Abstract
The fluoroquinolones (FQ) are used in the treatment of Mycobacterium tuberculosis, but the development of resistance could limit their effectiveness. FQ resistance (FQR) is a multistep process involving alterations in the type II topoisomerases and perhaps in the regulation of efflux pumps, but several of the steps remain unidentified. Recombinant plasmid pGADIV was selected from a genomic library of wild-type (WT), FQ-sensitive M. smegmatis by its ability to confer low-level resistance to sparfloxacin (SPX). In WT M. smegmatis, pGADIV increased the MICs of ciprofloxacin (CIP) by fourfold and of SPX by eightfold, and in M. bovis BCG it increased the MICs of both CIP and SPX by fourfold. It had no effect on the accumulation of 14C-labeled CIP or SPX. The open reading frame responsible for the increase in FQR, mfpA, encodes a putative protein belonging to the family of pentapeptides, in which almost every fifth amino acid is either leucine or phenylalanine. Very similar proteins are also present in M. tuberculosis and M. avium. The MICs of CIP and SPX were lower for an M. smegmatis mutant strain lacking an intact mfpA gene than for the WT strain, suggesting that, by some unknown mechanism, the gene product plays a role in determining the innate level of FQR in M. smegmatis.
Although the fluoroquinolones (FQ) are used against multidrug-resistant Mycobacterium tuberculosis (1), initial experience with ciprofloxacin (CIP) and ofloxacin (OFX) resulted in the rapid development of resistance (27, 34) and the use of the more effective sparfloxacin (SPX) is limited by toxicity (24). Nevertheless, newer drugs, such as moxifloxacin and gatifloxacin, have higher therapeutic ratios (8) and appear promising. Therefore, continued study of the mechanisms by which FQ resistance (FQR) develops in mycobacteria seems warranted.
Other bacteria, such as Escherichia coli, Streptococcus pneumoniae, and Staphylococcus aureus, develop FQR in a stepwise process, principally involving alterations in the A and B subunits of both gyrase and topoisomerase IV (30). However, there appear to be other elements involved in FQR, because a very high level of resistance may not be completely explained by mutations in topoisomerase genes and strains with a low level of resistance may lack mutations in these genes (39). The only other mechanism that has been implicated in FQR is the increased expression of FQ-transporting efflux pumps (29), which could play a role in both low-level and very high-level resistance (25).
Mycobacteria do not contain topoisomerase IV (7), and strains with moderate levels of resistance to CIP, OFX, or levofloxacin (LVX) (>3 μg) or SPX (>1 μg) all contain gyrA mutations (36). Mutations in gyrB have been found in FQR mutants selected in vitro (19, 42) but have not been described for FQR clinical isolates. No FQ-transporting pump has yet been described for M. tuberculosis, but increased expression of an efflux pump in M. smegmatis, LsrA, confers resistance to the hydrophilic FQ (35). While attempting to identify an M. smegmatis efflux pump that confers resistance to the hydrophobic FQ SPX, we instead found that a gene encoding a potential protein similar to E. coli McbG (10) confers resistance to both CIP and SPX and influences the baseline FQ MICs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type (WT), FQ-sensitive M. smegmatis strain mc2155 was used for genetic selections (14). Some plasmids were also tested for the ability to confer resistance in M. bovis BCG Pasteur. Mycobacteria were grown in 7H9 oleic acid-albumin-dextrose–0.05% Tween 80 (OAD-TW) liquid or on 7H10 OADC solid media. E. coli strain XL1-Blue was grown in Luria-Bertani media and used for the construction of libraries and plasmids. For routine selections, antibiotics were used at the following concentrations: carbenicillin, 50 μg/ml; and kanamycin (KAN), 20 μg/ml for mycobacteria and 25 μg/ml for E. coli. Concentrations of other antibiotics used are indicated in the text or in Table 1. Tests for levels of resistance to antibiotics and other compounds were performed with solid media.
TABLE 1.
Effect of pGADIV on FQ MICs
| Strain | FQ | MICa in
the presence of:
|
Relative change in the MIC (fold) | |
|---|---|---|---|---|
| pMD31 (vector) | pGADIVb | |||
| M. smegmatis mc2155 | CIP | 0.25 | 1.0 | 4 |
| CIP + CCCP | 0.25 | 1.0 | 4 | |
| SPX | 0.0625 | 0.5 | 8 | |
| SPX + CCCP | 0.0625 | 0.5 | 8 | |
| Norfloxacin | 2 | 4 | 2 | |
| OFX | 0.25 | 1.0–2.0 | 4–8 | |
| LVX | 0.0625 | 0.25–0.5 | 4–8 | |
| DU-6859a | 79 pg/ml | 158 pg/ml | 2 | |
| M. bovis BCG | CIP | 0.5 | 2 | 4 |
| SPX | 0.125 | 0.5 | 4 | |
| M. smegmatis mc2155 mfpA::aph (GM1) | CIP | 0.0625c | 0.25 | 0.25 |
| SPX | 0.0312c | 0.5 | 0.5 | |
Reported in micrograms per milliliter, unless otherwise indicated.
Containing ORF1, ORF2, and ORF3.
MIC in the presence of no plasmid.
DNA manipulations.
DNA isolation, cloning, and hybridization were performed with standard protocols (3). M. smegmatis genomic DNA was isolated as previously described (37). Genomic libraries were constructed in plasmid pMD31 using 3- to 6-kb fragments of M. smegmatis mc2155 genomic DNA generated by partial digestion with Sau3AI. The libraries were electroporated into E. coli strain XL1-Blue, KAN-resistant colonies were pooled, and plasmid DNA was isolated by alkaline lysis. The amplified libraries were then electroporated into WT M. smegmatis strain mc2155, with selection for resistance to KAN and CIP or KAN and SPX. For initial subcloning of the 3.2-kb insert of recombinant plasmid pGADIV, which was selected from the genomic libraries, fragments of various sizes generated by partial Sau3A digestion were ligated into pMD31. These sublibraries were amplified in E. coli and then electroporated into M. smegmatis mc2155, again with selection for resistance to both KAN and SPX. This selection produced pCM8, whose 2.1-kb insert was subcloned into pUC118 and pUC119 for sequencing. Plasmid pMD31 (9) was used to construct other subclones to be tested in mycobacteria.
E. coli was electroporated as described previously(13), and mycobacteria were electroporated using the protocols of Jacobs et al. (14). Some ligation mixtures were desalinated before electroporation (2). Ligations and restriction enzyme digestions were done with commercial enzymes as specified by the manufacturers or in standard protocols (3).
Allelic exchange.
Allelic exchange was performed essentially as described by Sander et al. (33). The aph gene from pYUB53 (35) was ligated into the PstI site within mfpA. The disrupted gene was ligated into rpsL suicide plasmid pYUB608 and then electroporated into streptomycin-resistant strain mc21255, the kind gifts of M. Pavelka (31). Strains with a disrupted mfpA gene were selected on 7H10 OADC plates containing streptomycin at 500 μg/ml and KAN at 20 μg/ml. Allelic exchange in Smr mfpA::aph strain GM1 was confirmed by Southern hybridization (3).
DNA sequencing.
Sequencing was performed manually with Sequenase 2.0 (U.S. Biochemicals) or with an ALFexpress sequencer (Pharmacia). Plasmid DNA was prepared for sequencing by alkaline lysis with lithium chloride (41). Single-stranded M13 DNA was produced with helper phage M13K07 (32).
Sequence analysis.
Analysis of DNA and implied protein sequences was performed using the MacVector package (Oxford Molecular) and the National Center for Biotechnology Information Blast site. Information on open reading frames (ORFs) in the M. tuberculosis genome was obtained from the National Center for Biotechnology Information genome website. Preliminary sequence data were obtained from The Institute for Genomic Research (TIGR) website at http://www.tigr.org.
FQ uptake studies.
SPX and CIP labeled with 14C were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (catalog numbers 3579 and 3638, respectively). The specific activities were 13.5 mCi/mmol for 2-14C-labeled CIP and 9 mCi/mmol for 2-14C-labeled SPX. Uptake studies were performed essentially as previously described (21), with counts per minute standardized to 5 mg (dry weight) of bacteria per assay.
Chemicals and drugs.
Acriflavine, CCCP (carbonyl cyanide m-chlorophenylhydrazone), CHH (2-chlorophenylhydrazine hydrochloride), chloramphenicol, CTAB (hexadecyltrimethylammonium bromide), ethidium bromide, KAN, nalidixic acid, norfloxacin, phenylmercuric acetate, reserpine, and tetracycline were obtained from Sigma or Aldrich Chemical Company. CIP was obtained from Miles; SPX was a gift from Rhone Poulenc USA; and LVX, OFX, and DU-6859a were kindly provided by Daichii Pharmaceuticals, Tokyo, Japan. Reserpine was dissolved in chloroform, CCCP was dissolved in N,N-dimethylformamide, nalidixic acid was dissolved in methanol, and the FQ were dissolved in 0.1 N NaOH. All other compounds were dissolved in distilled water.
RESULTS
Isolation of resistance-conferring plasmid pGADIV.
A genomic library was constructed in pMD31 using chromosomal DNA from WT M. smegmatis strain mc2155. The library was electroporated into WT mc2155 and plated on 7H10 OAD-TW containing KAN (20 μg/ml) and either SPX (0.125 μg/ml) or CIP (0.25 μg/ml). Five colonies were obtained on plates with KAN and CIP; four of these contained vector pMD31 without an insert, and the plasmid isolated from the fifth, which contained a 4.1-kb insert, did not confer CIP resistance upon retransformation of M. smegmatis mc2155. The single colony obtained on plates with KAN and SPX contained a plasmid with a 3.2-kb insert. This plasmid, named pGADIV, was retransformed into WT strain mc2155, with selection for KAN resistance. Compared to the WT mc2155 parent strain, the transformants showed increased resistance to FQ; the MIC of SPX increased by eightfold, those of CIP, OFX, and LVX increased by four- to eightfold, and those of norfloxacin and DU-6859a increased by twofold (Table 1). The increased FQR was not affected by either CCCP or reserpine, and there was no increase in resistance to nalidixic acid or compounds described as substrates for multidrug efflux pumps, including acriflavin, ethidium bromide, chloramphenicol, CTAB, CCCP, CHH, tetracycline, or phenylmercuric acetate (data not shown) (35). Plasmid pGADIV was stably maintained within M. smegmatis mc2155, and when it was electroporated into M. bovis BCG, it increased the MICs of both CIP and SPX by fourfold (Table 1).
Drug accumulation studies.
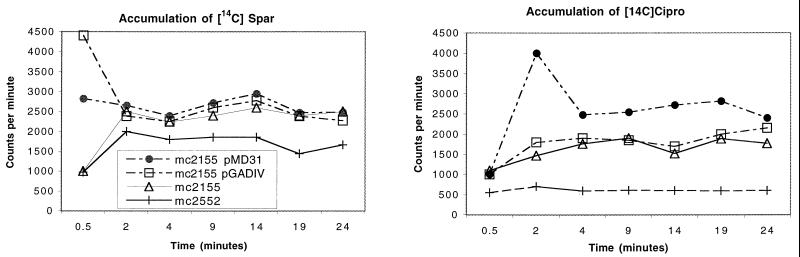
To determine whether pGADIV conferred resistance by decreasing the concentration of drug within bacteria, drug accumulation studies were performed using 14C-labeled SPX and CIP. As shown in Fig. 1, pGADIV had no effect on the accumulation of either drug within strain mc2155 and therefore does not appear to contain an efflux pump. The slight increase in SPX accumulation seen with pGADIV was more pronounced with vector pMD31 alone, and thus is not an effect of the pGADIV insert.
FIG. 1.
The presence of FQR-conferring plasmid pGADIV in M. smegmatis strain mc2155 has no effect on the accumulation of 14C-labeled SPX (Spar) or CIP (Cipro). M. smegmatis strain mc2552 (21) was used as a low-accumulation control.
Sequencing and subcloning of pGADIV.
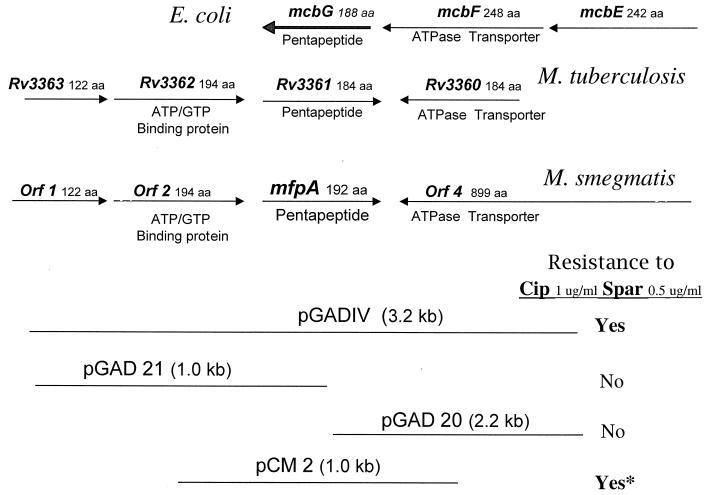
Shotgun subcloning of the 3.2-kb pGADIV insert yielded plasmid pCM8 (Fig. 2), whose 2.1-kb insert also conferred SPX resistance. Sequence data revealed that this insert contained the following (Fig. 2): ORF1, encoding a potential 121-amino-acid (aa) protein with homology to M. tuberculosis Rv3363c; ORF2, encoding a potential 193-aa protein similar to Rv3362c and ATP or GTP binding proteins; ORF3, encoding a potential 192-aa protein similar to Rv3361c and E. coli protein McbG; and the 3′ portion of ORF4, which would encode an 899-aa protein that is 73% identical to the putative ATPase transporter Rv1747. In the M. tuberculosis genome, the Rv3360 ORF also has similarity to the Rv1747 ORF but would encode a protein of only 122 aa (Fig. 3). All of the ORFs contained in pGADIV are also similar to ORFs present in the same order in the M. avium genome (contig 87; TIGR). The M. leprae genome contains nucleotide sequences similar to ORF2 and ORF3, but no corresponding proteins are found in the Leproma website (genolist.pasteur.fr/leproma), suggesting that they may be pseudogenes.
FIG. 2.
Subcloning of M. smegmatis genes contained in plasmid pGADIV and genomic arrangement of ORFs encoding similar proteins in E. coli and M. tuberculosis. ∗, plasmid pCM2 contains only one complete ORF, ORF3, subsequently named mfpA, and confers twofold less resistance to CIP (Cip) and SPX (Spar) than plasmid pGADIV. (Diagrams are not drawn to scale.)
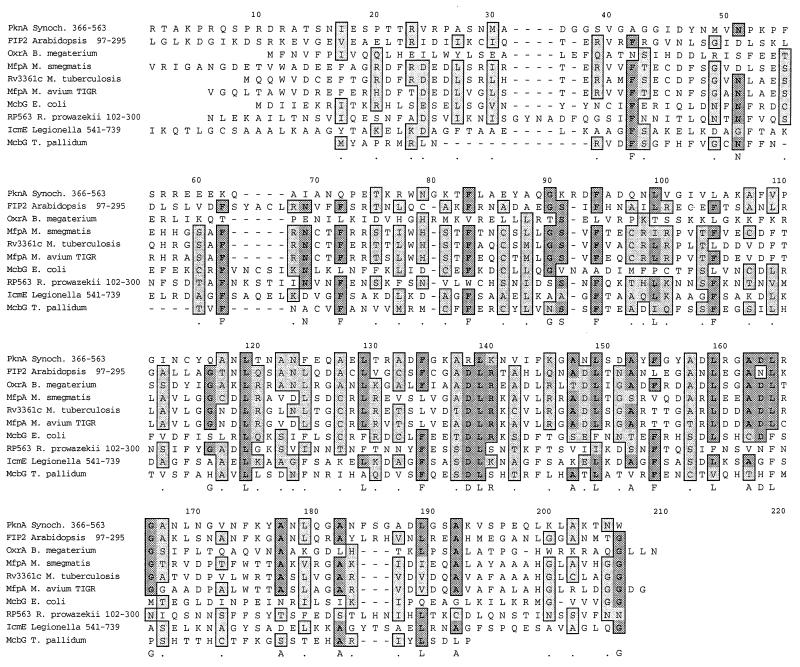
FIG. 3.
Clustal W alignment of M. smegmatis protein MfpA (ORF3, nucleotides [nt] 13826 to 14398 of TIGR contig 2943) with similar hypothetical proteins from M. tuberculosis (Rv3361c) and M. avium (nt 7330 to 7893 of TIGR contig 87). The other pentapeptide proteins (GenBank accession numbers) included are PknA (S76132), a Synechocystis sp. protein kinase of 574 aa; OxrA (T00033), a B. megaterium plasmid pOXTB2 oxetanocin A resistance protein of 185 aa; FIP2 (BAB10574), an A. thaliana AFH1 interacting protein of 298 aa; McbG, (P05530), an E. coli microcin B17 resistance protein of 187 aa; RP563 (A71661), an R. prowazekii hypothetical protein of 588 aa; IcmE (T18334), an L. pneumophila protein of 1,048 aa; and McbG (D71329), a T. pallidum protein of 149 aa. Dark grey highlighting indicates identical amino acids; light grey highlighting indicates similar amino acids.
Figure 2 shows the subcloning of the pGADIV insert to determine which ORF increased FQR. Plasmids pGAD20 and pGAD21 were constructed using a PstI site within ORF3. Plasmid pCM2 was constructed by ligating pMD31 with a 1-kb DNA fragment amplified from pGADIV by use of primers Lsr1 (5′ TTCTGGTTCATGTGGGACGAC) and Lsr2 (5′ GGAAGATCCGTCTCAAAGCC). It contains only one complete ORF, ORF3, and conferred twofold less FQR than pGADIV. The complete sequence of this region was subsequently obtained from the M. smegmatis genome project (TIGR) and used to confirm uncertain bases in our sequence data and to provide the entire sequence of ORF4.
Proteins similar to ORF3 protein.
As mentioned above, a Blast search determined that the putative protein encoded by ORF3 is similar to E. coli protein McbG, a protein that confers resistance to microcin B17. Further GenBank Entrez and Blast searches identified proteins similar to McbG in other bacteria, including Treponema pallidum, Legionella pneumophila, Rickettsia prowazekii, Bacillus megaterium, and Synechocystis sp., as well as in the plant Arabidopsis thaliana. From the alignment of these proteins in Fig. 3, there emerges a repeating consensus of leucines or phenylalanines occurring every fifth amino acid. This pattern has been previously described as pentapeptide repeats (5). The first amino acid in the 5-aa motif is often arginine; the second is often glycine; the third is often serine, cysteine, alanine, or threonine; and the fourth is often aspartate. Most of the proteins shown have one or two repeats with the submotif ADLRGA/T. The ORF3 gene was named mfpA, for mycobacterial FQR pentapeptide.
Construction and phenotype of an mfpA mutant strain.
To determine whether the MfpA protein plays a role in determining the intrinsic level of FQR in M. smegmatis, a mutant strain, GM1, was constructed so that its only mfpA gene is disrupted by a KAN resistance cassette. The mfpA disruption was stably maintained in mutant strain GM1, which was found to be twofold more sensitive to SPX and fourfold more sensitive to CIP than WT strain mc2155 (Table 1). Thus, it appears that in M. smegmatis the baseline MICs of the FQ are influenced by MfpA.
DISCUSSION
Using genetic selection for a gene conferring SPX resistance in multiple copies, we isolated mfpA, which encodes a potential protein similar to McbG of E. coli (10). When M. smegmatis contains mfpA on a multicopy plasmid, it is two- to eightfold more resistant to FQ but shows no increased resistance to nalidixic acid or several other compounds often transported by multidrug efflux pumps. Further evidence that MfpA is not an efflux pump was the absence of any effect on the accumulation of 14C-labeled CIP and SPX. In addition, the amino acid sequence of the MfpA peptide shows no similarity to any transporter but instead is similar to the recently described family of pentapeptide proteins (5). Plasmid pCM2, which contains only mfpA, conferred twofold less FQR than the original recombinant plasmid pGADIV, but it is unclear if this lower level of resistance is related to the level of mfpA expression from this plasmid or whether perhaps one of the upstream ORFs plays a role in the unknown FQR mechanism. The mfpA promoter has not been identified (11).
The similarity of MfpA to McbG is particularly interesting because McbG confers resistance to the peptide antibiotic microcin B17, whose target, like that of the FQ, is DNA gyrase (38). However, mutations conferring resistance to microcin B17 map in gyrB, while FQR mutations are most commonly found in gyrA (36). In E. coli, the gene for McbG is found next to the genes for McbE and McbF (10), in the plasmid-based operon that produces microcin B17, and McbEF and McbG have been shown to confer resistance to the bacteria producing the microcin. The resistance conferred by McbE and McbF is thought to be related to their role in exporting microcin B17 from the cytoplasm, but the separate mechanism of resistance conferred by McbG is unknown (10). One notion is that it somehow protects the gyrase (4). A subsequent study (22) found that the McbGEF genes also conferred resistance to SPX (eightfold), tosufloxacin (fourfold), and LVX (twofold) and but not to CIP, norfloxacin, temafloxacin, lomefloxacin, oxolinic acid, KAN, tetracycline, or chloramphenicol. The increased FQR was presumed to be the result of FQ export by the McbEF pump, but this action was not demonstrated. The M. smegmatis mfpA gene also had its greatest effect on resistance to SPX (eightfold) but, in addition, conferred resistance to CIP, OFX, LVX (fourfold), and norfloxacin (twofold).
McbG and MfpA are composed of pentapeptide repeats that were originally described for HglK (6), a protein in Anabaena that may be involved in the transport or assembly of heterocyst-specific glycolipids. Sixteen pentapeptide proteins of unknown function were found in the cyanobacterium Synechocystis (15), and one has been described for the plant Arabidopsis (16). An interesting pentapeptide protein is OxrA (26), a B. megaterium protein that confers resistance to the nucleoside oxetanocin A produced by this bacterium. The oxetanocins are potential antiviral agents that preferentially inhibit viral DNA polymerases (28).
It has been suggested that each pentapeptide repeat forms a beta sheet (5), and the proteins have been modeled as a superhelix with three beta-sheet pentapeptide repeats per turn. The presence in these proteins of regions where the hydrophobic amino acids leucine and phenylalanine alternate in the fifth position (Fig. 3) suggests that their structure may be more complex. The ADLRG motif may also represent a substructure or functional motif.
The family of leucine-rich proteins is characterized by protein-protein interactions (17). In some of these proteins, an alternating alpha-helix and beta-sheet structure results in a horseshoe-shaped protein that can cover an interacting protein, as the RNase inhibitor covers RNase A (18). Because both FQ and microcin B17 have gyrase as their target, it is tempting to imagine that the McbG homologues somehow protect gyrase. Alternatively, these proteins may confer resistance through hydrophobic interactions with structured molecules, such as quinolones, or the thiazoles and oxazoles in microcin B17 (40) and oxetanocin A. SPX is one of the more hydrophobic FQ, and strong hydrophobic interactions may explain the higher level of resistance to SPX conferred by MfpA. It is not clear why MfpA has no effect on the MIC of nalidixic acid.
The evolutionary function of these proteins is unknown. They could serve to protect bacteria from phytoalexins, which are toxins and signaling molecules produced by plants in response to bacterial or fungal infections (12, 40). Another possibility is that, like McbG, MfpA protects bacteria from a bacteriocin or other antibiotic synthesized by enzymes produced from nearby genes (20). While the M. smegmatis genome is not yet annotated, ORFs near the M. tuberculosis mfpA homologue are similar to genes encoding oxidoreductases, such as mitR (Rv3353c), which have been implicated in the synthesis of mitomycin C and other antibiotics (23). It is therefore conceivable that proteins encoded by surrounding genes are involved in the production of an antibiotic and that MfpA affords protection against this product, but there is currently no evidence to support this idea.
Because the mfpA mutant strain had a two- to fourfold lower level of resistance to the FQ, MfpA appears to play a role in determining the innate MICs of the FQ, at least in M. smegmatis. Although it remains to be demonstrated, this is likely to be true also in M. tuberculosis, both because of the similarity of M. tuberculosis MfpA and because the M. smegmatis mfpA gene carried on a plasmid increased the FQR of M. bovis BCG. Thus, mutations that alter MfpA or increase its expression could constitute one of the unknown steps in the development of FQR. At this time, however, although the ability of MfpA to confer FQR is an interesting observation, especially because of the unusual structure of the predicted protein, its significance in clinical FQR or in the relative efficacies of different FQ structures remains to be determined.
ACKNOWLEDGMENTS
We are indebted to Barbara Laughon, Mohamed Nasr, and the NIH AIDS Research and Reference Reagent Program for the synthesis and availability of 14C-labeled CIP and SPX. Preliminary sequence data were obtained from TIGR website at http://www.tigr.org.
This work was supported by CONICIT proyecto 960001322, ICGEB project CRP/VEN97-02, and Control de Enfermedades Endémicas project Ven/96/002 021-37.
REFERENCES
- 1.Alangaden G J, Lerner S A. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin Infect Dis. 1997;25:1213–1221. doi: 10.1086/516116. [DOI] [PubMed] [Google Scholar]
- 2.Atrazhev A M, Elliott J F. Simplified desalting of ligation reactions immediately prior to electroporation into E. coli. BioTechniques. 1996;21:1024. doi: 10.2144/96216bm12. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F, Brent R, Kingston R R, Moore D D, G. S J, Smith J A, Struhl K. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 4.Baba T, Schneewind O. Instruments of microbial warfare: bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 1998;6:66–71. doi: 10.1016/S0966-842X(97)01196-7. [DOI] [PubMed] [Google Scholar]
- 5.Bateman A, Murzin A G, Teichmann S A. Structure and distribution of pentapeptide repeats in bacteria. Protein Sci. 1998;7:1477–1480. doi: 10.1002/pro.5560070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black K, Buikema W J, Haselkorn R. The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaenasp. strain PCC 7120. J Bacteriol. 1995;177:6440–6448. doi: 10.1128/jb.177.22.6440-6448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas. Barry S C E, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Zhao X, Kreiswirth B N, Drlica K. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000;44:2581–2584. doi: 10.1128/aac.44.9.2581-2584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly-Wu M K, Jacobs W R, Hatfull G F. Superinfection immunity of mycobacteriophage L5: applications for genetic transformation of mycobacteria. Mol Microbiol. 1993;7:407–417. doi: 10.1111/j.1365-2958.1993.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrido M C, Herrero M, Kolter R, Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988;7:1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez M A, Smith I. Determinants of Mycobacterium gene expression. In: Hatfull G F, Jacobs W R Jr, editors. Molecular genetics of mycobacteria. Washington, D.C.: ASM Press; 2000. pp. 111–129. [Google Scholar]
- 12.Grayer R J, Kokubun T. Plant–fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry. 2001;56:253–263. doi: 10.1016/s0031-9422(00)00450-7. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coliand other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystissp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement) DNA Res. 1996;3:185–209. doi: 10.1093/dnares/3.3.185. [DOI] [PubMed] [Google Scholar]
- 16.Kieselbach T, Mant A, Robinson C, Schroder W P. Characterisation of an ArabidopsiscDNA encoding a thylakoid lumen protein related to a novel 'pentapeptide repeat' family of proteins. FEBS Lett. 1998;428:241–244. doi: 10.1016/s0014-5793(98)00517-1. [DOI] [PubMed] [Google Scholar]
- 17.Kobe B, Deisenhofer J. Proteins with leucine-rich repeats. Curr Opin Struct Biol. 1995;5:409–416. doi: 10.1016/0959-440x(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 18.Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186. doi: 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- 19.Kocagoz T, Hackbarth C J, Unsal I, Rosenberg E Y, Nikaido H, Chambers H F. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosisH37Ra. Antimicrob Agents Chemother. 1996;40:1768–1774. doi: 10.1128/aac.40.8.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y M, Milne J C, Madison L L, Kolter R, Walsh C T. From peptide precursors to oxazole and thiazole-containing peptide antibiotics: microcin B17 synthase. Science. 1996;274:1188–1193. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Takiff H E, Nikaido H. Active efflux of fluoroquinolones in Mycobacterium smegmatismediated by LfrA, a multidrug efflux pump. J Bacteriol. 1996;178:3791–3795. doi: 10.1128/jb.178.13.3791-3795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomovskaya O, Kawai F, Matin A. Differential regulation of the mcb and emr operons of Escherichia coli: role of mcbin multidrug resistance. Antimicrob Agents Chemother. 1996;40:1050–1052. doi: 10.1128/aac.40.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Y, Varoglu M, Sherman D H. Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulaeNRRL 2564. Chem Biol. 1999;6:251–263. doi: 10.1016/S1074-5521(99)80040-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin S J, Meyer J M, Chuck S K, Jung R, Messick C R, Pendland S L. Levofloxacin and sparfloxacin: new quinolone antibiotics. Ann Pharmacother. 1998;32:320–336. doi: 10.1345/aph.17178. [DOI] [PubMed] [Google Scholar]
- 25.Mazzariol A, Tokue Y, Kanegawa T M, Cornaglia G, Nikaido H. High-level fluoroquinolone-resistant clinical isolates of Escherichia colioverproduce multidrug efflux protein AcrA. Antimicrob Agents Chemother. 2000;44:3441–3443. doi: 10.1128/aac.44.12.3441-3443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita M, Tomita K, Ishizawa M, Takagi K, Kawamura F, Takahashi H, Morino T. Cloning of oxetanocin A biosynthetic and resistance genes that reside on a plasmid of Bacillus megateriumstrain NK84–0128. Biosci Biotechnol Biochem. 1999;63:563–566. doi: 10.1271/bbb.63.563. [DOI] [PubMed] [Google Scholar]
- 27.Muder R R, Brennen C, Goetz A M, Wagener M M, Rihs J D. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs medical center. Antimicrob Agents Chemother. 1991;35:256–258. doi: 10.1128/aac.35.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagahata T, Kitagawa M, Matsubara K. Effect of oxetanocin G, a novel nucleoside analog, on DNA synthesis by hepatitis B virus virions. Antimicrob Agents Chemother. 1994;38:707–712. doi: 10.1128/aac.38.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan X S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniaeby sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavelka M S, Jacobs W R. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar L, Fsihi H, de Rossi E, Riccardi G, Rios C, Cole S T, Takiff H E. Organization of the origins of replication of the chromosomes of Mycobacterium smegmatis, Mycobacterium leprae and Mycobacterium tuberculosis and isolation of a functional origin from M. smegmatis. Mol Microbiol. 1996;20:283–293. doi: 10.1111/j.1365-2958.1996.tb02617.x. [DOI] [PubMed] [Google Scholar]
- 33.Sander P, Meier A, Bottger E C. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol Microbiol. 1995;16:991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan E A, Kreiswirth B N, Palumbo L, Kapur V, Musser J M, Ebrahimzadeh A, Frieden T R. Emergence of fluoroquinolone-resistant tuberculosis in New York City. Lancet. 1995;345:1148–1150. doi: 10.1016/s0140-6736(95)90980-x. [DOI] [PubMed] [Google Scholar]
- 35.Takiff H E, Cimino M, Musso M C, Weisbrod T, Martinez R, Delgado M B, Salazar L, Bloom B R, Jacobs W R., Jr Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci USA. 1996;93:362–366. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takiff H E, Salazar L, Guerrero C, Philipp W, Huang W M, Kreiswirth B, Cole S T, Jacobs W R, Jr, Telenti A. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrBgenes and detection of quinolone resistance mutations. Antimicrob Agents Chemother. 1994;38:773–780. doi: 10.1128/aac.38.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Soolingen D, de Haas P E, Hermans P W, van Embden J D. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 1994;235:196–205. doi: 10.1016/0076-6879(94)35141-4. [DOI] [PubMed] [Google Scholar]
- 38.Vizan J L, Hernandez-Chico C, del Castillo I, Moreno F. The peptide antibiotic microcin B17 induces double-strand cleavage of DNA mediated by E. coliDNA gyrase. EMBO J. 1991;10:467–476. doi: 10.1002/j.1460-2075.1991.tb07969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C, Kreiswirth B N, Sreevatsan S, Musser J M, Drlica K. Fluoroquinolone resistance associated with specific gyrase mutations in clinical isolates of multidrug-resistant Mycobacterium tuberculosis. J Infect Dis. 1996;174:1127–1130. doi: 10.1093/infdis/174.5.1127. [DOI] [PubMed] [Google Scholar]
- 40.Yorgey P, Lee J, Kordel J, Vivas E, Warner P, Jebaratnam D, Kolter R. Posttranslational modifications in microcin B17 define an additional class of DNA gyrase inhibitor. Proc Natl Acad Sci USA. 1994;91:4519–4523. doi: 10.1073/pnas.91.10.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue C C, LaBash J D. Resistance to restriction digestion by plasmids prepared with a lithium-containing lysis buffer. BioTechniques. 1991;11:331. [PubMed] [Google Scholar]
- 42.Zhou J, Dong Y, Zhao X, Lee S, Amin A, Ramaswamy S, Domagala J, Musser J M, Drlica K. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J Infect Dis. 2000;182:517–525. doi: 10.1086/315708. [DOI] [PubMed] [Google Scholar]