PURPOSE
To provide precise age-specific risk estimates of cancers other than female breast and ovarian cancers associated with pathogenic variants (PVs) in BRCA1 and BRCA2 for effective cancer risk management.
METHODS
We used data from 3,184 BRCA1 and 2,157 BRCA2 families in the Consortium of Investigators of Modifiers of BRCA1/2 to estimate age-specific relative (RR) and absolute risks for 22 first primary cancer types adjusting for family ascertainment.
RESULTS
BRCA1 PVs were associated with risks of male breast (RR = 4.30; 95% CI, 1.09 to 16.96), pancreatic (RR = 2.36; 95% CI, 1.51 to 3.68), and stomach (RR = 2.17; 95% CI, 1.25 to 3.77) cancers. Associations with colorectal and gallbladder cancers were also suggested. BRCA2 PVs were associated with risks of male breast (RR = 44.0; 95% CI, 21.3 to 90.9), stomach (RR = 3.69; 95% CI, 2.40 to 5.67), pancreatic (RR = 3.34; 95% CI, 2.21 to 5.06), and prostate (RR = 2.22; 95% CI, 1.63 to 3.03) cancers. The stomach cancer RR was higher for females than males (6.89 v 2.76; P = .04). The absolute risks to age 80 years ranged from 0.4% for male breast cancer to approximately 2.5% for pancreatic cancer for BRCA1 carriers and from approximately 2.5% for pancreatic cancer to 27% for prostate cancer for BRCA2 carriers.
CONCLUSION
In addition to female breast and ovarian cancers, BRCA1 and BRCA2 PVs are associated with increased risks of male breast, pancreatic, stomach, and prostate (only BRCA2 PVs) cancers, but not with the risks of other previously suggested cancers. The estimated age-specific risks will refine cancer risk management in men and women with BRCA1/2 PVs.
INTRODUCTION
It is well established that pathogenic variants (PVs) in BRCA1 and BRCA2 (BRCA1/2) are associated with increased risks of breast and ovarian cancers in women for which reliable risk estimates are available.1 Accumulated evidence indicates that BRCA1/2 PVs are also associated with pancreatic cancer2-8 and male breast cancer risks3,6,9-13 and that BRCA2 PVs are associated with prostate cancer risk, particularly aggressive prostate cancer, whereas the association between BRCA1 PVs and prostate cancer risk is still debated.2,5,6,8,14-17 Associations with risks for other cancers have also been suggested, including colorectal, liver, and stomach cancers for BRCA1/2 PVs; cervical, corpus uteri, kidney, and testis cancers for BRCA1 PVs;3,4,6,8,18,19 and bone, brain, blood, and gallbladder cancers and malignant melanoma for BRCA2 PVs.2,5,6,8,20 However, these associations are based on studies with relatively small sample sizes, resulting in imprecise cancer risk estimates.
CONTEXT
Key Objective
The associations of pathogenic variants (PVs) in BRCA1 and BRCA2 with cancers other than female breast and ovarian cancers remain uncertain. Precise risk estimates are required to inform effective cancer risk management. This study investigates the associations between the risks of 22 cancers and BRCA1/2 PVs using data from 5,341 families segregating BRCA1 or BRCA2 PVs.
Knowledge Generated
BRCA1 and BRCA2 PVs are associated with increased risks of male breast, pancreatic, and stomach cancers; male BRCA2 carriers are also at increased prostate cancer risk. No associations were found with risks of other cancers. The cumulative risks to age 80 years ranged from 0.4% for male breast cancer to approximately 2.5% for pancreatic cancer for BRCA1 carriers and from approximately 2.5% for pancreatic cancer to 27% for prostate cancer for BRCA2 carriers.
Relevance
The findings provide age-specific cancer risk estimates and will allow for improved cancer risk assessment of male and female carriers.
The National Comprehensive Cancer Network and other guidelines recommend breast and ovarian cancer screening for BRCA1/2 carriers and prostate cancer screening particularly for BRCA2 carriers. Notably, National Comprehensive Cancer Network guidelines recently addressed testing and management for pancreatic cancer risk in BRCA1/2 carriers, but only in the presence of a positive family history of the disease.21,22 Overall, current guidelines suggest that men and women with BRCA1/2 PVs should consider participation in investigational screening studies and receive education regarding signs and symptoms of cancers possibly associated with BRCA1/2 PVs.21 The availability of more precise risk estimates will aid translation into evidence-based clinical guidelines for the cancer risk management in BRCA1/2 carriers and may guide treatment options for patients with cancer.
To inform clinical management strategies and optimize guidelines for cancer risk management in female and male BRCA1/2 carriers, we comprehensively assess the associations of BRCA1/2 PVs with risks of 22 cancers, other than female breast and ovarian cancers.
METHODS
Study Sample
Data on 7,618 families with at least one family member having a BRCA1 or BRCA2 PV were obtained from 26 study groups in the Consortium of Investigators of Modifiers of BRCA1/2 (Data Supplement, online only).23 Only families with a clearly PV identified were included.24 The majority of families (7,281) were ascertained through an index individual attending cancer family clinics, mainly because of having multiple affected relatives, and 337 families were ascertained through an index case with breast or ovarian cancer, unselected for family history. All index individuals were age ≥ 18 years. For each family member, data including familial relationship, BRCA1/2 PV status, sex, year of birth, and years or age at pedigree data collection, death, and cancer diagnoses were collected (Data Supplement). All participants provided written informed consent and participated in studies at the host institutions under ethically approved protocols.
Statistical Analysis
BRCA1 and BRCA2 families were analyzed separately. Complex segregation analysis,25 which considered the observed phenotype and observed or inferred genotype information of all family members, was used to estimate relative risks (RRs) for 22 first primary cancer sites, excluding female breast and ovarian cancers (Table 1). This involved comparing the observed cancer incidences for carriers with the age-, country- and birth cohort-specific population incidences (Cancer Incidence in Five Continents26); thus, the estimated RRs were equivalent to standardized incidence ratios. Noncarriers were assumed to develop the cancers according to population incidences. Pedigree likelihoods were constructed and maximized using the pedigree analysis software MENDEL.27
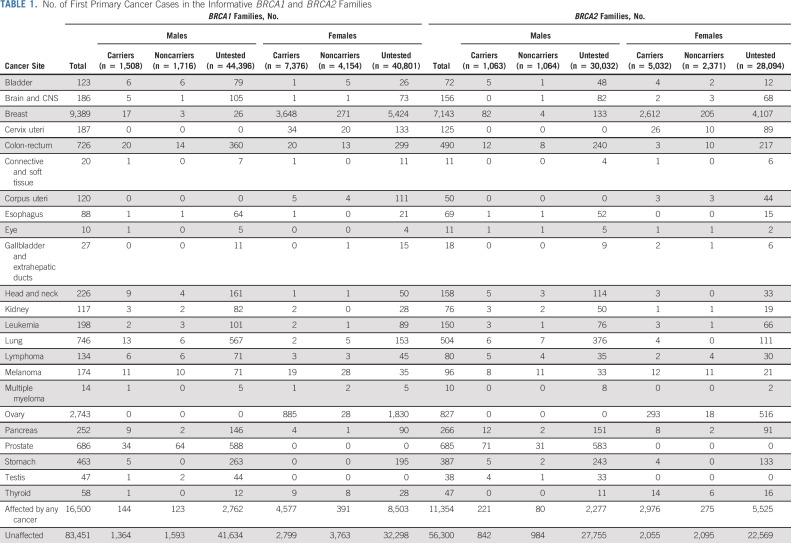
TABLE 1.
No. of First Primary Cancer Cases in the Informative BRCA1 and BRCA2 Families
Individuals were followed from birth until the age of the first primary cancer diagnosis, death, age at pedigree-data collection, risk-reducing mastectomy and/or salpingo-oophorectomy (if these occurred at least 1 year before breast or ovarian cancer diagnoses, respectively), or age 80 years, whichever occurred first. Missing year of birth and cancer diagnosis age were imputed (Data Supplement).
Each individual was assumed to be at risk of developing the cancer of interest, as well as breast or ovarian cancer. The RRs for female breast and ovarian cancers were assumed to be equal to previous estimates28; therefore, we only estimated the RR for the cancer of interest. We fitted models in which the RRs were assumed to be constant with age, birth cohort, sex, and study group and separate models with sex-specific RRs. For cancers with significant associations, we investigated whether the RRs varied by age. RRs from the best fitting models were used to estimate age-specific absolute risks on the basis of UK cancer incidences in year 2008-2012 (Data Supplement).
Because family ascertainment varied across study groups, we adjusted for the ascertainment of each family separately using an ascertainment-assumption-free approach.29-31 Pedigree likelihoods were computed conditional on any data that may be relevant to the ascertainment (Data Supplement). Noninformative families, in which no additional information beyond the data relevant to the ascertainment was available, were excluded from analysis. Since cancer family history was self-reported, we assessed the possibility of systematic under-reporting of specific cancers at the individual study group level and excluded any study groups in which under-reporting was likely relative to the population incidences (Data Supplement).
Sensitivity analyses under alternative inclusion, censoring, or ascertainment assumptions were performed for cancers that demonstrated associations: (1) stratifying by geographical region (Asian countries v others); (2) including study groups with possible cancer under-reporting; (3) excluding individuals with missing age at diagnosis; (4) individuals with risk-reducing bilateral mastectomy and/or salpingo-oophorectomy were still considered to be at risk of developing the other cancers, except breast and ovarian cancers; and (5) assuming the data relevant to the ascertainment for clinic-based families do not include the family history of cancer of interest. To account for population differences in melanoma skin pigmentation, we also conducted sensitivity analyses for melanoma by using (1) only families from Australia, Northern Europe, and North America; (2) only families in which probands self-identified as White European; and (3) only the families satisfying both (1) and (2).
All statistical tests were two-sided, and associations with a nominal P < .05 were considered statistically significant.
RESULTS
After ascertainment adjustment, 3,184 BRCA1 families and 2,157 BRCA2 families were informative for inclusion in the analysis, including 14,979 carriers, 9,296 noncarriers, and 153,323 untested individuals (Data Supplement). 61.3% of probands had self-reported ethnicity data. Of those, 77.0%, 11.5%, 4.7%, 3.3%, and 1.2% self-identified as White European, Asian, Ashkenazi Jewish, Hispanic, and Black, respectively. Prostate, lung, colorectal, stomach, and pancreatic cancers were the most common cancers in the data set, aside from breast and ovarian (Table 1). The age at diagnosis for each cancer by PV status is shown in the Data Supplement. After excluding study groups in which there was potential cancer under-reporting (Data Supplement), the proportions of families included in the estimation of cancer-specific risks varied from approximately 15% for lymphoma and multiple myeloma to > 90% for pancreatic and male breast cancers (Data Supplement).
Cancer Associations With BRCA1 PVs
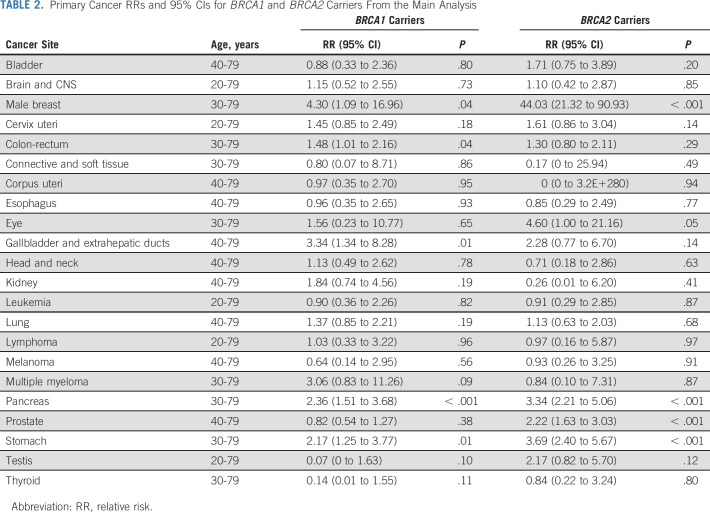
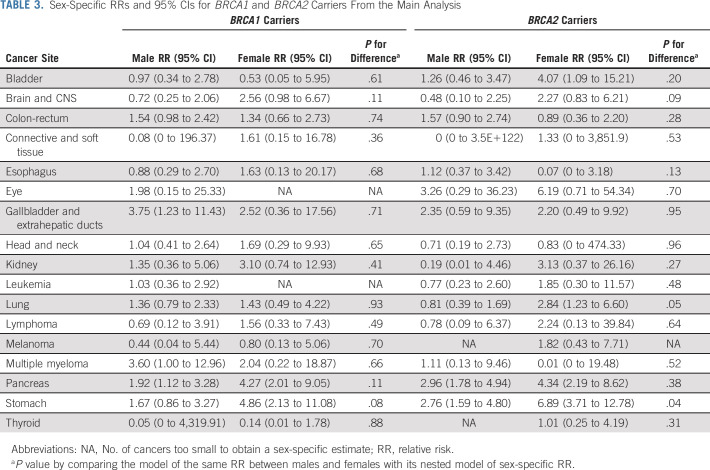
BRCA1 PVs were associated with male breast (RR = 4.30; 95% CI, 1.09 to 16.96), gallbladder (RR = 3.34; 95% CI, 1.34 to 8.28), pancreatic (RR = 2.36; 95% CI, 1.51 to 3.68), stomach (RR = 2.17; 95% CI, 1.25 to 3.77), and colorectal (RR = 1.48; 95% CI, 1.01 to 2.16) cancers (Table 2). No association was found for prostate cancer (RR = 0.82; 95% CI, 0.54 to 1.27). No difference in the RR estimates by sex was observed for any of the 17 non–sex-specific cancers (all P > .07; Table 3).
TABLE 2.
Primary Cancer RRs and 95% CIs for BRCA1 and BRCA2 Carriers From the Main Analysis
TABLE 3.
Sex-Specific RRs and 95% CIs for BRCA1 and BRCA2 Carriers From the Main Analysis
A model with RRs stratified by age 65 years (Data Supplement) provided a significantly better fit for stomach cancer: RR = 3.50 (95% CI, 2.01 to 6.10) for age < 65 years and higher than 0.61 (95% CI, 0.16 to 2.30) for age ≥ 65 years (P-heterogeneity = .01). For male breast cancer, a model with RRs stratified by age decade provided a better fit than the model with an age-constant RR (P = .03), but this was mainly driven by the lack of cases in the age group of 50-59 years (Data Supplement).
Cancer Associations With BRCA2 PVs
BRCA2 PVs were associated with increased risks of male breast (RR = 44.0; 95% CI, 21.3 to 90.9), stomach (RR = 3.69; 95% CI, 2.40 to 5.67), pancreatic (RR = 3.34; 95% CI, 2.21 to 5.06), and prostate (RR = 2.22; 95% CI, 1.63 to 3.03) cancers (Table 2). Female carriers had a higher risk of stomach cancer (RR = 6.89; 95% CI, 3.71 to 12.78) than male carriers (RR = 2.76; 95% CI, 1.59 to 4.80; P-heterogeneity = .04; Table 3).
A model with RRs stratified by age 65 years (Data Supplement) provided a significantly better fit for pancreatic cancer: RR = 4.92 (95% CI, 2.96 to 7.80) for age < 65 years and higher than 1.77 (95% CI, 0.87 to 3.58) for age ≥ 65 years (P-heterogeneity = .03). There was a suggestion that the prostate cancer RR was greater for age < 65 years (RR = 3.10; 95% CI, 2.00 to 4.79) than age ≥ 65 years (RR = 1.69; 95% CI, 1.09 to 2.62), but this model did not fit significantly better than the model with an age-constant RR (P = .06).
Sensitivity Analysis
The results are described in detail in the Data Supplement. There was no significant difference in the RR estimates by geographical region. The observed cancer associations were robust to all sensitivity analyses, except for colorectal and gallbladder cancers. No association was found for melanoma even when analyses were restricted to families from Australia, Northern Europe, and North America or families in which probands self-identified as White European.
Absolute Risks
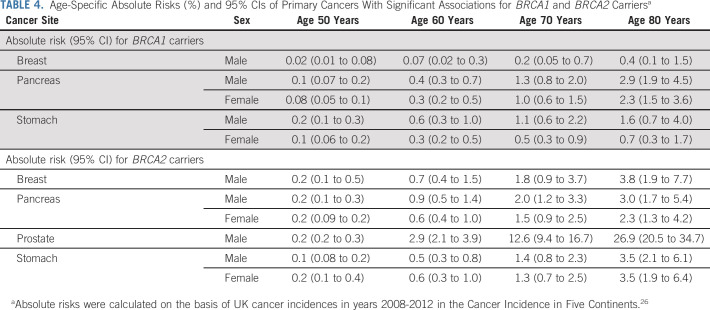
RRs from the main analysis best-fitting models were used to calculate age-specific absolute cancer risks (Table 4 and Fig 1). By age 80 years, the male breast cancer risk for BRCA1 and BRCA2 carriers was 0.4% (95% CI, 0.1 to 1.5) and 3.8% (95% CI, 1.9 to 7.7), respectively; the pancreatic cancer risk varied between 2.3% and 3.0% for both male and female BRCA1 and BRCA2 carriers; the stomach cancer risks were 1.6% (95% CI, 0.7 to 4.0) for male and 0.7% (95% CI, 0.3 to 1.7) for female BRCA1 carriers and approximately 3.5% for both male and female BRCA2 carriers. The prostate cancer risk associated with BRCA2 PVs was 26.9% (95% CI, 20.5 to 34.7) by age 80 years and 33.1% (95% CI, 25.5 to 42.2) by age 85 years.
TABLE 4.
Age-Specific Absolute Risks (%) and 95% CIs of Primary Cancers With Significant Associations for BRCA1 and BRCA2 Carriersa
FIG 1.
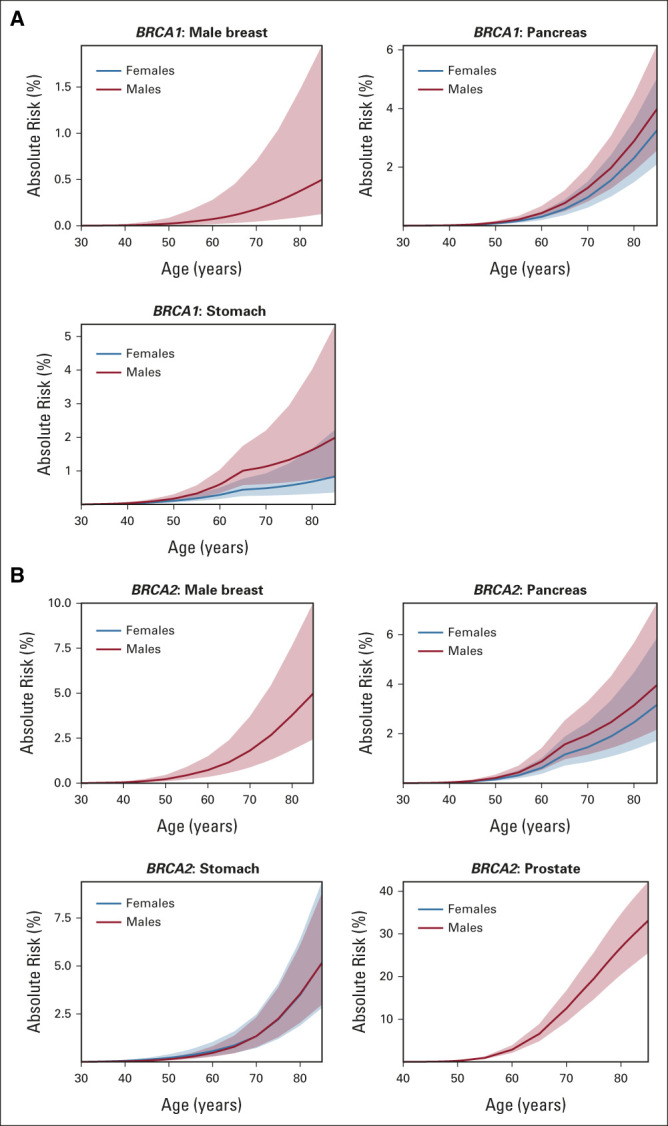
Age-specific absolute risks (%) and 95% CIs of primary cancers on the basis of UK cancer incidences in years 2008-2012 for (A) BRCA1 and (B) BRCA2 carriers. Solid lines are the age-specific absolute risk estimates, and ribbons are the relevant 95% CIs.
DISCUSSION
This study assessed the risks associated with BRCA1/2 PVs for 22 first primary cancers, other than female breast and ovarian cancers, and further clarified the cancer spectrum associated with BRCA1/2 PVs.
The associations of BRCA1/2 PVs with the risks of male breast and pancreatic cancers were confirmed and refined, as well as the association of prostate cancer with BRCA2 PVs, regardless of age and aggressiveness.
The lifetime male breast cancer risks were previously reported to be 2%-6% for BRCA1 and 7%-13% for BRCA2 carriers (Data Supplement).3,6,9-13 We estimated these risks to be somewhat lower, 0.4% (95% CI, 0.1 to 1.5) and 3.8% (95% CI, 1.9 to 7.7), respectively. The pancreatic cancer associations were consistent with previously reported RRs of 2-3 and lifetime risks of 1%-4% for BRCA1 carriers3,4,6 and RRs of 3-6 and lifetime risks of 3%-5% for BRCA2 carriers (Data Supplement).2,5-8 Notably, the RR was higher for BRCA2 carriers age < 65 years.
Previous retrospective studies reported prostate cancer RRs of 2-6 and absolute risks of 17%-31% by age 80 years for BRCA2 carriers (Data Supplement).2,5,6,8,14-17 Our estimated absolute risk by age 85 years was 33%, lower than the recently reported prospective estimate of 60% by Nyberg et al.32 However, after adjusting for possible increased prostate-specific antigen screening effects in the prospective study, their estimate was 41% (95% CI, 22 to 59), consistent with our estimate. The present estimate is unlikely to be subject to increased screening biases since prostate cancer family history was retrospectively collected, and increased screening in relatives is unlikely to have taken place before the identification of BRCA2 PVs. The reported associations of BRCA1 PVs with prostate cancer risk are inconsistent, with RRs of 0.4-4, most not statistically significant.3,4,6,8,14-18,32,33 This study confirms that BRCA1 PVs are not associated with overall prostate cancer risk.
Among the suggested associations with other cancers, the association between BRCA1/2 PVs and stomach cancer is under considerable debate.4,5 This study validated and further elucidated this association: there were associations with both BRCA1 and BRCA2 PVs, with RRs of 2.17 (3.50 for age < 65 years) and 3.69, respectively. Our estimates better refined the previously reported RRs of 2-7 for BRCA1 carriers3,6,8 and approximately 2.6 for BRCA2 carriers (Data Supplement).2,8 Notably, our findings showed that the stomach cancer RR for female BRCA2 carriers was higher than the estimate for male carriers although this translated in similar absolute risks, given the higher incidence of male stomach cancer in the general population. However, we cannot exclude the possibility that the higher female RR may be due to the misclassification of some ovarian cancers as stomach cancers.2,34
Data in the current study come from either epidemiologic studies or families undergoing PV screening collected at genetics centers. Although individual studies and clinical genetic centers, where possible, confirmed reported cancer diagnoses in families through medical records or registries as part of standard clinical practice, cancer confirmation information is not available centrally and it was not feasible to collect this at such a large scale. However, a key advantage of the present study is the large sample size, which results in RR estimates with greater precision. Only a small number of family-based studies reported cancer confirmation rates.2,4,5,8 Our RR estimates for stomach cancer, which may be susceptible to a greater degree of misclassification bias than other cancers,2,34 are not significantly different from the estimates from studies that reported cancer confirmation. However, the present RRs have similar or greater precision than the published estimates from studies with high cancer confirmation rates (Data Supplement).
In the present study, previously suggested associations of BRCA1/2 PVs with risks of other genitourinary cancers and melanoma2,8 were not replicated. Although associations of BRCA1 PVs with colorectal and gallbladder cancers were observed, the results were not robust in the sensitivity analyses performed.
Increased risks of bone and liver cancer have also been reported for BRCA1 or BRCA2 carriers.4-6 However, liver and bone are common metastatic sites for breast, prostate, or pancreatic cancers and could be the presenting cancer. Since no pathology confirmation data were available, we did not examine these associations in the main analysis. If we assume that the reported bone and liver cancers in the data set are indeed first primaries, the data suggest no association with BRCA1 PVs, but that BRCA2 carriers are at seven-fold increased risk of bone cancer and five-fold increased risk of liver cancer without significant differences between males and females (Data Supplement). However, no conclusion for these associations can be drawn without pathology confirmation.
Overall, the estimated age-specific relative and absolute risks suggest that, in addition to breast and ovarian cancers, the clinical management of BRCA1/2 carriers should focus on cancer sites, which now show robust associations, such as prostate (BRCA2 carriers only), pancreatic, and possibly stomach cancers. Notably, although rare, pancreatic and stomach cancers are associated with poor prognosis and their incidences have been rising over time, and thus, our results highlight the importance of screening for upper gastrointestinal tract malignancies for BRCA1 and BRCA2 carriers, particularly for age < 65 years. On the other hand, some cancers previously taken into consideration for screening for BRCA1/2 carriers, like melanoma, may be reconsidered, to further optimize cancer prevention screening strategies and eventually reduce carriers' distress. Given that the cancer risk associations were found for both male and female carriers, the results also suggest that male relatives of known BRCA1/2 carriers should be informed about their individual cancer risk and encouraged to be tested.35,36 It has been shown that knowing the germline BRCA1/2 PV status can influence treatment options for patients with cancer, leading to improved prognosis. For example, poly (ADP-ribose) polymerase inhibitor therapies that have been used successfully in the treatment of BRCA-related breast and ovarian cancers37 are now beginning to be used for pancreatic and prostate cancers,38,39 and in the near future, they might also be used for stomach cancer.40
To avoid biases in the risk estimates related to the ascertainment of clinic-based families, on the basis of multiple affected family members, we used a conservative ascertainment adjustment approach by conditioning on the family histories of cancers of breast and ovary and the cancer site under investigation. When only family history of female breast and ovarian cancers was considered in the ascertainment, the RR estimates were somewhat higher for most cancers but with narrower CIs (Data Supplement). Therefore, conditioning on the family history of the cancer of interest is unlikely to have led to substantial underestimation of risk. A notable exception was male breast cancer, where much higher RR estimates were obtained. However, this estimate is most likely biased because male breast cancer family history has been an important factor in considering BRCA1/2 germline genetic testing since the discovery of BRCA1/2.
This study has several limitations. First, this is a retrospective family-based study, with self-reported cancer family history, which may be inaccurate.41,42 Second, 7%-40% of reported cancer cases had missing age at diagnosis, with stomach cancer having the largest proportion. To minimize these potential biases, we performed sensitivity analyses excluding any study groups in which under-reporting was likely and any cases with missing age at diagnosis, and conclusions remained similar for most cancers. Third, we presented our results without any multiple testing adjustment. However, even using a false discovery rate adjustment, all the observed associations for BRCA2 carriers and the pancreatic cancer association for BRCA1 carriers had false discovery rates < 0.05. Fourth, the ethnicity of the family proband was not systematically collected by all studies because of variations in local data collection protocols. Among those with recorded ethnicity, in Asia-based studies, 97.7% of probands were Asian and in the rest of the studies 86.1%, 5.2%, 3.7%, 1.3%, and 1.1% of probands were White European, Ashkenazi, Hispanic, Black, and Asian, respectively. Therefore, the power to investigate the associations by all ethnic groups was limited. However, we did not find evidence of heterogeneity in the RRs by geographical region (Asia v others). Whether our risk estimates are applicable to non-European populations requires further investigation. Fifth, we did not have data on other genetic and environmental factors, so we were unable to investigate the modification effects of these factors; therefore, our risk estimates should be interpreted as the average risks across all potential genetic and environmental modifiers.
In conclusion, this study confirms that, aside from female breast and ovarian cancers, BRCA1/2 PVs are associated with increased risks of breast cancer in men, and pancreatic and stomach cancers in both sexes, and that only BRCA2 carriers are at elevated prostate cancer risk. BRCA1/2 PVs were not associated with the risks of any other cancers previously suggested. The association results and estimated age-specific risks will improve the cancer risk management for men and women with BRCA1/2 PVs.
ACKNOWLEDGMENT
We thank all the families and clinicians who contributed to the studies; GEMO study Collaborators; SWE-BRCA investigators; kConFab Investigators; Catherine M. Phelan for her contribution to CIMBA until she passed away on September 22, 2017; Sue Healey, in particular taking on the task of mutation classification with the late Olga Sinilnikova; Maggie Angelakos, Judi Maskiell, Gillian Dite, and Helen Tsimiklis; members and participants in the New York site of the Breast Cancer Family Registry; members and participants in the Ontario Familial Breast Cancer Registry; Vilius Rudaitis and Laimonas Griškevičius; Drs Janis Eglitis, Anna Krilova, and Aivars Stengrevics; Yuan Chun Ding and Linda Steele for their work in participant enrollment and biospecimen and data management; Bent Ejlertsen and Anne-Marie Gerdes for the recruitment and genetic counseling of participants; Alicia Barroso, Rosario Alonso, and Guillermo Pita; Ms JoEllen Weaver and Dr Betsy Bove; FPGMX: members of the Cancer Genetics group (IDIS): Ana Blanco, Miguel Aguado, Olivia Fuentes, and Ana Crujeiras; and IFE—Leipzig Research Centre for Civilization Diseases (Markus Loeffler, Joachim Thiery, Matthias Nüchter, and Ronny Baber). We thank all participants, clinicians, family doctors, researchers, and technicians for their contributions and commitment to the DKFZ study and the collaborating groups in Lahore, Pakistan (Noor Muhammad, Sidra Gull, Seerat Bajwa, Faiz Ali Khan, Humaira Naeemi, Saima Faisal, Asif Loya, and Mohammed Aasim Yusuf) and Bogota, Colombia (Diana Torres, Ignacio Briceno, and Fabian Gil). Genetic Modifiers of Cancer Risk in BRCA1/2 Mutation Carriers (GEMO) study is a study from the National Cancer Genetics Network UNICANCER Genetic Group, France. We would like to pay a tribute to Olga M. Sinilnikova, who with Dominique Stoppa-Lyonnet initiated and coordinated GEMO until she sadly passed away on June 30, 2014. The team in Lyon (Olga Sinilnikova, Mélanie Léoné, Laure Barjhoux, Carole Verny-Pierre, Sylvie Mazoyer, Francesca Damiola, and Valérie Sornin) managed the GEMO samples until the biological resource center was transferred to Paris in December 2015 (Noura Mebirouk, Fabienne Lesueur, and Dominique Stoppa-Lyonnet). We would like to thank all the GEMO collaborating groups for their contribution to this study: Coordinating Centre, Service de Génétique, Institut Curie, Paris, France: Muriel Belotti, Ophélie Bertrand, Anne-Marie Birot, Bruno Buecher, Sandrine Caputo, Chrystelle Colas, Anaïs Dupré, Emmanuelle Fourme, Marion Gauthier-Villars, Lisa Golmard, Marine Le Mentec, Virginie Moncoutier, Antoine de Pauw, Claire Saule, Dominique Stoppa-Lyonnet, and Inserm U900, Institut Curie, Paris, France: Fabienne Lesueur and Noura Mebirouk. Contributing Centers: Unité Mixte de Génétique Constitutionnelle des Cancers Fréquents, Hospices Civils de Lyon—Centre Léon Bérard, Lyon, France: Nadia Boutry-Kryza, Alain Calender, Sophie Giraud, and Mélanie Léone. Institut Gustave Roussy, Villejuif, France: Brigitte Bressac-de-Paillerets, Olivier Caron, Marine Guillaud-Bataille. Centre Jean Perrin, Clermont–Ferrand, France: Yves-Jean Bignon and Nancy Uhrhammer. Centre Léon Bérard, Lyon, France: Valérie Bonadona and Christine Lasset. Centre François Baclesse, Caen, France: Pascaline Berthet, Laurent Castera, and Dominique Vaur. Institut Paoli Calmettes, Marseille, France: Violaine Bourdon, Catherine Noguès, Tetsuro Noguchi, Cornel Popovici, Audrey Remenieras, and Hagay Sobol. CHU Arnaud-de-Villeneuve, Montpellier, France: Isabelle Coupier and Pascal Pujol. Centre Oscar Lambret, Lille, France: Claude Adenis, Aurélie Dumont, and Françoise Révillion. Centre Paul Strauss, Strasbourg, France: Danièle Muller. Institut Bergonié, Bordeaux, France: Emmanuelle Barouk-Simonet, Françoise Bonnet, Virginie Bubien, Michel Longy, and Nicolas Sevenet. Institut Claudius Regaud, Toulouse, France: Laurence Gladieff, Rosine Guimbaud, Viviane Feillel, and Christine Toulas. CHU Grenoble, France: Hélène Dreyfus, Christine Dominique Leroux, Magalie Peysselon, and Rebischung. CHU Dijon, France: Amandine Baurand, Geoffrey Bertolone, Fanny Coron, Laurence Faivre, Caroline Jacquot, and Sarab Lizard. CHU St-Etienne, France: Caroline Kientz, Marine Lebrun, and Fabienne Prieur. Hôtel Dieu Centre Hospitalier, Chambéry, France: Sandra Fert Ferrer. Centre Antoine Lacassagne, Nice, France: Véronique Mari. CHU Limoges, France: Laurence Vénat-Bouvet. CHU Nantes, France: Stéphane Bézieau and Capucine Delnatte. CHU Bretonneau, Tours and Centre Hospitalier de Bourges France: Isabelle Mortemousque. Groupe Hospitalier Pitié-Salpétrière, Paris, France: Florence Coulet and Mathilde Warcoin. CHU Vandoeuvre-les-Nancy, France: Myriam Bronner and Johanna Sokolowska. CHU Besançon, France: Marie-Agnès Collonge-Rame, Alexandre Damette. CHU Poitiers, Centre Hospitalier d'Angoulême and Centre Hospitalier de Niort, France: Paul Gesta. Centre Hospitalier de La Rochelle: Hakima Lallaoui. CHU Nîmes Carémeau, France: Jean Chiesa. CHI Poissy, France: Denise Molina-Gomes. CHU Angers, France: Olivier Ingster; CHU de Martinique, France: Odile Bera; Mickaelle Rose; Ilse Coene and Brecht Crombez; Alicia Tosar and Paula Diaque; Drs.Sofia Khan, Taru A. Muranen, Carl Blomqvist, Irja Erkkilä, and Virpi Palola; Hong Kong Sanatorium and Hospital; the Hungarian Breast and Ovarian Cancer Study Group members (Janos Papp, Aniko Bozsik, Timea Pocza, Zoltan Matrai, Miklos Kasler, Judit Franko, Maria Balogh, Gabriella Domokos, and Judit Ferenczi, Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary) and the clinicians and patients for their contributions to this study; the Oncogenetics Group (VHIO) and the High Risk and Cancer Prevention Unit of the University Hospital Vall d'Hebron, Miguel Servet Progam (CP10/00617), and the Cellex Foundation for providing research facilities and equipment; the ICO Hereditary Cancer Program team led by Dr Gabriel Capella; the ICO Hereditary Cancer Program team led by Dr Gabriel Capella; Dr Martine Dumont for sample management and skillful assistance; Ana Peixoto, Catarina Santos, and Pedro Pinto; members of the Center of Molecular Diagnosis, Oncogenetics Department and Molecular Oncology Research Center of Barretos Cancer Hospital; Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow-Up Study (which has received funding from the NHMRC, the National Breast Cancer Foundation, Cancer Australia, and the National Institute of Health [United States]) for their contributions to this resource, and the many families who contribute to kConFab; the KOBRA Study Group; Csilla Szabo (National Human Genome Research Institute, National Institutes of Health, Bethesda, MD); Lenka Foretova and Eva Machackova (Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute and MF MU, Brno, Czech Republic); and Michal Zikan, Petr Pohlreich, and Zdenek Kleibl (Oncogynecologic Center and Department of Biochemistry and Experimental Oncology, First Faculty of Medicine, Charles University, Prague, Czech Republic); Anne Lincoln and Lauren Jacobs; the participants in Hereditary Breast/Ovarian Cancer Study and Breast Imaging Study for their selfless contributions to our research; the NICCC National Familial Cancer Consultation Service team led by Sara Dishon, the laboratory team led by Dr Flavio Lejbkowicz, and the research field operations team led by Dr Mila Pinchev; the investigators of the Australia New Zealand NRG Oncology group; members and participants in the Ontario Cancer Genetics Network; Kevin Sweet, Caroline Craven, Julia Cooper, Amber Aielts, and Michelle O'Conor; Yip Cheng Har, Nur Aishah Mohd Taib, Phuah Sze Yee, Norhashimah Hassan, and all the research nurses, research assistants, and doctors involved in the MyBrCa Study for assistance in patient recruitment, data collection, and sample preparation; Philip Iau, Sng Jen-Hwei, and Sharifah Nor Akmal for contributing samples from the Singapore Breast Cancer Study and the HUKM-HKL Study, respectively; the Meirav Comprehensive breast cancer center team at the Sheba Medical Center; Christina Selkirk; Håkan Olsson, Helena Jernström, Karin Henriksson, Katja Harbst, Maria Soller, and Ulf Kristoffersson; from Gothenburg Sahlgrenska University Hospital: Anna Öfverholm, Margareta Nordling, Per Karlsson, and Zakaria Einbeigi; from Stockholm and Karolinska University Hospital: Anna von Wachenfeldt, Annelie Liljegren, Annika Lindblom, Brita Arver, and Gisela Barbany Bustinza; from Umeå University Hospital: Beatrice Melin, Christina Edwinsdotter Ardnor, and Monica Emanuelsson; from Uppsala University: Hans Ehrencrona, Maritta Hellström Pigg, and Richard Rosenquist; from Linköping University Hospital: Marie Stenmark-Askmalm, Sigrun Liedgren; Cecilia Zvocec, Qun Niu; Joyce Seldon and Lorna Kwan; Dr Robert Nussbaum, Beth Crawford, Kate Loranger, Julie Mak, Nicola Stewart, Robin Lee, Amie Blanco and Peggy Conrad and Salina Chan; Simon Gayther, Paul Pharoah, Carole Pye, Patricia Harrington, and Eva Wozniak; and Geoffrey Lindeman, Marion Harris, Martin Delatycki, Sarah Sawyer, Rebecca Driessen, and Ella Thompson for performing all DNA amplification.
Timothy R. Rebbeck
Honoraria: AstraZeneca (I)
Consulting or Advisory Role: AstraZeneca (I)
Andrew Lee
Employment: Illumina
Patents, Royalties, Other Intellectual Property: I am an inventor of the BOADICEA model that is licensed to Cambridge Enterprise (part of the University of Cambridge) for commercialization. I have received royalties from Cambridge Enterprise.
Norbert Arnold
Honoraria: AstraZeneca
Åke Borg
Honoraria: Roche, AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca
Sandrine Caputo
Research Funding: AstraZeneca/France (Inst)
Wendy K. Chung
Consulting or Advisory Role: Regeneron
Research Funding: Biogen
Chrystelle Colas
Consulting or Advisory Role: AstraZeneca/Merck, Pfizer
Miguel de la Hoya
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca
D. Gareth Evans
Honoraria: AstraZeneca
Tanja N. Fehm
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Daiichi Sankyo, MSD Oncology
Travel, Accommodations, Expenses: Roche
George Fountzilas
Stock and Other Ownership Interests: GENPREX Inc (I), ARIAD (I), Deciphera Pharmaceuticals Inc (I), Daiichi Sankyo, RFL Holdings, FORMYCON
Honoraria: AstraZeneca
Consulting or Advisory Role: Pfizer, Roche, Novartis
Speakers' Bureau: Roche (I), LEO Pharma (I), Pfizer (I)
Travel, Accommodations, Expenses: Merck (I), Pfizer (I), K.A.M Oncology/Hematology (I)
Megan Frone
Patents, Royalties, Other Intellectual Property: Receive royalties for being a codeveloper of CancerGene Connect
Eric Hahnen
Consulting or Advisory Role: AstraZeneca
Helen Hanson
Honoraria: Pfizer
Beth Y. Karlan
Consulting or Advisory Role: Roche Pharma AG, Merck, Mercy Bioanalytics, GRAIL
Research Funding: GOG Foundation (Inst), NCI-NRG Oncology (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: US and EU patent on gene signature
Other Relationship: Elsevier
Irene Konstantopoulou
Speakers' Bureau: AstraZeneca
Research Funding: AstraZeneca
Ava Kwong
Honoraria: Stryker, AstraZeneca Taiwan Limited, Roche Singapore, Pfizer, AstraZeneca Hong Kong Ltd
Joanne Ngeow Yuen Yie
Research Funding: AstraZeneca
Rita K. Schmutzler
Honoraria: AstraZeneca, Clovis, MSD/AstraZeneca
Consulting or Advisory Role: AstraZeneca, MSD/AstraZeneca
Research Funding: Amgen
Leigha Senter
Consulting or Advisory Role: AstraZeneca/Merck
Speakers' Bureau: AstraZeneca/Merck
Christian F. Singer
Honoraria: Novartis, AstraZeneca/MedImmune, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: AstraZeneca/MedImmune, Daiichi-Sankyo, Gilead Sciences, Sanofi/Aventis, Novartis
Speakers' Bureau: Novartis, AstraZeneca/MedImmune
Research Funding: Novartis, Sanofi, Myriad Genetics, Roche, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Novartis
Dominique Stoppa-Lyonnet
Honoraria: AstraZeneca/Merck (Inst)
Soo Hwang Teo
Speakers' Bureau: AstraZeneca, Pfizer, Roche
Sebastian A. Wagner
Consulting or Advisory Role: Bayer
Antonis C. Antoniou
Patents, Royalties, Other Intellectual Property: Inventor of the BOADICEA model, which has been licensed to Cambridge Enterprise for commercialization. May receive royalties if commercialization is realized.
No other potential conflicts of interest were reported.
See accompanying article on page 1590
DISCLAIMER
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the BCFR.
PRIOR PRESENTATION
Presented at the Familial Aspects of Cancers: Research and Practice conference, Kingscliff, New South Wales, Australia, September 1, 2021.
SUPPORT
Supported by grants from Cancer Research UK (C12292/A20861, C12292/A11174, and PPRPGM-Nov20\100002), the Gray Foundation; by grants from Fondazione AIRC (Associazione Italiana Ricerca sul Cancro) under IG 2018 ID. 21389, LILT (Lega Italiana per la Lotta Contro i Tumori) under IG 2019, P.I. Ottini Laura, and Italian Ministry of Education, Universities and Research–Dipartimenti di Eccellenza-L. 232/2016; and by the Victorian Cancer Agency Early Career Research Fellowship (ECRF19020) and the Picchi Award for Excellence in Cancer Research from the Victorian Comprehensive Cancer Centre. G.C.-T. is a National Health and Medical Research Council (NHMRC) Research Fellow. A.B.S. and M.T.P. were supported by NHMRC Funding (APP177524). T.R.R. was supported by NIH R01CA207365. M. Tischkowitz was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). BCFR: UM1 CA164920 from the National Cancer Institute. BFBOCC: Lithuania (BFBOCC-LT): Research Council of Lithuania grant SEN-18/2015. DEMOKRITOS: European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program of the General Secretariat for Research and Technology: SYN11_10_19 NBCA. Investing in knowledge society through the European Social Fund. DFKZ: German Cancer Research Center. EMBRACE: Cancer Research UK Grants C1287/A23382 and C1287/A26886. D.G.E. was supported by the Manchester NIHR Biomedical Research Centre (IS-BRC-1215-20007). Fiona Lalloo was supported by an NIHR grant to the Biomedical Research Centre, Manchester. The Investigators at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust were supported by an NIHR grant to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Ros Eeles and Elizabeth Bancroft were supported by Cancer Research UK Grant No. C5047/A8385. Ros Eeles was also supported by NIHR support to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. FPGMX: A.V. was supported by the Spanish Health Research Foundation, Instituto de Salud Carlos III (ISCIII), partially supported by FEDER funds through Research Activity Intensification Program (contract Grant Nos.: INT15/00070, INT16/00154, INT17/00133, INT20/00071) and through Centro de Investigación Biomédica en Red de Enferemdades Raras CIBERER (ACCI 2016: ER17P1AC7112/2018), Autonomous Government of Galicia (Consolidation and structuring program: IN607B), and by the Fundación Mutua Madrileña. GC-HBOC: German Cancer Aid (Grant Nos. 110837 and 113049, R.K.S.). GEMO: Ligue Nationale Contre le Cancer; the Association “Le cancer du sein, parlons-en!” Award, the Canadian Institutes of Health Research for the “CIHR Team in Familial Risks of Breast Cancer” program, the Fondation ARC pour la recherche sur le cancer (Grant No. PJA 20151203365) and the French National Institute of Cancer (INCa grants AOR 01 082, 2001-2003, 2013-1-BCB-01-ICH-1, and SHS-E-SP 18-015). HCSC: Spanish Ministry of Health PI15/00059, PI16/01292, and CB-161200301 CIBERONC from ISCIII (Spain), partially supported by European Regional Development FEDER funds. M.d.l.H. was supported by a grant from the Spanish Ministry of Science and Innovation, Plan Nacional de I + D + I 2013-2016, ISCIII (PI20/00110), and FEDER from Regional Development European Funds (European Union). HRBCP: Hong Kong Sanatorium and Hospital, Dr Ellen Li Charitable Foundation, The Kerry Group Kuok Foundation, National Institute of Health1R 03CA130065, and North California Cancer Center. kConFab: The National Breast Cancer Foundation, and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania, and South Australia, and the Cancer Foundation of Western Australia. KOHBRA: the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HI16C1127; 1020350; 1420190). NCI: the Intramural Research Program of the US National Cancer Institute, NIH, and by support services contract Nos. NO2-CP-11019-50, N02-CP-21013-63, and N02-CP-65504 with Westat Inc, Rockville, MD. OSUCCG: Ohio State University Comprehensive Cancer Center. SEABASS: Ministry of Science, Technology and Innovation, Ministry of Higher Education (UM.C/HlR/MOHE/06) and Cancer Research Initiatives Foundation. SWE-BRCA: the Swedish Cancer Society. UKFOCR: Cancer Research UK. WCP: B.Y.K. was funded by the American Cancer Society Early Detection Professorship (SIOP-06-258-01-COUN) and the National Center for Advancing Translational Sciences (NCATS), Grant No. UL1TR000124.
S.L. and V.S. contributed equally to this work as joint first authors. L.O. and A.C.A. contributed equally to this work as joint senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Shuai Li, Valentina Silvestri, Timothy R. Rebbeck, John L. Hopper, Bruno Buecher, D. Gareth Evans, Melissa C. Southey, Shan Wang-Gohrke, Douglas F. Easton, Georgia Chenevix-Trench, Laura Ottini, Antonis C. Antoniou
Financial support: Shuai Li, Rita K. Schmutzler, Melissa C. Southey, Shan Wang-Gohrke, Drakoulis Yannoukakos, Laura Ottini, Antonis C. Antoniou
Administrative support: John L. Hopper, Shan Wang-Gohrke, Drakoulis Yannoukakos
Provision of study materials or patients: Susan L. Neuhausen, Norbert Arnold, Muriel Belotti, Åke Borg, Bruno Buecher, Saundra S. Buys, Wendy K. Chung, Jackie Cook, Mary B. Daly, Miguel de la Hoya, Christoph Engel, D. Gareth Evans, Ulrike Faust, Tanja N. Fehm, Florentia Fostira, George Fountzilas, Megan Frone, Pilar Garre, Andrea Gehrig, Gord Glendon, David E. Goldgar, Mark H. Greene, Eric Hahnen, Ute Hamann, Louise Izatt, Esther M. John, Beth Y. Karlan, Irene Konstantopoulou, Ava Kwong, Fabienne Lesueur, Noura Mebirouk, Alfons Meindl, Hannah Musgrave, Joanne Ngeow Yuen Yie, Dieter Niederacher, Susan J. Ramus, Muhammad U. Rashid, Andreas Rump, Marta Santamariña, Rita K. Schmutzler, Leigha Senter, Saba Shariff, Christian F. Singer, Melissa C. Southey, Dominique Stoppa-Lyonnet, Christian Sutter, Soo Hwang Teo, Mary Beth Terry, Mads Thomassen, Marc Tischkowitz, Amanda E. Toland, Ana Vega, Shan Wang-Gohrke, Drakoulis Yannoukakos, Georgia Chenevix-Trench, Antonis C. Antoniou
Collection and assembly of data: Goska Leslie, Timothy R. Rebbeck, Susan L. Neuhausen, John L. Hopper, Henriette Roed Nielsen, Lesley McGuffog, Michael T. Parsons, Irene L. Andrulis, Norbert Arnold, Muriel Belotti, Åke Borg, Bruno Buecher, Saundra S. Buys, Sandrine Caputo, Wendy K. Chung, Chrystelle Colas, Sarah V. Colonna, Jackie Cook, Mary B. Daly, Antoine de Pauw, Hélène Delhomelle, Jacqueline Eason, Christoph Engel, D. Gareth Evans, Ulrike Faust, Tanja N. Fehm, Florentia Fostira, George Fountzilas, Megan Frone, Vanesa Garcia-Barberan, Pilar Garre, Marion Gauthier-Villars, Andrea Gehrig, Gord Glendon, David E. Goldgar, Lisa Golmard, Mark H. Greene, Eric Hahnen, Tiara Hassan, Julia Hentschel, Judit Horvath., Louise Izatt, Ramunas Janavicius, Yue Jiao, Esther M. John, Beth Y. Karlan, Sung-Won Kim, Irene Konstantopoulou, Ava Kwong, Anthony Laugé, Jong Won Lee, Fabienne Lesueur, Noura Mebirouk, Alfons Meindl, Emmanuelle Mouret-Fourme, Hannah Musgrave, Joanne Ngeow Yuen Yie, Dieter Niederacher, Sue K. Park, Inge Sokilde Pedersen, Juliane Ramser, Susan J. Ramus, Johanna Rantala, Muhammad U. Rashid, Florian Reichl, Julia Ritter, Marta Santamariña, Gunnar Schmidt, Rita K. Schmutzler, Leigha Senter, Saba Shariff, Christian F. Singer, Melissa C. Southey, Dominique Stoppa-Lyonnet, Christian Sutter, Yen Yen Tan, Soo Hwang Teo, Mary Beth Terry, Mads Thomassen, Marc Tischkowitz, Amanda E. Toland, Ana Vega, Sebastian A. Wagner, Shan Wang-Gohrke, Barbara Wappenschmidt, Bernhard H. F. Weber, Drakoulis Yannoukakos, Amanda B. Spurdle, Douglas F. Easton, Georgia Chenevix-Trench, Antonis C. Antoniou
Data analysis and interpretation: Shuai Li, Valentina Silvestri, John L. Hopper, Andrew Lee, Xin Yang, Bruno Buecher, Mary B. Daly, Miguel de la Hoya, D. Gareth Evans, Florentia Fostira, Lisa Golmard, Mark H. Greene, Ute Hamann, Helen Hanson, Alfons Meindl, Sue K. Park, Andreas Rump, Claire Saule, Christian F. Singer, Melissa C. Southey, Mary Beth Terry, Drakoulis Yannoukakos, Douglas F. Easton, Laura Ottini, Antonis C. Antoniou
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Timothy R. Rebbeck
Honoraria: AstraZeneca (I)
Consulting or Advisory Role: AstraZeneca (I)
Andrew Lee
Employment: Illumina
Patents, Royalties, Other Intellectual Property: I am an inventor of the BOADICEA model that is licensed to Cambridge Enterprise (part of the University of Cambridge) for commercialization. I have received royalties from Cambridge Enterprise.
Norbert Arnold
Honoraria: AstraZeneca
Åke Borg
Honoraria: Roche, AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca
Sandrine Caputo
Research Funding: AstraZeneca/France (Inst)
Wendy K. Chung
Consulting or Advisory Role: Regeneron
Research Funding: Biogen
Chrystelle Colas
Consulting or Advisory Role: AstraZeneca/Merck, Pfizer
Miguel de la Hoya
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca
D. Gareth Evans
Honoraria: AstraZeneca
Tanja N. Fehm
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Daiichi Sankyo, MSD Oncology
Travel, Accommodations, Expenses: Roche
George Fountzilas
Stock and Other Ownership Interests: GENPREX Inc (I), ARIAD (I), Deciphera Pharmaceuticals Inc (I), Daiichi Sankyo, RFL Holdings, FORMYCON
Honoraria: AstraZeneca
Consulting or Advisory Role: Pfizer, Roche, Novartis
Speakers' Bureau: Roche (I), LEO Pharma (I), Pfizer (I)
Travel, Accommodations, Expenses: Merck (I), Pfizer (I), K.A.M Oncology/Hematology (I)
Megan Frone
Patents, Royalties, Other Intellectual Property: Receive royalties for being a codeveloper of CancerGene Connect
Eric Hahnen
Consulting or Advisory Role: AstraZeneca
Helen Hanson
Honoraria: Pfizer
Beth Y. Karlan
Consulting or Advisory Role: Roche Pharma AG, Merck, Mercy Bioanalytics, GRAIL
Research Funding: GOG Foundation (Inst), NCI-NRG Oncology (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: US and EU patent on gene signature
Other Relationship: Elsevier
Irene Konstantopoulou
Speakers' Bureau: AstraZeneca
Research Funding: AstraZeneca
Ava Kwong
Honoraria: Stryker, AstraZeneca Taiwan Limited, Roche Singapore, Pfizer, AstraZeneca Hong Kong Ltd
Joanne Ngeow Yuen Yie
Research Funding: AstraZeneca
Rita K. Schmutzler
Honoraria: AstraZeneca, Clovis, MSD/AstraZeneca
Consulting or Advisory Role: AstraZeneca, MSD/AstraZeneca
Research Funding: Amgen
Leigha Senter
Consulting or Advisory Role: AstraZeneca/Merck
Speakers' Bureau: AstraZeneca/Merck
Christian F. Singer
Honoraria: Novartis, AstraZeneca/MedImmune, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: AstraZeneca/MedImmune, Daiichi-Sankyo, Gilead Sciences, Sanofi/Aventis, Novartis
Speakers' Bureau: Novartis, AstraZeneca/MedImmune
Research Funding: Novartis, Sanofi, Myriad Genetics, Roche, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Novartis
Dominique Stoppa-Lyonnet
Honoraria: AstraZeneca/Merck (Inst)
Soo Hwang Teo
Speakers' Bureau: AstraZeneca, Pfizer, Roche
Sebastian A. Wagner
Consulting or Advisory Role: Bayer
Antonis C. Antoniou
Patents, Royalties, Other Intellectual Property: Inventor of the BOADICEA model, which has been licensed to Cambridge Enterprise for commercialization. May receive royalties if commercialization is realized.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. : Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317:2402-2416, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Linkage Consortium : Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91:1310-1316, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Brose MS, Rebbeck TR, Calzone KA, et al. : Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94:1365-1372, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Easton DF: Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94:1358-1365, 2002 [DOI] [PubMed] [Google Scholar]
- 5.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. : Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J Med Genet 42:711-719, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risch HA, McLaughlin JR, Cole DE, et al. : Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98:1694-1706, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Ferrone CR, Levine DA, Tang LH, et al. : BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol 27:433-438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran A, O'Hara C, Khan S, et al. : Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer 11:235-242, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Tai YC, Domchek S, Parmigiani G, et al. : Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 99:1811-1814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easton DF, Steele L, Fields P, et al. : Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Hum Genet 61:120-128, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson D, Easton D: Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 68:410-419, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milne RL, Osorio A, Cajal TR, et al. : The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res 14:2861-2869, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Evans DG, Susnerwala I, Dawson J, et al. : Risk of breast cancer in male BRCA2 carriers. J Med Genet 47:710-711, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff T, Kauff ND, Mitra N, et al. : BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin Cancer Res 10:2918-2921, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gallagher DJ, Gaudet MM, Pal P, et al. : Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res 16:2115-2121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roed Nielsen H, Petersen J, Therkildsen C, et al. : Increased risk of male cancer and identification of a potential prostate cancer cluster region in BRCA2. Acta Oncol 55:38-44, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Oh M, Alkhushaym N, Fallatah S, et al. : The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 79:880-895, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Ford D, Easton DF, Bishop DT, et al. : Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 343:692-695, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Phelan CM, Iqbal J, Lynch HT, et al. : Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: Results from a follow-up study. Br J Cancer 110:530-534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struewing JP, Hartge P, Wacholder S, et al. : The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401-1408, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Daly MB, Pal T, Berry MP, et al. : Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 19:77-102, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Paluch-Shimon S, Cardoso F, Sessa C, et al. : Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol 27:v103-v110, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Chenevix-Trench G, Milne RL, Antoniou AC, et al. : An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: The Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res 9:104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium of Investigators of Modifiers of BRCA1/2: Eligibility. http://cimba.ccge.medschl.cam.ac.uk/eligibility/
- 25.Antoniou AC, Casadei S, Heikkinen T, et al. : Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371:497-506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer: Cancer incidence in five continents. http://ci5.iarc.fr [Google Scholar]
- 27.Lange K, Weeks D, Boehnke M: Programs for pedigree analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol 5:471-472, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Antoniou AC, Cunningham AP, Peto J, et al. : The BOADICEA model of genetic susceptibility to breast and ovarian cancers: Updates and extensions. Br J Cancer 98:1457-1466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannings C, Thompson EA: Ascertainment in the sequential sampling of pedigrees. Clin Genet 12:208-212, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Ewens WJ, Shute NC: A resolution of the ascertainment sampling problem. I. Theory. Theor Popul Biol 30:388-412, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Shute NC, Ewens WJ: A resolution of the ascertainment sampling problem. III. Pedigrees. Am J Hum Genet 43:387-395, 1988 [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg T, Frost D, Barrowdale D, et al. : Prostate cancer risks for male BRCA1 and BRCA2 mutation carriers: A prospective cohort study. Eur Urol 77:24-35, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agalliu I, Gern R, Leanza S, et al. : Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res 15:1112-1120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douglas FS, O'Dair LC, Robinson M, et al. : The accuracy of diagnoses as reported in families with cancer: A retrospective study. J Med Genet 36:309-312, 1999 [PMC free article] [PubMed] [Google Scholar]
- 35.Rauscher EA, Dean M, Campbell-Salome GM: “I am uncertain about what my uncertainty even is”: Men's uncertainty and information management of their BRCA-related cancer risks. J Genet Couns 27:1417-1427, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Silvestri V, Leslie G, Barnes DR, et al. : Characterization of the cancer spectrum in men with germline BRCA1 and BRCA2 pathogenic variants: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). JAMA Oncol 6:1-13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tutt ANJ, Garber JE, Kaufman B, et al. : Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 384:2394-2405, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mateo J, Lord CJ, Serra V, et al. : A decade of clinical development of PARP inhibitors in perspective. Ann Oncol 30:1437-1447, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadler ZK, Maio A, Chakravarty D, et al. : Therapeutic implications of germline testing in patients with advanced cancers. J Clin Oncol 39:2698-2709, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zheng K, Huang Y, et al. : PARP inhibitors in gastric cancer: Beacon of hope. J Exp Clin Cancer Res 40:211, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerber RA, Slattery ML: Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol 146:244-248, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Ziogas A, Anton-Culver H: Validation of family history data in cancer family registries. Am J Prev Med 24:190-198, 2003 [DOI] [PubMed] [Google Scholar]