEV1 as well as FEV1pred in moderate-to-severe COPD but not mild COPD, which indicated the different status of TB in different COPD severity and the possible role as potential biomarker merely in moderate-to-severe COPD. Future researches to determine whether TB could be served as biomarker for COPD and the mechanisms should be focused on some target patients with a certain disease severity.
Keywords: total bilirubin, COPD, lung function, GOLD stage, decline
Introduction
Serum total bilirubin (TB), an end product of heme degradation, is demonstrated to scavenge oxidation free radicals and thus suppress oxidative stress.1 Experimental evidence from animal models indicated that TB could protect respiratory tissues against environmental stressors by cytoprotective properties.2,3 Additionally, accumulating clinical studies supported that mild higher bilirubin levels could decrease the risk of cardiovascular disease (CVD) and CVD-related diseases.4–6 An increased total bilirubin level is also associated with a lower risk of chronic respiratory diseases, including lung cancer and chronic obstructive pulmonary disease (COPD), as well as all-cause mortality.7–9 Furthermore, Kirstin E. et al found that elevated bilirubin concentrations would decrease the acute exacerbation of COPD.10
A few longitudinal cohort studies have explored the relationship between total bilirubin and the rate of lung function decline. For example, Ah YL et al11 showed that serum bilirubin concentration was significantly related to the annual changes in FEV1, FVC, and FEV1 /FVC in general Koreans adjusted for sex, age, body mass index (BMI), and baseline lung function. Similarly, only in mild COPD, serum bilirubin is inversely related to the rate of FEV1 decline over 3 to 9 years.12 In a Swiss general population sample, per interquartile range increase of bilirubin exposure is concomitant with 1.1% increase in FEV1/FVC and 116.2 mL in FEF25-75% in smokers with high BMI.13 Also, David M. et al demonstrated that TB is significantly associated with higher FEV1 and FVC in baseline lung function in AIDS individuals.14 However, all these studies focused on the general or specific target population. And the data between bilirubin concentrations and the annual decline in lung function in all different GOLD stages of COPD have not been reported before. To fill this evidence gap, we hypothesized that higher TB level is associated with less annual lung function decline in COPD patients with different GOLD stages. To test the hypothesis, we pooled data from two observational and prospective cohort studies to assess the relationship between serum TB and lung function decline in the total COPD population and then further in different GOLD stage subgroups.
Methods
Study Subjects
The data were pooled from two population-based, multi-center, and prospective cohort studies, including the National Key Technology Research and Development Program of the 12th National 5-year Development Plan (2012–2015 Program; Trial registration number ChiCTR-OO-14004264) and the Early COPD (ECOPD; Trial registration number ChiCTR1900024643). The details of these two cohorts have been reported previously.15,16 Briefly, two cohorts recruited participants aged 20 years or older and completed bilirubin measurement, questionnaires, and lung function tests at baseline. Subjects included in this study completed spirometric measurements again for an interval of 7 to 9 years follow-up duration in 2012–2015 Program as well as 1 to 2 years in ECOPD. Only participants defined as COPD (postbronchodilator FEV1/FVC <0.7)17 with reliable data were included in our analysis. Participants were excluded if they had any comorbidity that might significantly influence bilirubin, such as hemolytic disorders, hepatobiliary diseases, or renal insufficiency by self-report. Additionally, TB concentrations >1.75 mg/dL for women and >2.34 mg/dL for men were also excluded for suspicious Gilbert syndrome, a benign hereditary disease caused by UGT1A1 genotypes.18 The original two studies were performed in line with the principles of the Declaration of Helsinki. Besides, the two studies were approved by the Ethics Committee of Scientific research project review of the First Hospital of Guangzhou Medical University (No.2013–37 for 2012–2015 Program and N0.2018–53 for ECOPD) and written informed consent was also attained from all the participants.
Bilirubin Measurement
At baseline, venous blood samples were collected from eligible participants required to fast for more than 8 hours by trained and professional staff. The samples will be stored at room temperature for at least 30 min and then quantified by biochemical assays performed by the clinical laboratory to test total bilirubin. Bilirubin concentrations were measured or transformed to the nearest 0.06 mg/dL (1 μmol/L).
Lung Function Measurement
Spirometry was performed by well-trained staff using a portable spirometer (CareFusion™ MasterScreen Pneumo, Germany) on the same day of bilirubin detection. Only at least three acceptable curves and two reproducible tests can be considered as acceptable for our study. We also performed postbronchodilator Spirometry (salbutamol sulfate aerosol, 400 μg 20min later) and then recorded and retrieved postbronchodilator FEV1, FVC, FEV1pred, FVCpred, and FEV1/FVC. Annual Lung function decline was calculated as the parameters at follow-up minus corresponding baseline values divided by the number of years for follow-up duration. All spirometric maneuvers and quality control were performed following European Respiratory Society/American Thoracic Society standards.19 The definition of GOLD stages in our study was in line with GOLD criteria for COPD, and we considered GOLD 3–4 COPD as severe COPD.17
Questionnaire
A questionnaire interview was carried out using a standardized questionnaire recommended by Zhong N et al.20 We abstracted information about demographic characteristics, risk factors (smoke, occupational exposure, comorbidities, and family history of respiratory diseases), and respiratory symptoms, including any one of wheeze, cough, sputum production, and dyspnea. We classified smoking status as never smoker, former smoker, and current smoker. Subjects who have smoked less than 100 cigarettes in the past are identified as never smokers. Current smokers are referred to someone who were smoking at baseline, and the others are regarded as former smokers. The definition of the smoking index is multiplying the number of packs of cigarettes smoked per day by the number of years the person smoked. We took subjects whose parents, siblings, or children had any of the listed respiratory diseases (chronic bronchitis, emphysema, asthma, COPD, cor pulmonale, bronchiectasis, lung cancer, interstitial lung disease, obstructive sleep apnea hypopnea syndrome) as having a family history of respiratory diseases.
Statistical Analysis
All variables are expressed as mean ± SD or number (%). Characteristics of participants among GOLD 1, GOLD 2, and GOLD 3–4 compared using One-Way analysis of variance (ANOVA) test for continuous variables and the χ2 or Fisher's exact test for categorical variables. The associations of TB with pulmonary function parameters at baseline or annual lung function decline were assessed using univariable and multivariable regression models. All linear regression models were adjusted for age, sex, BMI, smoking status, smoke index, passive smoker, occupational exposure, comorbidities, and family history of respiratory diseases. To ensure the authenticity of the data, there was no imputation for missing values of interest in our analysis. Data analysis was performed using IBM SPSS Version 25.0 and the statistical software R version 4.0.3. Two-sided p < 0.05 were considered statistically significant.
Results
Baseline Characteristics
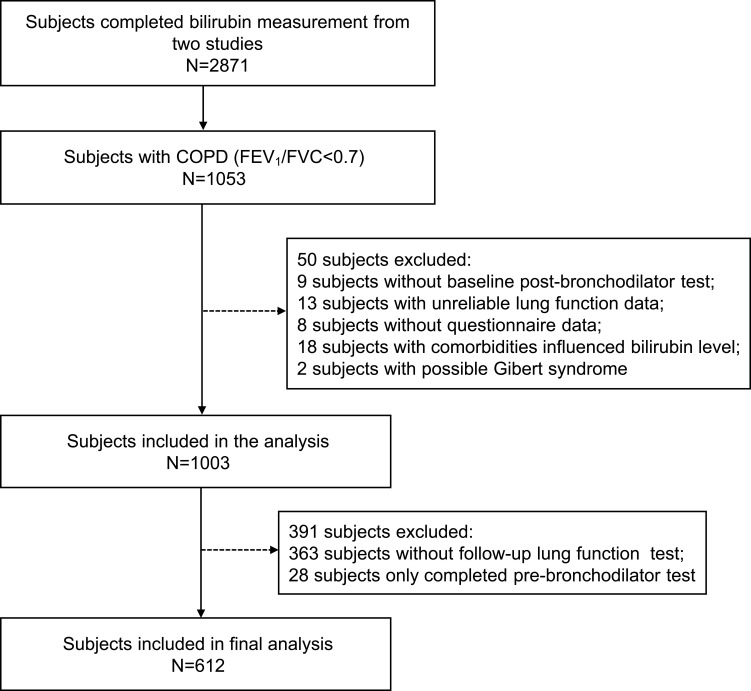
Of the 1053 patients with COPD, 442 were excluded for various reasons (Figure 1). The remaining 612 subjects were divided into three subgroups by GOLD stages. Characteristics of the total group and three subgroups are presented in Table 1. Compared with patients with mild COPD (GOLD 1), patients with moderate-to-severe COPD (GOLD 2, 3 and 4) were more likely to be old, female, breathless and have lower BMI, heavier smoking exposure, more comorbidities, more family history of respiratory diseases as well as worse lung function. There was no significant difference in TB levels among the three subgroups (P = 0.486). The mean follow-up duration for our patients was about two years.
Figure 1.
Flow chart of participant selection.
Note: COPD was diagnosed by postbronchodilator FEV1/FVC <0.7.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 1.
Baseline Characteristics of the Study Subjects
| Characteristics | Total Group (n=612) | GOLD 1 (n=285) | GOLD 2 (n=270) | GOLD 3–4 (n=57) | P values |
|---|---|---|---|---|---|
| Sex, male | 543(88.7) | 240(84.2) | 246(91.1) | 57(100) | 0.001 |
| Age, yr | 63.7±7.3 | 63.1±7.2 | 63.8±7.6 | 66.2±5.4 | 0.011 |
| BMI, kg/m 2 | 22.1±3.4 | 22.5±3.1 | 22.1±3.6 | 19.8±3.0 | <0.001 |
| Smoking status※ | <0.001 | ||||
| Never smoker | 99(16.2) | 59(20.7) | 37(13.7) | 3(5.3) | |
| Former smoker | 179(29.2) | 65(22.8) | 76(28.1) | 38(66.7) | |
| Current smoker | 333(54.4) | 160(56.1) | 157(58.1) | 16(28.1) | |
| Smoke index, pack-yrs | 30.9±29.8 | 29.0±30.0 | 29.5±26.9 | 46.7±36.9 | <0.001 |
| Passive smoker | 186(30.4) | 80(28.1) | 83(30.7) | 23(40.4) | 0.181 |
| Occupational exposure※ | 368(60.1) | 159(55.8) | 171(63.3) | 38(66.7) | 0.253 |
| Comorbidities | 414(67.6) | 165(57.9) | 205(75.9) | 44(77.2) | <0.001 |
| Family history of respiratory diseases# | 115(18.8) | 39(13.7) | 57(21.1) | 19(33.3) | 0.007 |
| Respiratory symptom | 375(61.3) | 139(48.8) | 187(69.3) | 49(86.0) | <0.001 |
| mMRC score※ | 0.47±0.73 | 0.27±0.60 | 0.62±0.91 | 0.98±0.88 | <0.001 |
| TB, μmol/L | 11.8±4.0 | 11.9±4.2 | 11.6±3.7 | 12.2±4.0 | 0.486 |
| Lung function | |||||
| FVC, L | 3.32±0.77 | 3.71±0.72 | 3.08±0.62 | 2.56±0.62 | <0.001 |
| FVCpred,% | 103.7±19.8 | 117.1±16.0 | 95.5±12.4 | 76.4±15.8 | <0.001 |
| FEV1, L | 1.96±0.59 | 2.37±0.47 | 1.71±0.35 | 1.04±0.23 | <0.001 |
| FEV1pred,% | 77.2±20.2 | 94.2±11.5 | 67.1±8.3 | 39.9±7.8 | <0.001 |
| FEV1/FVC,% | 58.6±9.4 | 64.2±4.6 | 56.3±8.2 | 41.4±7.2 | <0.001 |
| Follow-up duration, yr | 1.8±1.9 | 2.0±2.1 | 1.6±1.6 | 2.0±2.2 | 0.095 |
Notes: Data are presented as the n (%) for or the mean ± SD. Characteristics of participants among GOLD 1, GOLD 2 and GOLD 3–4 compared using One-Way analysis of variance (ANOVA) test for continuous variables and the χ2 or Fisher exact test for categorical variables. ※There is one subject excluded in the analysis of smoking status, occupational exposure and mMRC score variables respectively due to missing values of interest. #Means that seven people were excluded for unknown family history of respiratory diseases. Pack-yrs is a unit of smoking index. It indicates multiplying the number of packs of cigarettes smoked per day by the number of years the person smoked. P values marked bold indicate that the value is statistically significant. BMI was calculated by dividing the weight in kilograms by the square of height in meters (kg/m2).
Abbreviations: GOLD, the Global Initiative for Chronic Lung Disease; mMRC, modified Medical Research Council dyspnea; BMI, Body Mass Index; TB, Total Bilirubin; %, percent; FVC, Forced vital capacity; FVC, % predicted, the ratio of FVC to its predicted value; FEV1, Forced expiratory volume in the first second; FEV1%predicted, the ratio of FEV1 to its predicted value; FEV1/FVC, the ratio of FEV1 to FVC.
Associations of Total Bilirubin with Baseline Lung Function Parameters
Univariable and multivariable linear regressions of baseline pulmonary function parameters such as FEV1, FEV1pred, FVC, FVCpred, and FEV1/FVC on TB concentrations are shown in Table 2. After adjustment for age, sex, BMI, smoking status, smoke index, passive smoker, occupational exposure, comorbidities, and family history of respiratory diseases, TB level (μmol/L) was significantly related to FEV1 and FVC in the total COPD patients (β 0.02, 95% CI 0.001~0.02, P = 0.025 and β 0.02, 95% CI 0.002~0.03, P = 0.022, respectively). Stratified by GOLD stages, the relationship between TB and FEV1 was only present in GOLD 1 stage (β 0.01, 95% CI 0.001~0.02, P = 0.032), whereas FVC became tending to be slightly associated with bilirubin (P = 0.054).
Table 2.
Univariable and Multivariable Linear Regression Analysis for TB and Baseline Lung Function Parameters in the Total Group and Subgroups by GOLD Stages
| Univariable | Multivariable | |||
|---|---|---|---|---|
| β Estimate (95% CI) | P values | β Estimate (95% CI) | P values | |
| Total group | ||||
| FEV1, L | 0.008(−0.003~0.02) | 0.157 | 0.02(0.001~0.02) | 0.025 |
| FEV1pred,% | 0.15(−0.25~0.55) | 0.465 | 0.32(−0.06~0.69) | 0.097 |
| FVC, L | 0.01(−0.001~0.03) | 0.063 | 0.02(0.002~0.03) | 0.022 |
| FVCpred,% | 0.15(−0.24~0.54) | 0.458 | 0.26(−0.11~0.63) | 0.170 |
| FEV1/FVC,% | −0.01(−0.20~0.17) | 0.893 | 0.07(−0.10~0.25) | 0.416 |
| GOLD 1 | ||||
| FEV1, L | 0.01(−0.003~0.02) | 0.123 | 0.01(0.001~0.02) | 0.032 |
| FEV1pred,% | 0.16(−0.15~0.48) | 0.308 | 0.25(−0.06~0.04) | 0.117 |
| FVC, L | 0.02(−0.002~0.04) | 0.076 | 0.01(0.00~0.03) | 0.054 |
| FVCpred,% | 0.16(−0.28~0.60) | 0.473 | 0.24(−0.17~0.66) | 0.254 |
| FEV1/FVC,% | −0.03(−0.16~0.10) | 0.660 | 0.03(−0.1~0.15) | 0.686 |
| GOLD 2 | ||||
| FEV1, L | 0.004(−0.01~0.02) | 0.451 | 0.01(−0.003~0.01) | 0.215 |
| FEV1pred,% | 0.05(−0.22~0.32) | 0.716 | 0.08(−0.19~0.35) | 0.561 |
| FVC, L | 0.004(−0.02~0.02) | 0.702 | 0.01(−0.01~0.02) | 0.515 |
| FVCpred,% | −0.05(−0.45~0.35) | 0.791 | −0.05(−0.45~0.36) | 0.825 |
| FEV1/FVC,% | 0.07(−0.19~0.34) | 0.580 | 0.09(−0.18~0.35) | 0.517 |
| GOLD 3–4 | ||||
| FEV1, L | 0.01(−0.007~0.02) | 0.284 | 0.01(−0.01~0.03) | 0.298 |
| FEV1pred,% | 0.16(−0.36~0.69) | 0.537 | 0.27(−0.30~0.84) | 0.343 |
| FVC, L | 0.03(−0.012~0.07) | 0.155 | 0.03(−0.02~0.07) | 0.222 |
| FVCpred,% | 0.61(−0.45~1.66) | 0.253 | 0.69(−0.48~1.86) | 0.241 |
| FEV1/FVC,% | −0.21(−0.69~0.27) | 0.389 | −0.11(−0.63~0.41) | 0.667 |
Notes: P values marked bold indicate that the value is statistically significant. Multivariable linear regression analysis models were adjusted for age, sex, BMI, smoking status, smoke index, passive smoker, occupational exposure, comorbidities and family history of respiratory diseases. β estimates from Multivariate linear regression analysis were the unstandardized β estimates. Other abbreviations see Table 1.
Abbreviation: CI, confidence interval.
Total Bilirubin and Annual Lung Function Decline
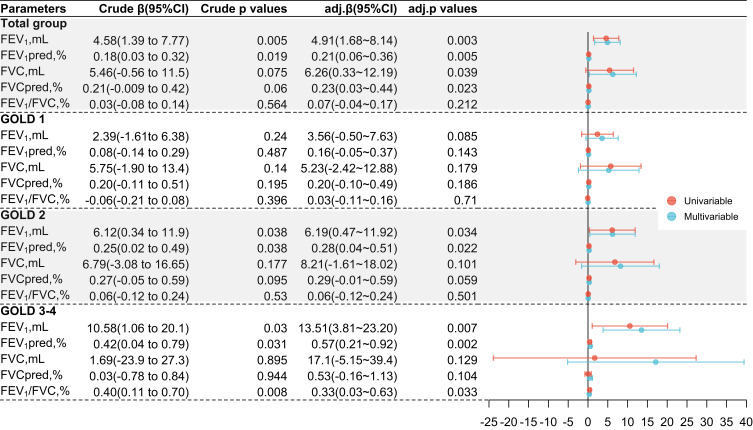
Totally, higher bilirubin concentrations were associated with a reduced rate of decline in lung function, which is consistent in the univariable and multivariable regression analysis approximately (Figure 2). In the total COPD group, we found that per 1 μmol/L increase in TB will reduce 4.91 mL/L decline in FEV1 (P = 0.003), 0.21% decline in FEV1pred (P = 0.005), 6.26 mL/L decline in FVC (P = 0.039), and 0.23% in FVCpred (P = 0.023). However, following stratification according to GOLD stages and multi-adjustments, the similar associations of FEV1 and FEV1pred with TB merely existed in GOLD 2 and GOLD 3–4 patients. Besides, bilirubin only showed a protective effect on the rate of decline in airflow limitation (FEV1/FVC) in severe COPD (GOLD 3–4).
Figure 2.
Linear regression analysis for the relationship between bilirubin and annual lung function decline, by overall COPD population and stratified by GOLD stages.
Notes: Annual Lung function decline was calculated as the parameters at follow-up minus corresponding baseline values divided by the number of years for follow-up duration. Multivariable linear regression analysis models were adjusted for age, sex, BMI, smoking status, smoke index, passive smoker, occupational exposure, comorbidities and family history of respiratory diseases. Per unit increase of TB in all these models is 1 μmol/L. Crude β means the unstandardized β estimate from univariable analysis, Crude P values mean the P values from univariable analysis, adj.β means the unstandardized β estimate from the multivariable analysis, adj.P values mean the P values from multivariable analysis. Abbreviations see abbreviation section in Table 1.
Discussion
In the pooled data from two prospective and longitudinal cohort studies, we found that TB was positively associated with FEV1 in the total COPD population at baseline. Besides, TB also significantly reduced the rate of FEV1 and FEV1pred decline in the total COPD group at follow-up duration after adjustment for age, sex, BMI, smoking status, smoke index, passive smoker, occupational exposure, comorbidities, as well as family history of respiratory diseases. Surprisingly, when stratifying by disease severity according to GOLD stages, relationships significantly persisted only in patients with moderate-to-severe COPD. To our best knowledge, our study was the first to explore TB and lung function in the target COPD population with different GOLD stages.
What we found between TB and FEV1 were in line with those of Ah YL et al11 who found that higher TB concentration was associated with higher FEV1 and less annual FEV1 decline in the general Koreans and those of Apperley S et al12 who also showed that consistent associations in mild-to-moderate COPD after adjusting for age, sex, race, BMI and baseline measures of lung function. All these demonstrations indicated the potential protective role of TB played on lung function. We could reasonably speculate about the mechanisms of mild higher TB level protecting lung function. Summarily, the most important heme oxygenase that influences the biochemical production of bilirubin is heme oxygenase-1 (HO-1),21 which would be up-regulated by oxidative stress22 and hypoxia.23,24 HO-1 is expressed in pulmonary cells including type II pneumocytes and alveolar macrophages.25 COPD is a chronic pathological process accompanied by endogenously or exogenously produced oxidative stress and inflammation.26,27 Higher HO-1 levels expressed in COPD would increase TB concentrations. Furthermore, bilirubin has been reported to be an endogenous bioactive substance possessing substantial antioxidant activities by several experiments.28–30 Thus, TB may improve the lung function in COPD in this way.
Another extended finding was that the significant protection effects merely persisted in moderate-to-severe COPD. It may be implied that only when oxidative stress increased to a certain degree can bilirubin perform a function against oxidation. We can get some evidence from the current studies. A Mendelian Randomization analysis using UK Biobank showed that smokers with genetically raised serum bilirubin levels have lower rates of lung cancer and these relationships are strongest in current heavy smokers (20 or more cigarettes per day),31 whereas Hyun et al found that high serum TB was not significantly associated with the presence of COPD in never-smokers.32 Likewise, our data showed that patients in GOLD 2 and GOLD 3–4 had more smoking exposure (more smoking index, pack-yrs). As we know, heavy cigarettes exposure aggravated inflammation and oxidative damage of COPD, which would be suppressed by bilirubin reduction to attenuate pulmonary injury.33 Differently from previous studies,11,13 we found no significant association between TB and airflow limitation (FEV1/FVC) in the total group. The conflicting results may be attributed to the differences of adjusted covariates in the proportion of sex, distribution of age, BMI, smoking status and index, comorbidities, as well as family history of respiratory diseases.
Our study also has a few limitations. First, the sample size in moderate-to-severe COPD was small, so we could not respectively evaluate the relationship of TB and GOLD 3 COPD or GOLD 4 COPD, given that the participants were community-based. However, GOLD 3 and GOLD 4 were usually incorporated as severe COPD in clinical epidemiology. Second, we only collected one blood sample, which did not allow for continuous evaluation of intraindividual variability, although bilirubin levels are demonstrated to keep stable after adolescence.34 Besides, we could not exclude the influence of alcohol intake on TB for lacking the related information. However, we have excluded other factors affecting bilirubin as we can by incorporating hepatic disease into comorbidities, which may be caused by heavy alcohol consumption.
Conclusions
In conclusion, we confirmed again that TB is inversely associated with annual lung function decline in FEV1 and FEV1pred in COPD. And that association persists only in moderate-to-severe COPD adjusted for multi-covariates, which implicated the different functional status of TB being in different degrees of COPD severity. It is also suggested that TB may be a biomarker for only moderate-to-severe COPD but not mild COPD. We should focus more on the specific COPD patients with a certain disease severity when validating bilirubin as a biomarker of COPD potentially in future studies, given the protective effects of TB. Besides, further researches are also needed to investigate the mechanisms of TB action on different severity levels of COPD to explain the different influences on lung function decline.
Acknowledgments
The authors thank for the contributions of all the subjects who agreed to donate their information for analysis. Besides, the authors thank Bijia Lin, Shaodan Wei and Xiaopeng Ling (State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Nanshan Medicine Innovation Institute of Guangdong Province) for their help in collecting the data.
Funding Statement
The study was funded by The Nature Key Research and Development Program (2016YFC1304101), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01s155), the independent project of the State Key Laboratory of Respiratory Diseases (SKLRD-QN-201913), the National Science Foundation of China (81970045), and Zhong Nanshan Medical Development Foundation of Guangdong Province (ZNSA-202003, ZNSA-2020012, ZNSA-2020013).
Abbreviations
TB, total bilirubin; COPD, chronic obstructive pulmonary disease; GOLD, the Global Initiative for Chronic Lung Disease; mMRC, Modified Medical Research Council dyspnea; BMI, body mass index; FVC, forced vital capacity; FVC, %predicted, the ratio of FVC to its predicted value; FEV1, forced expiratory volume in the first second; FEV1,%predicted, the ratio of FEV1 to its predicted value; FEV1/FVC, the ratio of FEV1 to FVC; ANOVA, analysis of variance; SD, standard deviation; CI, confidence interval; CVD, cardiovascular disease; ECOPD, the early COPD; HO-1, oxygenase-1.
Data Sharing Statement
The datasets used and analyzed in this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The original two studies were performed in line with the principles of the Declaration of Helsinki. All the participants from two studies gave written informed consent and two studies were approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No.2013-37 and No. 2018-53 respectively).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors declare they have no real or potential competing interests in this work.
References
- 1.Stocker R, Yamamoto Y, Mcdonagh A, Glazer A, Ames B. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science. 1987;235:43. [DOI] [PubMed] [Google Scholar]
- 2.Wang H-D, Yamaya M, Okinaga S, et al. Bilirubin Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Rats. Am J Respir Crit Care Med. 2002;165(2):406–411. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarty MF. “Iatrogenic Gilbert syndrome”–a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69(5):974–994. [DOI] [PubMed] [Google Scholar]
- 5.Vitek L, Jirsa M, Brodanova M, et al. Gilbert syndrome and ischemic heart disease a protective effect of elevated bilirubin levels. Atherosclerosis. 2008;198:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Schwertner HA, Vitek L, Schwertner HA. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198(1):1–11. [DOI] [PubMed] [Google Scholar]
- 7.Horsfall LJ, Rait G, Walters K, et al. Serum Bilirubin and Risk of Respiratory Disease and Death. JAMA. 2011;305:691–697. [DOI] [PubMed] [Google Scholar]
- 8.Monroy-Iglesias MJ, Moss C, Beckmann K, et al. Serum Total Bilirubin and Risk of Cancer: a Swedish Cohort Study and Meta-Analysis. Cancers. 2021;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim JE, Kimm H, Jee SH. Combined effects of smoking and bilirubin levels on the risk of lung cancer in Korea: the severance cohort study. PLoS One. 2014;9(8):e103972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown KE, Sin DD, Voelker H, et al. Serum bilirubin and the risk of chronic obstructive pulmonary disease exacerbations. Respir Res. 2017;18(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leem AY, Kim HY, Kim YS, Park MS, Chang J, Jung JY. Association of serum bilirubin level with lung function decline: a Korean community-based cohort study. Respir Res. 2018;19(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apperley S, Park HY, Holmes DT, et al. Serum Bilirubin and Disease Progression in Mild COPD. Chest. 2015;148(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curjuric I, Imboden M, Adam M, et al. Serum bilirubin is associated with lung function in a Swiss general population sample. Eur Respir J. 2014;43(5):1278–1288. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald DM, Zanotto AD, Collins G, et al. Associations between baseline biomarkers and lung function in HIV-positive individuals. AIDS. 2019;33(4):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F, Zhou YM, Peng JQ, et al. Rationale and design of the Early Chronic Obstructive Pulmonary Disease (ECOPD) study in Guangdong, China: a prospective observational cohort study. J Thorac Dis. 2021;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agusti A, Vogelmeier C, Papi A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: 2021 report. Available from: https://goldcopd.org/2021-gold-reports/. Accessed July 1, 2021.
- 18.Duzenli T, Maden O, Tanoglu A, Kaplan M, Yazgan Y. Associations between Gilbert’s syndrome and personality characteristics. Trends Psychiatry Psychother. 2021;43(2):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 20.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. [DOI] [PubMed] [Google Scholar]
- 21.Tenhunen R, Marver HS, Schmid R. Microsomal Heme Oxygenase. Biol Chem. 1969;244(23):6388–6394. [PubMed] [Google Scholar]
- 22.Carter EP, Garat C, Imamura M. Continual emerging roles of HO-1: protection against airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L24–L25. [DOI] [PubMed] [Google Scholar]
- 23.Christou H, Morita T, Hsieh C-M, et al. Prevention of Hypoxia-Induced Pulmonary Hypertension by Enhancement of Endogenous Heme Oxygenase-1 in the Rat. Circ Res. 2000;86:1224–1229. [DOI] [PubMed] [Google Scholar]
- 24.Minamino T, Christou H, Hsieh C-M, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. PNAS. 2001;98(15):8798–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007;36(2):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266–273. [DOI] [PubMed] [Google Scholar]
- 27.Harijith A, Natarajan V, Fu P. The Role of Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Lung Architecture Remodeling. Antioxidants(Basel). 2017;6(4):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Guo M, Liu N, et al. Bilirubin neurotoxicity is associated with proteasome inhibition. Cell Death Dis. 2017;8(6):e2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitek L. Bilirubin as a signaling molecule. Med Res Rev. 2020;40(4):1335–1351. [DOI] [PubMed] [Google Scholar]
- 30.Stocker R, Glazert AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Medical Sciences. 1987;84(2):5918–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horsfall LJ, Burgess S, Hall I, Nazareth I. Genetically raised serum bilirubin levels and lung cancer: a cohort study and Mendelian randomisation using UK Biobank. Thorax. 2020;75(11):955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Hong Y, Lim MN, et al. Inflammatory biomarkers and radiologic measurements in never-smokers with COPD: a cross-sectional study from the CODA cohort. Chron Respir Dis. 2018;15(2):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J, Zhao H, Fan G, Li J. Bilirubin treatment suppresses pulmonary inflammation in a rat model of smoke-induced emphysema. Biochem Biophys Res Commun. 2015;465(2):180–187. [DOI] [PubMed] [Google Scholar]
- 34.Wilding P, Rollasox JG, Robinson D. Patterns of change for various biochemical constituents detected in well population screening. Clin chim Acta. 1972;1:375–387. [DOI] [PubMed] [Google Scholar]