Abstract
Objective
To describe a step-by-step guide to robot-assisted anterior partial prostatectomy (RA-APP) for isolated magnetic resonance imaging (MRI)-detected anterior prostate cancer (APC).
Patients and Methods
After Institutional Review Board approval, over an 8-year period (2008–2015), 17 consenting patients were enrolled in a prospective, single-arm, single-centre, Idea, Development, Evaluation, Assessment and Long-term evaluation of innovative surgery (IDEAL) phase 2a study. The inclusion criteria comprised pre-urethral, low–intermediate risk APC diagnosed by MRI and targeted biopsies. Patient position and port placement were identical to the transperitoneal RA radical prostatectomy procedure. Three steps of dissection were identified in the following order: (i) retrograde apical, after dorsal venous plexus division, transition zone (TZ) enucleation, and distal peripheral zone (PZ) sectioning; (ii) antegrade, at the bladder neck (BN) after anterior BN sectioning, TZ enucleation up to the verumontanum; and (iii) lateral dissections, including anterolateral PZ sectioning without incision of the endopelvic fascia. We report the incidence of perioperative complications. The RA completion of prostatectomy in four cases with cancer recurrence was performed at 0.3, 2.5, 2 and 2 years, respectively.
Results
The RA-APP comprised en bloc excision of the anterior part of the prostate comprising of the anterior fibromuscular stroma, BN, prostate adenoma (TZ and median lobe) along with the proximal prostate urethra, PZ apical anterior horns, anterior aspect of the distal (sub-montanal) urethra, and anterior BN. The posterolateral parts of the PZ and distal (sub-montanal) urethra and periprostatic tissues were preserved intact. The bladder opening was sutured to the anterior sphincteric urethra wall and PZ lateral edges. The technique was feasible in all cases with no conversion to an open procedure. Perioperative complications were only Clavien–Dindo grade II. RA completion of prostatectomy was feasible in the four cases with cancer recurrence.
Conclusion
PZ prostate-sparing RA-APP for isolated APC is feasible and safe, and represents an option for highly selected men with APCs as an alternative to other focal ablative therapy.
Keywords: focal therapy, prostate MRI, robot-assisted surgery, #PCSM, #ProstateCancer
Introduction
Complications of radical prostatectomy have been well documented, such as erectile dysfunction, chronic urinary incontinence, and the occasional bladder neck (BN) contracture. These complications arise because of the close proximity of the neurovascular bundle and the rhabdosphincter to the surgical dissection plane, which increases the risk of injury to these structures. Potentially these structures could be preserved if the surrounding prostatic tissue was indeed benign. Recent studies using MRI have shown great promise in detecting and locating focal areas of prostatic cancer. For prostate cancer localised in the anterior prostate, transition zone (TZ) and/or in anterior fibromuscular stroma (AFMS), an en bloc template surgical excision of this part of the gland could preserve intact the posterior-lateral parts of the distal urethra, peripheral zone (PZ), and periprostatic tissues. This in turn would impact functional results, such as stress incontinence and erectile function. Short-term oncological and functional outcomes were recently published [1]. The objective of the present study was to describe a step-by-step guide to robot-assisted anterior partial prostatectomy (RA-APP) for isolated MRI-detected anterior prostate cancer (APC).
Patients and Methods
This single-arm single-centre Idea, Development, Evaluation, Assessment and Long-term evaluation of innovative surgery (IDEAL) phase 2a study for APP was approved by our Institutional Review Board. Detailed information was given to the patients and signed consent was requested. Inclusion and exclusion criteria have been previously described [1]. Inclusion criteria comprised an MRI-targeted biopsy confirmed predominant APC, based on MRI findings, i.e. at a distance of at least 17 mm (biopsy core length) from the rectal surface, with a negative posterior 12 systematic biopsy series. Between January 2008 and November 2015, 28 patients fulfilled entry criteria and 17 (60%) signed the consent form. Therefore, 17 patients were enrolled and underwent RA-APP at our institution. RA-APP completion of prostatectomy in four cases with cancer recurrence was performed at 0.3, 2.5, 2 and 2 years, respectively.
Perioperative data included estimated blood loss, operative time, intraoperative and postoperative complications (Table 1). Five surgeons performed at least one RA-APP. Three surgeons (I.S.G., G.P.H., M.M.D.) did only one case. One surgeon completed two cases (V.F.), while one surgeon completed 12 cases (A.V.). All surgeons had experience of ≥50 RA radical prostatectomies. The RA completion of prostatectomies in the four cases with cancer recurrence were performed by two surgeons (A.V. and V.F.).
Table 1.
Perioperative and postoperative clinical data of the 17 patients who underwent RA-APP [1].
| Variable | Value |
|---|---|
| Operative time, min, median (IQR) | 150 (148–188) |
| Fluid loss (blood and urine), mL, median (IQR) | 300 (200–400)* |
| Bladder catheter removal, days, median (IQR) | 7 (6–7) |
| Postoperative complications, n (%) | |
| Clavien–Dindo grade II | 2 (12), urinary infection treated with antibiotics |
| Clavien–Dindo grade II | 1 (6), transient anastomotic leak (case #2) that resolved spontaneously with 10-day catheter drainage |
| Clavien–Dindo grade II | 1 (6), transient intestinal ileus (case #1) that resolved with trans-anal exsufflation tube. |
After the first five consecutive surgeries in a 18-month period, there were no transfusions. IQR, interquartile range.
Results
Oncological perioperative and functional results have been previously described [1].
Step-by-step Instructions (accompanying video)
Patient Positioning and RA Approach
All procedures were performed by one of five surgeons (A.V., I.S.G., M.M.D., G.P.H. and V.F.) using a Da Vinci® S surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
Patient positioning and port placement were identical to the transperitoneal RA radical prostatectomy procedure [2,3] After dropping the bladder, the prevesical space was developed. The surgical protocol comprised en bloc template excision of the anterior part of the prostate comprising of the AFMS, prostate adenoma TZ and median lobe with the proximal urethra, anterior part of the distal (sub-montanal) urethra, and the anterior BN. The posterolateral PZ and periprostatic tissues were preserved intact (Fig. 1).
Fig. 1.
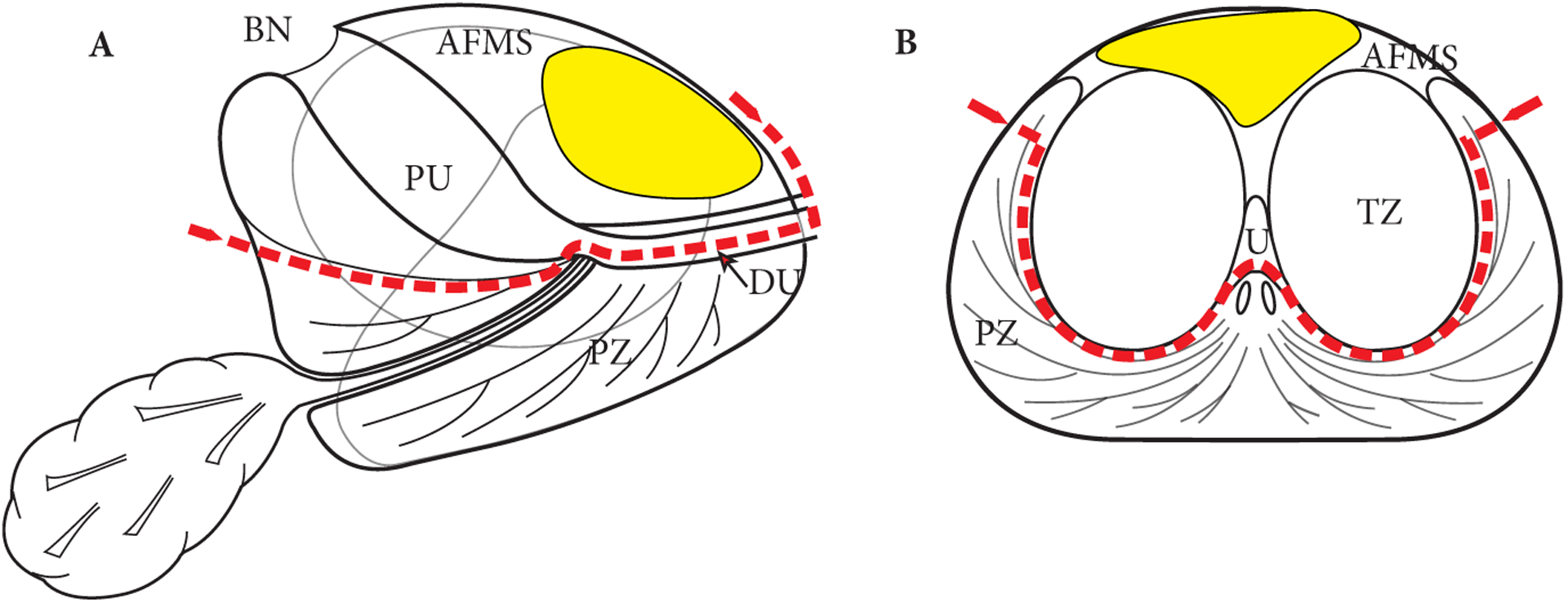
Schematic view of the prostate. (A) Sagittal and (B) Transverse at the mid-gland. The red dotted line shows the dissection plane of the APP. The average APC is depicted in yellow. The protocol comprises en bloc template excision of the anterior part of the prostate including the AFMS, prostate adenoma (TZ and median lobe) with the proximal urethra (PU), the anterior part of the distal (sub-montanal) urethra (DU), the most anterior apical parts of the PZ, and the anterior BN.
Three steps of dissection were identified as follows: Step 1, retrograde dissection of the PZ and TZ at the apex; Step 2, antegrade dissection of the TZ lobe at the BN; and Step 3, lateral sectioning of the PZ. This strategy ensured adequate exposure of the en bloc template excision, even in cases of distortion of the zonal anatomy due to retraction of the gland anteriorly and in cases of bleeding.
Step 1 - Retrograde Dissection of the PZ and TZ at the Apex
The prostate was exposed without removing the fatty tissue anterior to the prostate to avoid anterior positive margins at this level (Fig. 2). A 1-cm opening of the reflection of the endopelvic fascia was made on each side at the prostate-urethral junction. The neurovascular bundles were not exposed, as the plane of dissection remains anterior to the 3 and 9 o’clock location, and lateral to the urethra. The dorsal venous complex was secured and divided (Fig. 3), which facilitated access to the anterior and lateral urethral wall. The anterior half of urethra was transected at the apex. The lateral walls of distal urethra length were divided at the 3 and 9 o’clock positions with cold scissors along its course using a retrograde approach (Fig. 4A). Incision lines were traced with cauterisation on both sides at the anterolateral periprostatic fasciae surfaces, to facilitate PZ dissection at the mid gland, allowing further dissection as anterior retraction and displacement during the procedure of the prostate gland (Fig. 2).
Fig. 2.
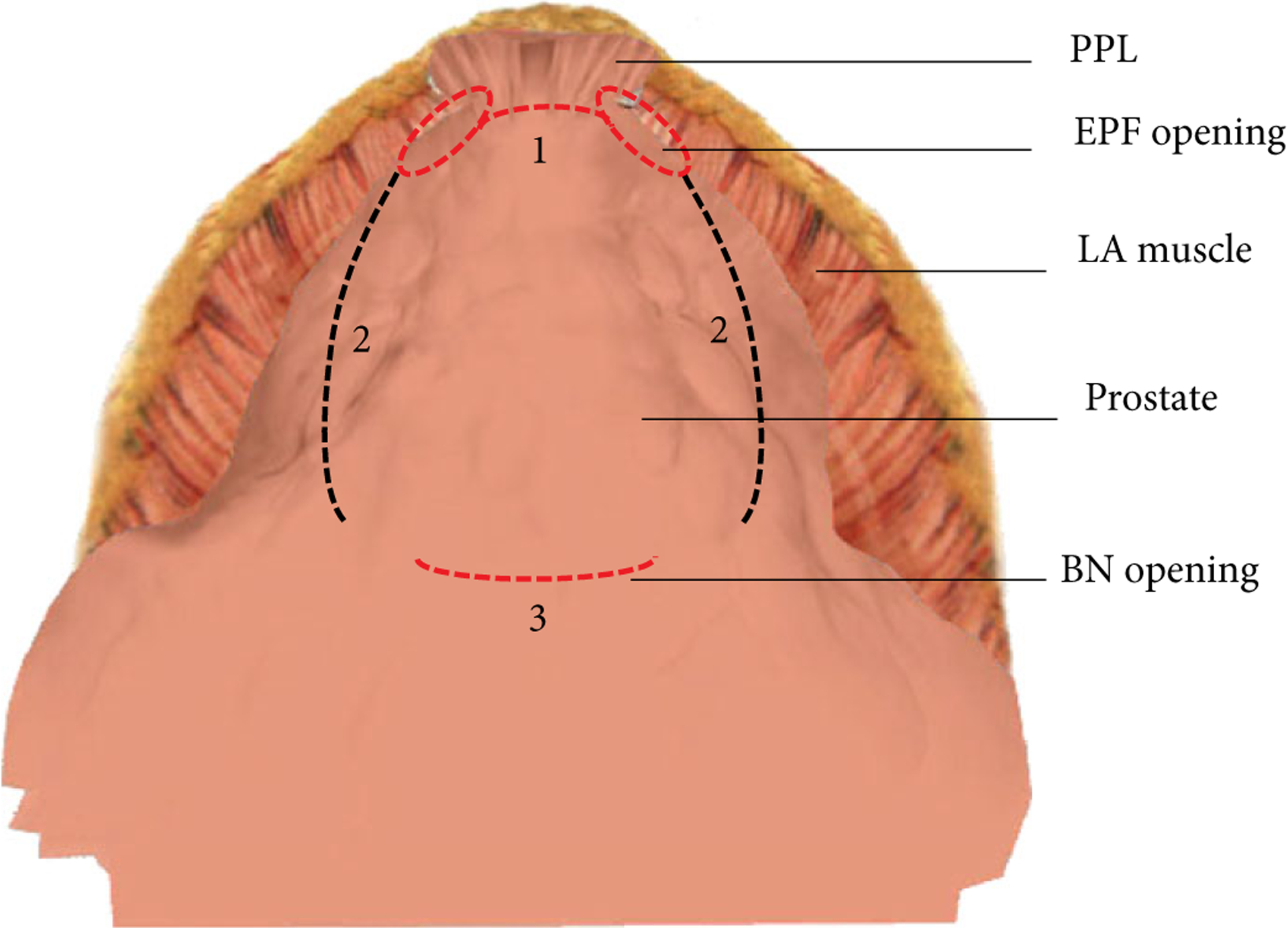
Intraoperative exposure of the anterior prostate surface covered by anterior fascia showing: 1. Endopelvic fascia (EPF) opening, a 1-cm opening of the reflexion of the EPF lateral to the pubo-prostatic ligaments is necessary to give access to lateral urethral wall and striated sphincter. 2. BN opening. 3. Incision lines traced with cauterisation on both sides at the anterolateral periprostatic fascia surfaces as landmarks to facilitate PZ sectioning at the mid-gland. LA, levatori ani; PPL. pubo-prostatic.
Fig. 3.
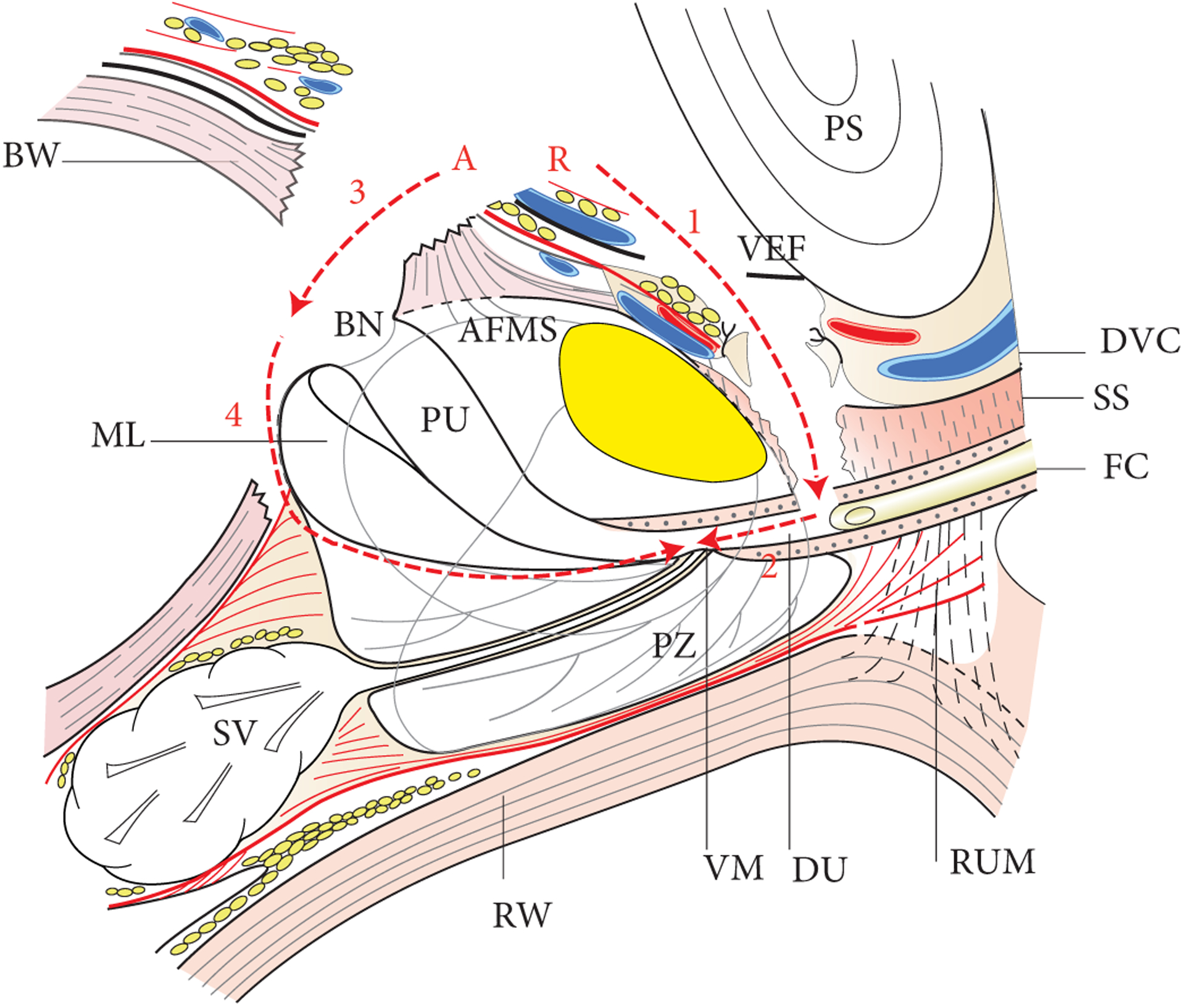
Sagittal section of prostate, bladder, seminal vesicles, urethra and periprostatic fascia. The average APC is depicted in yellow. The red dotted line shows the dissection plane of the APP. Step 1 (1), retrograde (R): division of dorsal venous complex (DVC) (1 - arrow) and anterior half of the urethra at the apex (2 - arrow). Step 2, antegrade (A): division of the anterior BN (3 - arrow), division of the posterior neck and median lobe enucleation (4 - arrow). BW, bladder wall; DA, detrusor apron; DU, distal urethra; EPF, endopelvic fascia; FC, Foley catheter; ML, median lobe; PRS, pre-rectal space; PS, pubic symphysis; PU, proximal urethra; RUM, recto-urethralis muscle; RW, rectum wall; SS, striated sphincter; SV, seminal vesicles; VM, verumontanum.
Fig. 4.
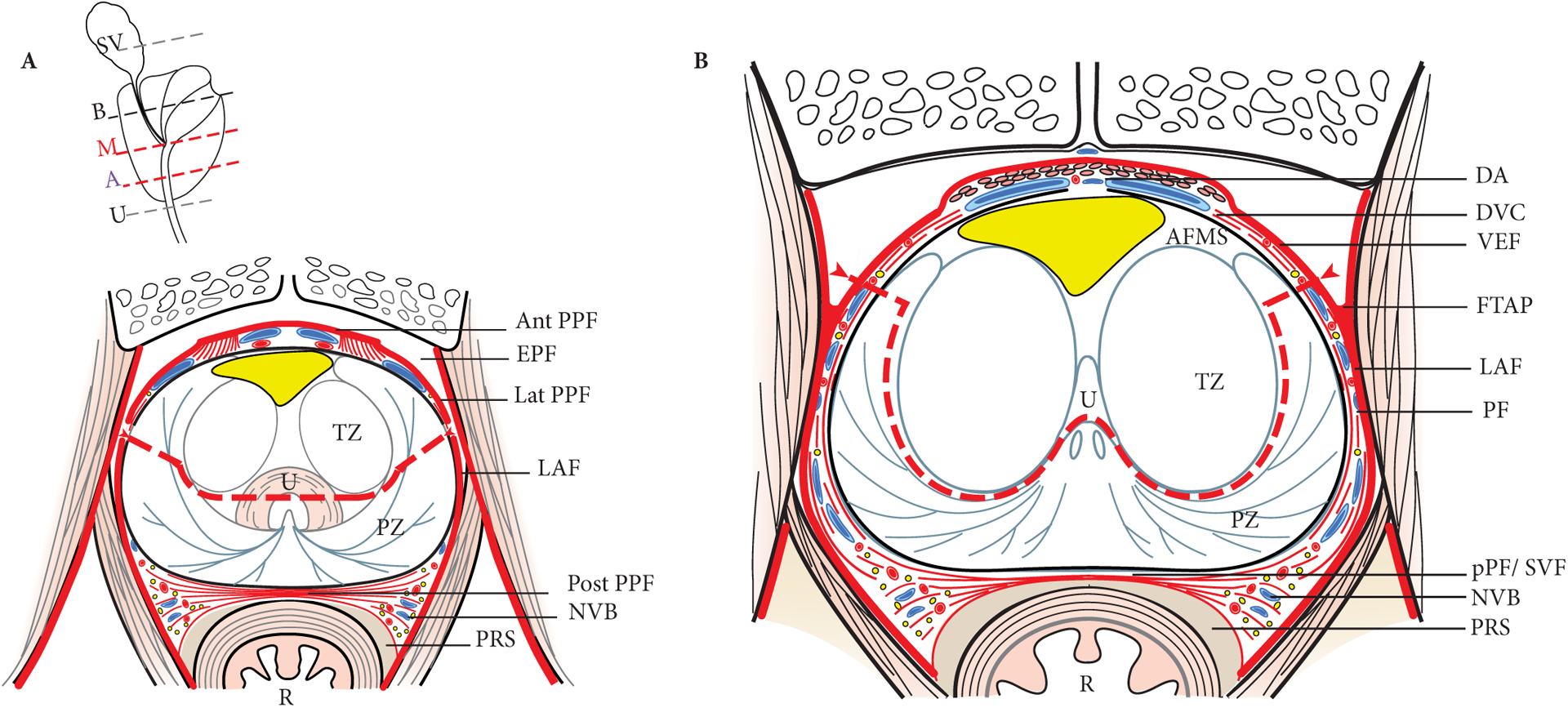
Transverse section of prostate and periprostatic fasciae at the level of the apex (A) and mid-gland (B). The red dotted lines show the dissection plane of the APP. The lateral parts of the PZ are divided at the base and mid-gland, and along a plane (arrows) that is above the level of the reflexion of the endopelvic fascia [EPF and fascial tendinous arch of the pelvis (FTAP)] on the prostate. The average APC is depicted in yellow. Ant PPF, anterior periprostatic fascia; DA detrusor apron; DVC, dorsal venous complex; LAF, levatori ani fascia; Lat PPF, lateral periprostatic fascia; NVB, neurovascular bundle; Post PPF (DF), posterior periprostatic fascia (Denonvilliers’ fascia); PRS, pre-rectal space; R, rectum; U, urethra.
The apical PZ lobes were divided, at the 3 and 9 o’clock positions with cold scissors using a retrograde approach without significant bleeding requiring haemostasis. The apical TZ lobes were enucleated up to the verumontanum using a retrograde approach. The verumontanum and posterior half of urethra were maintained intact.
Step 2 - Antegrade Dissection of TZ lobe at the BN
The anterior and posterior BN was divided (Fig. 3). The BN was then opened slightly proximal to the prostate to avoid positive margins. The specimen was retracted anteriorly. The ureteric orifices were exposed. The posterior BN incision was made at the edge of the median and lateral lobes of the adenoma, up to the glandular surface of the adenoma. The adenoma enucleation plane was developed bluntly and sharply, posteriorly up to the verumontanum. The anterior and anterolateral aspects of the adenoma lobes were not enucleated, keeping the anterior attachments to the AFMS intact. The PZ anterolateral lobes were divided at the mid-gland (Fig. 4B). The pre-seminal space outside the prostate was not entered. The anterior and anterolateral aspects of the adenoma were not dissected, which allowed us to keep the anterior attachments to the AFMS intact (Fig. 3).
Step 3 - Lateral Sectioning of the PZ
The lateral parts of the PZ were divided at the mid-gland, and specifically excised along a coronal plane anterior to the level of the reflection of the endopelvic fascia on the prostate. This was joined to the previous retrograde adenoma enucleation plane and PZ sectioning at the mid-gland along the incision line (Fig. 4B). At this step, the en bloc anterior prostate was only attached by the urethra wall, at the upper limit of the verumontanum, which was divided. The retrograde and antegrade dissections joined at the verumontanum. The PZ lateral parts were divided anterolaterally. The proximal urethra posterior wall was divided with cold scissors at the upper limit of the verumontanum. The specimen was placed in a bag and extracted for pathological evaluation.
Inspection of the remaining part of the PZ was made to assess haemostasis in the prostatic bed, and to evaluate if any residual adenoma lobules were remaining. The edges of the PZ were located (Fig. 5A).
Fig. 5.
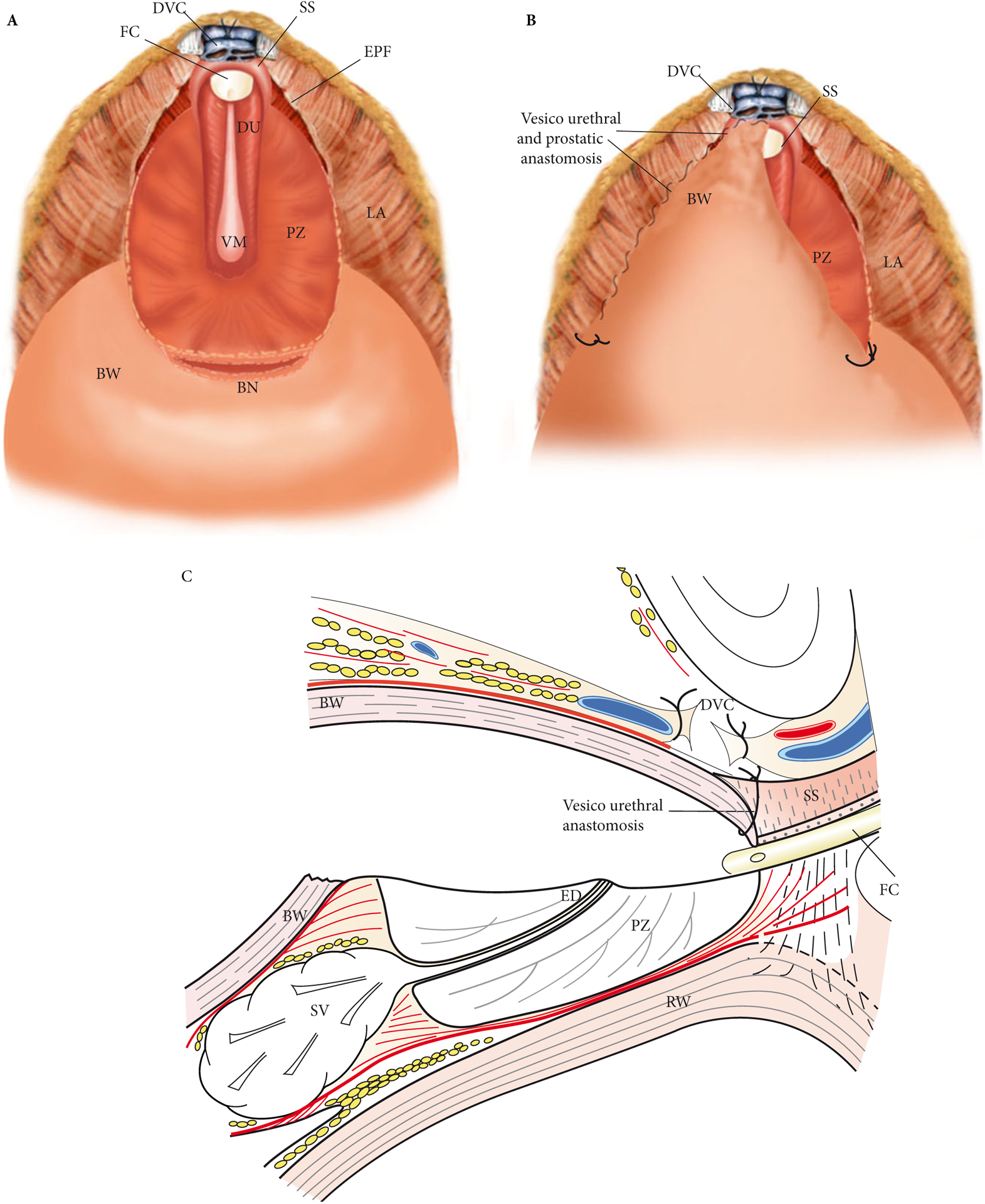
(A) Intraoperative exposure of the remaining PZ. The verumontanum (VM) and distal posterior half of the urethra (DU) are left intact. (B) Intraoperative view of the vesicourethral anastomosis, which is made by suturing the edges of the bladder opening, advancing an anterior bladder flap to the lateral PZ edges and to the anterior half of the urethra with two running sutures. (C) Schematic sagittal view of the vesico-urethral anastomosis. BW, bladder wall; DVC, dorsal venous complex; DU, distal urethra; ED, ejaculatory ducts; EPF, endopelvic fascia; FC, Foley catheter; LA, levatori ani muscle; RW, rectum wall; SS, striated sphincter; SV, seminal vesicles.
Bladder Suturing to the Urethra and PZ
Reconstruction was performed by suturing the edges of the bladder opening, and advancing an anterior bladder flap to the anterior remaining half of the urethra using two 3/0 V-lock running sutures (Fig. 5B). The BN was then sutured to the edges of the PZ. The sutures were placed at the angles of the BN to facilitate exposure. Once the anterior half of the urethra was sutured, the edges of the PZ were then sutured to the bladder opening on each side to obtain a watertight urethro-prostato-vesical anastomosis (Fig. 5C). An 18-F Foley catheter was left in situ for 7 days without irrigation. A bulb suction drain was placed in the retropubic space.
Operative Results
The template en bloc RA-APP was technically feasible in all 17 cases. A cystogram was taken at day 7 for the first three cases, which revealed an anastomotic leak in one patient (case #2). No intraoperative complications were identified. Perioperative complications included Clavien–Dindo grade II only. These complications were as follows: anastomotic leak (two cases, 12%), urinary tract infection (one case, 6%), and transient intestinal ileus (one case, 6%) (Table 1).
RA Completion of Prostatectomies
The RA-APP was performed in four cases due to cancer recurrence [1]. In these cases complete nerve-sparing prostatectomy was performed as a transperitoneal RA procedure. The anterior aspect of the bladder wall was divided at the level of the PZ upper limit at a distance of 3 cm from the urethral rhabdosphincter. The endopelvic fascia was incised laterally, and a margin of bladder wall covering the PZ (previously sutured to the PZ lateral edges) was excised to ensure removal of the APC recurrence site. The posterior BN was then divided and posterior and lateral dissection of the PZ performed. The urethral wall was incised at the prostato-urethral junction at the level of the previous bladder suturing.
Discussion
Our initial experience with RA-APP showed that the technique is feasible and safe. None of the participating surgeons felt that the RA-APP was more difficult than standard RA prostatectomy, all of whom had expertise in RA prostatectomy, provided they followed the surgical steps in order.
Technical Points of View about the RA-APP
We feel that the order of the three steps of dissection we described needs to be followed: Step 1, retrograde apical; Step 2, antegrade at BN; and Step 3, lateral dissections. The apical dissection from the apex to the level of the verumontanum is unusual to urological surgeons and needs to be performed when the anterior part of the prostate is still attached to the rest of the PZ. The surgeon can at this step rely on the prostate contours at the prostato-urethral junction and then lateral part of the PZ to divide the gland along a coronal plane, along the TZ enucleation plane medially and through the PZ anterolateral horns laterally. The landmark is the posterior half of the distal urethra. The division plane should stay at the 3 and 9 o’clock positions from this landmark. The verumontanum is a fixed point in prostate anatomy, and on average is 1.5 cm from the prostato-urethral junction and 1.5 cm from the posterior PZ surface, regardless of prostate weight or BPH enlargement (unpublished personal data). Therefore, division of the PZ anterolateral horns laterally can rely on this landmark and ensure enough safety benign tissue margins for cancer, mainly anterior to the TZ. The other steps are more familiar to urologists, i.e. antegrade adenoma enucleation, plane development and lateral division anterior to endopelvic fascia surfaces, even in cases of distortion of the zonal anatomy due to retraction of the gland anteriorly.
We also identified prostate volume (should be >42 mL) as an important factor for success of the procedure [1]. Hence, the technical challenge of PP is not at the apex or at the anterolateral aspect of the gland where the dissection planes are similar to radical prostatectomy; the challenge is to ensure negative margins posterolaterally at the PZ site. The larger the prostate gland volume, the longer the thickness of safety margin of benign tissue posterior to the APC. The specific role of prostate volume in the technique was not prospectively evaluated. However, as for open simple prostatectomy, the enucleation plane is easier to find and develop in high-volume glands (> 40–50 mL).
Difficulties or Specific Potential Complications during RA Completion of Prostatectomy in Four Cases due to Cancer Recurrence
We were not concerned about the posterolateral inter-prostato-rectal dissection, as these planes were not entered during the PP surgery. However, we discussed using a transperitoneal approach through the rectovesical pouch of Douglas to perform posterior seminal vesicles and inter-prostato-rectal dissection and choose that approach for the first case. We did not encounter difficulties at this part of the dissection and therefore decided not do it for the three other cases and to stay with the posterior BN opening approach. However, due to the small size of the residual prostate (PZ only), RA completion of prostatectomy was judged as difficult, as the prostate lateral contours, fused to the bladder wall, were not easy to see and palpate. BN suturing was not judged difficult.
Limitations of the study include the small sample size. The decision to analysis the results after 17 patients was established empirically (median follow-up >24 months reached) [1]. However, some potential surgical difficulties may not have been encountered in these 17 cases and in the four cases who had completion prostatectomy.
Conclusion
PZ prostate sparing RA-APP for isolated APC is feasible and safe, and represents an option for highly selected men with APCs as an alternative to other focal ablative therapy.
Supplementary Material
Video S1. 5 min 47 s (available at: https://www.dropbox.com/scl/fi/svotq3uwun86ntr/PAP%20short%20movie.mov?oref=wn&sm=1)
Acknowledgment
We thank Nelson N. Stone, MD for his assistance in manuscript editing.
Abbreviations:
- AFMS
anterior fibromuscular stroma
- APC
anterior prostate cancer
- APP
anterior partial prostatectomy
- BN
bladder neck
- PZ
peripheral zone
- RA
robot-assisted
- TZ
transition zone
Footnotes
Conflicts of Interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Villers A, Puech P, Flamand V et al. Partial prostatectomy for anterior cancer: short-term oncologic and functional outcomes. Eur Urol 2016. [Epub ahead of print]. DOI: 10.1016/j.eururo.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biffl WL, Spain DA, Reitsma AM et al. Responsible development and application of surgical innovations: a position statement of the Society of University Surgeons. J Am Coll Surg 2008; 206: 1204–9 [DOI] [PubMed] [Google Scholar]
- 3.Montorsi F, Wilson TG, Rosen RC et al. Best practices in robot-assisted radical prostatectomy: recommendations of the Pasadena Consensus Panel. Eur Urol 2012; 62: 368–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. 5 min 47 s (available at: https://www.dropbox.com/scl/fi/svotq3uwun86ntr/PAP%20short%20movie.mov?oref=wn&sm=1)


