PURPOSE
Proportionate minority representation in clinical trials is an important step toward addressing health care inequities. Given the paucity of data on this topic in urologic oncology, we sought to quantify the enrollment of minority patients in clinical trials studying prostate, kidney, and bladder/urothelial cancers.
METHODS
The ClincialTrials.gov database was queried for completed phase II and III interventional trials in prostate, kidney, and bladder cancers. The SEER database was used to calculate the US prevalence of these genitourinary cancers. Representation quotients (RQ) were calculated to describe the relative proportion of each racial/ethnic group enrolled in clinical trials over the proportion of persons from each group among national cancer cases by cancer type.
RESULTS
Of 341 trials that met initial eligibility criteria, only 169 (49.7%) reported data on race or ethnicity. Aggregate RQs from 2000 to 2017 showed that White patients were continually over-represented in trials for all cancer types. Black and Asian patients were poorly represented across all cancer types. When stratified by 3-year increments, the RQs remained stable for all races, from 2000 to 2017. When stratified by ethnicity, Hispanic patients were under-represented across all cancer types in the study period. When examining representation by funding source, we found that US government–funded clinical trials proportionally enroll the most diverse patient populations over those funded by academic institutions and industry. Interestingly, more than 50% of the trials examined did not report race nor ethnicity, highlighting a crucial flaw in investigator compliance with federal clinical trial mandates.
CONCLUSION
Clinical trials targeting prostate, kidney, and bladder cancers continue to under-represent racial/ethnic minority patients. On the basis of the incidence of these cancers within minority populations, efforts should focus on creating racially and ethnically inclusive cancer research.
INTRODUCTION
Racial and ethnic disparities affect outcomes across medicine, and the field of oncology is no exception. The American Cancer Society's annual cancer statistics publication highlights a stark difference in cancer incidence and mortality between racial and ethnic communities.1 One well-documented disparity is that of prostate cancer in Black men.2,3 Between 2012 and 2017, the incidence of prostate cancer in Black men was 173 per 100,000, which was markedly higher than the incidence in Asian (52.9), Hispanic (86.8), Native American (68.0), and White (97.1) counterparts. When adjusting for age, Black men diagnosed with prostate cancer had a mortality rate of 38.7 per 100,000, more than twice that of their Asian (8.6), Hispanic (15.7), Native American (18.7), and White (18.0) counterparts.1 The cause of these differences is likely multifactorial, including differences in access to and delivery of quality care, environment, and cultural behaviors. However, one controllable factor in cancer intervention is the enrollment of minority populations in clinical trials. Minority enrollment is imperative as failure to study differences in populations undermines the generalizability of research results.
CONTEXT
Key Objective
Do genitourinary cancer clinical trials proportionally enroll minority patients? This original work examines the enrollment of minority populations in clinical trials in urologic oncology, as well as the impact of funding source on enrollment. It contains 169 eligible trials; to our knowledge, it is the largest and most comprehensive analysis of its kind to date.
Knowledge Generated
Our analysis revealed that Black, Asian, and Hispanic patients were generally poorly enrolled across cancer types and across funding source, with the exception of government-funded prostate cancer trials. More than half of the trials examined failed to report race or ethnicity data, highlighting the lack of investigator compliance.
Relevance
Awareness of patterns in patient enrollment should be highlighted to emphasize the importance of enrolling diverse cohorts in trials for the betterment of the diversity of patients that exist nationwide and globally.
To regulate diversity and inclusion in clinical trial participants, the National Institutes of Health (NIH) Revitalization Act of 1993 introduced the Clinical Research Equity for Women and Minorities.4 This addendum required that there be greater emphasis on including women and racial/ethnic minorities in clinical trials funded by the NIH. Almost 30 years have passed since the introduction of this mandate, but current studies indicate that across many cancer types, the relative representation of racial and ethnic minorities remains low.5
Genitourinary cancers represent a large proportion of cancer cases, with an estimated 20.7% of new cancer diagnoses in 2021.6 Because of the known under-representation of minority men in prostate cancer trials, we conduct an expanded investigation of racial and ethnic representation across the three most prevalent genitourinary cancers.7 In this study, we aim to examine the racial and ethnic representation among the population of patients enrolled in phase II and III clinical trials for prostate, kidney, and bladder/urothelial cancers. We hypothesize that racial and ethnic minorities will be under-represented in clinical trials across all funding mechanisms and all cancer types.
METHODS
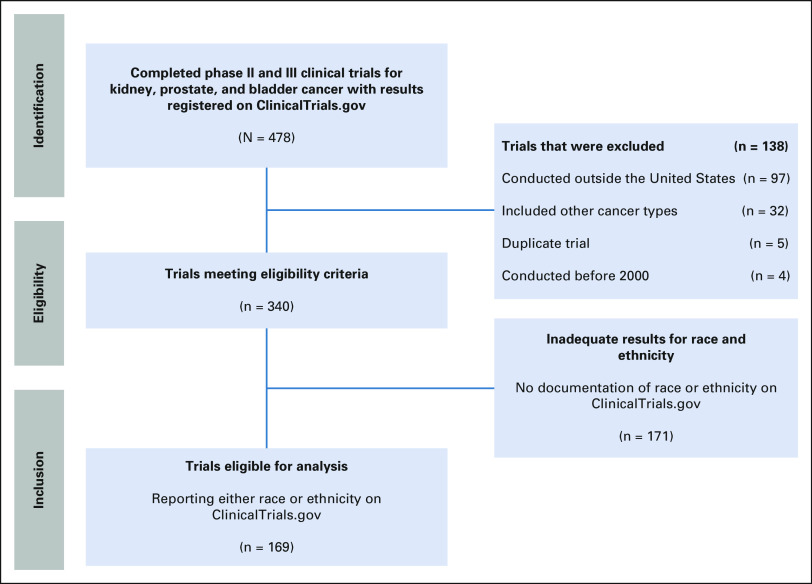
The ClinicalTrials.gov registry was queried for completed phase II and III interventional trials in urologic oncology between January 2000 and December 2017 for prostate, kidney, and bladder/urothelial cancer. Studies that targeted urothelial carcinoma were included in the bladder cancer cohort. The title, inclusion criteria, and description of each trial were examined to ensure that only studies focused solely on urologic oncology were collected. Trials that reported neither race nor ethnicity, that enrolled, or were conducted outside of the United States were excluded (Fig 1). The data collected did not include patient identifying information and therefore waived the requirement for informed consent and institutional review board approval.
FIG 1.
Flowchart of identified clinical trials in urologic oncology.
Race and ethnicity were identified using the defined terms as demonstrated on the ClinicalTrials.gov database, typically White/Caucasian, Black/African American, Asian, American Indian, or Alaska Native (NA), Native Hawaiian, or Pacific Islander, multiracial, or unknown/did not report. For the purposes of this study Asian and Native Hawaiian/Pacific Islander were combined to Asian/Pacific Islander (AAPI). Ethnicity was defined as Hispanic/Latino or non-Hispanic/Latino. To align with the creation of the ClinicalTrials.gov database (February 2000), we used SEERStat software (version 8.3.8) and the SEER 21 registry accessing data for cancer cases diagnosed from 2000 to 2017 for patients age 20 years and older.
To examine enrollment by funding source, trials were classified by the primary funding entity listed on ClinicalTrials.gov. Sources were defined as funded by the US government (NIH, Veterans Affairs, or the US Food and Drug Administration), academic as in funded by an academic center, or industry, defined as a trial funded by a pharmaceutical or biotechnology company.
To assess the minority enrollment in each clinical trial, we elected to create a representation quotient (RQ) to describe the proportion of representation for each race and cancer type similar to the enrollment fraction by Murthy et al.8 This was calculated as the number of patients enrolled in a trial divided by the US cancer cases in each racial or ethnic group per SEER data. An RQ of 1.0 described proportionate racial representation, compared with cancer cases in each racial group. An RQ > 1.0 described an over-representation, and an RQ of < 1.0 described an under-representation of a population in a trial.
To analyze temporal changes in enrollment, we examined the racial and ethnic composition of enrollees at 3-year increments from 2000-2017, labeled as follows: (2000: 2000-2002; 2003: 2003-2005; 2006: 2006-2008; 2009: 2009-2011; 2012: 2012-2014; and 2015: 2015-2017). We conducted an additional subanalysis to examine the role that funding sources has on the enrollment of racial and ethnic minorities into clinical trials. The null hypothesis was that the proportions of each race and ethnicity enrolled in urologic oncology trials were equal to the proportion of cancer in these populations.
RESULTS
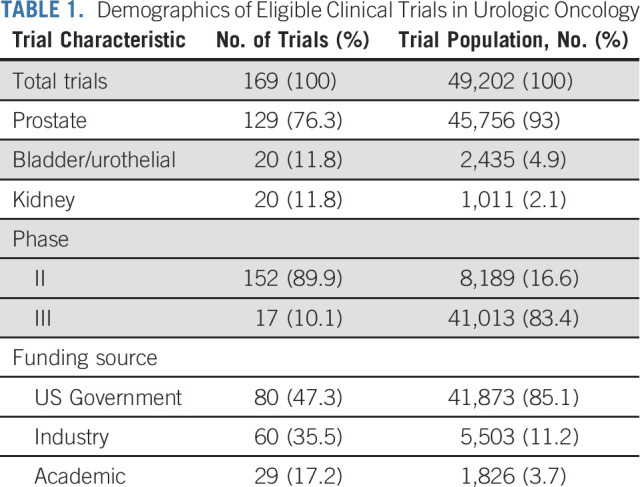
Of 341 trials that met initial eligibility criteria, only 169 (49.7%) reported data on race or ethnicity. These 169 eligible trials had a total of 49,202 enrolled patients. Descriptive statistics were analyzed for the cohort (Table 1). One hundred and fifty-two (152) trials were in phase II and 17 were phase III. Overall, 80 trials were funded by the US government (76 NIH and four US federal agencies), 60 by industry, and 29 by academic institutions.
TABLE 1.
Demographics of Eligible Clinical Trials in Urologic Oncology
Representation by Cancer Type
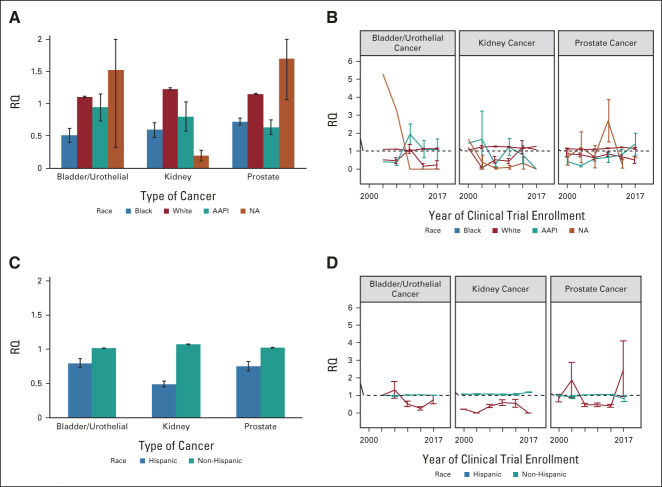
When aggregating all trials over the study period (2000-2017; Figs 2A and 2B), White patients were consistently over-represented for each cancer type (RQ: bladder [B] = 1.10 ± 0.01 [SE], kidney [K] = 1.23 ± 0.02, and prostate [P] = 1.15 ± 0.01) with maximum representation in 2015 (RQ: K = 1.27) and minimum in 2009 (RQ: B = 1.02 ± 0.04). Black patients were consistently under-represented across all cancer types (RQ: B = 0.51 ± 0.11, K = 0.59 ± 0.11, P = .72 ± 0.50) with maximum representation in 2012 (K: 1.22 ± 0.36) and minimum representation in 2003 (K: 0.06 ± 0.06). AAPIs were consistently under-represented in kidney and prostate cancer (RQ: B = 0.94 ± 0.21, K = 0.80 ± 0.22, P = .63 ± 0.11), but almost proportionally represented in bladder trials, with maximum representation in 2009 (B: 1.93 ± 0.58) and minimum with no representation of AAPI in 2015 kidney cancer trials (RQ: K = 0). Representation across time can be seen in Figure 2. Interestingly, NA patients have had vast variability in the representation in clinical trials for each cancer type. NAs were under-represented in kidney trials, but drastically over-represented in bladder and prostate trials (RQ: B = 1.52 ± 1.20, K = 0.19 ± 0.08, P = 1.70 ± 0.64) with maximum presentation in 2015 (RQ: P = 10.92 ± 10.29) and minimum representation from 2009-2015 (RQ: B = 0, K = 0).
FIG 2.
Patient representation by race and ethnicity. (A) Aggregated representation by race across different urologic oncologic cancer types. Error bars represent the SE for each racial group. (B) Representation by race across the study period (2000-2017). (C) Aggregated representation by ethnicity across different urologic oncologic cancer types. Error bars represent SE for each ethnicity. (D) Representation by ethnicity across the study period (2000-2017). AAPI, Asian/Pacific Islander; NA, Alaska Native; RQ, representation quotient.
Representation by Ethnicity
When aggregated across the study period, representation of patients by ethnicity (Figs 2C and 2D) revealed that non-Hispanic patients were proportionally represented (RQ: B = 1.01 ± 0.004, K = 1.08 ± 0.005, P = 1.02 ± 0.007) across all cancer types. Maximum representation was seen in kidney trials in 2015 (RQ: K = 1.19 ± 0.01), and minimum representation in prostate trials in 2015 (RQ: P = .83 ± 0.17). Conversely, Hispanic patients were consistently under-represented in kidney trials aggregated across the entire study period (RQ: B = 0.80 ± 0.07, K = 0.49 ± 0.04, P = .75 ± 0.07). Interestingly, there was a gross over-representation of Hispanic patients in 2006 in bladder trials (RQ: B = 1.32 ± 0.47) and prostate trials in 2003 and 2015 (max; RQ: P2003 = 1.89 ± 1.00 and P2015 = 2.53 ± 1.58) respectively. This is likely because of the small number of qualifying studies conducted during these two years. Minimum representation was seen in kidney trials conducted 2003 and 2015 with an RQ of 0 for both years.
Representation by Funding Source
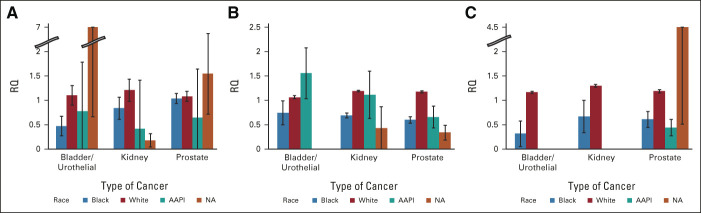
Racial representation of trial enrollees by funding source revealed that White patients were proportionally represented across all cancer types and funding sources (Fig 3). US government–funded clinical trials enrolled White patients proportionally (RQ: B = 1.10 ± 0.02, K = 1.21 ± 0.03, P = 1.08 ± 0.02) across all cancer types. Black patients were proportionally represented in prostate cancer trials, but less so in bladder and kidney trials (RQ: B = 0.47 ± 0.20, K = 0.84 ± 0.23, P = 1.04 ± 0.10). AAPIs were under-represented in trials across all cancer types (RQ: B = 0.78 ± 0.29, K = 0.41 ± 0.16, P = .64 ± 0.23). NAs were under-represented in kidney trials funded by the NIH, but over-represented in bladder and prostate trials (RQ: B = 3.44 ± 2.79, K = 0.18 ± 0.14, P = 1.54 ± 0.83). Trials funded by industry sponsors revealed over-representation of White patients (RQ: B = 1.06 ± 0.03, K = 1.19 ± 0.02, P = 1.18 ± 0.02) across all cancer types. Both Black (RQ: B = 0.74 ± 0.25, K = 0.69 ± 0.05, P = .60 ± 0.07) and NA (RQ: B = 0, K = 0.43 ± 0.43, P = .34 ± 0.15) patients were under-represented in kidney and prostate trials and bladder cancer trials. Interestingly, AAPIs were over-represented for bladder and kidney trials and under-represented in prostate trials funded by industry sponsors (RQ: B = 1.56 ± 0.52, K = 1.11 ± 0.49, P = .66 ± 0.22). Representation in trials funded by academic entities revealed proportionate representation of White patients across all cancer types; however, Black patients (RQ: B = 0.32 ± 0.26, K = 0.67 ± 0.33, P = .61 ± 0.16) were poorly enrolled across all cancer types. AAPIs were poorly enrolled in prostate cancer trials (RQ: P = .44 ± 0.17), and completely absent from kidney and bladder cancer trials. NAs were also absent from bladder and kidney trials, but grossly over-represented (RQ: P = 4.65 ± 4.15) in academic prostate trials on the basis of a single institutional trial that enrolled 61 NA patients, considerably more than the mean enrollment of this population in other trials (0.15 patients).
FIG 3.
Patient representation by funding source: (A) NIH and federal agency, (B) industry, and (C) academic. Error bars represent the SE. AAPI, Asian/Pacific Islander; NA, Alaska Native; RQ, representation quotient.
DISCUSSION
To our knowledge, this study represents the largest, most comprehensive review of minority enrollment in clinical trials in urologic oncology. Minority populations were poorly represented across time for all cancer types and all funding sources. Trials examining kidney cancer fared the worst in enrolling minorities, whereas prostate cancer trials fared best. This trend may mirror general awareness of disparities in prostate cancer, resulting in greater effort to recruit diverse populations to those trials, and conversely lack of such awareness in other cancer types. Additionally, funding source was associated with improved enrollment of minority populations, with government sponsorship demonstrating the most proportionate enrollment of minorities, whereas academic institutions failed to enroll minority patients proportional to national cancer cases. This emphasizes the influence of trial funding source and reinforces the impact that legislation (such as the NIH Revitalization Act) and medical society recommendations have on minority enrollment. Similar legislation enacted over academic and industry funded trials may aid in enrolling more diverse populations by requiring investigators to recruit with minority populations in mind.
Although our study assesses the state of diversity in clinical trials in urologic oncology specifically, our results are consistent with work examining minority enrollment in other medical specialties. Nazha et al9 reviewed enrollment of minority populations in trials for immunotherapy and found that among 582 patients treated with nivolumab in non–small-cell lung cancer trials, Black and Asian patients made up only 2.7% and 2.9% of patients, respectively. Similarly, in a study examining enrollment in large, randomized, multicenter prostate, lung, colon, and ovarian screening trials, < 6% of the almost 155,000 patients were either Black, Hispanic, or Asian, despite nationwide recruitment in both academic and community settings.10 In 2020, the US Food and Drug Administration (FDA) Drug Trials Snapshots Summary Report noted that 4,922 patients participated in clinical trials that led to the approval of 16 new oncologic therapeutics. Overall, 73% were White, 5% were Black/African American, 14% Asian, and 6% were Hispanic/Latino.11 Although only 44% of these patients were from the United States, it still highlights the disparity that exists within clinical trial enrollees within the United States and worldwide.
Diversity in clinical research exists in many dimensions ranging from race, ethnicity, sex, and age to comorbidity and socioeconomic status. Ideally, participants of a clinical trial reflect that of the general population and/or those most affected by the disease in question. The importance of diverse populations in clinical trials can be demonstrated by the black box warnings of carbamazepine, and potential Steven-Johnson's syndrome in patients with the HLA-B*1502 gene, found to be more prevalent in those of East Asian ancestry.12 However, it is also important to acknowledge the role that social variables and environmental stressors play in varying populations of patients. As we advance in the direction of precision medicine, understanding the complex nature of human health, behavior, and intersectionality in differing populations of people is imperative. Clinical trials offer an opportunity to examine this in detail, but only if the trials contain diverse, representative cohorts.
There are multiple potential barriers to effective recruitment. In some cases, investigator bias may contribute to poor recruitment of racial and ethnic minorities to clinical trials.13 In a qualitative study examining the perceptions of investigative teams at various EMPaCT (Enhancing Minority Participation in Clinical Trials) cancer centers, Niranjan et al13 revealed that recruitment of minority patients was heavily influenced by negative stereotypes and perceived negative outcomes by members of the team. In a separate study, Williams et al found that lack of access to the study population, lack of experience in recruiting, and cultural differences between the patients and the investigative teams were barriers to recruitment.14 These cultural challenges include language barriers and differences in cultural norms and understanding, which can likely be overcome by recruiting diverse individuals to the research team.15 Conversely, historical patient mistrust in the medical community widens the gap in racial and ethnic disparities in cancer research and beyond.16-19 In a national sample of respondents, Corbie-Smith et al20 revealed that African Americans were more likely than White patients to believe that their physicians would fail to be transparent when explaining a research study and expose them to unnecessary harm. These studies emphasize how stereotypes of both the investigators and patients limit the potential for clinical trial enrollment.
Trials that exclude populations on the basis of a disease process that disproportionally affects a specific community may also serve as a barrier to enrollment. Sickle cell disease or trait, for example, may exclude many patients of African ancestry. In interventional studies, it would be important to understand the interactions that biochemical agents have on specific cohorts not only in response to therapy, but also to understand the occurrence of adverse events. Ultimately, exclusion of comorbidities creates a barrier to access.17 Although exclusion criteria are often selected on the basis of maintaining patient safety and quality of the study, more transparency in how these criteria are selected is necessary.21 Generalizable results must apply to the entire population despite comorbidities, not simply the ideal patient.
Despite current challenges in addressing trial participation, several entities focus on aiding investigative teams in conducting inclusive and diverse clinical trials. The NCI Community Oncology Research Program (NCORP) is a national network of investigative teams designed to engage community oncologists and their minority and underserved patients in cancer research across 46 sites nationwide.22 This allows greater access to patient populations who span a spectrum of age, race, ethnicity, socioeconomic status, and geographic location. Similarly, the NCI Minority-Based Clinical Community Oncology Program (MBCCOP) funds resources to institutions whose patient population is at least 40% racial or ethnic minority, resulting in minority participation as high as 67% in the cooperative groups for which MBCCOP was associated.23 Finally, the Multi-Regional Clinical Trials Center (MRCTC) at Brigham and Women's Hospital and Harvard have developed a handbook to guide investigators at every level of the clinical trials process in ensuring diverse and inclusive trial design, enrollment, and execution. The center has published case studies documenting successful organizational enhancements that improve minority representation, as well as therapeutic case studies documenting differential responses to treatments in varying ancestral populations.24
We acknowledge some limitations to our study. The first lies in the variability of demographics reporting. Of 340 eligible clinical trials, 50.3% (n = 171) reported neither race nor ethnicity data on the ClinicalTrials.gov database. This deficiency in reporting highlights a significant flaw in investigator compliance as required by legislation. The FDA Amendments Act of 2007 (FDAAA 801) and Final Rule require investigators to publish their results data to ClinicalTrials.gov, including demographic information.25 Failing to uphold obligations in reporting is not only a barrier to this study, but also to physicians and patients who use the website as a resource for shared decision making in the implementation of care. Finally, the number of American Indian/Native American patients enrolled was relatively small. The results for this group are inherently less robust, but this could also further emphasize lack of access to health care that exists within American Indian/Alaska Native populations and in Native Reserve communities.26
In conclusion, clinical trials targeting prostate, kidney, and bladder cancers continue to under-represent racial/ethnic minority patients. On the basis of the incidence of these cancers within minority populations, efforts should target creating and improving racially and ethnically inclusive cancer research. To better serve the plurality of patients, the medical community at every level of the clinical trials process must be proactive in safely and inclusively investigating the medical needs of under-represented and underserved individuals.
Peter A. Pinto
Patents, Royalties, Other Intellectual Property: Philips/In Vivo Inc pays royalties to NIH for a licensing agreement, and NIH then pays royalties to Dr Pinto. NIH and Philips have a CRADA. NIH has intellectual property in the field, including among other patents and patent applications, Patent: System And Method For Prostate Cancer Detection And Distribution Mapping US Patent number: 8,447,384 and System And Method For Computer Aided Cancer Detection Using T2-weighted And High-value Diffusion-weighted Magnetic Resonance Imaging US Patent number: 10,215,830 with inventors/author, Brad Wood and Peter Pinto. NIH and Philips (In Vivo Inc) have a licensing agreement. NIH does not endorse or recommend any commercial products, processes, or services. The views and personal opinions of authors expressed herein do not necessarily state or reflect those of the US Government, nor reflect any official recommendation nor opinion of the NIH or NCI.
No other potential conflicts of interest were reported.
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
SUPPORT
Supported by the NIH Intramural Program (grant number: Z99 CA999999), and through the NIH Medical Research Scholars Program a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, and the Colgate-Palmolive Company.
AUTHOR CONTRIBUTIONS
Conception and design: Jeunice Owens-Walton, Cheyenne Williams, Alexis Rompré-Brodeur, Mark W. Ball
Financial support: Mark W. Ball
Administrative support: Peter A. Pinto, Mark W. Ball
Provision of study materials or patients: Mark W. Ball
Collection and assembly of data: Jeunice Owens-Walton, Cheyenne Williams, Mark W. Ball
Data analysis and interpretation: Cheyenne Williams, Alexis Rompré-Brodeur, Peter A. Pinto, Mark W. Ball
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Minority Enrollment in Phase II and III Clinical Trials in Urologic Oncology
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter A. Pinto
Patents, Royalties, Other Intellectual Property: Philips/In Vivo Inc pays royalties to NIH for a licensing agreement, and NIH then pays royalties to Dr Pinto. NIH and Philips have a CRADA. NIH has intellectual property in the field, including among other patents and patent applications, Patent: System And Method For Prostate Cancer Detection And Distribution Mapping US Patent number: 8,447,384 and System And Method For Computer Aided Cancer Detection Using T2-weighted And High-value Diffusion-weighted Magnetic Resonance Imaging US Patent number: 10,215,830 with inventors/author, Brad Wood and Peter Pinto. NIH and Philips (In Vivo Inc) have a licensing agreement. NIH does not endorse or recommend any commercial products, processes, or services. The views and personal opinions of authors expressed herein do not necessarily state or reflect those of the US Government, nor reflect any official recommendation nor opinion of the NIH or NCI.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Odedina FT, Akinremi TO, Chinegwundoh F, et al. : Prostate cancer disparities in Black men of African descent: A comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer 4:S2, 2009. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGinley KF, Tay KJ, Moul JW: Prostate cancer in men of African origin. Nat Rev Urol 13:99-107, 2016 [DOI] [PubMed] [Google Scholar]
- 4.United States. Congress. House. Committee on Energy and Commerce. Subcommittee on Health and the Environment : NIH Revitalization Act: Hearing Before the Subcommittee on Health and the Environment of the Committee on Energy and Commerce, House of Representatives, One Hundred Third Congress, First Session, on H.R. 4, a bill to amend the Public Health Service Act … February 3, 1993. Washington, U.S. G.P.O. : For Sale by the U.S. G.P.O., Supt. of Docs. Congressional Sales Office, 1993 [Google Scholar]
- 5.Chen MS Jr, Lara PN, Dang JH, et al. : Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer 120:1091-1096, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer facts & figures 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan AS, Palmer NR, Fergus KB, et al. : Minority recruitment trends in phase III prostate cancer clinical trials (2003 to 2014): Progress and critical areas for improvement. J Urol 201:259-267, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Murthy VH, Krumholz HM, Gross CP: Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291:2720-2726, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Nazha B, Mishra M, Pentz R, et al. : Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Ed Book 39:3-10, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Pinsky PF, Ford M, Gamito E, et al. : Enrollment of racial and ethnic minorities in the prostate, lung, colorectal and ovarian cancer screening trial. J Natl Med Assoc 100:291-298, 2008 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration : 2020 drug trials snapshots summary report. https://www.fda.gov/media/145718/download [Google Scholar]
- 12.Ferrell PB Jr, McLeod HL: Carbamazepine: HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 9:1543-1546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niranjan SJ, Martin MY, Fouad MN, et al. : Bias and stereotyping among research and clinical professionals: Perspectives on minority recruitment for oncology clinical trials. Cancer 126:1958-1968, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Williams IC, Corbie-Smith G: Investigator beliefs and reported success in recruiting minority participants. Contemp Clin Trials 27:580-586, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Egleston BL, Pedraza O, Wong YN, et al. : Characteristics of clinical trials that require participants to be fluent in English. Clin Trials 12:618-626, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreer LE, Weston J, Owsley C: Strategic planning for recruitment and retention of older African Americans in health promotion research programs. J Health Dispar Res Pract 7:14-33, 2014 [PMC free article] [PubMed] [Google Scholar]
- 17.Ford JG, Howerton MW, Lai GY, et al. : Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 112:228-242, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Rogers CR, Rovito MJ, Hussein M, et al. : Attitudes toward genomic testing and prostate cancer research among Black men. Am J Prev Med 55:S103-S111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washington HA: Medical Apartheid: The Dark History of Medical Experimentation on Black Americans From Colonial Times to the Present (ed 1). New York, NY, Doubleday, 2006 [Google Scholar]
- 20.Corbie-Smith G, Thomas SB, St George DM: Distrust, race, and research. Arch Intern Med 162:2458-2463, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt AF, Groenwold RH, van Delden JJ, et al. : Justification of exclusion criteria was underreported in a review of cardiovascular trials. J Clin Epidemiol 67:635-644, 2014 [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute: NCI Community Oncology Research Program (NCORP) . https://www.cancer.gov/research/infrastructure/clinical-trials/ncorp [DOI] [PMC free article] [PubMed]
- 23.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. : Increasing minority participation in cancer clinical trials: The minority-based community clinical oncology program experience. J Clin Oncol 23:5247-5254, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Brigham and Women’s Hospital Division of Global Health Equity : Diversity, Inclusion and Equity in Clinical Research, Cambridge, MA, The Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard, 2021 [Google Scholar]
- 25.DeVito NJ, Bacon S, Goldacre B: Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: A cohort study. Lancet 395:361-369, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Willging CE, Sommerfeld DH, Jaramillo ET, et al. : Improving Native American elder access to and use of health care through effective health system navigation. BMC Health Serv Res 18:464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]