PURPOSE
Chemotherapy outcomes in older patients with Philadelphia (Ph) chromosome–negative B-acute lymphoblastic leukemia (ALL) are very poor. Here, we evaluated blinatumomab as induction and consolidation therapy followed by prednisone, vincristine, 6-mercaptopurine, and methotrexate (POMP) maintenance chemotherapy in this patient population.
PATIENTS AND METHODS
Patients were treated at National Clinical Trial Network sites. Eligibility criteria included age ≥ 65 years and newly diagnosed Ph chromosome–negative B-ALL. Patients received blinatumomab as induction for one-two cycles until attainment of response (complete remission (CR) and CR with incomplete count recovery). Patients then received three cycles of consolidation with blinatumomab followed by 18 months of POMP maintenance chemotherapy. Eight doses of intrathecal methotrexate were administered as central nervous system prophylaxis.
RESULTS
Twenty-nine eligible patients were enrolled. The median age was 75 years, and the median bone marrow blast count at diagnosis was 87%. Cytogenetic risk was poor in 10 patients (34%), and five of 14 patients (36%) tested had the Ph-like ALL gene signature. Nineteen patients (66%; 95% CI, 46 to 82) achieved CR. Kaplan-Meier 3-year disease-free survival and overall survival estimates were 37% (95% CI, 17 to 57) and 37% (95% CI, 20 to 55), respectively.
CONCLUSION
Blinatumomab was well tolerated and effective in the treatment of older patients with newly diagnosed Ph chromosome–negative B-ALL, including patients with poor-risk cytogenetics. The 3-year disease-free survival and overall survival results are encouraging and suggest that this approach should be further explored.
INTRODUCTION
The prognosis of older patients with acute lymphoblastic leukemia (ALL) remains poor, and novel therapeutic approaches are clearly needed. Intensive combination chemotherapy has been the standard of care for adults with ALL. However, older adults with ALL (defined as age ≥ 60 years) have been difficult to treat with standard chemotherapy, and their 5-year survival rate is estimated to be 6%-20% with high rates of induction and treatment-related mortality.1-3 During the past decade, multiple clinical trials have attempted to improve outcomes with early dose intensification of myelosuppressive agents, but none have resulted in improved overall survival (OS) in the older patient cohorts.4-10 Thus, the incorporation of novel agents is a priority. CD19 is expressed on the majority of precursor B-ALLs and represents an attractive therapeutic target. The anti-CD19 bispecific T-cell engager antibody construct blinatumomab has demonstrated significant activity in both relapsed/refractory ALL and minimal residual disease (MRD)–positive B-ALL.11,12 Therefore, we evaluated blinatumomab as a single agent in the up-front treatment of patients with newly diagnosed Philadelphia chromosome (Ph)–negative B-ALL. This trial design was novel in which blinatumomab was used as monotherapy during induction and consolidation in the frontline setting. The response rate was assessed, but 3-year OS was the primary end point.
CONTEXT
Key Objective
To test the anti-CD19 bispecific T-cell engager antibody blinatumomab as induction and consolidation therapy followed by prednisone, vincristine, 6-mercaptopurine, and methotrexate maintenance chemotherapy in older patients with newly diagnosed Philadelphia chromosome–negative B-acute lymphoblastic leukemia (ALL).
Knowledge Generated
Blinatumomab is well tolerated as induction and consolidation therapy in this patient population with encouraging 3-year disease-free and overall survival.
Relevance
Blinatumomab represents a promising therapy for older patients with newly diagnosed Philadelphia chromosome–negative B-ALL.
PATIENTS AND METHODS
Patients were treated at National Clinical Trial Network sites from June 2015 to September 2017. The primary objective was to estimate 3-year OS. An investigational new drug application was approved by the US Food and Drug Administration (FDA), and the Protocol (online only) was approved by a central institutional review board. All patients provided informed consent. Eligibility criteria included newly diagnosed Ph chromosome–negative B-ALL with no evidence of central nervous system disease and no history or presence of clinically relevant central nervous system pathology, age ≥ 65 years, and adequate organ function (creatinine ≤ 1.5 mg/dL, AST and ALT ≤ 3.0 × institutional upper limits of normal [IULN], total bilirubin ≤ 2 × IULN, and alkaline phosphatase ≤ 2.5 × IULN). Cytogenetic and molecular testing were performed, and cytogenetic risk was described by National Comprehensive Cancer Network criteria.13 Bone marrow samples were analyzed centrally at the University of New Mexico for the Ph-like signature using a 15-gene signature low-density array (LDA card).14 An absolute peripheral blast count of < 25 K/µL at the time of the first infusion of blinatumomab was required. Prephase dexamethasone (10-20 mg/m2 once daily) for 3-5 days was given to patients with bone marrow blasts ≥ 50%, peripheral blood blasts 15 K/µL or higher, or elevated LDH.
Treatment
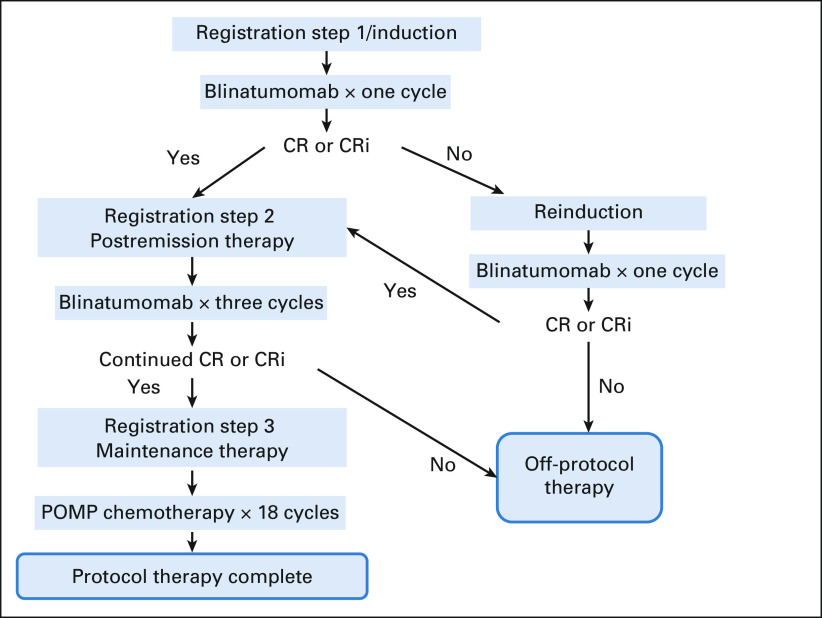
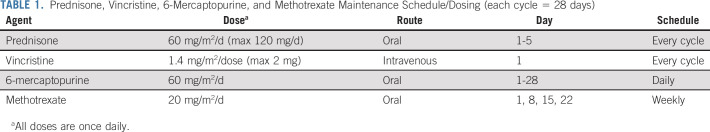
Patients received blinatumomab for induction for one and two cycles until attainment of complete remission (CR) or CR with incomplete count recovery (CRi). Patients then received three cycles of blinatumomab postremission therapy followed by 18 months of maintenance prednisone, vincristine, 6-mercaptopurine, and methotrexate (POMP; Fig 1 and Table 1). During induction, blinatumomab was administered at doses of 9 mcg once daily on days 1-7 and 28 mcg once daily on days 8-28. For reinduction and postremission therapy, blinatumomab was administered at a dose of 28 mcg once daily on days 1-28. Patients received dexamethasone once before each cycle, 20 mg intravenous 1 hour before the start of blinatumomab for each cycle and before day 8 of cycle 1 when the blinatumomab dose was escalated. Doses of intrathecal methotrexate (12 mg) were administered as central nervous system prophylaxis every 4-6 weeks for a total of eight doses. Intrathecal methotrexate was given a minimum of 2 days apart from blinatumomab administration. Dose modifications are outlined in the full Protocol. MRD was assessed centrally by eight-color flow cytometry during pretreatment at the University of Washington,15 on day 35 of induction cycle 1 and on day 35 of reinduction. MRD negativity was defined as < 0.01% on the basis of the sensitivity of this method.
FIG 1.
Treatment schema. Eight doses of IT methotrexate were given as CNS prophylaxis. CR, complete remission; CRi, CR with incomplete count recovery; IT, intrathecal; POMP, prednisone, vincristine, 6-mercaptopurine, and methotrexate.
TABLE 1.
Prednisone, Vincristine, 6-Mercaptopurine, and Methotrexate Maintenance Schedule/Dosing (each cycle = 28 days)
Response and Outcomes
Response was assessed at the completion of one and two cycles of blinatumomab. CR was defined as < 5% marrow blasts with no evidence of extramedullary disease and recovery of counts (absolute neutrophil count > 1,000/µL and platelet count > 100,000/µL). CRi is the same as CR, but the absolute neutrophil count is ≤ 1,000/µL and/or platelets ≤ 100,000/µL. Relapse was defined as (1) appearance of leukemic blasts in the peripheral blood, (2) appearance of extramedullary disease, or (3) ≥ 5% blasts in the bone marrow not attributable to another cause (ie, recovery of normal cells after chemotherapy-induced aplasia). OS was measured from the day of registration on trial until the date of death, with patients last known to be alive censored at their date of last contact. The primary analysis analyzed 3-year OS as a binary end point (yes if the patient was alive at 3 years after registration and no if the patient died before 3 years). Disease-free survival (DFS) was measured from the date of CR/CRi until relapse or death, with patients last known to be alive and relapse-free censored at their date of last contact. Toxicities were graded according to NCI CTCAE version 4.0.
Statistics
Primary objective.
Historical estimates of OS in this population provide 3-year OS ranging from 6%-20%.2,4 On the basis of the historical data, the current regimen would be of no further interest if it yielded a true 3-year OS rate of 10% or less (null hypothesis).
Analyses and power justifications.
Up to 26 eligible patients were to be accrued. An interim analysis for futility was performed among the first 11 eligible patients. If fewer than five CRs or CRis were observed, then the trial would be close to accrual. Accrual continued while the CR data were being reviewed. The final analysis tested whether the binary observed 3-year OS was improved over the historical rate of 10% with a one-sided binominal test at the 0.04 level. In the event of censoring, 3-year OS was estimated using the method of Kaplan-Meier to construct a one-sided 90% CI using the log-log transformation. Accounting for the interim analysis, this design has a critical level (probability of erroneously concluding the regimen warrants further study) of 0.03 if the true response rate is 50% and the binary 3-year OS is 10%. This design has a power (probability of correctly concluding the regimen warrants further study) of 0.89 if the true response rate is 70% and the true binary 3-year OS is 33%.
RESULTS
Patient Characteristics
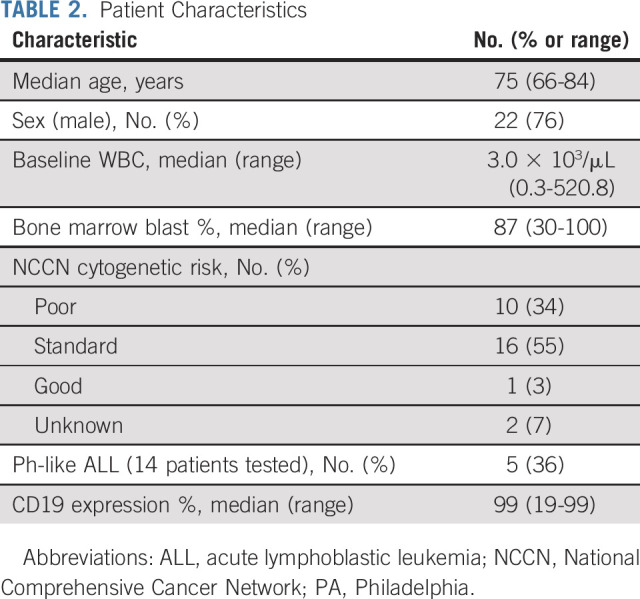
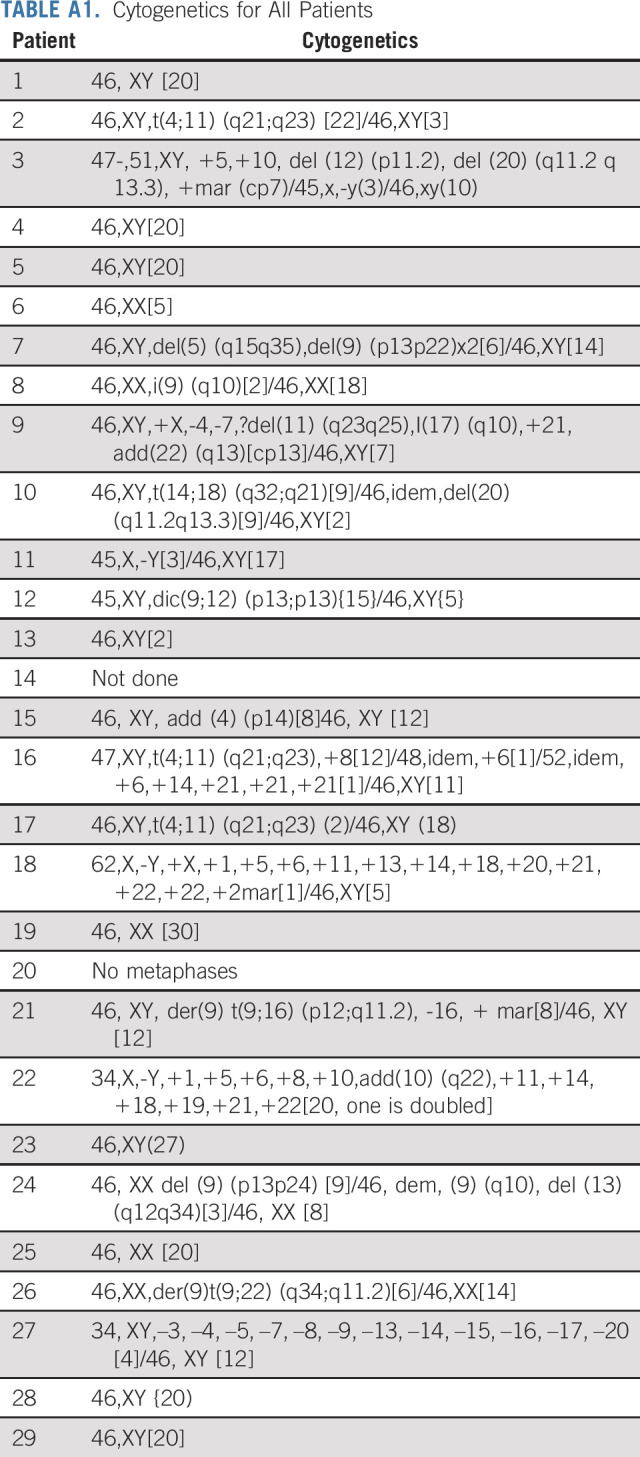
Of 31 patients enrolled, 29 were eligible. The median age was 75 (range 66-84) years; 22 (76%) were male; the median baseline white blood cell count was 3.0 × 103/µL (range 0.3-520.8) at registration; the median bone marrow blast count percentage was 87% (range 30-100; Table 2). Three patients received hydroxyurea or steroids before treatment initiation as cytoreduction (one patient received steroids alone, one patient received steroids and hydroxyurea to reduce the peripheral blast count before receiving blinatumomab, and one patient received vincristine and steroids to reduce the blast count before receiving blinatumomab). Cytogenetic risk at diagnosis was poor (34% of patients, n = 10), standard (55% of patients; n = 16), good (3% of patients; n = 1), and unknown (7% of patients, n = 2). Appendix Table A1 (online only) lists the full cytogenetic details for all patients. Fourteen patients had testing sent for the Ph-like signature, and five (36%) were positive. Three of these five patients (60%) had high CRLF2 expression; a fourth patient had a low-level P2RY8-CRLF2 fusion; a fifth patient was low positive by LDA analysis but had the mixed lineage leukemia (MLL) signature. The LDA assay that detects expression profiles associated with other recurring translocations identified three of 14 evaluable patients (21%) with a MLL signature.
TABLE 2.
Patient Characteristics
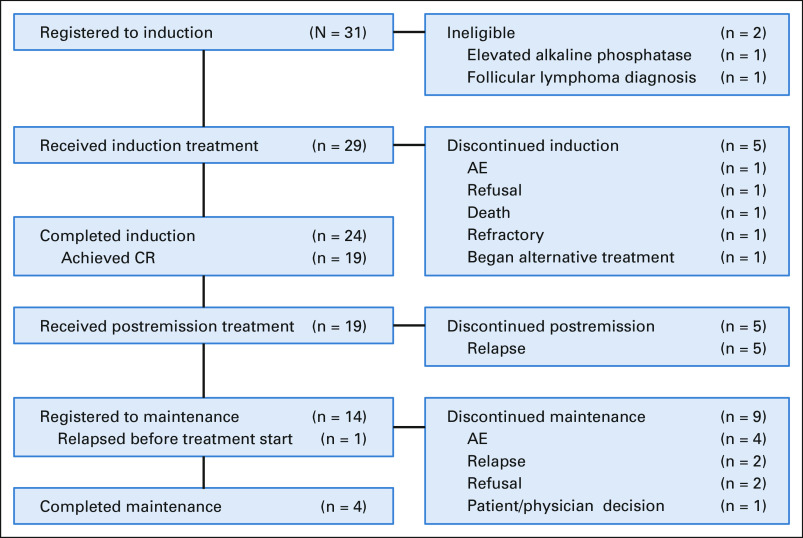
Treatment received: A flow diagram is provided (Fig 2). Patients received a median of four cycles of blinatumomab (range 2-5). Patients received a median of four cycles of POMP maintenance therapy (range 1-18 cycles) with three patients completing POMP maintenance.
FIG 2.
Flow diagram. AE, adverse event; CR, complete remission.
Toxicities
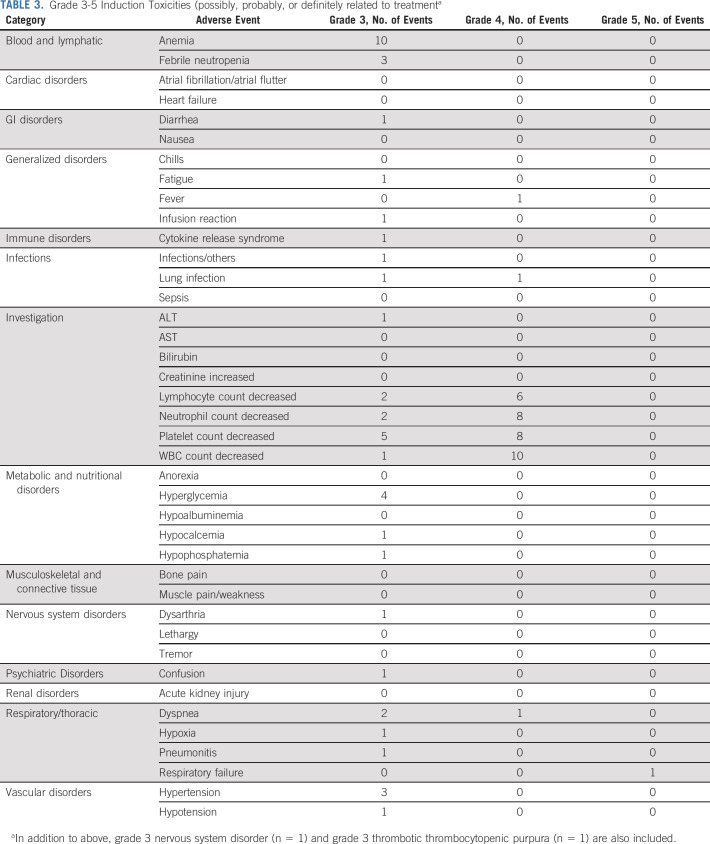
Grade 3-5 nonhematologic toxicities during induction related to treatment are listed in Table 3. The most common grade 3 and 4 toxicities were hyperglycemia (4 of 29; 14%), dyspnea (3 of 29; 10%), febrile neutropenia (3 of 29; 10%), hypertension (3 of 29; 10%), and lung infection (2 of 29; 7%). One patient developed grade 3 cytokine release syndrome, and another developed grade 3 neurotoxicity. Both were considered by the site to be probably related to treatment. No patients died during the first 28 days of treatment. One patient died 34 days after beginning induction therapy. This patient died of respiratory failure, with B-cell ALL contributory and joint infection possibly contributory.
TABLE 3.
Grade 3-5 Induction Toxicities (possibly, probably, or definitely related to treatmenta
Response/Outcomes
The overall response rate (CR + CRi) was 66% (exact binomial 95% CI, 46 to 82), all CRs. CR rates were not significantly different between poor-risk cytogenetics (7 of 10; 70%) versus other risk cytogenetics (standard and good risk combined; 10 of 17; 59%; Fisher's exact P = .69). Of the five patients with Ph-like ALL, three patients (60%) achieved CR and MRD negativity (by multicolor flow cytometry). Thirteen of the 19 responders have available MRD data post-treatment with 12 patients (92%) achieving MRD negativity (by multicolor flow cytometry). One patient required two cycles of blinatumomab to achieve CR. One patient proceeded to allogeneic hematopoietic stem-cell transplant. The median follow-up time was 3.14 years, and the median duration on trial was 170 days. The Kaplan-Meier 3-year OS estimate was 37% (lower one-sided 90% CI, 26; Fig 3). The median DFS and OS in the five Ph-like patients were 1.27 years and 2.61 years, respectively (Appendix Figures A1A and A1B, online only). This was not significantly different from the nine patients who tested negative for the Ph-like signature (1.56 years and 1.57 years, respectively). The median DFS for poor risk versus other cytogenetic risk groups was 0.69 years and 2.38 years, respectively; log-rank P = .24 (Appendix Figure A2A, online only). The median OS for poor risk versus other cytogenetic risk groups was 1.33 years and 2.48 years, respectively; log-rank P = .19 (Appendix Figure A2B). By age, the median DFS for patients age < 75 years versus ≥ 75 years was 2.51 years (95% CI, 0.16 to not reached) versus 1.27 years (95% CI, 0.06 to 3.27), respectively. The median OS for patients age < 75 years versus ≥ 75 years was 2.61 years (95% CI, 0.79 to not reached) and 1.94 years (95% CI, 0.28 to 3.63), respectively. Ten of 29 patients were alive at 3 years; two withdrew consent for follow-up before 3 years. Considering these two patients as failures, the binary 3-year OS is 34% (95% CI, 18 to 54), significantly higher than the historical rate of 10% (one-sided P < .001). The Kaplan-Meier 3-year DFS estimate was 37% (95% CI, 17 to 57; Fig 3). All patients in CR/CRi who died did so after progression. No baseline factors including bone marrow blast percentage, peripheral blast percentage, CD19 expression (by percentage or mean fluorescence intensity), or presence of a CD19-negative subpopulation were associated with response. However, the sample size was small, and there was probably not sufficient power to detect an association unless it was dramatic.
FIG 3.
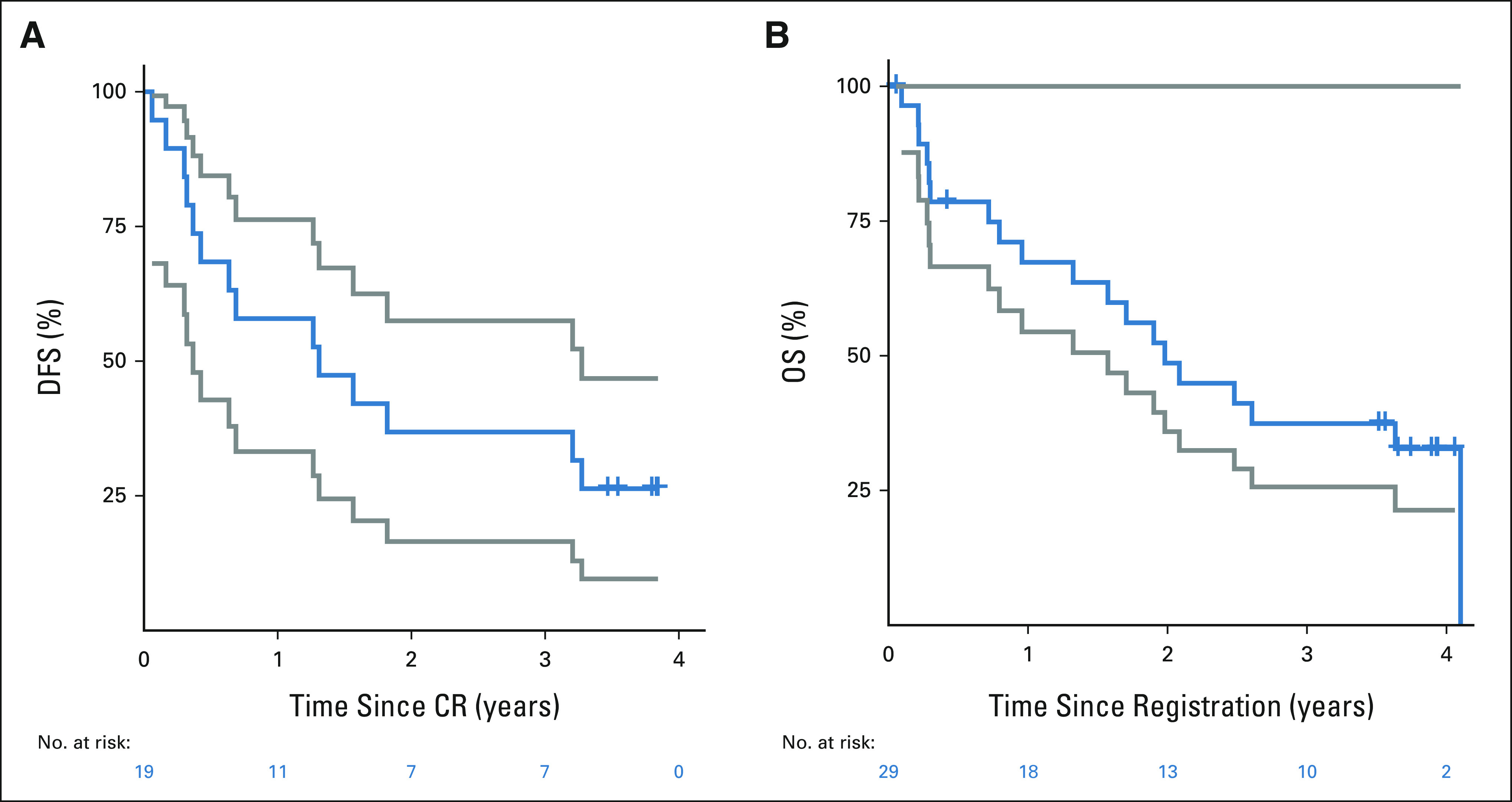
(A) Kaplan-Meier DFS (blue) with two-sided 95% CI (gray), and (B) Kaplan-Meier OS (blue) with one-sided 90% CI (gray). CR, complete remission; DFS, disease-free survival; OS, overall survival.
At the time of relapse, of the 13 patients with available data, two patients developed extramedullary disease and one patient had a switch to myeloid lineage. Seven of the patients had CD19-negative lymphoblasts at the time of relapse, and one patient had CD19 dim lymphoblasts.
DISCUSSION
Historically, the outcomes of older adults with ALL have been poor. Unfortunately, these outcomes have not improved over time, even with dose intensification. In consecutive CALGB trials, the OS at 3 years in patients age ≥ 60 years was 12%, as compared with 57% in patients age < 30 years and 38% in patients age 30-59 years.4 Fortunately, the majority of ALLs in older patients are of the B-cell phenotype, and novel B-cell antigen-directed therapies, therefore, represent new treatment options for these patients.
Blinatumomab and the anti-CD22 antibody-drug conjugate inotuzumab ozogamicin (IO) have demonstrated impressive single-agent activity, even in the relapsed/refractory setting,11,16 and thus likely represent better agents in the up-front setting, with the goal of omitting or decreasing chemotherapy.
To our knowledge, this is the first multi-institutional trial to evaluate blinatumomab as a single agent in patients with newly diagnosed Ph chromosome–negative ALL. The advantage of this treatment was the very limited toxicity (no deaths in the first month), and the OS was encouraging as compared with historical controls. This was a small study but was performed within a cooperative group setting, thus illustrating the generalizability of such an approach. In addition, there was a high median age and high-risk population of patients (on the basis of cytogenetics, MLL status, and Ph-like status). The CR/CRi rate was high at 66% despite the median bone marrow blast percentage being 87%. This is more favorable than blinatumomab in relapsed/refractory patients with high bone marrow blast percentage where the CR/CRi rate was closer to 30%. Finally, this trial design is unique in evaluating blinatumomab as monotherapy and true chemotherapy-free induction and consolidation. Patients did receive POMP maintenance chemotherapy.
Despite the improved outcomes, there is need for further improvement. Of note, a large proportion of the relapses were CD19-negative. The reason for this high rate may be targeting of CD19 in the up-front setting (with only low-dose maintenance chemotherapy) versus other mechanisms of resistance in the relapsed/refractory setting after chemotherapy. There were also cases of myeloid lineage switch in patients with MLL-rearranged disease.
The high incidence of CD19-negative relapses in our study suggests that sequencing other agents (ie, targeting CD22) may be beneficial. Current approaches include sequencing blinatumomab and inotuzumab in the treatment of older patients with newly diagnosed ALL (clinical trial A041703) and combining inotuzumab with mini-hyper–cyclophosphamide, vincristine, dexamethasone (CVD) plus blinatumomab.
CD22 is almost uniformly expressed on B-ALLs, and IO is an anti-CD22 antibody-drug conjugate that has been FDA-approved in the relapsed/refractory setting on the basis of the pivotal INNOVATE trial.16 Inotuzumab has now been evaluated as induction therapy (as a single agent) followed by chemotherapy-based consolidation and maintenance in patients age ≥ 56 years in the INITIAL-1 trial.17 These results have been encouraging with a 100% CR rate and a 1-year OS of 82.4%. The drug has also been combined with low-intensity chemotherapy (mini-hyper–CVD) for older patients with Ph chromosome–negative ALL.18 Mini-hyper–CVD is a lower intensity version of conventional hyperCVAD.18 The median age on this trial was 68 years, and the 2-year progression-free survival was encouraging at 59%. The median OS was not reached, and there was a plateau of both progression-free survival and OS. A limitation of this trial is the relatively younger median age (68 years, as compared with 75 years on our trial). This approach also included 12% treatment-related deaths and an 8% incidence of veno-occlusive disease (with veno-occlusive disease contributing to 2% of deaths).18 A subsequent trial added four cycles of blinatumomab to mini-hyper–CVD inotuzumab in older adults with newly diagnosed Philadelphia chromosome–negative B-ALL.19 This approach also led to impressive results with a 98% overall response rate and a 4-year OS rate of 50%. Given these encouraging results, a randomized US Intergroup trial is planned to evaluate this combination, but patients will be allowed to receive fewer cycles of chemotherapy treatment (in an attempt to decrease treatment-related mortality) and proceed to maintenance if they are in a molecular remission and move to blinatumomab if they remain MRD-positive.
The US Intergroup recently completed a randomized trial of chemotherapy with or without blinatumomab (ECOG 1910) in patients up to age 70 years, and these results are eagerly awaited (longer follow-up is needed). This trial included a more intensive chemotherapy backbone and so was limited to 70 years although the chemotherapy regimen was modified by age. Other encouraging approaches in the older patients have included the addition of the bcl-2 inhibitor (venetoclax) to mini-hyper–CVD.20 Finally, chimeric antigen receptor T-cell therapy (CAR T-cell therapy) was recently FDA-approved in patients age > 26 years, and several ongoing trials are testing new platforms, which will hopefully make this treatment safer in older patients; this may become another therapy to evaluate in the future.
Other novel agents are currently being evaluated in the relapsed setting and may be moved to the up-front setting, pending review of outcomes. This includes drugs such as AMG404, the programmed cell death protein 1 inhibitor, which is being evaluated in combination with blinatumomab in the relapsed/refractory setting. Other potential approaches in the up-front setting may include the incorporation of ruxolitinib or other tyrosine kinase inhibitors for Ph-like ALL and of menin inhibitors for MLL-rearranged ALL. Of note, a recent trial of the combination of dasatinib and blinatumomab in older patients with Ph chromosome–positive ALL produced an OS and a DFS of 87.8% and 79.8%, respectively, and further follow-up is eagerly awaited.21,22
In the future, treatment is likely to include combinations of multiple novel agents, but clinical trials will be needed to determine the best approach for each subset of patients. We may be moving toward largely chemotherapy-free approaches in older patients with ALL, as is already the case for acute promyelocytic leukemia.23
ACKNOWLEDGMENT
The authors thank the study participants and study personnel for their invaluable support during the study.
APPENDIX
FIG A1.
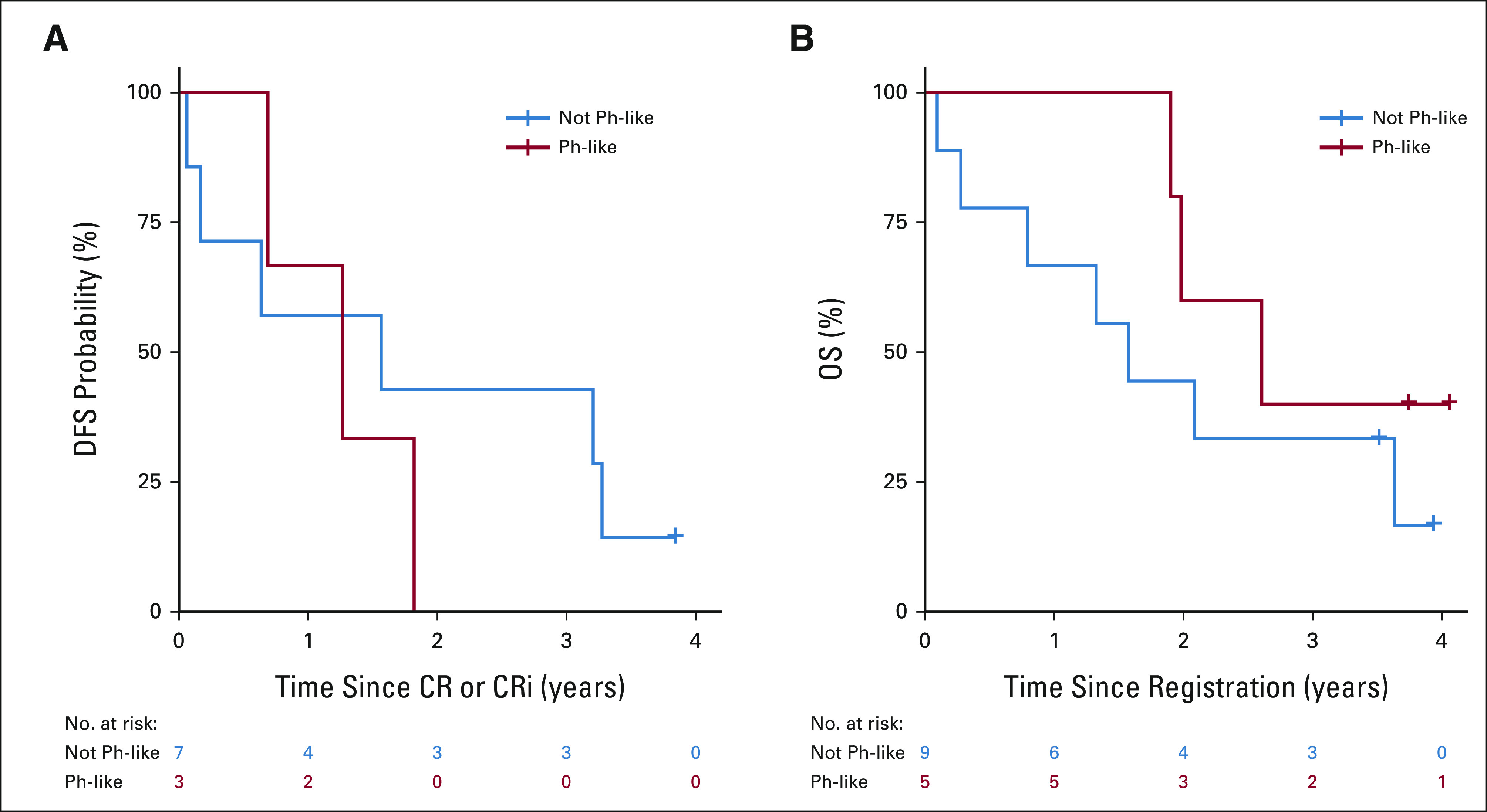
(A) DFS by Ph-like status. (B) OS by Ph-like status. DFS, disease-free survival; OS, overall survival; Ph, Philadelphia.
FIG A2.
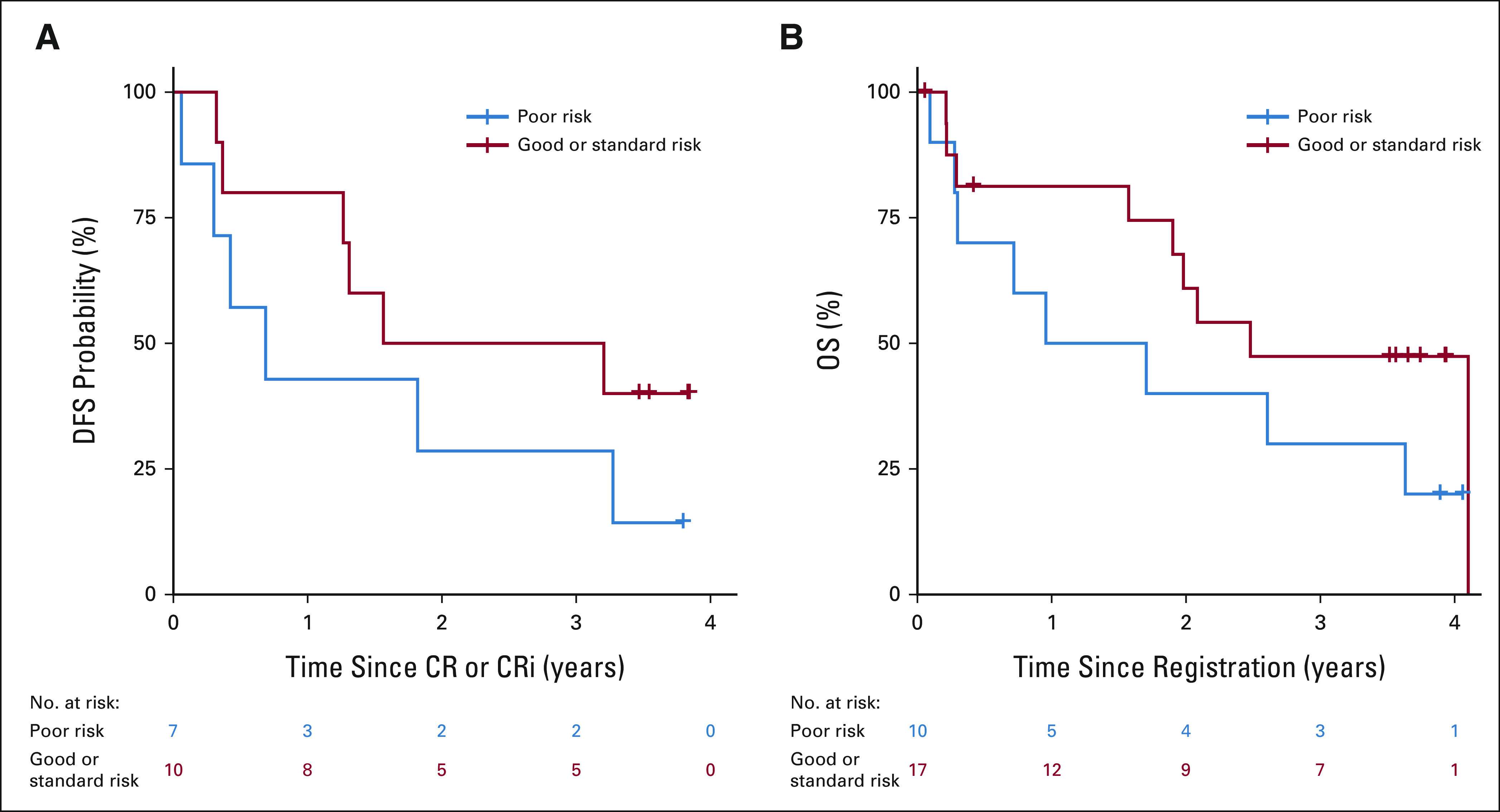
(A) DFS by cytogenetic risk. (B) OS by cytogenetic risk. DFS, disease-free survival; OS, overall survival.
TABLE A1.
Cytogenetics for All Patients
Anjali S. Advani
Honoraria: Pfizer
Consulting or Advisory Role: Novartis, GlycoMimetics, Kite, a Gilead company, Seattle Genetics, Amgen, Beam Therapeutics, Jazz Pharmaceuticals, Nkarta, Taiho Oncology
Research Funding: Pfizer (Inst), Millennium (Inst), KaloBios (Inst), Seattle Genetics (Inst), AbbVie (Inst), GlycoMimetics (Inst), MacroGenics (Inst), Pfizer (Inst), Amgen (Inst), Trillium Therapeutics (Inst), Seattle Genetics (Inst), Immunogen (Inst), SERVIER (Inst), Kite/Gilead (Inst), Incyte (Inst), OBI Pharma (Inst)
Anna Moseley
Consulting or Advisory Role: BioSight
Kristen M. O'Dwyer
Consulting or Advisory Role: Beam Therapeutics
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BioLineRx (Inst), BioSight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis, Kite, a Gilead company (Inst), MacroGenics (Inst)
Travel, Accommodations, Expenses: Amgen, Amgen
Min Fang
Consulting or Advisory Role: UnityDx
Research Funding: Nucleix (Inst)
Matthew J. Wieduwilt
Stock and Other Ownership Interests: Reata Pharmaceuticals
Honoraria: Gilead Sciences, Servier
Consulting or Advisory Role: Gilead Sciences, Servier
Research Funding: NCI (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Ibrahim Aldoss
Honoraria: Amgen, AbbVie, Kite, a Gilead company, Agios, Jazz Pharmaceuticals
Consulting or Advisory Role: Amgen
Speakers' Bureau: Pfizer
Research Funding: AbbVie, MacroGenics
Jae H. Park
Consulting or Advisory Role: Amgen, Novartis, Pfizer, Kite, a Gilead company, Autolus, Takeda, Servier, Incyte, Intellia Therapeutics, Innate Pharma, Artiva, Bristol Myers Squibb/Celgene/Juno, Kura Oncology, Kura Oncology, Kura Oncology, Minerva Biotechnologies, Precision Biosciences, AffyImmune Therapeutics, BeiGene
Research Funding: Juno Therapeutics (Inst), Genentech/Roche (Inst)
Rebecca B. Klisovic
Consulting or Advisory Role: Novartis, Visante, AbbVie, Bristol Myers Squibb, Pfizer
Research Funding: Pfizer, ARIAD, Bristol Myers Squibb, Avillion, Novartis
Travel, Accommodations, Expenses: Novartis
Maria R. Baer
Research Funding: AbbVie (Inst), FORMA Therapeutics (Inst), Kite, a Gilead company (Inst), Takeda (Inst), Kura Oncology (Inst), Ascentage Pharma (Inst)
Wendy Stock
Honoraria: Amgen, Pfizer
Consulting or Advisory Role: Agios, Amgen, Astra Zeneca, Beam, Glaxo Smith Kline, Jazz, Kite, Kronos, Kura, Morphosys, Newave, Pfizer, Pluristem, Servier, Syndax
Patents, Royalties, Other Intellectual Property: Royalties for a chapter in UpToDate
Megan Othus
Consulting or Advisory Role: GlycoMimetics, Cascadia Labs, Merck, Daiichi Sankyo, BioSight
Other Relationship: Celgene, GlycoMimetics
Cheryl L. Willman
Patents, Royalties, Other Intellectual Property: I have three patents for genomic diagnostics. They are all held by the University of New Mexico and have been licensed by TriCore Reference Laboratories. One generates minimal annual royalties (< $5,000 US dollars)
Mark R. Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutics, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: BioSight
Richard M. Stone
Honoraria: Prime Oncology, Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Agios, Celgene, Novartis, Actinium Pharmaceuticals, Arog, Astellas Pharma, MacroGenics, Takeda, BioLineRx, Daiichi-Sankyo, Trovagene, GEMoaB, Syntrix Biosystems, ElevateBio, Syndax, Syros Pharmaceuticals, BerGenBio, Janssen, Innate Pharma, Foghorn Therapeutics, Aprea Therapeutics, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Boston Pharmaceuticals, Onconova Therapeutics, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Harry P. Erba
Consulting or Advisory Role: Agios, Astellas Pharma, Amgen, Celgene, Daiichi Sankyo, GlycoMimetics, Immunogen, Incyte, Jazz Pharmaceuticals, MacroGenics, Novartis, AbbVie/Genentech, Janssen Oncology, Pfizer, Trillium Therapeutics, Takeda, Kura Oncology
Speakers' Bureau: Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, AbbVie/Genentech
Research Funding: AbbVie, Agios (Inst), Amgen (Inst), Daiichi Sankyo (Inst), FORMA Therapeutics (Inst), Gilead/Forty Seven (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst), MacroGenics (Inst), Novartis (Inst), PTC Therapeutics (Inst), AbbVie (Inst), GlycoMimetics (Inst), ALX Oncology (Inst)
Other Relationship: GlycoMimetics, Celgene
Uncompensated Relationships: Daiichi Sankyo
No other potential conflicts of interest were reported.
DISCLAIMER
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CLINICAL TRIAL INFORMATION
PRIOR PRESENTATION
Presented at American Society of Hematology Annual Meeting 2018: Advani A, Moseley A, O'Dwyer K, Wood B, Fang M, Wieduwilt M, Aldoss I, Park J, Klisovic R, Baer M, Stock W, Bhave R, Othus M, Litzow M, Stone R, and Erba HP. Results of SWOG 1318: A Phase II Trial of Blinatumomab Followed By Pomp (Prednisone, Vincristine, Methotrexate, 6-Mercaptopurine) Maintenance in Elderly Patients with Newly Diagnosed Philadelphia Chromosome Negative B-Cell Acute Lymphoblastic Leukemia. Blood 132:33(suppl 1), abst. 33, 2018. https://ashpublications.org/blood/article/132/Supplement%201/33/264657/Results-of-SWOG-1318-A-Phase-2-Trial-of.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under award numbers grants U10CA180888, U10CA180819, U10CA180821, U10CA180820, UG1CA233230, UG1CA233290, UG1CA233247, UG1CA233327, UG1CA232760, UG1CA233253, and UG1CA233180.
AUTHOR CONTRIBUTIONS
Conception and design: Anjali S. Advani, Kristen M. O'Dwyer, Matthew J. Wieduwilt, Jae H. Park, Wendy Stock, Megan Othus, Cheryl L. Willman, Elad Sharon, Harry P. Erba
Financial support: Elad Sharon
Administrative support: Elad Sharon
Provision of study materials or patients: Ibrahim Aldoss, Jae H. Park, Rebecca B. Klisovic, Maria R. Baer, Wendy Stock, Cheryl L. Willman, Mark R. Litzow, Richard M. Stone, Elad Sharon
Collection and assembly of data: Anjali S. Advani, Anna Moseley, Kristen M. O'Dwyer, Brent L. Wood, Ibrahim Aldoss, Jae H. Park, Rebecca B. Klisovic, Wendy Stock, Rupali R. Bhave, Richard C. Harvey, Cheryl L. Willman, Mark R. Litzow, Richard M. Stone, Elad Sharon, Harry P. Erba
Data analysis and interpretation: Anjali S. Advani, Anna Moseley, Kristen M. O'Dwyer, Brent L. Wood, Min Fang, Matthew J. Wieduwilt, Ibrahim Aldoss, Jae H. Park, Rebecca B. Klisovic, Maria R. Baer, Wendy Stock, Megan Othus, Richard C. Harvey, Cheryl L. Willman, Mark R. Litzow, Richard M. Stone, Elad Sharon, Harry P. Erba
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SWOG 1318: A Phase II Trial of Blinatumomab Followed by POMP Maintenance in Older Patients with Newly Diagnosed Philadelphia Chromosome–Negative B-Cell Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anjali S. Advani
Honoraria: Pfizer
Consulting or Advisory Role: Novartis, GlycoMimetics, Kite, a Gilead company, Seattle Genetics, Amgen, Beam Therapeutics, Jazz Pharmaceuticals, Nkarta, Taiho Oncology
Research Funding: Pfizer (Inst), Millennium (Inst), KaloBios (Inst), Seattle Genetics (Inst), AbbVie (Inst), GlycoMimetics (Inst), MacroGenics (Inst), Pfizer (Inst), Amgen (Inst), Trillium Therapeutics (Inst), Seattle Genetics (Inst), Immunogen (Inst), SERVIER (Inst), Kite/Gilead (Inst), Incyte (Inst), OBI Pharma (Inst)
Anna Moseley
Consulting or Advisory Role: BioSight
Kristen M. O'Dwyer
Consulting or Advisory Role: Beam Therapeutics
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BioLineRx (Inst), BioSight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis, Kite, a Gilead company (Inst), MacroGenics (Inst)
Travel, Accommodations, Expenses: Amgen, Amgen
Min Fang
Consulting or Advisory Role: UnityDx
Research Funding: Nucleix (Inst)
Matthew J. Wieduwilt
Stock and Other Ownership Interests: Reata Pharmaceuticals
Honoraria: Gilead Sciences, Servier
Consulting or Advisory Role: Gilead Sciences, Servier
Research Funding: NCI (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Ibrahim Aldoss
Honoraria: Amgen, AbbVie, Kite, a Gilead company, Agios, Jazz Pharmaceuticals
Consulting or Advisory Role: Amgen
Speakers' Bureau: Pfizer
Research Funding: AbbVie, MacroGenics
Jae H. Park
Consulting or Advisory Role: Amgen, Novartis, Pfizer, Kite, a Gilead company, Autolus, Takeda, Servier, Incyte, Intellia Therapeutics, Innate Pharma, Artiva, Bristol Myers Squibb/Celgene/Juno, Kura Oncology, Kura Oncology, Kura Oncology, Minerva Biotechnologies, Precision Biosciences, AffyImmune Therapeutics, BeiGene
Research Funding: Juno Therapeutics (Inst), Genentech/Roche (Inst)
Rebecca B. Klisovic
Consulting or Advisory Role: Novartis, Visante, AbbVie, Bristol Myers Squibb, Pfizer
Research Funding: Pfizer, ARIAD, Bristol Myers Squibb, Avillion, Novartis
Travel, Accommodations, Expenses: Novartis
Maria R. Baer
Research Funding: AbbVie (Inst), FORMA Therapeutics (Inst), Kite, a Gilead company (Inst), Takeda (Inst), Kura Oncology (Inst), Ascentage Pharma (Inst)
Wendy Stock
Honoraria: Amgen, Pfizer
Consulting or Advisory Role: Agios, Amgen, Astra Zeneca, Beam, Glaxo Smith Kline, Jazz, Kite, Kronos, Kura, Morphosys, Newave, Pfizer, Pluristem, Servier, Syndax
Patents, Royalties, Other Intellectual Property: Royalties for a chapter in UpToDate
Megan Othus
Consulting or Advisory Role: GlycoMimetics, Cascadia Labs, Merck, Daiichi Sankyo, BioSight
Other Relationship: Celgene, GlycoMimetics
Cheryl L. Willman
Patents, Royalties, Other Intellectual Property: I have three patents for genomic diagnostics. They are all held by the University of New Mexico and have been licensed by TriCore Reference Laboratories. One generates minimal annual royalties (< $5,000 US dollars)
Mark R. Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutics, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: BioSight
Richard M. Stone
Honoraria: Prime Oncology, Medscape, Research to Practice, DAVA Pharmaceuticals
Consulting or Advisory Role: Amgen, AbbVie, Agios, Celgene, Novartis, Actinium Pharmaceuticals, Arog, Astellas Pharma, MacroGenics, Takeda, BioLineRx, Daiichi-Sankyo, Trovagene, GEMoaB, Syntrix Biosystems, ElevateBio, Syndax, Syros Pharmaceuticals, BerGenBio, Janssen, Innate Pharma, Foghorn Therapeutics, Aprea Therapeutics, GlaxoSmithKline, CTI BioPharma Corp, Bristol Myers Squibb, Boston Pharmaceuticals, Onconova Therapeutics, Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Agios (Inst), AbbVie/Genentech (Inst)
Harry P. Erba
Consulting or Advisory Role: Agios, Astellas Pharma, Amgen, Celgene, Daiichi Sankyo, GlycoMimetics, Immunogen, Incyte, Jazz Pharmaceuticals, MacroGenics, Novartis, AbbVie/Genentech, Janssen Oncology, Pfizer, Trillium Therapeutics, Takeda, Kura Oncology
Speakers' Bureau: Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, AbbVie/Genentech
Research Funding: AbbVie, Agios (Inst), Amgen (Inst), Daiichi Sankyo (Inst), FORMA Therapeutics (Inst), Gilead/Forty Seven (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst), MacroGenics (Inst), Novartis (Inst), PTC Therapeutics (Inst), AbbVie (Inst), GlycoMimetics (Inst), ALX Oncology (Inst)
Other Relationship: GlycoMimetics, Celgene
Uncompensated Relationships: Daiichi Sankyo
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute : SEER stat fact sheet: Acute lymphocytic leukemia. Bethesda, MD, 2012. http://seer.cancer.gov/statfacts/html/alyl.html [Google Scholar]
- 2.O’Brien S, Thomas DA, Ravandi F, et al. : Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer 113:297-2101, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sive J, Buck G, Fielding A, et al. : Outcomes in older adults with acute lymphoblastic leukemia (ALL): Results from the international MRC UKALL XII/ECOG 2993 trial. Br J Haematol 157:463-471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stock W: personal communication. Data from 5 CALGB trials 1988-2001
- 5.Larson RA: The US trials in adult acute lymphoblastic leukemia. Ann Hematol 83:S127-S128, 2004. (suppl I) [DOI] [PubMed] [Google Scholar]
- 6.Larson RA, Dodge RK, Burns CP, et al. : A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood 85:2025-2037, 1995 [PubMed] [Google Scholar]
- 7.Larson RA, Dodge RK, Linker CA, et al. : A randomized control trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood 92:1556-1564, 1998 [PubMed] [Google Scholar]
- 8.Bassan R, Pogliani E, Casula P, et al. : Risk-oriented post remission strategies in adult acute lymphoblastic leukemia: Prospective confirmation of anthracycline activity in standard-risk groups. Hematol J 2:117-126, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Todeschini G, Tecchio C, Meneghini V, et al. : Estimated 6-year event-free survival of 55% in 60 consecutive adult acute lymphoblastic leukemia patients treated with an intensive phase II protocol based on high induction dose of daunorubicin. Leukemia 12:144-149, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Thomas DA, O’Brien S, Faderl S, et al. : Anthracycline dose intensification in adult acute lymphoblastic leukemia: Lack of benefit in the context of the fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen. Cancer 116:4580-4589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarjian H, Stein A, Gokbuget N, et al. : Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376:836-847, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokbuget N, Dombret H, Bonifacio M, et al. : Blinatumomab for minimal residual disease in adults in B-cell precursor acute lymphoblastic leukemia. Blood 131:1522-1531, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown PA, Shah B, Advani A, et al. : Acute lymphoblastic leukemia version 2.2021. J Natl Comp Cancer Netw 19:1079-1109, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Roberts K, Gu Z, Payne-Turner D, et al. : High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol 35:394-401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borowitz MJ, Wood BL, Devidas M, et al. : Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children’s Oncology Group study AALL 0232. Blood 126:964-971, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. : Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 375:740-753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stelljes M, Raffel S, Wasch R, et al. : First results of an open label phase II study to evaluate the efficacy and safety of Inotuzumab ozogamicin for induction therapy followed by a conventioan chemotherapy based consolidation and maintenance therapy in patients aged 56 years and older with acute lymphoblastic leukemia (INITIAL-1 trial). Am Soc Hematol 136, 2020. (abstr 267) [Google Scholar]
- 18.Kantarjian H, Ravandi F, Short NJ, et al. : Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: A single-arm phase 2 study. Lancet Oncol 19:240-248, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short N, Kantarjian H, Ravandi F, et al. : Reduced-intensity chemotherapy with mini-hyper-CVD plus Inotuzumab, with or without blinatumomab in older adults with newly diagnosed Philadelphia chromosome negative B-ALL: Results from a phase 2 study. Blood 136:15-17, 2020. (suppl 1) [Google Scholar]
- 20.Luskin MR, DeAngelo DJ: Mini-hyperCVD combinations for older adults: Results of recent trials and a glimpse into the future. Clin Lymphoma Myeloma Leuk 20:544-547, 2020. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 21.Foa R, Bassan R, Vitale A, et al. : Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia. N Engl J Med 383:1613-1623, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Chiaretti S, Bassan R, Vitale A, et al. Updated results of the GIMEMA LAL2116, D-ALBA trial, for newly diagnosed adults with Ph+ ALL. EHA Library 324520, S112, 2021
- 23.Lo-Coco F, Avvisati G, Vignetti M, et al. : Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369:111-121, 2013 [DOI] [PubMed] [Google Scholar]