Abstract
RWJ-270201 is a novel cyclopentane inhibitor of influenza A and B virus neuraminidases (NAs). We compared the ability of RWJ-270201 to inhibit NA activity of clinical influenza isolates and viruses with defined resistance mutations with that of zanamivir and oseltamivir carboxylate. In NA inhibition assays with influenza A viruses, the median 50% inhibitory concentration (IC50) of RWJ-270201 (approximately 0.34 nM) was comparable to that of oseltamivir carboxylate (0.45 nM) but lower than that of zanamivir (0.95 nM). For influenza B virus isolates, the IC50 of RWJ-270201 (1.36 nM) was comparable to that of zanamivir (2.7 nM) and less than that of oseltamivir carboxylate (8.5 nM). A zanamivir-resistant variant bearing a Glu119-to-Gly (Glu119→Gly) or Glu119→Ala substitution in an NA (N2) remained susceptible to RWJ-270201 and oseltamivir carboxylate. However, a zanamivir-selected variant with an Arg292→Lys substitution in an NA (N2) showed a moderate level of resistance to RWJ-270201 (IC50 = 30 nM) and zanamivir (IC50 = 20 nM) and a high level of resistance to oseltamivir carboxylate (IC50 > 3,000 nM). The zanamivir-resistant influenza B virus variant bearing an Arg152→Lys substitution was resistant to each NA inhibitor (IC50 = 100 to 750 nM). The oseltamivir-selected variant (N1) with the His274→Tyr substitution exhibited resistance to oseltamivir carboxylate (IC50 = 400 nM) and to RWJ-270201 (IC50 = 40 nM) but retained full susceptibility to zanamivir (IC50 = 1.5 nM). Thus, drug-resistant variants with substitutions in framework residues 119 or 274 can retain susceptibility to other NA inhibitors, whereas replacement of functional residue 152 or 292 leads to variable levels of cross-resistance. We conclude that RWJ-270201 is a potent inhibitor of NAs of wild-type and some zanamivir-resistant or oseltamivir-resistant influenza A and B virus variants.
Until recently, the M2 ion channel (21) inhibitors, amantadine and rimantadine, were the only antiviral agents available for the management of influenza A virus infections. Because influenza B viruses lack the M2 protein, they are not susceptible to these drugs. Another limitation of amantadine and rimantadine is their propensity to rapidly select resistant strains in vitro and in vivo (10). In addition, certain influenza A virus strains isolated before these drugs were used contain amantadine-resistant M2 protein (10). Each of the five single-amino-acid substitutions that have been found in the transmembrane domain of the M2 protein confers a high level of resistance to amantadine and rimantadine (10). The resistant strains seem to be genetically stable, fully pathogenic, and transmissible to close contacts. Recent attempts to identify M2 ion channel inhibitors that are effective against resistant viruses have been unsuccessful (18).
Neuraminidase (NA) inhibitors are a new class of anti-influenza drugs. Two inhibitors, zanamivir and oseltamivir, have been approved for use in humans (6). Because of its low bioavailability, zanamivir is delivered topically by inhalation. Oseltamivir (GS4104), the ethyl ester prodrug form of oseltamivir carboxylate (GS4071), is the first NA inhibitor that is bioavailable after oral administration. The novel NA inhibitor RWJ-270201 is also bioavailable upon oral administration (1) and is currently undergoing clinical evaluation.
The NA inhibitors were rationally designed to specifically block the active center of the influenza virus NA. Despite the low level of amino acid sequence homology between influenza A and B viruses, the active center is formed by amino acid residues conserved among types and subtypes of influenza viruses. Some of these residues directly interact with the substrate (functional residues), and others provide a structural scaffold for the functional residues (framework residues) (5).
The framework and functional residues are presumed to be essential for optimal enzyme function. Most interactions between zanamivir or oseltamivir carboxylate and residues in the NA active center are similar to those with the natural substrate, neuraminic acid (15, 25). However, zanamivir and oseltamivir carboxylate also rely on interactions with conserved residues of NA that differ from those between the neuraminic acid and the enzyme. It was anticipated that resistance to NA inhibitors would be conferred by substitutions at framework residues rather than at functional residues. However, substitutions in both the functional and the framework residues were acquired by influenza A and B viruses after in vitro passage in the presence of the NA inhibitors and have been identified also in viruses recovered from treated patients (Table 1). An understanding of the molecular interactions between the present NA inhibitors and the mutated target enzyme is important for the development of more-effective antiviral agents.
TABLE 1.
Influenza viruses with drug-resistant enzymes selected in the presence of NA inhibitors
| Residue in NA active centera | Substitution | NA type/subtype | NA inhibitorb | Type of selectionb | Reference(s) | Mutants used in present study |
|---|---|---|---|---|---|---|
| Glu119 | Gly | A/N2 | Zanamivir | In vitro | 9 | + |
| Gly | A/N9 | Zanamivir | In vitro | 16 | − | |
| Gly | B | Zanamivir | In vitro | 16 | − | |
| Ala | A/N2 | Zanamivir | In vitro | 9 | + | |
| Asp | A/N2 | Zanamivir | In vitro | 9 | + | |
| Val | A/N2 | Oseltamivir | In vitro | 14, 29 | − | |
| Arg292 | Lys | A/N2 | Zanamivir | In vitro | 9 | + |
| Lys | A/N9 | 6-Carboxamide derivative of zanamivir | In vitro | 17 | − | |
| Lys | A/N2 | Oseltamivir | In vitro | 22 | − | |
| Lys | A/N2 | Oseltamivir | In vivo | 14, 29 | − | |
| Lys | A/N2 | RWJ-270201 | In vitro | 3 | − | |
| His274 | Tyr | A/N1 | Oseltamivir | In vitro | 28 | − |
| Tyr | A/N1 | Oseltamivir | In vivo | 7, 29 | + | |
| Arg152 | Lys | B | Zanamivir | In vivo | 8 | + |
Position of amino acid in the N2 enzyme.
The viruses with the mutant NAs were selected in the presence of NA inhibitor in vitro or were isolated from patients treated with an NA inhibitor.
The aims of the present study were to compare the antiviral potency of the novel NA inhibitor RWJ-270201 with that of zanamivir and oseltamivir carboxylate and to evaluate the cross-resistance patterns of drug-resistant variants.
MATERIALS AND METHODS
Compounds.
The NA inhibitors oseltamivir carboxylate (GS4071), zanamivir (GG167), and RWJ-270201 were provided by BioCryst, Birmingham, Ala. They were resuspended in distilled water and stored at −20°C before they were further diluted for use in the enzyme assay.
Viruses.
Nineteen clinical isolates of influenza A (H1N1 and H3N2) and B virus were recovered from patients at the University of Virginia Health Sciences Center during the 1991 to 1999 influenza seasons. Clinical isolates (wild-type viruses) were propagated twice in Madin-Darby canine kidney (MDCK) cells by a standard procedure before they were used in the NA inhibition assay. The oseltamivir-resistant virus variant was recovered from a volunteer experimentally infected with the influenza A/Texas/36/91 (H1N1) strain and treated with oseltamivir (7). This clinical isolate was propagated three times in MDCK cells. Zanamivir-selected virus variants and corresponding wild-type viruses were from the repository at St. Jude Children's Research Hospital, Memphis, Tenn. (Table 1).
NA inhibition assays.
Whole viruses from clarified cell culture supernatants were used as a source of NA activity. We used a modified fluorometric assay based on that of Potier et al. (17a) to measure influenza virus NA activity and its inhibition by antiviral drugs. The assay measures 4-methylumbelliferone released from the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA) (Sigma, St. Louis, Mo.) by the enzymatic activity of influenza virus enzyme.
The NA activity for each virus was determined before each test of NA inhibition because of variation in the NA activity among the viruses. Titration of NA activity was performed with serial half-log dilutions of each virus to determine a working dilution of virus. Virus and substrate (final concentration of MUNANA, 75 μM) were mixed and incubated at 37°C for 30 min. Free 4-methylumbelliferone diluted in 0.9% NaCl (5, 10, 20, and 40 μl of 40 μM solution) was used in parallel to determine the linear range of measurements. Reactions were stopped with the addition of 150 μl of 0.1 M glycine buffer (pH 10.7) containing 25% ethanol. Fluorescence was read with an HTS 7000 Bio Assay Reader (Perkin-Elmer, Norwalk, Conn.); the excitation wavelength was 365 nm, and the emission wavelength was 460 nm.
The concentration of drug required to inhibit NA activity by 50% (IC50) was determined by testing serial dilutions (10 to 0.00001 μM) of the drug against a working dilution of virus which was estimated prior to the assay. Equal volumes of the virus and inhibitor were mixed and incubated at room temperature for 30 min. After the substrate was added to the reaction mixture, the mixture was then incubated for 1 h at 37°C. Each reaction was stopped by addition of the stop solution described above, and the fluorescence of each combination of compound and virus was measured. The IC50s were obtained by extrapolation of data from the resulting graph that indicated the relationship between log inhibitor concentration and percent fluorescence inhibition.
RESULTS
Inhibitory effects of RWJ-270201 on NA activity of influenza viruses.
We compared the ability of RWJ-270201 to inhibit the NA activity of recent clinical isolates of influenza A and B viruses with that of zanamivir and oseltamivir carboxylate. RWJ-270201 demonstrated potency against the NA activities of both influenza A and B viruses with IC50s ranging from 0.26 to 1.95 nM (Table 2). RWJ-270201 was comparable to oseltamivir carboxylate in inhibiting the NA activity of the 11 clinical isolates of influenza A virus, but RWJ-270201 was approximately two- to fourfold more potent than zanamivir against the NA activity of the same isolates (Table 2). Against the NA activity of the eight influenza B virus isolates, RWJ-270201 was comparable to zanamivir and approximately five- to ninefold more potent than oseltamivir carboxylate (Table 2).
TABLE 2.
Inhibition of NA activity of clinical isolates by zanamivir, oseltamivir carboxylate, or RWJ-270201
| Virus type/ subtypea | No. of isolates | Medianb (range) IC50 (nM)
|
||
|---|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Zanamivir | ||
| A/H1N1 | 5 | 0.34 (0.26–0.43) | 0.45 (0.45–0.60) | 0.95 (0.73–1.05) |
| A/H3N2 | 6 | 0.60 (0.47–0.87) | 0.37 (0.27–0.45) | 2.34 (1.85–3.13) |
| B | 8 | 1.36 (1.08–1.95) | 8.50 (5.33–18.33) | 2.70 (2.00–3.10) |
To isolate influenza A (H1N1 and H3N2) and B viruses from patients, we initially grew the viruses in MDCK cells, and the viruses were propagated twice in this cell culture system before testing.
The median IC50s were determined for each virus in the NA inhibition assay.
Assessment of virus resistance and cross-resistance in NA inhibition assay.
Typically, the variability between measurements of the activity (IC50s) for the same virus in the same experimental conditions did not exceed fourfold. Therefore, the virus is considered as showing reduced susceptibility if its IC50 value increases by at least 8- to 10-fold in comparison to the wild-type virus (9, 22).
We determined whether zanamivir-resistant or oseltamivir-resistant virus variants exhibited cross-resistance to the other inhibitors. Zanamivir-resistant virus variants in which the framework residue Glu119 was replaced with Gly or Ala retained full susceptibility to RWJ-270201 and oseltamivir carboxylate (Table 3). In addition, the zanamivir-resistant virus variant with Asp119 was fully susceptible to oseltamivir carboxylate, but the IC50 of RWJ-270201 against this virus variant was approximately ninefold higher than that against the wild-type virus. The virus variant (A/H1N1) with the framework substitution His274 to Tyr (His274→Tyr) was recovered from a patient treated with oseltamivir, and, not surprisingly, this virus variant was resistant to oseltamivir carboxylate (IC50 = 400 nM). The IC50 (40 nM) of RWJ-270201 against the virus variant was approximately 80-fold greater than the IC50 of RWJ-270201 against the wild-type virus. Therefore, the virus variant with the His274→Tyr substitution demonstrated a moderate level of resistance to RWJ-270201 in the NA inhibition assay. In contrast, zanamivir was fully active against this virus variant (Table 3).
TABLE 3.
Cross-resistance of zanamivir-resistant or oseltamivir-resistant enzymes
| Residue in NA active centera and positionb | Amino acid | IC50 (nM)c
|
||
|---|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Zanamivir | ||
| Framework | ||||
| 119 | Glu (wt)d | 1.1 | 0.4 | 2.5 |
| 119 | Gly | 1.8 | 0.5 | 100 |
| 119 | Ala | 1.6 | 1.1 | 50 |
| 119 | Asp | 9.5 | 0.5 | 150 |
| 274 | His (wt) | 0.5 | 1.0 | 1.0 |
| 274 | Tyr | 40 | 400 | 1.5 |
| Functional | ||||
| 292 | Arg (wt) | 1.1 | 0.4 | 2.5 |
| 292 | Lys | 30 | 3,750 | 20 |
| 152 | Arg (wt) | 1.4 | 4 | 3.5 |
| 152 | Lys | 570 | 750 | 100 |
According to Colman et al. (5).
Position of amino acid in the N2 enzyme.
IC50 in NA inhibition assays. The median IC50s of three or more measurements are shown.
Amino acid at the indicated position in the wild-type (wt) NA.
The zanamivir-resistant virus variant with an Arg292→Lys substitution exhibited a moderate level of resistance to RWJ-270201 (approximately 27-fold increase) compared to wild type but a high level of resistance to oseltamivir carboxylate (approximately 9,400-fold increase) (Table 3). The Arg152→Lys substitution in the NA of influenza B virus led to cross-resistance to all three inhibitors; the IC50s were greater than 100 nM (Table 3).
DISCUSSION
Our results show that RWJ-270201 is a potent inhibitor of the NAs of influenza A and B viruses. This novel inhibitor was approximately threefold more potent than zanamivir in inhibiting NA activity of A/H1N1 clinical isolates, approximately fourfold more potent than zanamivir in inhibiting NA activity of A/H3N2 clinical isolates, and approximately sixfold more potent than oseltamivir carboxylate in inhibiting NA activity of influenza B virus clinical isolates (Table 2). These results confirm those previously reported (1, 2). The in vitro antiviral activity of RWJ-270201 reflects its antiviral effects in experimentally infected animals (2, 19, 20) and in humans with influenza infections (13).
In cross-resistance studies, the novel inhibitor was fully active against certain zanamivir-resistant and partially active against oseltamivir carboxylate-resistant virus variants (Table 3). Overall, the virus variants with substitutions at framework residues 119 or 274 of the NA appeared to retain full susceptibility to at least one of the three NA inhibitors. In contrast, the replacement of the functional residues at positions 152 and 292 led to variable levels of resistance to each of the NA inhibitors. The biological importance of a variable level of resistance is unknown at present and requires further in vivo investigation. Our findings based on results of the NA inhibition assay are in agreement with the statement that despite certain similarities in their structure, zanamivir, oseltamivir carboxylate, and RWJ-270201 (Fig. 1) interact differently with residues of the NA active center (1, 15, 25). The resulting differences in interactions have implications for cross-resistance of virus variants to these agents. The guanidino group of zanamivir interacts with the conserved Glu119 in the active center pocket which was unoccupied by the neuraminic acid (25). Similarly, RWJ-270201 contains a guanidino group that can occupy the same pocket, although the orientation of the group is different from that of the guanidino group of zanamivir (1). The zanamivir-resistant virus variants (Glu119→Gly or Glu119→Ala) were susceptible to RWJ-270201 (Table 3). In addition, one virus variant (Glu119→Asp) was highly resistant to zanamivir, whereas its susceptibility to RWJ-270201 was only ninefold less than that of the parental NA to the same compound (Table 3). Our results also confirmed the previous finding that the substitution at position 119 (Glu→Gly) results in a substantial decrease in susceptibility to zanamivir in NA inhibition assays but in little or no change in susceptibility to oseltamivir carboxylate (16). The virus variants with either the Glu119→Ala or Glu119→Asp substitution in the NA were also susceptible to oseltamivir carboxylate under the conditions of this study.
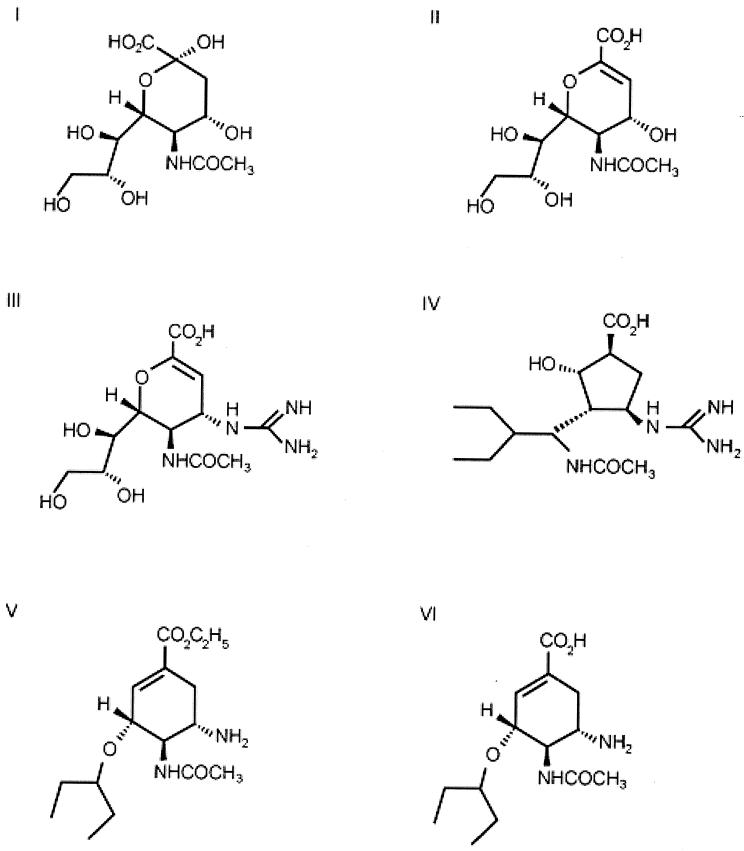
FIG. 1.
Structures of NA substrate and inhibitors. (I) Neuraminic acid; (II) Neu5Ac2en (DANA); (III) zanamivir (4-guanidino-Neu5Ac2en) (GG167); (IV) RWJ-270201 (BCX-1812); (V) oseltamivir (GS4104); (VI) oseltamivir carboxylate (GS4071).
The side chain volume of the framework residue at position 274 of N1 exerts different effects on the binding affinities of oseltamivir carboxylate and zanamivir. Thus, replacement of His274 with residues bearing side chains with larger volumes (Tyr or Phe), but not with smaller volumes, leads to oseltamivir resistance (28). It was postulated that the side chain of the amino acid at position 274 could interfere with the reorientation of the side chain of the conserved Glu276; this reorientation is necessary for the successful binding of oseltamivir carboxylate to the NA active center (22, 27). A similar rearrangement of the side chain of Glu276 was reported for RWJ-270201, which has a hydrophobic group similar to that of oseltamivir (Fig. 1) (1). The His274→Tyr substitution led to a reduction in susceptibility to RWJ-270201, although the reduction was not as great as that observed for oseltamivir carboxylate (Table 3). Glu276 normally interacts with hydroxyl groups of the glycerol side chain of neuraminic acid (26). Zanamivir maintains the glycerol side chain of the natural substrate, and in contrast to oseltamivir carboxylate and RWJ-270201, zanamivir does not require the reorientation of the side chain of Glu276 for successful binding. Not surprisingly, the virus variant with the His274→Tyr substitution was as susceptible to zanamivir as the wild-type virus (Table 3). However, the only way to be certain about the effect of the changes in the NA active site on resistance to a particular NA inhibitor is to determine the structure of the compound with both the wild-type and the mutant enzyme and look for differences.
The replacement of Arg at position 292 was detected in enzymes of viruses selected in the presence of RWJ-270201, oseltamivir carboxylate, zanamivir, or the 6-carboxamide derivative of zanamivir (Table 1). Of note, this variant is the most common one recovered from adults or children treated with oseltamivir (14, 28). The virus variants bearing the Arg292→Lys substitution had a low level of resistance (10- to 30-fold) to RWJ-270201 and zanamivir (3) and a high level of resistance (up to 5,000- to 30,000-fold) to oseltamivir carboxylate (17, 22). According to Tai et al. (22) and Varghese et al. (27), the profound effect of the Arg292→Lys substitution on the enzyme interaction with oseltamivir carboxylate is also the result of the ability of Lys292 to block the reorientation of the side chain of Glu276. We confirmed that this substitution led to a low level of virus resistance to zanamivir (8-fold) and a moderate level of resistance to RWJ-270201 (27-fold), despite the apparent need for reorientation for the interaction of RWJ-270201. The reorientation of the side chain of Glu276 could be energetically less favorable in influenza B virus NAs than in influenza A virus NAs, because the region surrounding Glu276 is hydrophobic in influenza B viruses and hydrophilic in influenza A viruses (23).
Differences in binding of inhibitors to influenza A and B virus NAs have been reported previously (1, 23). In our experiments, the NAs of clinical isolates of influenza B virus were less susceptible to oseltamivir carboxylate than to RWJ-270201 (Table 2), despite the fact that both require reorientation of the Glu276 side chain for binding to the NA active center (1, 15). We cannot exclude the possibility that some influenza B viruses are more susceptible to oseltamivir carboxylate than to zanamivir or RWJ-270201 in NA inhibition assays due to variability of the NA sequences within the same virus type.
The functional residue Arg152 interacts with the acetamido group, which is one of the four main groups of the natural substrate, neuraminic acid. This group is also present in all three NA inhibitors (Fig. 1). Consistent with the important role of residue 152 in catalysis, the Arg152→Lys substitution led to resistance to all the available NA inhibitors.
In immunocompetent adults, influenza virus causes disease of a short duration. The in vitro data and clinical trial experience to date predict that virus resistance conferred by substitution in the NA active center in response to treatment with NA inhibitors will probably not be a problem in this population (3, 4, 11, 12). A low incidence of oseltamivir-resistant virus variants with enzymes bearing substitution in the NA active center was reported for viruses recovered from drug-treated young and middle-aged adults (<2%) (7, 14, 24). The incidence of drug resistance was higher in children treated with this NA inhibitor (5.5%), although shedding was not detected beyond day 9 (28). However, drug resistance could play a more important role in the treatment of immunocompromised patients with influenza virus infection (8).
The availability of the NA inhibitors, which target the NA of influenza A and B viruses, and amantadine and rimantadine, which target the M2 protein of influenza A viruses, provides more options for the control of influenza virus infection. Our studies indicate that the novel NA inhibitor RWJ-270201 has potent inhibitory activity against NAs of influenza A and B viruses and a unique pattern of activity against resistant variants. Therefore, RWJ-270201 has the potential to become a valuable anti-influenza drug.
ACKNOWLEDGMENTS
This work was supported in part by grant AI-45782 from the National Institute of Allergy and Infectious Diseases (L.V.G.) and by a grant from the R. W. Johnson Pharmaceutical Research Institute (L.V.G.).
We thank Y. S. Baby (BioCryst) for helpful discussion and Douglas W. Schallon (University of Virginia, Charlottesville) for his excellent technical assistance.
REFERENCES
- 1.Babu Y S, Chand P, Bantia S, Kotian P, Dehghani A, El Kattan Y, Lin T H, Hutchison T L, Elliott A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 2.Bantia S, Parker C D, Ananth S L, Horn L L, Andries K, Chand P, Kotian P L, Dehghani A, El-Kattan Y, Lin T, Hutchinson T L, Montgomery J A, Kellog D L, Babu Y S. Comparison of the anti-influenza activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45:1162–1167. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantia S, Ananth S, Horn L, Parker C, Gulati U, Chand P, Babu Y, Air G. Generation and characterization of a mutant of influenza A virus selected with a neuraminidase inhibitor RWJ-270201. Antivir Res. 2000;46:A60. [Google Scholar]
- 4.Barnett J M, Cadman A, Gor D, Dempsey M, Walters M, Candlin A, Tisdale M, Morley P J, Owens I J, Fenton R J, Lewis A P, Claas E C, Rimmelzwaan G F, De Groot R, Osterhaus A D. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob Agents Chemother. 2000;44:78–87. doi: 10.1128/aac.44.1.78-87.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman P M, Hoyne P A, Lawrence M C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993;67:2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubareva L V, Kaiser L, Hayden F G. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 7.Gubareva, L. V., L. Kaiser, M. N. Matrosovich, Y. Soo-Hoo, and F. G. Hayden. 2001. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J. Infect. Dis. 183 [DOI] [PubMed]
- 8.Gubareva L V, Matrosovich M N, Brenner M K, Bethell R C, Webster R G. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis. 1998;178:1257–1262. doi: 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- 9.Gubareva L V, Robinson M J, Bethell R C, Webster R G. Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J Virol. 1997;71:3385–3390. doi: 10.1128/jvi.71.5.3385-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden F G. Amantadine and rimantadine—clinical aspects. In: Richman D D, editor. Antiviral drug resistance. New York, N.Y: John Wiley & Sons Ltd.; 1996. pp. 59–77. [Google Scholar]
- 11.Hayden F G, Gubareva L V, Monto A S, Klein T, Elliott M J, Hammond J M, Sharp S J, Ossi M J. Inhaled zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 12.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 13.Hayden F G, Jennings L, Robson R, Schiff G, Jackson H, Rana B, McClelland G, Ipe D, Roberts N, Ward P. Oral oseltamivir in human experimental influenza B infection. Antivir Ther. 2000;5:205–213. [PubMed] [Google Scholar]
- 14.Jackson H C, Roberts N, Wang Z M, Belshe R. Management of influenza. Use of new antivirals and resistance in perspective. Clin Drug Investig. 2000;20:447–454. [Google Scholar]
- 15.Kim C U, Lew W, Williams M A, Wu H, Zhang L, Chen X, Escarpe P A, Mendel D B, Laver W G, Stevens R C. Structure-activity relationship studies of novel carbocyclic influenza neuraminidase inhibitors. J Med Chem. 1998;41:2451–2460. doi: 10.1021/jm980162u. [DOI] [PubMed] [Google Scholar]
- 16.McKimm-Breschkin J L. Resistance of influenza viruses to neuraminidase inhibitors. Antivir Res. 2000;47:1–17. doi: 10.1016/s0166-3542(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 17.McKimm-Breschkin J L, Sahasrabudhe A, Blick T J, McDonald M, Colman P M, Hart G J, Bethell R C, Varghese J N. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac2en-derived inhibitors. J Virol. 1998;72:2456–2462. doi: 10.1128/jvi.72.3.2456-2462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Potier M, Mameli L, Belisle M, Dallaire L, Melancon S B. Fluorimetric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-d-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 18.Scholtissek C, Quack G, Klenk H D, Webster R G. How to overcome resistance of influenza A viruses against adamantane derivatives. Antivir Res. 1998;37:83–95. doi: 10.1016/s0166-3542(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 19.Sidwell R, Smee D F, Huffman J H, Barnard D L, Bailey K W, Morrey J D, Babu Y S. In vivo influenza-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smee D F, Huffman J H, Morrison A C, Barnard D L, Bush K, Sidwell R W. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activity. Antimicrob Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugrue R J, Hay A J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai C Y, Escarpe P A, Sidwell R W, Williams M A, Lew W, Wu H, Kim C U, Mendel D B. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob Agents Chemother. 1998;42:3234–3241. doi: 10.1128/aac.42.12.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor N R, Cleasby A, Singh O, Skarzynski T, Wonacott A J, Smith P W, Sollis S L, Howes P D, Cherry P C, Bethell R, Colman P, Varghese J. Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 2. Crystallographic and molecular modeling study of complexes of 4-amino-4H-pyran-6-carboxamides and sialidase from influenza virus types A and B. J Med Chem. 1998;41:798–807. doi: 10.1021/jm9703754. [DOI] [PubMed] [Google Scholar]
- 24.Treanor J J, Hayden F G, Vrooman P S, Barbarash P, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills R G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 25.Varghese J N, Epa V C, Colman P M. Three-dimensional structure of the complex of 4-guanidino-Neu5Ac2en and influenza virus neuraminidase. Protein Sci. 1995;4:1081–1087. doi: 10.1002/pro.5560040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varghese J N, McKimm-Breschkin J L, Caldwell J B, Kortt A A, Colman P M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992;14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 27.Varghese J N, Smith P W, Sollis S L, Blick T J, Sahasrabudhe A, McKimm-Breschkin J L, Colman P M. Drug design against a shifting target: a structural basis for resistance to inhibitors in a variant of influenza virus neuraminidase. Structure. 1998;6:735–746. doi: 10.1016/s0969-2126(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z M, Tai C Y, Mendel D B. Studies on the mechanism by which mutations at His274 alter sensitivity of influenza A virus neuraminidase type 1 to GS4071 and zanamivir. Antivir Res. 2000;46:A60. doi: 10.1128/AAC.46.12.3809-3816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitley R J, Hayden F G, Reisenger K S, Young N, Dutkowski R, Ipe D, Mills R G, Ward P. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]