Abstract
Background
Anemia is a disorder by which the body’s red blood cells are inadequate to fulfill The physiological needs of the body. The World Health Organization (WHO) defines anemia as having a hemoglobin (Hb) level of less than 120 g/l for nonpregnant women and 110 g/l for pregnant women. It has serious implications for human health as well as negative social and economic consequences like decreased workforce, impaired learning, and stunted child development. As these women are highly vulnerable to different micro and macro-nutritive deficiency associated with rapid physical, mental and psychological development, particular attention should be given to a young woman (15–24). Therefore this study assesses the magnitude and determinants of anemia among young women in sub-Saharan Africa (SSA).
Methods
This was a secondary data analysis based on the Demographic and Health Surveys (DHS) data conducted in sub-Saharan Africa. We pooled the most recent DHS surveys done in 31 sub-Sahara Africa and a total weighted sample of 88, 832 young women (15–24 years) were included. At bivariable analysis, variables with a p-value of ≤0.2 were selected for multivariable analysis, and at the multivariable analysis variables with a p-value of ≤0.05 were considered as a significant factor associated with anemia among young women (15–24 years).
Results
The pooled prevalence of anemia among young women (15–24) in sub-Sahara Africa was 42.17% [95%CI: 41.85, 42.50]. Young women of aged 20–24 years [AOR = 0.92, 95%CI: 0.89–0.95], women from rich household [AOR = 0.83, 95%CI: 0.80–0.87], young women with primary [AOR = 0.7, 95%CI: 0.67–0.72], secondary [AOR = 0.72, 95%CI: 0.69–0.75] and higher educational status [AOR = 0.58, 95%CI: 0.53–0.64], married women [AOR = 1.12, 95%CI: 1.08–1.17], divorced/separated/widowed women [AOR = 1.16, 95%CI: 1.08–1.25], women who use modern contraceptive [AOR = 0.65, 95%CI: 0.62–0.67], young women who ever had terminated pregnancy [AOR = 1.22, 95%CI: 1.14–1.29], overweight young woman [AOR = 0.79, 95%CI: 0.76–0.82] and young women from female-headed household [AOR = 0.94, 95%CI: 0.91–0.97] were the individual-level factors that significantly associated with anemia of young women. Meanwhile, being a rural dweller [AOR = 0.82, 95%CI: 0.79–0.85] and high community educational level [AOR = 0.87, 95%CI: 0.70–0.97] were the community level determinant of anemia. Interclass correlation coefficient (ICC), Median Odds Ratio (MOR) and Percentage change in variance (PCV) were done for the assessment of the random effect model of the multilevel analysis. The ICC value in the null model was 0.05, which indicates that 5% of the variation in anemia among young women in sub-Saharan Africa was attributed to community-level factors.
Conclusion
The prevalence of anemia among young women in this study was higher compared with reports from the previous studies. Divorced/separated/widowed women, married women and women with ever terminated pregnancy, young women with primary, secondary and higher educational achievement, being rural dwellers, young women aged 20–24 years, being from rich households and women who used modern contraceptives were factors that significantly associated with anemia among young women. Therefore, particular attention should be given to those higher-risk women including, young women with a history of a terminated pregnancy, those from rural areas and young women aged 15–19 years to reduce the burden of anemia among these young women as the continuity of the future generation depends on the health of young women.
Background
Anemia is a condition in which the body’s red blood cells are insufficient to meet its physiological need. Anemia affects 1.62 billion individuals worldwide, accounting for 24.8% of the population [1]. According to the World Health Organization (WHO) report, the prevalence of anemia among reproductive-age women is 57.1%, with the highest prevalence in Central (61%) and West Africa (61%) and the lowest in South Africa (34%) [2]. The World Health Organization (WHO) defines anemia as having a hemoglobin (Hb) level of less than 120 g/l for nonpregnant women and 110 g/l for pregnant women aged 15 years and above [3]. Anemia has serious implications for human health as well as long-term negative social and economic impacts [4]. The WHO estimates that nearly two billion people are anemic accounts for one million deaths per year of which three-quarters occur in low and middle-income countries [5]. Anemia is one of the most common nutritional deficiency diseases that affect more than a quarter of the world’s population [6–9]. It also impairs blood oxygen circulation by reducing the volume of red blood cells leads to negative consequences for maternal and child health [10]. It also results in stunted child development, impaired learning and decreased work efficiency [10, 11]. Anemia harms the health of women in reproductive age and pregnant women, under-five and preschool children, adolescents and young women [6, 9, 12–15]. Besides, the increased burden of anemia among adolescent and young women is associated with a period of rapid physical growth, reproductive maturation and cognitive transformations, which leads to higher demands for macro and micronutrients [16].
Anemia reduces worker productivity, particularly in developing countries where physical labor is the norm [17, 18]. Because of these negative effects, the WHO divides anemia level as it is not a public health problem (prevalence≤4.9%), mild public health concern (5 to 19.9%), moderate public health concern (20.0 to 39.9%) and extreme public health concern (prevalence ≥40.0%) depending on its impact on the community [7, 11 19, 20]. Different studies have identified that the age of the respondent, marital status, educational level, nutritional status, wealth status, source of drinking water and type of toilet facility were the factors that significantly associated with anemia among young women [12, 21–25]. Despite the implementation of control programmers including iron supplementation, deworming and insecticide-treated bed net distribution, anemia remains a major concern among young women in sub-Saharan Africa (SSA) [11] and with the current trends of anemia, it is difficult to achieve sustainable development goals of anemia (reduction of anemia of reproductive age woman by 50%) by 2030. As eradication of all forms of malnutrition is one of the main targets of SDGs and through the reduction of malnutrition anemia prevalence can be reduced. It is associated with an increased risk of death with poor cognitive abilities [7–9] and severe cases, lower aerobic exercise capacity and heart failure [21]. Even though studies are estimating the magnitude of anemia among reproductive-age women and children, the researcher gives less attention to the impact of anemia on the health of young women. As these women are highly vulnerable to different micro and macro-nutritive deficiency because of rapid physical, mental and psychological development, particular attention should be given to this population. Besides, some previously published works and reports indicated that the health impact and economic burden of anemia are persisting high in sub-Saharan Africa. Therefore this study assesses the magnitude and determinants of anemia among young women in sub-Saharan Africa.
Methods
Data source
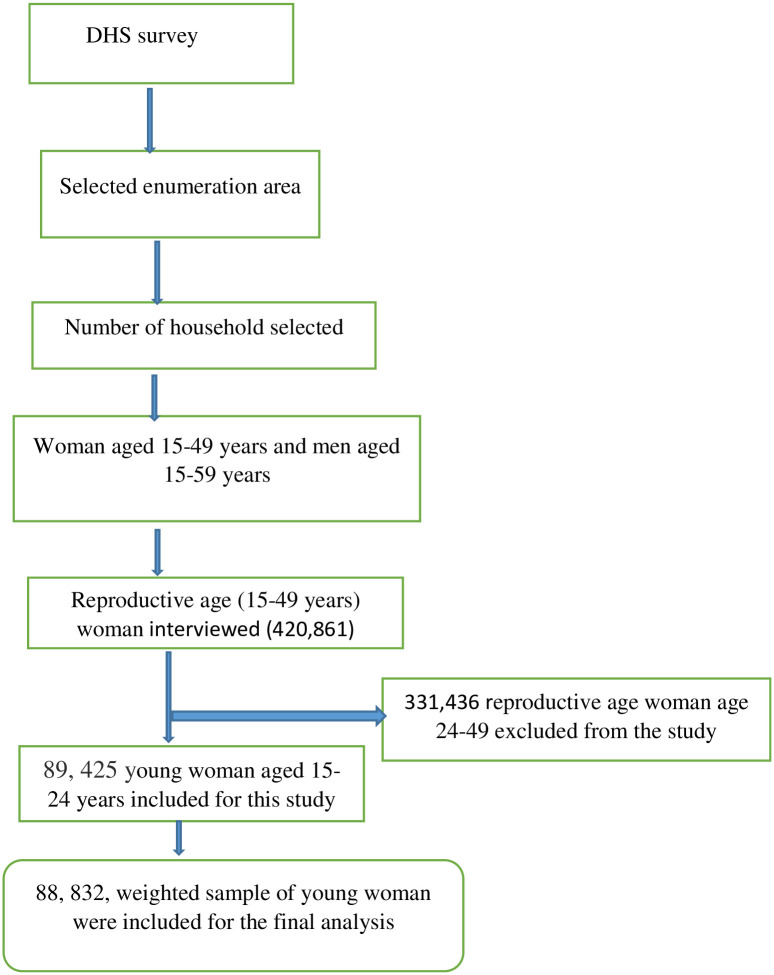
A dataset from the most recent Demographic and Health Surveys (DHS) conducted in Sub-Saharan African countries (SSA) was used for this analysis. Young women aged 15–24 years from 31 SSA countries participating in the DHS that performed anemia testing were included. We pooled the most recent DHS surveys and a total weighted sample of 88, 832 young women (15–24 years) were included. The data from the DHS survey naturally form hierarchical nature of households within the cluster, household members within each household, interviewed women and men as a subset of household members and children of each interviewed woman. An enumeration area with a probability proportionate to the EA size was selected at the first stage and then a specified number of households per cluster were chosen at random from a newly produced household list with an equal probability at the second stage of sampling procedures. All women of 15–49 years and all men aged 15–59 years who were either permanent residents of selected households or visitors residing in the household the night before the survey were eligible for interview (Fig 1). From these national DHS survey data information on the basic health indicators including mortality, morbidity, family planning utilization, fertility, maternal and child health were collected. The survey year and total weighted sample included for this study were presented in Table 1.
Fig 1. Flow diagram showing the sampling procedures of the DHS data.
Table 1. Survey year and total weighted sample included from each country.
| Country | Survey year | Weighted sample | |
|---|---|---|---|
| East Africa | Ethiopia | 2016 | 5796 |
| Burundi | 2016/17 | 3554 | |
| Tanzania | 2015/16 | 5315 | |
| Uganda | 2016 | 2588 | |
| Mozambique | 2011 | 5456 | |
| Zimbabwe | 2015 | 3639 | |
| Zambia | 2018 | 5566 | |
| Madagascar | 2008 | 3274 | |
| Rwanda | 2014/15 | 2611 | |
| Malawi | 2015/16 | 3402 | |
| Central Africa | Cameroon | 2018 | 2847 |
| Democratic Republic of Congo | 2013/14 | 3806 | |
| Congo | 2011/12 | 2141 | |
| Gabon | 2012 | 2096 | |
| São Tomée Príncipe | 2008/9 | 972 | |
| West Africa | Benin | 2017/18 | 3113 |
| Burkina Faso | 2010 | 3266 | |
| Ivory Coast | 2011/12 | 1876 | |
| Gambia | 2013 | 1986 | |
| Ghana | 2014 | 1601 | |
| Guinea | 2018 | 2125 | |
| Mali | 2018 | 1871 | |
| Niger | 2012 | 1733 | |
| Nigeria | 2018 | 5006 | |
| Senegal | 2010/2011 | 2395 | |
| Sierra Leone | 2019 | 2827 | |
| Togo | 2013/14 | 1731 | |
| South Africa | Lesotho | 2014 | 1393 |
| Namibia | 2013 | 1716 | |
| South Africa | 2016 | 952 | |
| Swaziland | 2005 | 2161 |
Variables of the study
Dependent variable
First, we dichotomized anemia in young women (15–24 years) into non-anemic which coded as “0” and anemic (by considering and merging mild, moderate, and severe anemia as anemic) which was coded as “1”. In the DHS data, women’s hemoglobin level was measured using HemoCue and the values were adjusted for altitude and smoking [26].
Independent variables
The independent variables were identified from different literature, which incorporates both the individual and community level variables. Age of respondent, educational level, marital status, wealth status, type of toilet facility, source of drinking water, body mass index (BMI), use of mosquito bed net, sex of household head, distance from the health facility, ever born children, modern contraceptive use, Cigarette smoking, ever terminated pregnancy and media exposure were the individual-level variables included. Whereas, residence, community poverty level and community educational level were community-level variables included for this study. Community poverty level and community educational level were created by aggregating their respective individual-level factor (wealth index) and (highest educational status), respectively. The median value was used to classify community poverty level as low (<50%) and high (≥50) as the variable was not normally distributed (Table 2).
Table 2. Description and measurement of independent variables.
| Independent variables and their description/categorization | |
|---|---|
| Individual-level variables | |
| Age Group | The current age of the women and re-coded into two categories with values of “0” for 15–19, “1” for 20–24. |
| Wealth Index | The datasets contained a wealth index that was created using principal components analysis coded as “poorest”, “poorer”, “Middle”, “Richer”, and “Richest in the DHS data set.” For this study we recoded it in to three categories as “poor” (includes the poorest and the poorer categories), “middle”, and “rich” (includes the richer and the richest categories) |
| Distance to the health facility | Re-coded in two categories with a value of “0” if the woman perceived as it is not a big problem to get a medical help from the health facility and “1” if the woman perceived as it is a big problem to get a medical help from the nearby health facility. |
| Media exposure | A composite variable obtained by combining whether a respondent reads newspaper/magazine, listen to radio, and watch television with a value of “0” if a women were not exposed to at least one of the three medias, and “1” if a woman has access/exposure to at least one of the three medias. |
| Educational status | This is the minimum educational level a woman achieved and re-coded in to three groups with a value of “0” for no education, “1” for primary education, and “2” for secondary and above (combining secondary and higher education categories together). |
| Marital status | This was the current marital status of women and recoded in two categories with a value of “0” for unmarried (includes those who were never in union, divorced, widowed, and separated), and “1” for “married” (includes those living with partner and those who are married) |
| Sex of household | The variable sex of household head was recorded as male and female in the dataset and we used without change. |
| Cigarate smoking | The variable Cigarate smoking was recoded as “0” for woman who never smoke Cigarate and “1” for woman who smokes Cigarate. |
| Body mass index | The variable body mass index was recoded as “0” for women of BMI 18.5–24.5 (Normal), “1” for women of BMI <18.5 (underweight) and “2”for woman with BMI of >24.5 (overweight). |
| Modern Contraceptive usage | Recoded in to two categories with value of 0 for “no” if a women don’t use any of the modern contraceptive methods, and 1 for “Yes” if a women use any of the modern contraceptive methods. of either of or combination of the following methods (female sterilization, implant, intrauterine device (IUD), injectable, oral contraceptive, emergency contraceptive, condom, lactational amenorrhea and periodic abstinence) |
| Ever terminated pregnancy | The variable ever terminated pregnancy was categorized in to two categories as “0” for woman who have no any form of terminated pregnancy and “1” for woman have ever terminated pregnancy. |
| Toilet facility | The variable toilet facility was categorized in to two categories “0” which stands from unimproved toilet facility flush to somewhere else, flush don’t know where, pit latrine-without slab/open pit, no facility/bush/field, bucket toilet, hanging toilet/latrine and others) and “1” for improved toilet facility (flush-to piped sewer system, flush-to septic tank, pit latrine, unspecified, ventilated improved pit (VIP), pit latrine-with slab and composite toilet) |
| Source of drinking water | Source of drinking water was categorized as “0” for unimproved (unprotected well, unprotected spring, surface water, tanker truck, car with small tank, bottled water and other) and “1” for those who have improved water sources (piped in to dwelling, piped to yard/plot, pubic tab/stand pip, protected well, protected spring, rain water and bottled water). |
| Total children ever born | The variable total children ever born was categorized in to three categories “0” for those who have no child, “1” for those woman with 1–5 children and “2” for woman with more than 5 children. |
| Community level variables | |
| Community of poverty level | Measured by proportion of households in the poor (combination of poorer and poorest) wealth quintile derived from data on wealth index. Then it was categorized based on national median value as: low (communities in which <50% of women had poor socioeconomic status) and high (communities in which ≥50% of women had poor socioeconomic status) poverty level. |
| Community educational level | Measured by the proportion of educated women (combination of primary, secondary and higher education). It was categorized based on national media value as: low (community in which < 50% of women had no education) and high (community with ≥ 50% of women had educational attainment). |
| Type of place of residence | The variable place of residence recorded as rural and urban in the dataset was used without change. |
Data management and analysis
Stata version 14 software was used for data extraction, recoding and analysis. The data were weighted before any statistical analysis to restore the representativeness of the data and to get a reliable estimate and standard error. Descriptive statistics were done using frequencies and percentages. For this analysis we cannot use the standard logistic regression analysis as the hierarchal nature of the DHS data violates the independent assumptions of the standard binary logistic regression model and because of this, we fitted a multilevel mixed-effects generalized linear model (with the assumption that individual specific-effect are uncorrelated with the independent variables). At first, we were considering both the individual level, household level, and community level analysis. But when we see the analysis result there is no significant clustering effect at the household level and for the above-mentioned reason, we were going to use the two-level i.e. the individual and community level analysis. To balance the size of clusters and detect the random effect efficiently, we excluded an EA with less than 10 observation per cluster and the maximum number of individual observed were 213 per cluster. While conducting a multilevel logistic regression analysis four models; the null model containing only the outcome variable, a model I and II containing individual and community level variables, respectively, and model III, which contains both individual and community level variables were fitted. Intraclass Correlation Coefficient, Median Odds Ratio and Percentage Change in Variance were checked to assess the clustering effect. Since these models were nested, we used deviance to check the model comparison and the model with the lowest deviance were chosen. Both bivariable and multivariable multilevel logistic regression was done and variables with a p-value of ≤0.2 in the bivariable analysis were considered for multivariable analysis. Finally, variables with P-value ≤0.05 in the multivariable analysis were identified as significant factors associated with anemia among young women (15–24 years).
Results
Sociodemographic characteristic of study participants
A weighted sample of 88,832 young women aged 15–24 years were included for this study. More than half (53.62%) of the participants were aged 15–19 years and about 45.99% of them were from rich households. Considering educational status, nearly 45% of the participants had achieved secondary level education and the majority (83.72%) of the participants didn’t use modern contraceptives. The majority (72.52%) of the participants had been exposed to different media and more than half (53.01%) of them had access to an improved toilet facility. The majority (61.17%) of the participants were from rural dwellers and about 97.41% of them were from the community of higher educational level (Table 3).
Table 3. Sociodemographic characteristics of study participants (N = 88, 832).
| Variable | Frequency | Percentage | |
|---|---|---|---|
| Respondent age | 15–19 | 47629 | 53.62 |
| 20–24 | 41203 | 46.38 | |
| Wealth status | Poor | 30931 | 34.82 |
| Middle | 17052 | 19.20 | |
| Rich | 40850 | 45.99 | |
| Educational status | No | 15986 | 18.00 |
| Primary | 30623 | 34.47 | |
| Secondary | 39435 | 44.39 | |
| Higher | 2788 | 3.14 | |
| Distance to health facility | Big problem | 32580 | 36.68 |
| Not big problem | 56252 | 63.32 | |
| Modern contraceptive use | No | 74371 | 83.72 |
| Yes | 14461 | 16.28 | |
| Marital status | Unmarried | 50730 | 57.11 |
| Married | 34520 | 38.86 | |
| Divorced/separated/ divorced | 3583 | 4.03 | |
| Ever terminated pregnancy | No | 83788 | 94.32 |
| Yes | 5044 | 5.68 | |
| Cigarette smoking | No | 88376 | 99.49 |
| Yes | 456 | 0.51 | |
| Body mass index | Normal | 59734 | 67.24 |
| Underweight | 11950 | 13.45 | |
| Overweight | 17149 | 19.30 | |
| Media exposure | No | 24410 | 27.48 |
| Yes | 64423 | 72.52 | |
| Sex of house hold head | Male | 63318 | 71.28 |
| Female | 25514 | 28.72 | |
| Toilet facility | Improved | 47094 | 53.01 |
| Unimproved | 41738 | 46.99 | |
| Source of drinking water | Unimproved | 41273 | 46.46 |
| Improved | 47559 | 53.54 | |
| Total children ever born | No | 51600 | 58.09 |
| 1–5 | 37183 | 41.86 | |
| >5 | 49.60 | 0.06 | |
| Residence | Urban | 34497 | 38.83 |
| Rural | 54335 | 61.17 | |
| Community level education | Low | 86535 | 97.41 |
| High | 2300 | 2.59 | |
| Community poverty level | Low | 44013 | 49.55 |
| High | 44819 | 50.45 | |
Prevalence of anemia among young women in sub-Saharan Africa
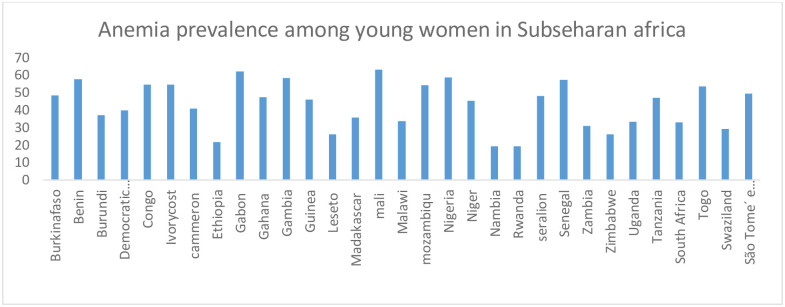
The pooled weighted prevalence of anemia among young women (15–24) in sub-Sahara Africa was 42.17% [95%CI: 41.85, 42.50] ranged from 21.71% in Ethiopia to 62.95% in Mali (Fig 2).
Fig 2. Showing the weighted prevalence of anemia among young women in sub-Sahara Africa.
Random effect analysis
Interclass correlation coefficient (ICC), MOR and PCV were done to assess the random effect model of the multilevel analysis. The ICC value in the null model was 0.05, which indicates that community-level factors were attributed to 5% of the variation in anemia among young women in sub-Sahara Africa. Also, the highest MOR value 1.13 in the null model indicated that there is a higher clustering of anemia among young women. The percentage change in variance (PCV) in the final model, which is 42% indicated that the individual and community level factors together explained about 42% of the variation in anemia among young women (Table 4).
Table 4. Random effect model and model fitness for the assessment of anemia among young women in sub-Saharan Africa.
| Parameter | Null model | Model I | Model II | Model III |
|---|---|---|---|---|
| Community-level variance | 0.015343 | .0152306 | .0143298 | .0135648 |
| ICC | 0.05 | 0.046 | .0433 | .0411 |
| MOR | 1.13 | 1.23 | 1.22 | 1.21 |
| PCV | Reff | 0.073 | 0.066 | 0.42 |
| Model fitness | ||||
| Log likelihood | -60867.956 | -60057.849 | -60775.791 | -59894.816 |
| Deviance | 121734.812 | 12015.698 | 121551.582 | 119789.632 |
Determinants of anemia among young women in sub-Saharan Africa
In fitting of the final model age of the respondent, educational status, ever terminated pregnancy, nutritional status, current marital status, modern contraceptive use, household wealth status, type of toilet facility, source of drinking water, total children ever born, sex of household head, community-level education and residence were the significant determinant of anemia among young women. Young women aged 20–24 years had 8% [AOR = 0.92, 95%CI: 0.89–0.95) lower risk of being anemic compared with a woman aged 15–19 years. Women from rich households had 0.83 [AOR = 0.83, 95%CI: 0.80–0.87] times lower odds of developing anemia compared with young women from poor households. Looking at educational status, young women with primary [AOR = 0.7, 95%CI: 0.67–0.72], secondary [AOR = 0.72, 95%CI: 0.69–0.75] and higher educational status [AOR = 0.58, 95%CI: 0.53–0.64] had lower odds of anemia compared with uneducated young women. Married women had 1.12 [AOR = 1.12, 95%CI: 1.08–1.17] times higher odds of developing anemia compared with unmarried women. Similarly, divorced/separated/widowed women had 1.16[AOR = 1.16, 95%CI: 1.08–1.25] times higher odds of anemia compared with unmarried women. Interestingly, women who use modern contraceptives had 0.65[AOR = 0.65, 95%CI: 0.62–0.67] times lower odds of developing anemia compared with a young woman who did not use modern contraceptives. Young women who ever had terminated pregnancy had a 1.22[AOR = 1.22, 95%CI: 1.14–1.29] times higher risk of developing anemia compared with a woman with no history of pregnancy terminated. Overweighed young women had a 21% [AOR = 0.79, 95%CI: 0.76–0.82] lower chance of developing anemia compared with women with normal body mass index. Female-headed households had 0.94[AOR = 0.94, 95%CI: 0.91–0.97] times lower odds of developing anemia compared with male-headed households. Young women with total children of 1 to 5 [AOR = 1.09, 95%CI: 1.04–1.14] and greater than 5 [AOR = 2.20, 95%CI: 1.28–3.78] had higher odds of having anemia compared with women with no children. Rural dweller young women had 18% [AOR = 0.82, 95%CI: 0.79–0.85] lower risk of developing anemia compared with urban young women. Considering community-level education, women from the community of higher educational status had 0.87[AOR = 0.87, 95%CI: 0.70–0.97] times lower odds of being anemic compared with women from the community of low educational level (Table 5).
Table 5. Multilevel analysis for determinants of anemia among young women (15–24) in sub-Saharan Africa.
| Variables | Anemia status | COR (95%CI) | AOR (95%CI) | ||
|---|---|---|---|---|---|
| Non-anemic | Anemic | ||||
| Age | 15–19 | 27408 | 23959 | 1 | 1 |
| 20–24 | 20221 | 17244 | 0.96(0.94, 0.99) | 0.92(0.89, 0.95)* | |
| Marital status | Unmarried | 30264 | 20466 | 1 | 1 |
| Married | 19106 | 15413 | 1.21(1.17, 1.24) | 1.12(1.08, 1.17)* | |
| Divorced/widowed/separate | 1997 | 1584 | 1.15(1.08, 1.24) | 1.16(1.08, 1.25)* | |
| Educational status | No education | 7802 | 8184 | 1 | 1 |
| Primary education | 18263 | 12360 | 0.63(0.61, 0.66) | 0.7(0.67, 0.72)* | |
| Secondary education | 23499 | 15936 | 0.62(0.60, 0.65) | 0.72(0.69, 0.75)* | |
| Higher education | 1803 | 985 | 0.46(0.43, 0.51) | 0.58(0,53, 0.64)* | |
| Wealth status | Poor | 17253 | 13678 | 1 | 1 |
| Middle | 9619 | 7402 | 0.93(0.90, 0.95) | 0.97(0.93, 1.01) | |
| Rich | 24465 | 16385 | 0.79(0.77, 0.82) | 0.83(0.80, 0.87)* | |
| Distance to health facility | Big problem | 18510 | 14070 | 1 | 1 |
| Not big problem | 32857 | 23395 | 0.91(0.88, 0.93) | 0.97(0.94, 1.00) | |
| Modern contraceptive use | No | 41750 | 32621 | 1 | 1 |
| Yes | 9617 | 4844 | 0.63(0.60, 0.65) | 0.65(0.62, 0.67)* | |
| Ever terminated pregnancy | No | 48706 | 35082 | 1 | 1 |
| Yes | 2661 | 2383 | 1.26(1.19, 1.33) | 1.22(1.14, 1.29)* | |
| Body mass index | Normal | 6642 | 5307 | 1 | 1 |
| Underweight | 33842 | 25891 | 1.03(0.99, 1.08) | 1.02(0.98, 1.06) | |
| Overweight | 10883 | 6266 | 0.74(0.71, 0.76) | 0.79(0.76, 0.82) | |
| Cigarette smoking | No | 51096 | 37280 | 1 | 1 |
| Yes | 271 | 185 | 0.95(0.78, 1.16) | 0.97(0.79, 1.18) | |
| Toilet facility | Improved | 27780 | 19295 | 1 | 1 |
| Unimproved | 23568 | 18170 | 1.17(1.14, 1.20) | (1.01, 1.07)* | |
| Current breast feed | No | 39772 | 28409 | 1 | 1 |
| Yes | 11595 | 9056 | 1.10(1.07, 1.14) | 0.97(0.93, 1.01) | |
| Media exposure | No | 13949 | 10461 | 1 | 1 |
| Yes | 37419 | 27004 | 0.91(0.88, 0.93) | 1.03(0.99, 1.06) | |
| Sex of house hold head | Male | 36115 | 27203 | 1 | 1 |
| Female | 15252 | 10262 | 0.88(0.85, 0.89) | 0.94(0.91, 0.97)* | |
| Source of drinking water | Unimproved | 23329 | 17944 | 1 | 1 |
| Improved | 28038 | 19522 | 0.87(0.85, 0.89) | 0.97 (0.94, 1.01) | |
| Total children ever born | No | 30309 | 21291 | 1 | 1 |
| 1–5 | 21042 | 16142 | 1.10(1.07, 1.13) | 1.09(1.04, 1.14)* | |
| >5 | 17 | 33 | 2.66(1.56, 4.54) | 2.20(1.28, 3.78)* | |
| Community educational level | Low | 50344 | 36188 | 1 | 1 |
| High | 1023 | 1277 | 1.75(1.61, 1.91) | 0.87(0.70, 0.97)* | |
| Community poverty level | Low | 25593 | 18420 | 1 | 1 |
| High | 25774 | 19045 | 1.01(0.97, 1.04) | 1.01(0.967, 1.03) | |
| Residence | Urban | 19537 | 14960 | 1 | 1 |
| Rural | 31830 | 22505 | 1.04(1.02, 1.07) | 0.82(0.79, 0.85)* | |
*P-value≤0.05
Discussion
The prevalence of anemia among young women in sub-Sahara Africa was 42.17% [95%CI: 41.85%, 42.50%]. The finding of this study indicated that anemia is a major public health problem among young women according to the WHO classification of anemia for public health significance. Anemia prevalence among young women was higher than the studies conducted in Ethiopia, East Africa, Rwanda, Nepal and Chinese [12, 25, 27–33]. Meanwhile, the pooled prevalence of anemia in this study was lower than the prevalence reported in Ghana [34], Cameron [34], Congo democratic republic [34], Burkina Faso [34] and Asia [22]. This might be attributed to differences in the study settings and wider participants’ age group as we included only young women (15–24 years) [21]. In addition, these variations might be due to differences in cutoffs value used in the different organizations which are used to define anemia, geographic location as well as time differences [35].
The difference in the prevalence of anemia might also be due to the differences in socio-demographic characteristics, culture and adolescents’ behavioral and feeding habits or practices [33]. In the multilevel analysis age of respondent, educational status, ever terminated pregnancy, nutritional status, current marital status, modern contraceptive use, household wealth status, type of toilet facility, source of drinking water, total children ever born, sex of household head, community-level education and residence were the significant determinant of anemia among young women. Young women aged 20–24 years had lower odds of being anemic compared with their counterparts. This finding was supported by the study conducted in Asia [22]. A higher prevalence of anemia among early younger women could be due to the adverse effect of lower dietary iron intake and additional demand for iron imposed by iron loss during menstruation, pregnancy and lactation [13]. Because it is a phase of rapid growth, proper nutrition is essential for achieving full growth potential and failure to acquire optimal nutrition may result in delayed and stunted child growth as well as impaired organ remodeling. Because young women need additional micro and macronutrient, they are in danger of developing a variety of health problems, including anemia, if they do not receive appropriate nutrition [36].
Women who used modern contraceptives had lower odds of developing anemia supported by studies conducted in Ethiopia and Pennsylvania [24, 25, 31]. This might be associated with hormonal contraceptives are important to reduce excessive bleeding during the menstrual period that ultimately reduces blood loss over time [37, 38]. Young married women had a higher risk of developing anemia compared with unmarried women supported by previous studies conducted in Ethiopia [24, 25]. This might be due to as married women can get repeated pregnancy and this frequent pregnancy might lead to increased risk of hemorrhage before, during and after delivery that exposes them to a higher risk of anemia [39]. Women from rich households had lower odds of developing anemia compared with women from poor households. This finding was supported by the reports in Eastern Ethiopia [21] and Asia [22]. Young women with a primary and higher level of education had a lower risk of developing anemia compared with uneducated women, which was supported by other studies conducted in Ethiopia [12, 28] and Asia [22]. This is because young women who have some level of formal education can be aware of anemia during their life and take some preventive measures like eating iron-rich food and taking iron tablets during pregnancy [28]. This might also be associated with as educated females utilize adequate medical care and have good knowledge about appropriate nutrition and personal hygiene, which might be directly related to the risk of developing anemia [40]. Educated young women consume iron-rich food, utilize adequate healthcare facilities and manage a hygienic household environment, which is associated with a reduction in anemia among young women [41]. Young women with improved water sources have lower risk of developing anemia compared with a woman with unimproved water sources. This finding was in agreement with another study done in Japan [27] and Ethiopia [25]. Similarly, the likelihood of having anemia was greater among women with unimproved toilet facilities compared with a woman with improved toilet facility. This finding was supported by another studies conducted in Ethiopia and LaoPDR [25, 27].
This might be associated with the probability of environmental contamination of drinking water with parasites that causes anemia increases because of the inaccessibility of appropriate toilet facilities, which might, in turn, increase the risk of anemia [27].
In the present studies, women with ever terminated pregnancies had a higher risk of developing anemia compared with their counterparts. The finding is in agreement with another study [27]. In this study women who had ever children born of greater than five were more likely to develop anemia compared with women with no children, which was supported by a study conducted in Japan [27]. This might be associated with the physiological stress of several pregnancies, along with low-quality meals, parasites and poor sanitation poses significant hazards to mothers and their children. Being rural dwellers had a lower risk of developing anemia compared to the urban dweller, which was supported by other studies conducted in India [42], Malawi [43], and sub-Sahara Africa [44]. This might be because traditional staple foods consumed in rural households are more diversified and rich in iron content compared to those food taken by most urban dwellers and such diversified food patterns might have a positive role in meeting the adequacy of iron requirements for the young women [42]. Considering the nutritional status, overweight young women had a lower odds of developing anemia compared to women with normal nutritional statues, which is supported by studies conducted in China [32]. This might because as food insecurity is the major problem in developing countries leads to food deficiency and poor quality of diet, which finally end up with different health problems including anemia.
As anemia of young women is fatal because of rapid physical and mental growth and menstruation-associated risks, policymakers should give particular attention to this public health problem by taking into consideration the variation of anemia among different clusters by using the random effect analysis. Since the random effect analysis of anemia among young women indicated that there is a significant clustering in anemia and those woman in higher risk clusters have a higher risk of developing anemia which is only addressed by the random effect analysis of multi-level model that provides suitable epidemiological information on area-level variance and clustering. The multilevel random effect analysis implied to this study identified anemia high and low-risk community which is important to policy and decision-maker to give priority to this high-risk group to take possible intervention strategies early. Also, the random effect analysis estimate of anemia of young women showed that there is a variation in the occurrence of anemia among young women in different randomly selected clusters/communities, therefore, the estimate of random effect analysis is very crucial to identify a young woman who lived in higher anemia risk cluster and to give priority to that higher risk population group.
Strength and limitation of the study
Since this study was based on weighted large representative data the statistical power of the study is high. We also use an appropriate statistical approach to accommodate the hierarchical nature of the data and also the unobserved heterogeneity of this study is accounted as we used random parameter approaches. This study had limitations in that the DHS survey was based on respondents’ self-report and might have the possibility of recall bias. In addition as this study was based on cross-sectional collected DHS data, we are unable to show the temporal relationship between anemia among young women and independent variables.
Conclusion
The prevalence of anemia among young women in sub-Sahara Africa was higher than reports in previous studies. In this study age of the respondent, educational status, ever terminated pregnancy, nutritional status, current marital status, modern contraceptive use, household wealth status, type of toilet facility, source of drinking water, total children ever born, sex of household head, community-level education and residence were the significant determinant of anemia among young women in SSA. Therefore, program planners should focus on the provision of iron, folate and other micronutrients through different intervention programs including supplementation and fortification to reduce these devastating nutritional-related high prevalence of anemia. In addition, there should be improved water access and improved sanitation including toilet facilities to reduce the anemia burden among young women in SSA. Besides, particular attention should be given to those higher-risk women including, a young woman with a history of terminated pregnancy, those from poor economic status, and low educational achievement to reduce the burden of anemia among these young women for the betterment of the continuity of generations.
Acknowledgments
We greatly acknowledge MEASURE DHS for granting access to the Demographic and Health Surveys data.
Abbreviations
- CI
Confidence Interval
- CSA
Central Statistical Agency
- DHS
Demographic Health Survey
- EA
Enumeration Area
- GDP
Gross Domestic Product
- ICC
Intraclass Correlation Coefficient
- LLR
Likelihood Ratio
- LMIC
low and middle-income country
- PCV
Proportional change in Variance
- SSA
sub-Saharan Africa
- WHO
World Health Organization
Data Availability
All the relevant data were included in the manuscript. However, it is ethically not acceptable to share the DHS data set to third parties and anyone who want the data set can access from the Measure DHS program at www.dhsprogram.com, through legal requesting. The authors had no special access privileges others would not have.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Organization WH. Global anaemia prevalence and number of individuals affected. WHO, Geneva. 2008.
- 2.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. The Lancet Global Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geneva S, Organization WH. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Document Reference WHO. NMH/NHD/MNM/11.1. http://www.who.int/entity/vmnis/indicators/haemoglobin; 2011
- 4.Organization WH. The global prevalence of anaemia in 2011 [Internet]. World Health Organization Geneva, Switzerland. 2015.
- 5.Osungbade KO, Oladunjoye AO. Anaemia in developing countries: burden and prospects of prevention and control. Anemia. 2012;3:116–29. [Google Scholar]
- 6.De Benoist B, Cogswell M, Egli I, McLean E. Worldwide prevalence of anaemia 1993–2005; WHO Global Database of anaemia. 2008. [DOI] [PubMed]
- 7.Salhan S, Tripathi V, Singh R, Gaikwad HS. Evaluation of hematological parameters in partial exchange and packed cell transfusion in treatment of severe anemia in pregnancy. Anemia. 2012;2012. doi: 10.1155/2012/608658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barooti E, Rezazadehkermani M, Sadeghirad B, Motaghipisheh S, Tayeri S, Arabi M, et al. Prevalence of iron deficiency anemia among Iranian pregnant women; a systematic review and meta-analysis. Journal of reproduction & infertility. 2010;11(1):17. [PMC free article] [PubMed] [Google Scholar]
- 9.Baig-Ansari N, Badruddin SH, Karmaliani R, Harris H, Jehan I, Pasha O, et al. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food and nutrition bulletin. 2008;29(2):132–9. doi: 10.1177/156482650802900207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achadi E, Ahuja A, Bendech MA, Bhutta ZA, De-Regil LM, Fanzo J, et al. Global nutrition report 2016: From promise to impact: Ending malnutrition by 2030: International Food Policy Research Institute; 2016.
- 11.Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S. Anaemia in low-income and middle-income countries. The lancet. 2011;378(9809):2123–35. doi: 10.1016/S0140-6736(10)62304-5 [DOI] [PubMed] [Google Scholar]
- 12.Tura MR, Egata G, Fage SG, Roba KT. Prevalence of Anemia and Its Associated Factors Among Female Adolescents in Ambo Town, West Shewa, Ethiopia. Journal of Blood Medicine. 2020;11:279. doi: 10.2147/JBM.S263327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public health nutrition. 2009;12(4):444–54. doi: 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 14.Premalatha T, Valarmathi S, Srijayanth P, Sundar J, Kalpana S. Prevalence of anemia and its associated factors among adolescent school girls in Chennai, Tamil Nadu, India. Epidemiol an open access journal. 2012;2(1). [Google Scholar]
- 15.Deshpande NS, Karva D, Agarkhedkar S, Deshpande S. Prevalence of anemia in adolescent girls and its co-relation with demographic factors. International Journal of Medicine and Public Health. 2013;3(4). [Google Scholar]
- 16.Engidaw MT, Wassie MM, Teferra AS. Anemia and associated factors among adolescent girls living in Aw-Barre refugee camp, Somali regional state, Southeast Ethiopia. PloS one. 2018;13(10):e0205381. doi: 10.1371/journal.pone.0205381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JD, Brownlie T IV. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. The Journal of nutrition. 2001;131(2):676S–90S. doi: 10.1093/jn/131.2.676S [DOI] [PubMed] [Google Scholar]
- 18.Gardner GW, Edgerton VR, Senewiratne B, Barnard RJ, Ohira Y. Physical work capacity and metabolic stress in subjects with iron deficiency anemia. The American journal of clinical nutrition. 1977;30(6):910–7. doi: 10.1093/ajcn/30.6.910 [DOI] [PubMed] [Google Scholar]
- 19.Organization WH. The World Health Report 2001: Mental health: new understanding, new hope. 2001.
- 20.Caballero B. Encyclopedia of human nutrition: Elsevier; 2005. [Google Scholar]
- 21.Addis Alene K, Mohamed Dohe A. Prevalence of anemia and associated factors among pregnant women in an urban area of Eastern Ethiopia. Anemia. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunuwar DR, Singh DR, Chaudhary NK, Pradhan PMS, Rai P, Tiwari K. Prevalence and factors associated with anemia among women of reproductive age in seven South and Southeast Asian countries: Evidence from nationally representative surveys. PloS one. 2020;15(8):e0236449. doi: 10.1371/journal.pone.0236449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tezera R, Sahile Z, Yilma D, Misganaw E, Mulu E. Prevalence of anemia among school-age children in Ethiopia: a systematic review and meta-analysis. Systematic reviews. 2018;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worku MG, Tesema GA, Teshale AB. Prevalence and determinants of anemia among young (15–24 years) women in Ethiopia: A multilevel analysis of the 2016 Ethiopian demographic and health survey data. PloS one. 2020;15(10):e0241342. doi: 10.1371/journal.pone.0241342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teshale AB, Tesema GA, Worku MG, Yeshaw Y, Tessema ZT. Anemia and its associated factors among women of reproductive age in eastern Africa: A multilevel mixed-effects generalized linear model. Plos one. 2020;15(9):e0238957. doi: 10.1371/journal.pone.0238957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutstein SO, Rojas G. Guide to DHS statistics. Calverton, MD: ORC Macro. 2006;38.
- 27.Keokenchanh S, Kounnavong S, Tokinobu A, Midorikawa K, Ikeda W, Morita A, et al. Prevalence of Anemia and Its Associate Factors among Women of Reproductive Age in Lao PDR: Evidence from a Nationally Representative Survey. Anemia. 2021;2021. doi: 10.1155/2021/8823030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebre A, Mulugeta A. Prevalence of anemia and associated factors among pregnant women in North Western zone of Tigray, Northern Ethiopia: a cross-sectional study. Journal of nutrition and metabolism. 2015;2015. doi: 10.1155/2015/165430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam S, Min H, Kim H, Jeong H-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: Evidence from recent national survey data. PloS one. 2019;14(6):e0218288. doi: 10.1371/journal.pone.0218288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakizimana D, Nisingizwe MP, Logan J, Wong R. Identifying risk factors of anemia among women of reproductive age in Rwanda–a cross-sectional study using secondary data from the Rwanda demographic and health survey 2014/2015. BMC public health. 2019;19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekhar DL, Murray-Kolb LE, Kunselman AR, Weisman CS, Paul IM. Differences in risk factors for anemia between adolescent and adult women. Journal of women’s health. 2016;25(5):505–13. doi: 10.1089/jwh.2015.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Li Y, Hu P, Ma J, Song Y. Prevalence of anemia and its associated factors among Chinese 9-, 12-, and 14-year-old children: results from 2014 Chinese national survey on students constitution and health. International journal of environmental research and public health. 2020;17(5):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z, Sudfeld CR, Cheng Y, Qi Q, Li S, Elhoumed M, et al. Anemia and associated factors among adolescent girls and boys at 10–14 years in rural western China. BMC public health. 2021;21(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasutake S, He H, Decker MR, Sonenstein FL, Astone NM. Anemia among adolescent and young women in low-and-middle-income countries. International Journal of Child Health and Nutrition. 2013;2(2):105–12. [Google Scholar]
- 35.Bekele A, Tilahun M, Mekuria A. Prevalence of anemia and Its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch town, Gamo Gofa Zone, Ethiopia: A Cross-sectional study. Anemia. 2016;2016. doi: 10.1155/2016/1073192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das JK, Salam RA, Thornburg KL, Prentice AM, Campisi S, Lassi ZS, et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Annals of the New York Academy of Sciences. 2017;1393(1):21–33. doi: 10.1111/nyas.13330 [DOI] [PubMed] [Google Scholar]
- 37.Hillard PA. Menstrual suppression: current perspectives. International journal of women’s health. 2014;6:631. doi: 10.2147/IJWH.S46680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstetrics & Gynecology. 2003;101(4):653–61. doi: 10.1016/s0029-7844(03)00014-0 [DOI] [PubMed] [Google Scholar]
- 39.Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. Effect of high parity on occurrence of anemia in pregnancy: a cohort study. BMC pregnancy and childbirth. 2011;11(1):1–7. doi: 10.1186/1471-2393-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Win HH, Ko MK. Geographical disparities and determinants of anaemia among women of reproductive age in Myanmar: analysis of the 2015–2016 Myanmar Demographic and Health Survey. WHO South-East Asia journal of public health. 2018;7(2):107–13. doi: 10.4103/2224-3151.239422 [DOI] [PubMed] [Google Scholar]
- 41.Habib N, Abbasi S-U-RS, Aziz W. An Analysis of Societal Determinant of Anemia among Adolescent Girls in Azad Jammu and Kashmir, Pakistan. Anemia. 2020;2020. doi: 10.1155/2020/1628357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey VL, Mahendra Dev S, Jayachandran U. Impact of agricultural interventions on the nutritional status in South Asia: A review. Food Policy. 2016;62:28–40. doi: 10.1016/j.foodpol.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamu AL, Crampin A, Kayuni N, Amberbir A, Koole O, Phiri A, et al. Prevalence and risk factors for anemia severity and type in Malawian men and women: urban and rural differences. Population Health Metrics. 2017;15(1):12. doi: 10.1186/s12963-017-0128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moschovis PP, Wiens MO, Arlington L, Antsygina O, Hayden D, Dzik W, et al. Individual, maternal and household risk factors for anaemia among young children in sub-Saharan Africa: a cross-sectional study. BMJ open. 2018;8(5):e019654. doi: 10.1136/bmjopen-2017-019654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data were included in the manuscript. However, it is ethically not acceptable to share the DHS data set to third parties and anyone who want the data set can access from the Measure DHS program at www.dhsprogram.com, through legal requesting. The authors had no special access privileges others would not have.