Abstract
The traditional method of preparing hydrogen and oxygen as efficient clean energy sources mainly relies on the use of platinum, palladium, and other precious metals. However, the high cost and low abundance limit wide application of such metals. As such, one challenging issue is the development of low-cost and high-efficiency electrocatalysts for such purposes. In this study, we synthesized Co–FeS2/CoS2 heterostructures via a hydrothermal method for efficient hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Benefitting from their unique three-dimensional hierarchical nanostructures, Co-doped FeS2, and CoS2 formed heterostructures on Co–FeS2 petals, which bestowed remarkable electrocatalytic properties upon Co–FeS2/CoS2 nanostructures. Co–FeS2/CoS2 effectively catalyzed the OER with an overpotential of 278 mV at a current density of 10 mA cm−2 in 1 M KOH solution, and also is capable of driving a current density −10 mA cm−2 at an overpotential of −103 mV in 0.5 M H2SO4 solution. The overpotential of the OER and HER only decreased by 5 mV and 3 mV after 1000 cycles. Our Co–FeS2/CoS2 materials may offer a promising alternative to noble metal-based electrocatalysts for water splitting.
Here we report a facile solvothermal synthesis of Co–FeS2/CoS2 heterostructures with remarkable electrocatalytic properties.
1. Introduction
In response to global energy and environmental problems, researchers have made tremendous efforts to explore and develop high-performance and low-cost electrocatalysts for HER and OER to replace platinum, palladium and other precious metals.1–5 Non-noble metal electrocatalysts such as Fe, Co, Ni, Mo, and their sulfides,6–8 phosphides9–12 or their alloys13–17 have been investigated widely as electrocatalysts over the past decades. Among them, the low-cost, earth-abundant iron sulfide has attracted much attention as an electrocatalytic material due to its excellent catalytic activity.7,18 However, the catalytic performance of iron sulfide is limited by its low surface area and lack of active sites.19 If the non-noble metals electrocatalysts are grown on carbon material substrates, doping with homologous elements not only reduces the catalytic resistance but also exposes more active sites.20–22 Furthermore, the formation of epitaxial heterostructures can regulate the energy barrier between the two interfaces to reduce the catalytic kinetic energy.23–25
In this work, we prepared Co–FeS2/CoS2 heterostructures electrocatalysts on a carbon cloth (CC) by using sulfur powder and thiourea as sulfur sources and show that they have excellent catalytic performance. The unique 3D hierarchical nanostructures give it a high surface area and the doping of cobalt reduced the kinetic energy barrier of the catalytic reaction of FeS2. The bumps heterostructures of CoS2 further exposed more active sites and adjusted the kinetic energy barrier for catalytic reaction at the two interface junctions, which led to the remarkable electrocatalytic properties. The as-synthesized Co–FeS2/CoS2 materials has an exceptional overpotential of 278 mV at a current density of 10 mA cm−2 in 1 M KOH solution and the Tafel slope is only 73 mV dec−1. The overpotential is −103 mV at a current density of −10 mA cm−2 in 0.5 M H2SO4 solution, and the Tafel slope is only 56 mV dec−1. In addition, the overpotential of OER and HER only decreased by 5 mV and 3 mV after 1000 cycles. Our Co–FeS2/CoS2 materials may be a promising alternative to noble metal-based electrocatalysts for water splitting applications.
2. Experimental section
2.1. Chemicals and materials
WOS1009 carbon cloth (CC) was supplied by CeTech Co., Ltd. FeSO4·7H2O, was purchased from Shanghai Titan Scientific Co., Ltd. CH3CSNH2 was purchased from Aladdin Ltd. Co(NO3)·6H2O, sulfur power (S), thiourea (SC(NH2)2), Na2S·9H2O, C2H5OH, H2SO4, and KOH were purchased from Nanjing Chemical Reagent Co., Ltd. Ultrapure water was obtained using a Millipore pure water filter (Millipore Q, USA).
2.2. Synthesis of Co–FeS2/CoS2 heterostructures
In this experiment, the carbon cloth (1.8 cm × 2.2 cm) was first ultrasonically clean for 15 minutes using ultrapure water and anhydrous ethanol and then dried. Subsequently, FeSO4·7H2O (1.2 mM), Co(NO3)2·6H2O (0.156 mM), and SC(NH2)2 (1.8 mM) were added to a 30 mL reaction kettle followed by 25 mL of ultrapure water and stirred for 15 minutes to form a transparent homogeneous solution. Sulfur powder (0.72 mM) was then introduced to the above reactor and stirred at a low speed for 15 minutes. After the stirring was stopped, the magnetic stirrer was removed and sulfur powder film was formed on the liquid surface. The clean and dried carbon cloth was inserted vertically into reaction kettle solution and maintained at 180 °C for 8 hours. After the reactor cooled down to room temperature, the solution was removed, and then samples were washed using ultrapure water and absolute ethanol.
2.3. Materials characterization
The crystal phase properties of the samples were analyzed with a Bruker D8 Advance X-ray diffractometer (XRD) using Cu Kα radiation at 40 kV and 40 mA, for 2θ ranging from 20° to 70°, with a scan rate of 0.1° per second. Raman spectra were obtained by Raman spectroscopy (JY T64000) excited at 514.5 nm of a 100 μW Ar+ laser. Scanning electron microscopy (FE-SEM; JSM-7000F) was used to obtain the surface morphology of the sample. The energy dispersive spectrometer (EDS; Inca x-stream 034A0) was used to confirm the elemental composition of the sample. Transmission electron microscope (TEM) and high resolution transmission electron microscope (HRTEM) images were obtained by using a JEOL type JEM2100 instrument at an accelerating voltage of 200 kV. The chemical compositions of samples were determined by using X-ray photoelectron spectroscopy (XPS) analysis (PHI5000 Versaprobe).
2.4. Electrochemical measurements
Electrochemical measurements were performed with a CHI760E electrochemical analyzer (CH Instruments, Chenhua Co., Shanghai, China). A conventional three-electrode cell was used, including the sample as a working electrode, a calomel electrode as a reference electrode, and a graphite rod as a counter electrode. 1 M KOH and 0.5 M H2SO4 solution were used as the electrolyte solution, nitrogen was bubbled into the solution for 30 minutes before testing. In this paper, the positive scan curve is selected as the LSV curve (between 0 and 0.8 V, 2 mV s−1) for OER and the negative scan curve is selected as the LSV curve for HER (between −0.8 and −0.2 V, 2 mV s−1). The Tafel slope is calculated from the logarithmic relationship between overpotential and current density based on the LSV curve. The electrochemical active surface areas (ECSA) were calculated from the double layer charging curves using cyclic voltammograms (CVs) at different scan rates of 2–160 mV s−1 in potential range from 0 to 0.20 V vs. RHE for HER. Electrochemical impedance spectroscopy (EIS) was performed with a frequency range of 105–0.1 Hz. The stability assessment was performed by 1000 cycles of the testing at a scan rate of 100 mV s−1.
3. Results and discussion
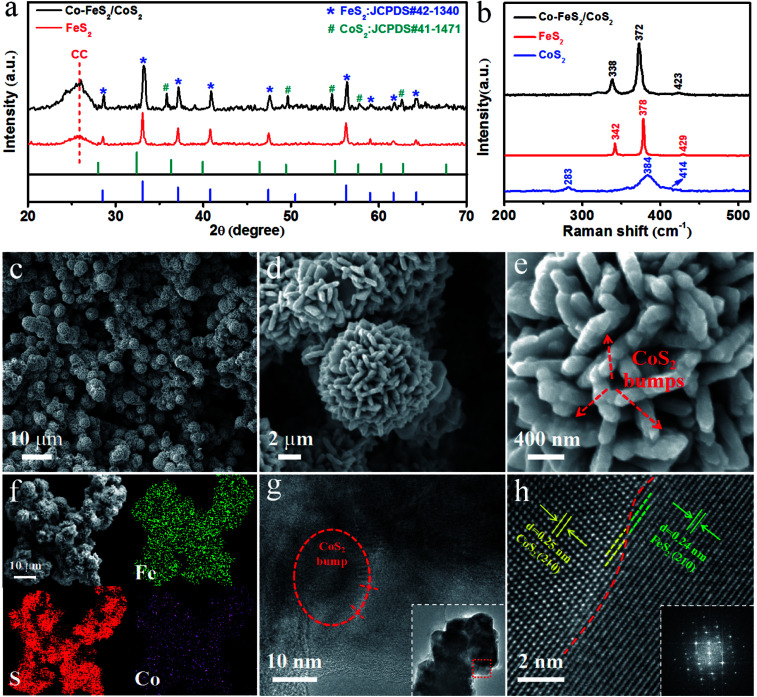
In the experiment, we prepared Co–FeS2/CoS2 heterostructures with superior electrocatalytic performance through the selection of sulfur sources, regulation of cobalt content, optimization of loading, addition and optimization of dual sulfur sources (as can be seen in ESI†). Fig. 1a shows the X-ray diffraction (XRD) patterns for Co–FeS2/CoS2 heterostructures and FeS2. The peaks at 28.5°, 33.1°, 37.1°, 40.8°, 47.5° and 56.3° can be indexed to the (111), (200), (210), (211), (220), (311) planes of FeS2 (JCPDS#42-1340).19,26 The peaks at 35.9°, 49.5°, 54.7°, 57.7° and 62.7° correspond to the planes of (210), (221), (311), (222) and (321) of CoS2 (JCPDS#41-1471).8,27 From the XRD pattern, it can be seen that the Co–FeS2/CoS2 heterostructures exhibit good crystallinity. Fig. 1b shows the Raman spectra of the Co–FeS2/CoS2 heterostructures, FeS2 and CoS2. The Raman spectrum for the Co–FeS2/CoS2 heterostructures shows a broad primary peak centered around 372 cm−1, which is likely the result of contributions from the most intense FeS2 peaks at 378 cm−1 and the primary CoS2 peak at 384 cm−1.28
Fig. 1. (a) XRD patterns for Co–FeS2/CoS2 and FeS2; (b) Raman spectra for Co–FeS2/CoS2, FeS2 and CoS2; (c)–(e) progressively enlarged SEM images of Co–FeS2/CoS2; (f) SEM image and corresponding EDX elemental mapping images of Fe, S, Co for Co–FeS2/CoS2; (g) TEM image of Co–FeS2/CoS2 heterostructures, the inset is TEM image of Co–FeS2/CoS2 nanopetals; (h) HRTEM image of Co–FeS2/CoS2 heterostructures, the inset is corresponding SAED pattern of Co–FeS2/CoS2 heterostructures.
As shown in Fig. 1c, Co–FeS2/CoS2 exhibits a microflower-like morphology with diameters ranging from 4 to 6 micrometer. Fig. 1d shows the nano-petals structures on the Co–FeS2/CoS2 heterostructures, the nano-petals crosslinked together to form a 3D microflower-like structures,29 which not only increase the specific surface area but also accelerate the outward diffusion rate of the generated gas in the solution for water splitting.30,31Fig. 1e is a high-resolution SEM image showing the bumps of CoS2 on the nano-petals of the Co–FeS2/CoS2 heterostructures; Co-doped FeS2 decreased the kinetic energy barrier of the catalytic reaction, synergistic catalysis by heterostructures of Co–FeS2/CoS2 further improves electrocatalytic activity.10,32–34Fig. 1f shows the SEM image and the energy-dispersive X-ray (EDX) spectrum elemental mapping images of Fe, S and Co, for the Co–FeS2/CoS2 heterostructures, revealing the distribution of the three elements. Fig. 1g is a TEM image of Co–FeS2/CoS2 heterostructures, the inside of the ellipse is a bump of CoS2 which further increased the surface area of the sample and adjusted the kinetic energy. Fig. 1h is a high resolution TEM (HRTEM) image of Co–FeS2/CoS2 heterostructures, the interplanar spacing (210) of FeS2 is 0.24 nm,19,35 the interplanar spacings (210) of the CoS2 bump are 0.25 nm,36,37 and the inset showed the selected area electron diffraction (SAED) pattern, which shows the monocrystallinity of the sample. All these potentially explain the excellent electrocatalytic performance of the Co–FeS2/CoS2 heterostructures.
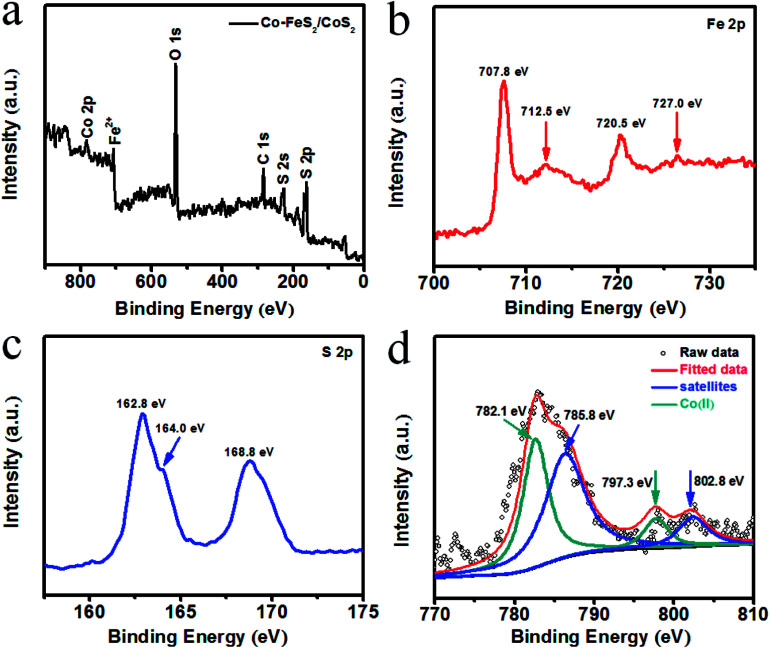
Fig. 2a presents the full XPS survey spectrum of the Co–FeS2/CoS2 heterostructures, also confirming the presence of Fe, S and Co elements. Fig. 2b shows the XPS spectrum of Fe (2p); the characteristic peaks of Co–FeS2/CoS2 were detected at 707.8 (Fe 2p3/2) and 720.5 eV (Fe 2p1/2) due to the Fe2+ of the FeS2 structure.7 The peaks at 712.5 and 727.0 eV can be attributed to a small amount of Fe2+ being oxidized to Fe3+.38 The XPS spectra of Co–FeS2/CoS2 were measured in the S (2p) region, as shown in Fig. 2c. The binding energies of S (2p) at 162.8 and 164.0 eV belong to S22− of FeS2.39,40 There were some oxidized S species found (168.8 eV) in Co–FeS2/CoS2.41,42 As shown in Fig. 2d, the Co 2p spectrum can be deconvoluted into four peaks: the Co 2p3/2 and Co 2p1/2 peaks at 782.1 and 797.3 eV can be attributed to Co2+ bound to oxygen,43,44 while another two peaks at 785.8 and 802.8 eV are ascribed to higher oxidized cobalt species (Co3+).45
Fig. 2. (a) XPS survey spectrum for Co–FeS2/CoS2; XPS spectra of the Co–FeS2/CoS2 from (b) Fe 2p, (c) S 2p, (d) Co 2p.
We further analyzed the main process of formation of Co–FeS2/CoS2 heterostructures. We speculate the following reaction process: solid sulfur powder attached to the carbon cloth at 180 °C is melted into small droplets. At the sulfur powder droplets and solution interface, due to the severe excess in sulfur powder compared to Fe2+, Co2+, and S2−. Co2+ was incorporated into the reaction of Fe2+, S2−, and sulfur powder droplets (S0). When Co–FeS2 was formed, Co2+ further reacted with sulfur powder droplets (S0)/S2− to form CoS2, thus forming the Co–FeS2/CoS2 heterostructures.
To our knowledge, Co–FeS2/CoS2 heterostructures has not yet been explored for the electrocatalytic splitting of water into hydrogen and oxygen. Unique 3D hierarchical nanostructures not only increase the surface area but also facilitates the release of hydrogen and oxygen from the electrode surface. The superior oxygen evolution performance of the Co–FeS2/CoS2 heterostructures were mainly attributed to the presence of the CoS2 phase and the formation of heterostructures with the Co–FeS2.46 In addition, density functional theory calculation revealed that sulfur was responsible for the active sites for proton adsorption and reduction; the high catalytic activity was stemmed from a large reduction of the kinetic energy barrier of H atom adsorption on FeS2 surface upon Co doping in the iron pyrite structure.18,19 The formation of heterostructures for Co–FeS2 and CoS2 further lowers the kinetic energy barrier of the reaction to gain superior electrocatalytic performance.23–25
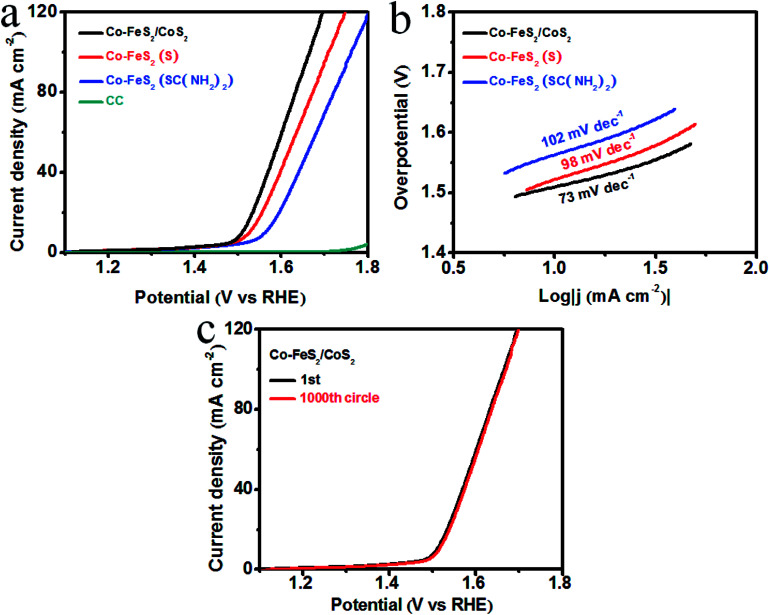
We used a typical three-electrode system with a sweeping rate of 2 mV s−1 to test the OER electrocatalytic activity and stability of the Co–FeS2/CoS2 heterostructures. For comparison study, the electrocatalytic activity of Co–FeS2 synthesized using sulfur powder or thiourea was also tested. Fig. 3a shows the linear sweep voltammetry (LSV) curves, showing that bare CC almost has no OER activity. Co–FeS2/CoS2 heterostructures shows superior OER activity, with an overpotential of only 278 mV required to drive 10 mA cm−2. This is better than the overpotential of 292 mV for Co–FeS2 (S) and 333 mV for Co–FeS2 (SC(NH2)2). Fig. 3b shows that the Tafel slope of Co–FeS2/CoS2 is 73 mV dec−1, which is superior to the measured 98 mV dec−1 of Co–FeS2 (S) and 102 mV dec−1 of Co–FeS2 (SC(NH2)2). As shown in Fig. 3c, the overpotential of Co–FeS2/CoS2 is only 283 mV after 1000 cycles, which signifies Co–FeS2/CoS2 heterostructures have good electrochemical stability in strongly alkaline solutions.
Fig. 3. (a) LSV curves of Co–FeS2/CoS2, Co–FeS2 (S), Co–FeS2 (SC(NH2)2), and bare CC for OER in 1 M KOH solution; (b) corresponding Tafel plots; (c) LSV curves of Co–FeS2/CoS2 before and after 1000 CV cycles.
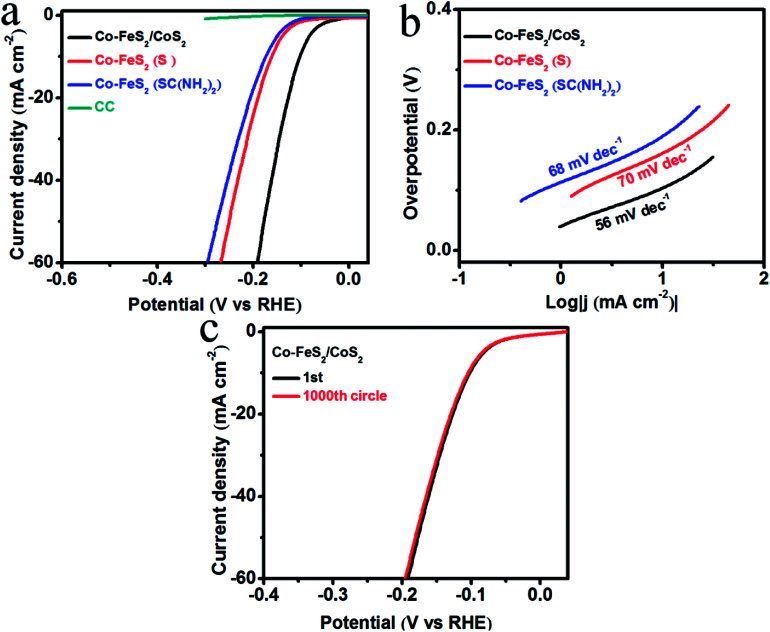
HER activity was analyzed by measuring the LSV curves of Co–FeS2/CoS2 and bare CC in 0.5 M H2SO4 solution. In the polarization curve of Fig. 4a, the blank substrate of CC shows negligible HER activity over the measured voltage range, indicating that the HER performance of the CC has little contribution. The Co–FeS2/CoS2 heterostructures grown on the CC substrate achieved geometric current densities of −10 mA cm−2 at much lower overpotential of −103 mV versus the reversible hydrogen electrode (RHE) compared to that of Co–FeS2 (−161 mV for (S) and −173 mV for SC(NH2)2). As shown in Fig. 4b, Co–FeS2/CoS2 heterostructures have a Tafel slope of 56 mV dec−1, smaller than those for Co–FeS2 70 mV dec−1 for (S) and 68 mV dec−1 for SC(NH2)2. To investigate the origin of the superior activity of Co–FeS2/CoS2 heterostructures, we further estimated the ECSA by calculating its non-faradaic double-layer capacitance using cyclic voltammetry measurement, since ECSA value is linearly proportional to Cdl.46–48 As shown in Fig. S10a–d,† cyclic voltammograms were measured in the non-faradaic capacitance current range, the Cdl value of hierarchical Co–FeS2/CoS2 is 86 mF cm−2 is larger than that of Co–FeS2(S) (37 mF cm−2) and Co–FeS2(SC(NH2)2) (20 mF cm−2). The result indicates that hierarchical architecture and bump feature can maximize the exposure of accessible active sites, which contributes to excellent electrocatalytic performance of Co–FeS2/CoS2 heterostructures. In addition, as shown in Fig. S10e,† the reaction kinetics is verified by EIS, the hierarchical Co–FeS2/CoS2 heterostructures present a smaller semicircle than Co–FeS2(S) and Co–FeS2(SC(NH2)2), which can be associated with interfacial charge transfer process, a lower value corresponds to a faster electron transfer rate. This result demonstrated further the faster catalytic kinetics of Co–FeS2/CoS2 heterostructures.49–51 In addition to catalytic activity, stability is another critical factor to evaluate a good electrocatalyst, we investigated the stability of Co–FeS2/CoS2 heterostructures via 1000 cycles scanning. As shown in Fig. 4c, the Co–FeS2/CoS2 electrode lost only 3 mV of overpotential at −10 mV cm−2 after 1000 cycles. Furthermore, we analyzed the characterization results of the Co–FeS2/CoS2 heterostructures before and after the 1000 cycles. As shown in Fig. S11,† it was found that the XRD patterns, XPS spectra, SEM, and TEM images of Co–FeS2/CoS2 heterostructures did not significantly changed. The Co–FeS2/CoS2 heterostructures also has excellent electrochemical stability in 0.5 M H2SO4 solution, which is better than the previously reported non-noble metal-based sulfide electrocatalyst, presented in Table 1.
Fig. 4. (a) LSV curves of Co–FeS2/CoS2, Co–FeS2/CoS2 (S), Co–FeS2 (SC(NH2)2), and bare CC for HER in 0.5 M H2SO4 solution; (b) corresponding Tafel plots; (c) LSV curves of Co–FeS2/CoS2 before and after 1000 cycles.
Summary of HER and OER activity of electrocatalysts.
| Catalyst | Electrolyte | η j (mV vs. RHE) | Tafel slope (mV dec−1) | η j (mV vs. OER) | Reference |
|---|---|---|---|---|---|
| FeS2 | 0.1 M KOH | η −10 = −96 | 78 | — | 19 |
| FeS2 | 0.5 M H2SO4 | η −10 = −139 | 66 | — | 7 |
| Fe0.68Co0.32S2 | 0.5 M H2SO4 | η −10 = −166 | 51 | — | 38 |
| CoS2 | 0.5 M H2SO4 | η −10 = −145 | 51.6 | — | 8 |
| CoS2 | 0.5 M H2SO4 | η −100 = −140 | 70.1 | — | 27 |
| CoS2 | 0.1 M KOH | — | — | η 10 = 290 | 52 |
| Co–FeS 2 /CoS 2 | 0.5 M H 2 SO 4 | η −10 = −103 | 56 | η 10 = 278 | This work |
4. Conclusions
In summary, by optimizing experiment conditions, Co–FeS2/CoS2 heterostructures were successfully prepared by the hydrothermal route with excellent OER and HER electrocatalytic performance. Their unique three-dimensional structure not only increases the surface area but also facilitates the release of hydrogen and oxygen from the electrode surface. Co-doped FeS2 and CoS2 formed heterostructures on the petals of Co–FeS2, which change the energy barrier of the catalytic reaction to gain excellent electrocatalytic performance. This study not only provides a low-cost, stable and earth-abundant iron-based electrocatalyst for efficient water splitting, it will also provide an exciting new method for the rational design and scalable preparation of three-dimensional polynary heterostructures as electrocatalysts.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the National Basic Research Program of China (2018YFA0209101), the National Science Foundations of China (No. 61205057, No. 11574136), Qing Lan Project, the ‘1311 Talent Plan’ Foundation of Nanjing University of Posts and Telecommunications, Six Talent Peaks Project in Jiangsu Province (JY-014), and State Key Laboratory of High Performance Computing, National University of Defense Technology.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05237a
References
- Tang C. Gan L. Zhang R. Lu W. Jiang X. Asiri A. M. Ternary FexCo1-xP nanowire array as a robust hydrogen evolution reaction electrocatalyst with pt-like activity: experimental and theoretical insight. Nano Lett. 2016;16:6617–6621. doi: 10.1021/acs.nanolett.6b03332. [DOI] [PubMed] [Google Scholar]
- Xiong W. Guo Z. Li H. Zhao R. Wang X. Rational bottom-up engineering of electrocatalysts by atomic layer deposition: a case study of FexCo1-xSy-based catalysts for electrochemical hydrogen evolution. ACS Energy Lett. 2017;2:2778–2785. doi: 10.1021/acsenergylett.7b01056. [DOI] [Google Scholar]
- Zou X. Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015;44:5148–5180. doi: 10.1039/C4CS00448E. [DOI] [PubMed] [Google Scholar]
- Wang J. Cui W. Liu Q. Xing Z. Asiri A. M. Sun X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016;28:215–230. doi: 10.1002/adma.201502696. [DOI] [PubMed] [Google Scholar]
- Li Y. Zhang H. Jiang M. Zhang Q. He P. Sun X. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting. Adv. Funct. Mater. 2017;27:1702513. doi: 10.1002/adfm.201702513. [DOI] [Google Scholar]
- Yu X. Feng Y. Jeon Y. Guan B. Lou X. W. Paik U. Formation of Ni-Co-MoS2 nanoboxes with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 2016;28:9006–9011. doi: 10.1002/adma.201601188. [DOI] [PubMed] [Google Scholar]
- Chen Y. Xu S. Li Y. Jacob R. J. Kuang Y. Liu B. FeS2 Nanoparticles embedded in reduced graphene oxide toward robust, high-performance electrocatalysts. Adv. Energy Mater. 2017;7:1700482. doi: 10.1002/aenm.201700482. [DOI] [Google Scholar]
- Faber M. S. Dziedzic R. Lukowski M. A. Kaiser N. S. Ding Q. Jin S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro-and nanostructures. J. Am. Chem. Soc. 2014;136:10053–10061. doi: 10.1021/ja504099w. [DOI] [PubMed] [Google Scholar]
- Wang Y. Kong B. Zhao D. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today. 2017;15:26–55. doi: 10.1016/j.nantod.2017.06.006. [DOI] [Google Scholar]
- Lu X. Q. Yan X. Devaramani S. Self-supported rectangular CoP nanosheet arrays grown on carbon cloth as an efficient electrocatalysts for hydrogen evolution reaction over a variety of pH values 1. New J. Chem. 2017;41:2436–2442. doi: 10.1039/C6NJ03887E. [DOI] [Google Scholar]
- Ouyang C. Wang X. Wang S. Phosphorus-doped CoS2 nanosheet arrays as ultra-efficient electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2015;51:14160–14163. doi: 10.1039/C5CC05541E. [DOI] [PubMed] [Google Scholar]
- Wang D. Zhang D. Tang C. Zhou P. Wu Z. Hydrogen evolution catalyzed by cobalt promoted molybdenum phosphide nanoparticles. Catal. Sci. Technol. 2016;6:1952–1956. doi: 10.1039/C5CY01457C. [DOI] [Google Scholar]
- Zhu X. Jin T. Tian C. Lu C. Liu X. Zeng M. In situ coupling strategy for the preparation of FeCo alloys and Co4N hybrid for highly efficient oxygen evolution. Adv. Mater. 2017;29:1704091. doi: 10.1002/adma.201704091. [DOI] [PubMed] [Google Scholar]
- Fang W. Liu D. Lu Q. Sun X. Asiri A. M. Nickel promoted cobalt disulfide nanowire array supported on carbon cloth: an efficient and stable bifunctional electrocatalyst for full water splitting. Electrochem. Commun. 2016;63:60–64. doi: 10.1016/j.elecom.2015.10.010. [DOI] [Google Scholar]
- Bachvarov V. Lefterova E. Rashkov R. Electrodeposited NiFeCo and NiFeCoP alloy cathodes for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy. 2016;41:12762–12771. doi: 10.1016/j.ijhydene.2016.05.164. [DOI] [Google Scholar]
- Zhang X. Zhang X. Xu H. Wu Z. Wang H. Liang Y. Iron-doped cobalt monophosphide nanosheet/carbon nanotube hybrids as active and stable electrocatalysts for water splitting. Adv. Funct. Mater. 2017;27:1606635. doi: 10.1002/adfm.201606635. [DOI] [Google Scholar]
- Han X. P. Wu X. Y. Zhong C. Deng Y. d. NiCo2S4, nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Nano Energy. 2017;31:541–550. doi: 10.1016/j.nanoen.2016.12.008. [DOI] [Google Scholar]
- Wang D. Y. Gong M. Chou H. L. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets-carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015;137:1587–1592. doi: 10.1021/ja511572q. [DOI] [PubMed] [Google Scholar]
- Miao R. Dutta B. Sahoo S. He J. Zhong W. Cetegen S. A. Mesoporous iron sulfide for highly efficient electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 2017;139:13604–13607. doi: 10.1021/jacs.7b07044. [DOI] [PubMed] [Google Scholar]
- Yu J. Cheng G. Luo W. Ternary nickel–iron sulfide microflowers as a robust electrocatalyst for bifunctional water splitting. J. Mater. Chem. A. 2017;5:15838–15844. doi: 10.1039/C7TA04438K. [DOI] [Google Scholar]
- Wu Z. Wang X. Huang J. Gao F. A Co-doped Ni–Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J. Mater. Chem. A. 2018;6:167–178. doi: 10.1039/C7TA07956G. [DOI] [Google Scholar]
- Ao K. Li D. Yao Y. Fe-doped Co9S8, nanosheets on carbon fiber cloth as pH-universal freestanding electrocatalysts for efficient hydrogen evolution. Electrochim. Acta. 2018;264:157–165. doi: 10.1016/j.electacta.2018.01.080. [DOI] [Google Scholar]
- Kuo T. Chen W. Liao H. Improving hydrogen evolution activity of earth-abundant cobalt-doped iron pyrite catalysts by surface modification with phosphide. Small. 2017;13:1603356. doi: 10.1002/smll.201603356. [DOI] [PubMed] [Google Scholar]
- Zhu C. Wang A. L. Xiao W. Chao D. Zhang X. In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv. Mater. 2018;30:1705516. doi: 10.1002/adma.201705516. [DOI] [PubMed] [Google Scholar]
- Ganesan P. Sivanantham A. Shanmugam S. CoS2-TiO2 hybrid nanostructures: efficient and durable bifunctional electrocatalysts for alkaline electrolyte membrane water electrolyzers. J. Mater. Chem. A. 2018:1075–1085. doi: 10.1039/C7TA09096J. [DOI] [Google Scholar]
- Xu L. Hu Y. Zhang H. Confined synthesis of FeS2 nanoparticles encapsulated in carbon nanotube hybrids for ultrastable lithium-ion batteries. ACS Sustainable Chem. Eng. 2016;4:4251–4255. doi: 10.1021/acssuschemeng.6b00741. [DOI] [Google Scholar]
- Zhang H. Li Y. Zhang G. Xu T. Wan P. Sun X. A metallic CoS2 nanopyramid array grown on 3D carbon fiber paper as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A. 2015;3:6306–6310. doi: 10.1039/C5TA00707K. [DOI] [Google Scholar]
- Faber M. S. Lukowski M. A. Ding Q. Kaiser N. S. Jin S. Earth-abundant metal pyrites (FeS2, CoS2, NiS2, and their Alloys) for highly efficient hydrogen evolution and polysulfide reduction electrocatalysis. J. Phys. Chem. C. 2014;118:21347–21356. doi: 10.1021/jp506288w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Z. Tang C. Y. Zhou P. Liu Z. H. Enhanced hydrogen evolution catalysis from osmotic swelled ammoniated MoS2. J. Mater. Chem. A. 2015;3:13050–13056. doi: 10.1039/C5TA02010G. [DOI] [Google Scholar]
- Yu J. Cheng G. Luo W. Hierarchical NiFeP microflowers directly grown on Ni foam for efficient electrocatalytic oxygen evolution. J. Mater. Chem. A. 2017;5:11229–11235. doi: 10.1039/C7TA02968C. [DOI] [Google Scholar]
- Wu X. Y. Han X. P. Ma X. Y. Zhang W. Morphology-Controllable Synthesis of Zn-Co-Mixed Sulfide Nanostructures on Carbon Fiber Paper Toward Efficient Rechargeable Zinc–Air Batteries and Water Electrolysis. ACS Appl. Mater. Interfaces. 2017;9:12574–12583. doi: 10.1021/acsami.6b16602. [DOI] [PubMed] [Google Scholar]
- Zhang H. Li X. Hähnel A. Naumann V. Lin C. Azimi S. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018;28:1706847. doi: 10.1002/adfm.201706847. [DOI] [Google Scholar]
- Song H. Oh S. Yoon H. Kim K. H. Ryu S. Oh J. Bifunctional NiFe inverse opal electrocatalysts with heterojunction Si solar cells for 9.54%-efficient unassisted solar water splitting. Nano Energy. 2017;42:1–7. doi: 10.1016/j.nanoen.2017.10.028. [DOI] [Google Scholar]
- Yu H. Xue Y. Hui L. Zhang C. Li Y. Zuo Z. Efficient hydrogen production on a 3D flexible heterojunction material. Adv. Mater. 2018:1707082. doi: 10.1002/adma.201707082. [DOI] [PubMed] [Google Scholar]
- Zhu L. Richardson B. J. Yu Q. Anisotropic growth of iron pyrite FeS2 nanocrystals via oriented attachment. Chem. Mater. 2015;27:150427111020001. [Google Scholar]
- Guo Y. Gan L. Shang C. A cake-style CoS2@MoS2/RGO hybrid catalyst for efficient hydrogen evolution. Adv. Funct. Mater. 2017;27:1602699. doi: 10.1002/adfm.201602699. [DOI] [Google Scholar]
- Zhang H. Li Y. Zhang G. Highly crystallized cubic cattierite CoS2, for electrochemically hydrogen evolution over wide pH range from 0 to 14. Electrochim. Acta. 2014;148:170–174. doi: 10.1016/j.electacta.2014.09.164. [DOI] [Google Scholar]
- Huang S. Y. Sodano D. Leonard T. Luiso S. Fedkiw P. S. Cobalt-doped iron sulfide as an electrocatalyst for hydrogen evolution. J. Electrochem. Soc. 2017;164:276–282. doi: 10.1149/2.0761704jes. [DOI] [Google Scholar]
- Zheng M. Ding Y. Yu L. Du X. Zhao Y. In situ grown pristine cobalt sulfide as bifunctional photocatalyst for hydrogen and oxygen evolution. Adv. Funct. Mater. 2017;27:1605846. doi: 10.1002/adfm.201605846. [DOI] [Google Scholar]
- Ma X. Y. Zhang W. Deng Y. D. Zhong C. Phase and composition controlled synthesis of cobalt sulfide hollow nanospheres for electrocatalytic water splitting. Nanoscale. 2018;10:4816–4824. doi: 10.1039/C7NR09424H. [DOI] [PubMed] [Google Scholar]
- Fang L. Zhang Y. Guan Y. Zhang H. Wang S. Wang Y. Specific synthesis of CoS2 nanoparticles embedded in porous Al2O3 nanosheets for efficient hydrogen evolution and enhanced lithium storage. J. Mater. Chem. A. 2017;5:2861–2869. doi: 10.1039/C6TA10700A. [DOI] [Google Scholar]
- Jiang J. Lu S. Gao H. Zhang X. Yu H. Q. Ternary FeNiS2 ultrathin nanosheets as an electrocatalyst for both oxygen evolution and reduction reactions. Nano Energy. 2016;27:526–534. doi: 10.1016/j.nanoen.2016.07.032. [DOI] [Google Scholar]
- Zhang R. Wang X. Yu S. Ternary NiCo2Px nanowires as pH-universal electrocatalysts for highly efficient hydrogen evolution reaction. Adv. Mater. 2016;29:1605502. doi: 10.1002/adma.201605502. [DOI] [PubMed] [Google Scholar]
- Nakayama M. Kotaro F. Kobayakawa T. A binder-free thin film anode composed of Co2+-intercalated buserite grown on carbon cloth for oxygen evolution reaction. Electrochem. Commun. 2017;84:24–27. doi: 10.1016/j.elecom.2017.09.012. [DOI] [Google Scholar]
- Ma M. Zhu G. Xie F. Qu F. Liu Z. Du G. Homologous catalysts based on Fe-doped CoP nanoarrays for high-performance full water splitting under benign conditions. ChemSusChem. 2017;10:3188–3192. doi: 10.1002/cssc.201700693. [DOI] [PubMed] [Google Scholar]
- Hua Y. Jiang H. Jiang H. Hierarchical porous CoS2 microboxes for efficient oxygen evolution reaction. Electrochim. Acta. 2018;278:219–225. doi: 10.1016/j.electacta.2018.05.028. [DOI] [Google Scholar]
- Hao S. Yang L. Liu D. Integrating natural biomass electro-oxidation and hydrogen evolution: using a porous Fe-doped CoP nanosheet array as a bifunctional catalyst. Chem. Commun. 2017;53:5710–5713. doi: 10.1039/C7CC01680H. [DOI] [PubMed] [Google Scholar]
- Wang Y. Ni Y. Liu B. Vertically oriented CoO@FeOOH nanowire arrays anchored on carbon cloth as a highly efficient electrode for oxygen evolution reaction. Electrochim. Acta. 2017;257:356–363. doi: 10.1016/j.electacta.2017.10.011. [DOI] [Google Scholar]
- Wang X. Li F. Li W. Hollow bimetallic cobalt-based selenide polyhedrons derived from metal-organic framework: an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A. 2017:17982–17989. doi: 10.1039/C7TA03167J. [DOI] [Google Scholar]
- Teng Y. Wang X. D. Chen H. Y. Iron-assisted engineering of molybdenum phosphide nanowires on carbon cloth for efficient hydrogen evolution in a wide pH range. J. Mater. Chem. A. 2017:22790–22796. doi: 10.1039/C7TA07895A. [DOI] [Google Scholar]
- Gao Z. Gao Q. Liu Z. High-efficiency hydrogen evolution catalyzed by iron phosphide nanocrystals. RSC Adv. 2016;6:114430–114435. doi: 10.1039/C6RA24186G. [DOI] [Google Scholar]
- Yang J. Yang Z. Li L. H. Highly efficient oxygen evolution from CoS2/CNT nanocomposites via a one-step electrochemical deposition and dissolution method. Nanoscale. 2017;9:6886–6894. doi: 10.1039/C7NR01293D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.