Abstract
Background:
Coarctation of the aorta (CoA) typically requires repair, but re-interventions and vascular complications occur, particularly with associated defects like bicuspid aortic valve (BAV). Magnetic resonance imaging (MRI) may identify anatomic and hemodynamic factors contributing to clinical complications.
Purpose:
To investigate 4D flow MRI characteristics in pediatric CoA to determine parameters for long-term clinical surveillance.
Study type:
Retrospective.
Population:
CoA (n = 21), CoA with BAV (n = 24), BAV alone (n = 29), and healthy control (n = 25).
Field strength/sequence:
A 1.5 T, 3D CE IR FLASH MRA, 4D flow MRI using 3D time resolved PC-MRI with velocity encoding.
Assessment:
Thoracic aorta diameters were measured from 3D CE-MRA. Peak systolic velocities and wall shear stress were calculated and flow patterns were visualized throughout the thoracic aorta using 4D flow. Repair characteristics, re-interventions, and need for anti-hypertensive medications were recorded.
Statistics:
Descriptive statistics, ANOVA with post hoc t-testing and Bonferroni correction, Kruskal-Wallis H, intraclass correlation coefficient, Fleiss' kappa.
Results:
Patients with CoA with or without repair had smaller transverse arch diameters compared to BAV alone and control cohorts (P < 0.05), higher peak systolic flow velocities and wall shear stress compared to controls in the transverse arch and descending aorta (P < 0.05), and flow derangements in the descending aorta. The most common CoA repairs were extended end-to-end anastomosis (n = 22/45, 48.9%, age at repair 1 ± 2 years, seven re-interventions) and stent/interposition graft placement (n = 10/45, 22.2%, age at repair 12 ± 3 years, one re-intervention). Anti-hypertensive medications were prescribed to 33.3% (n = 15/45) of CoA and 34.4% of BAV alone patients (n = 10/29).
Data conclusions:
Despite repair, CoA alters hemodynamics and flow patterns in the transverse arch and descending aorta. These findings may contribute to vascular remodeling and secondary complications. 4D flow MRI may be valuable in risk stratification, treatment selection and postintervention assessment. Long-term, prospective studies are warranted to correlate patient and MRI factors with clinical outcomes.
Evidence level:
3 TECHNICAL EFFICACY: Stage 3.
Keywords: 4D flow, cardiac, coarctation of the aorta, congenital heart disease, pediatrics
Coarctation of the aorta occurs in 5%-7% of patients with congenital heart disease.1 Along with other left sided obstructive lesions, it is frequently associated with bicuspid aortic valve (BAV), which is the most common congenital cardiovascular abnormality affecting 1%–2% of the general population.2, 3 Patients deemed to have hemodynamically significant coarctation typically undergo early surgical repair.1 Although there are well-established surgical techniques developed for coarctation repair, there is risk for re-intervention, particularly in the setting of other associated defects such as BAV.3 Additionally, patients require life-long follow-up due to potential late complications related to the intervention and/or secondary to arteriopathies such as aortic aneurysm, dissection, hypertension, and brain aneurysms.1, 4-6 MRI can potentially play an important role in surveillance for these complications; however, there is limited data regarding hemodynamic changes postcoarctation repair and their associations with co-factors such as BAV.7, 8
The ability to visualize and quantify flow-dependent parameters in patients with vascular disease may provide an understanding of the complex hemodynamic mechanisms resulting in clinically significant outcomes. Focal lesions like coarctation may have effects on global aortic hemodynamics serving as a potential mechanism for the development of secondary aortopathy. Four-dimensional (4D) flow MRI studies have shown that changes in aortic 3D blood flow characteristics, such as deranged helix or vortex flow and elevated peak velocities, can be associated with the development of aortic dilation and aneurysms.9-11 Hemodynamic alterations can result in changes in aortic wall shear stress, which have been shown in BAV patients to promote endothelial cell dysfunction and may ultimately lead to vascular remodeling and aortic wall degeneration.12-15 Outside of BAV, assessments of complex aortic diseases with 4D flow MRI have been typically described in small studies or case reports in the pediatric population.8, 16, 17 In a small subgroup analysis by Allen et al, coarctation of the aorta was suggested to alter hemodynamics in both the ascending and descending aorta, but detailed hemodynamic analysis in a larger study is warranted.12
The aim of this 4D flow MRI study was to systematically investigate hemodynamic changes in patients with congenital coarctation of the aorta and the additional effects of BAV compared to healthy controls with trileaflet aortic valves.
Materials and Methods
Study Population
All studies performed were in accordance with our institutional review board (IRB) standards. Informed consent was obtained per IRB requirements for the 4D flow acquisition in patients otherwise undergoing clinically indicated cardiac MRI in this HIPAA-compliant study. Consent was provided by legal guardians for patients under the age of 18. IRB approval included clinical chart review with data housed in a de-identified HIPAA-compliant database.
The pediatric 4D flow institutional database was retrospectively queried from 2011 to 2018 for all unique patients with the diagnosis of coarctation of the aorta with and without BAV. Cohorts of patients with BAV alone and with normal trileaflet aortic valves were also identified and analyzed for comparison. The BAV alone and normal trileaflet aortic valve datasets have been used previously for other institutional studies. Patients were excluded if they had a genetic syndrome or underwent aortic valve repair. Characteristics of patient cohorts (control n = 25, coarctation n = 21, BAV n = 29, coarctation + BAV n = 24) are described in Table 1. Since patients had varying numbers of scans at different ages and follow-up times, the most recent MRI for each unique patient was evaluated. Clinical chart review was performed to record repair characteristics and re-interventions for coarctation cohorts (n = 45) and the need for anti-hypertensive medications in coarctation and BAV cohorts (n = 74).
TABLE 1.
Comparison of Patient Characteristics and Arch Dimensions
| Control (TAV) | Coarctation | BAV | Coarctation + BAV | ||
|---|---|---|---|---|---|
| N = 25 | N= 21 | N = 29 | N= 24 |
P value* |
|
| Age atscan (years) | 15 ±4 | 16 ± 5 | 15 ±4 | 16± 11 | 0.20 |
| Male, Female | 13, 12 | 12,9 | 23, 6 | 16, 8 | - |
| BSA(m2) | 1.6± 0.2 | 1.6 ± 0.3 | 1.7± 0.4 | 1.5 ± 0.5 | 0.33 |
| Ej ec tion fr ac tion (%) | 57 ±7 | 58 ± 5 | 57 ± 5 | 58 ±3 | 0.87 |
| AoRZ-score | 0.5 ±0.8 | 0.4 ± 1.5 | 2.6± 1.5 | 1.9 ± 1-7 | <0.05 |
| Mid-AAo Z-score | −0.5 ±0.7 | −1.0± 1.3 | 3.2 ± 2.1 | 0.6±1.9 | <0.05 |
| Trans Arch Z-score | −0.1 ±0.7 | −1.5 ± 1.4 | 0.4 ± 1.2 | −1.7 ± 1.6 | <0.05 |
| DAoDimensionMin(mm) | 15 ±2.2 | 14 ± 4.3 | 16 ±2.4 | 16 ±4.7 | 0.43 |
| DAoDimensionMax(mm) | 17 ± 3.0 | 17 ± 3.7 | 18 ±2.7 | 18 ±4.9 | 0.19 |
TAV = trileaflet aortic valve; BAV = bicuspid aortic valve; BSA = body surface area; AoR = aortic root; AAo = ascending aorta;
Trans Arch = transverse arch; DAo= descending aorta.
P-values are from AN OVA testing to identify differences among all cohorts.
MR Imaging
All subjects underwent standard-of-care cardiothoracic MRI using a 1.5 T MRI system (Aera, Siemens, Erlangen, Germany). Two-dimensional (2D) breath-held and electrocardiogram (ECG)-gated cine balanced steady-state free precession (bSSFP) were acquired in two chamber, three chamber, four chamber, and ventricular short-axis planes (temporal resolution [TR] = 3.1 msec; echo time [TE] = 1.3 msec; section thickness = 6 mm for young adults and 5 mm for children; field of view = 320 mm × 240 mm for young adults and 280–300 mm × 188–225 mm for children).
Global cardiac function via ejection fraction was calculated using the short-axis 2D cine bSSFP images (Medis Suite MR, Leiden, The Netherlands). 3D contrast enhanced (gadofosveset trisodium 0.03 mmol/kg or gadobutrol 0.15 mmol/kg), ECG gated, and respiratory navigator triggered inversion recover-prepared (IR-FLASH) MR angiography (CE-MRA) was also performed (adults and adolescents (>12 years): TR = 3.2 msec, TE 1.2 msec, pixel size 1.5 × 1.5 mm, field of view = 340 mm × 310 mm, slice thickness 1.5 mm, flip angle 18°; children (6–12 years): TR = 3.3 msec, TE 1.3 msec, pixel size 1.3 × 1.3 mm, field of view = 300 mm × 280 mm, slice thickness 1.3 mm, flip angle 18°; infants (0–1 year): TR = 3.6 msec, TE 1.5 msec, pixel size 1.0 × 1.0 mm, field of view = 220 mm × 200 mm, slice thickness 0.9 mm, flip angle 18°).
4D flow MRI allowed for the in vivo measurement of time-resolved 3D blood flow velocities with full coverage of the thoracic aorta. 4D flow MRI consisted of prospectively ECG triggered and high-efficiency respiratory navigator-gated 3D time-resolved PC-MRI with three-directional velocity encoding.18-20 4D flow MRI pulse sequence parameters were set based on patient size, age, and peak velocities: TE = 2.3–3.0 msec, TR = 37.6–45.6 msec, field of view = 250–350 mm × 156–360 mm, spatial resolution = 1.9–4.4 mm × 1.4–2.2 mm × 1.4–2.7 mm, velocity sensitivity = 80–400 cm/sec to avoid aliasing, and parallel imaging (GeneRalized Autocalibrating Partial Parallel Acquisition, GRAPPA) with an acceleration factor of R = 2–5.
Data Analysis: Aortic Dimensions
Thoracic aortic diameters were measured from the 3D CE-MRA exams using a dedicated 3D workstation with multiplanar reformatting capabilities (Vitrea, Vital Images, Minneapolis, MN, USA). Maximum orthogonal aortic diameters were measured from inner-edge to inner-edge at the sinuses of Valsalva, mid-ascending aorta (mid-AAo), distal transverse aortic arch, and descending aorta (DAo) at the level of the left atrium. All dimensions were measured by our 3D MRI lab technologists blinded to clinical data. To evaluate interobserver reliability, a second observer (V.G., 10 years MR experience) independently measured aortic arch dimensions in 20% of randomly chosen patients. To account for the range of patient age and body size, aortic z-scores were calculated for each patient based on body surface area (BSA) as defined by the Mosteller equation using EchoIMS (Merge Healthcare, Chicago, IL, USA).21 Z-scores are not available for DAo dimensions, so the largest and smallest absolute diameters were used for comparison between groups.
Data Analysis: Aortic 4D Flow MRI
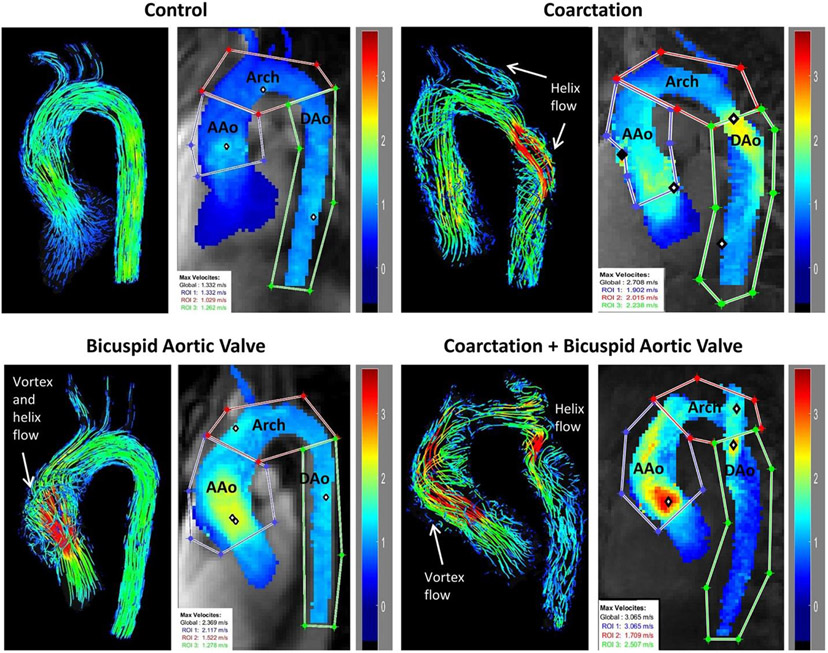
Data analysis included corrections for phase offset errors (eddy currents, Maxwell terms) and velocity aliasing using a custom-built tool programmed in Matlab (Mathworks, Natick, MA, USA).22 3D aortic segmentation using 3D PC-MRA images (Mimics, Materialise, Leuven, Belgium) was used to mask the 4D flow velocity data and to calculate a peak systolic velocity maximum intensity projection (MIP), where the maximum peak systolic velocity magnitude was projected onto a sagittal-oblique plane through the thoracic aorta.23 As illustrated in Fig. 1, region of interest analysis (regions drawn by H.B., 2 years of cardiovascular MR and data analysis experience) was used to quantify aortic peak systolic velocities in the AAo, ransverse arch, and proximal DAo.
FIGURE 1.
4D pathline images comparing flow patterns and maximum intensity projections (MIPs) of aortic peak systolic velocities in the ascending aorta (AAo), transverse arch (Arch), and descending aorta (DAo).
Aortic blood flow visualization was performed by color-coded 3D pathlines (EnSight, Canonsburg, PA, USA). Helix and vortex secondary flow patterns in the AAo and DAo were graded by three observers (L.D., 3 years MR experience; H.S., 2 years MR experience; H.B., 2 years MR experience) based on previously reported qualitative methods.24-26 A four-point scale was used as none (0), mild (1): one 360° turn, moderate (2): two 360° turns, and severe (3): three or more 360° turns. Vortex flow was defined as particles revolving around an axis orthogonal to the vessel centerline. Helix flow was defined as particles revolving around the longitudinal axis of the vessel centerline (i.e. corkscrew-like pattern). To evaluate interobserver reliability, the three observers independently graded flow throughout the arch in 30% of randomly chosen patients.
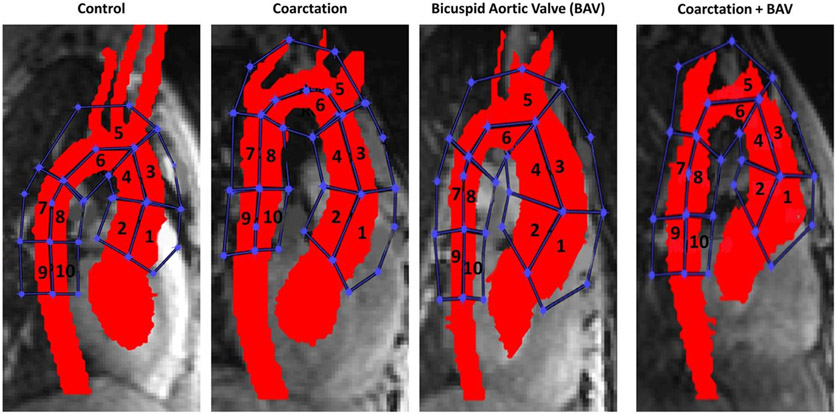
Wall shear stress for each cohort was derived from the velocity data for each analysis region (drawn by H.B., 2 years of cardiovascular MR and data analysis experience) by interpolating the local velocity derivative on the lumen contour using b-splines.27 Wall shear stress was calculated along 10 segments of the aortic wall including the AAo, transverse arch, and DAo as illustrated in Fig. 2.
FIGURE 2.
Ten regions were analyzed for wall shear stress in each cohort. Wall shear stress was increased in the coarctation group compared to controls in regions 5, 6, 7, and 8. Wall shear stress was increased in the coarctation + bicuspid aortic valve (BAV) group compared to BAV alone in regions 5 and 6.
Statistics
Patient characteristics were summarized using descriptive statistics that included means, standard deviations, medians, and interquartile ranges. Parameters were assessed for normality using a Lilliefors test. ANOVA was used for parametric comparison of groups. A two-sided P-value < 0.05 was considered statistically significant. If significance was found, post hoc t-testing was performed. Bonferroni correction was applied to post hoc analysis with P-value < 0.008 considered statistically significant (P < 0.05/6). For evaluation of flow grading, Kruskal–Wallis H testing was used to compare groups. Intraclass correlation coefficient (ICC) was calculated to determine interobserver variability in thoracic aortic arch dimension measurements. Fleiss’ kappa statistic (0.01–0.20 = poor agreement, 0.21–0.40 = fair agreement, 0.41–0.60 = moderate agreement, 0.61–0.80 = substantial agreement, and 0.81–1.00 = near perfect agreement) was calculated to determine interobserver variability in flow grading. All statistical analysis was performed using R version 3.6.1 (R Core Team 2019, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics and Arch Dimensions
There were no significant differences between control, coarctation, BAV, or coarctation + BAV cohorts in age at scan (15 ± 4 years, 16 ± 5 years, 15 ± 4 years, 16 ± 11 years; P = 0.20), BSA (1.6 ± 0.2 m2, 1.6 ± 0.3 m2, 1.7 ± 0.4 m2, 1.5 ± 0.5 m2; P = 0.33) or ejection fraction (57% ± 7%, 58% ± 5%, 57% ± 5%, 58% ± 3%, P = 0.87) (Table 1). There were statistically significant differences between control, coarctation, BAV, and coarctation + BAV cohorts in aortic root (0.5 ± 0.8, 0.4 ± 1.5, 2.6 ± 1.5, 1.9 ± 1.7, P < 0.05), AAo (−0.5 ± 0.7, −1.0 ± 1.3, 3.2 ± 2.1, 0.6 ± 1.9, P < 0.05), and transverse arch (−0.1 ± 0.7, −1.5 ± 1.4, 0.4 ± 1.2, −1.7 ± 1.6, P < 0.05) z-scores (Table 1). Post hoc analysis showed that aortic root and AAo z-scores were significantly greater in the BAV alone vs. controls with trileaflet aortic valves and coarctation + BAV vs. coarctation alone (all P < 0.008). Transverse arch z-scores were significantly smaller in the coarctation cohorts compared to controls and BAV alone even after repair (all P < 0.008). ICC for measurements between observers was 0.98 (C.I. 0.93–0.99) indicating excellent reliability.
Coarctation Cohort Characteristics
Repair characteristics for coarctation patients are summarized in Table 2. The most common initial coarctation repair in both cohorts was extended end-to-end anastomosis with or without a reverse subclavian flap (n = 22/45, 48.9%) with an average age at repair of 1 ± 2 years (Table 2). The second most common type of repair was placement of a stent or interposition graft at the site of coarctation (n = 10/45, 22.2%) with a much older average age at initial repair of 12 ± 3.2 years (Table 2). Seven out of eight re-interventions occurred in patients who initially had repair via extended end-to-end anastomosis and/or reverse subclavian flap repair, with six of these patients having initial repair at <6 months of age. The other patient requiring re-intervention had stent placement at 16 years of age and subsequent stent migration requiring re-positioning. Anti-hypertensive medications were being used by 33.3% (n = 15/45) of patients with a history of coarctation with or without BAV (Table 2). A similar percentage of patients were on anti-hypertensives in the BAV alone cohort (n = 10/29, 34.4%, not shown in table format).
TABLE 2.
Clinical Characteristics and Outcomes of Coarctation Cohorts
| Coarctation (N = 21) |
Coarctation + BAV (N = 24) |
Age at Repair (Years) |
|
|---|---|---|---|
| Type of initial coarctation repair (n) | |||
| Extended end-to-end anastomosis | 7 | 12 | 1 ± 2 |
| Reverse subclavian flap repair | 1 | 3 | 0.24 ± 0.23 |
| End-to-end anastomosis + reverse subclavian flap repair | 2 | 1 | <0.01 ± 0.01 |
| Patch aortoplasty | 1 | 0 | 7a |
| Stent/interposition graft | 4 | 6 | 12 ± 3.2 |
| Unknown | 4 | 1 | 0.6 ± 0.8 |
| No surgery | 2 | 1 | N/A |
| Re-interventions (n) | 3 | 5 | |
| Anti-hypertensive medications (n) | 7 | 8 |
Reported age is for the one patient who had this type of repair. BAV = bicuspid aortic valve.
Arch Velocities and Flow Grades
Scan times for 4D flow were between 8 minutes and 12 minutes. Aortic arch velocities and composite flow grades (vortex plus helix) throughout the aortic arch are shown in Table 3. There were statistically significant differences in peak systolic velocities between control, coarctation, BAV, and coarctation + BAV cohorts in the AAo (1.6 ± 0.1 m/sec, 1.8 ± 0.1 m/sec, 2.3 ± 0.5 m/sec, 1.9 ± 0.3 m/sec, P < 0.05), transverse arch (1.3 ± 0.1 m/sec, 1.9 ± 0.3 m/sec, 1.6 ± 0.1 m/sec, 1.8 ± 0.2 m/sec, P < 0.05), and DAo (1.4 ± 0.03 m/sec, 1.8 ± 0.2 m/sec, 1.5 ± 0.1 m/sec, 1.7 ± 0.3 m/sec, P < 0.05). Peak systolic flow velocities were increased in the coarctation cohort compared to controls throughout the aortic arch but with statistical significance in the transverse arch and DAo (post hoc P = 0.02, P < 0.008, and P < 0.008 for the AAo, transverse arch, and DAo, respectively). In the AAo, the peak systolic flow velocity was significantly increased in the BAV alone cohort compared to control and coarctation cohorts (all post hoc P < 0.008).
TABLE 3.
Comparison of Aortic Arch Velocities and Flow Characteristics
| Control (TAV) |
Coarctation | BAV | Coarctation + BAV |
|||
|---|---|---|---|---|---|---|
| N = 25 | N = 21 | N = 29 | N = 24 | P value | ||
| Peak Systolic Velocity (m/s) | AAo | 1.6 ± 0.1 | 1.8 ± 0.1 | 2.3 ± 0.5 | 1.9 ± 0.3 | <0.05 |
| Trans | 1.3 ± 0.1 | 1.9 ± 0.3 | 1.6 ± 0.1 | 1.8 ± 0.2 | <0.05 | |
| Arch | ||||||
| DAo | 1.4 ± 0.03 | 1.8 ± 0.2 | 1.5 ± 0.1 | 1.7 ± 0.3 | <0.05 | |
| Composite Flow Derangement3 | AAo | 0 | 0 | 3 (3-4) | 3 (0-4) | <0.05 |
| DAo | 0 | 1 (0-3) | 0 | 1 (1-5) | <0.05 |
TAV = trileaflet aortic valve; BAV = bicuspid aortic valve; AAo = ascending aorta; Trans Arch = transverse arch; DAo = descending aorta.
Helicity plus vorticity.
There were statistically significant differences in flow derangement grading between control, coarctation, BAV, and coarctation + BAV cohorts in the AAo (0, 0, 3 (3–4), 3 (0–4), P < 0.05) and DAo (0, 1 (0–3), 0, 1 (1–5), P < 0.05). Based on flow grading, an increased amount of flow derangement was present in the AAo in the BAV cohorts and in the DAo in the coarctation cohorts (Table 3). Interobserver agreement was moderate for aortic arch flow grading with a kappa value of 0.54 (C.I. 0.50–0.58) between the three observers.
Figure 1 shows a comparison of aortic peak velocity MIPs and 4D flow pathline visualizations in a representative subject for each of the four cohorts (control, coarctation, BAV, coarctation + BAV). The coarctation patients had elevated peak velocities throughout the arch compared to the control patient and complex high-velocity flow jet patterns in the transverse arch and proximal DAo near the sites of surgical repair (Fig. 1, right). In the coarctation + BAV patient, substantial helix and vortex flow derangement can be seen in both the AAo and DAo (Fig. 1, bottom right). In comparison, flow derangement in the BAV alone patient was confined to the AAo (Fig. 1, bottom left) and in the coarctation alone patient was confined to the DAo (Fig. 1, top right). The control subject had no flow derangement (Fig. 1, top left).
Wall Shear Stress
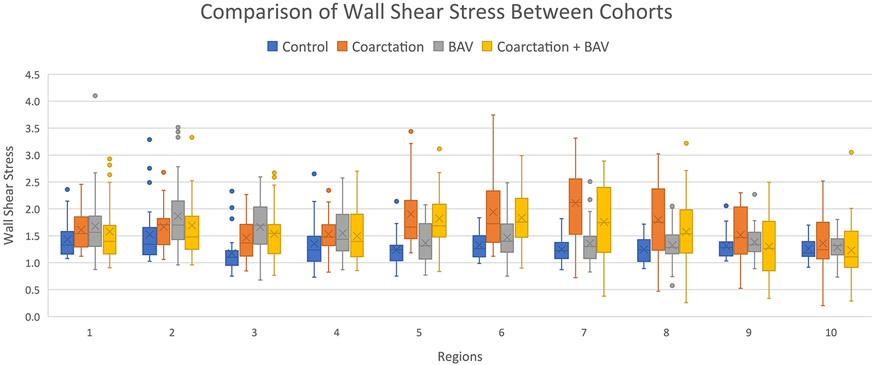
Figure 2 illustrates the 10 regions of interest along the aortic arch analyzed for wall shear stress in each cohort. The max wall shear stress values for each region and cohort are shown in Fig. 3. Wall shear stress was increased along the transverse arch and proximal DAo in the coarctation group vs. controls (region 5, region 6, region 7, region 8, P < 0.008) and along the transverse arch and proximal DAo in the coarctation + BAV group vs. BAV alone (region 5, region 6, P < 0.008). Figure 4 provides an illustration summarizing the results throughout the aortic arch.
FIGURE 3.
Box plots with interquartile ranges of the max wall shear stress values for each aortic arch region and cohort. Wall shear stress was increased in the coarctation group compared to controls in regions 5–8. Wall shear stress was increased in the coarctation + bicuspid aortic valve (BAV) group compared to BAV alone in regions 5 and 6.
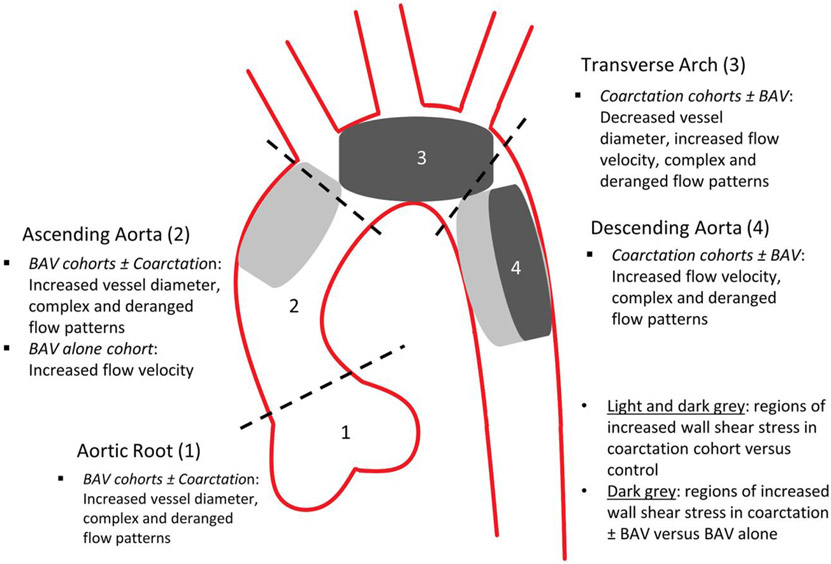
FIGURE 4.
Diagram showing summary of results by aortic arch region. BAV = bicuspid aortic valve.
Discussion
MRI surveillance is important to monitor progression of aortic disease and effects of repair.7, 8, 28 This study demonstrated that coarctation of the aorta can alter hemodynamics and flow patterns throughout the aortic arch and these changes may require further consideration of anatomic, surgical, and physiologic patient factors. 4D flow analysis may offer additional benefits to conventional MRI, particularly in patients with complex disease, by allowing visualization of flow derangements before and after repair and evaluation of advanced parameters like wall shear stress to better understand clinical complications. Currently, it is common for children with congenital heart disease repairs to undergo echocardiograms on an annual/periodic basis. There is not a current consensus on the frequency these patients should have cross-sectional imaging, particularly in the pediatric population.
As potentially expected based on the typical location for coarctation of the aorta and sites of surgical repair, patients with coarctation predominantly had hemodynamic and qualitative flow changes in the transverse and descending aortic arch. This included increased peak systolic velocities, complex flow derangements and increased wall shear stress. When the coarctation + BAV cohort was compared to the BAV cohort alone to account for any changes that may be due to BAV, the wall shear stress was increased in the coarctation patients along the transverse arch (including along the ostia of the head and neck vessels) and along the proximal DAo suggesting a relationship between peak systolic velocities and wall shear stress. Despite repair, coarctation cohorts continued to have decreased transverse arch diameters compared to healthy controls and BAV alone.
Recent literature suggests that the caliber of the vessel may have more effect on hemodynamic measures and flow changes (eg, backward compression waves) than curvature or “shape” of the vessel.29 In the Quail et al study, increased backward compression waves were seen in patients with a large AAo narrowing to a relatively smaller distal arch and in patients with transverse arch hypoplasia.29 This would suggest, based on the data in this study, that the patients in the coarctation + BAV cohort should be evaluated for these pathologic wave reflections in future studies. The possible relationship between vessel caliber, increased velocities, wall shear stress, and flow derangements and the development of complications such as aneurysms in these regions should be evaluated in larger cohorts, as these factors may help determine when re-interventions are needed.
It is well reported that patients with coarctation need long-term follow-up due to associated morbidities, including systemic hypertension.1, 4-6, 28 These are typically thought of as “late findings” and repaired patients have a shorter life expectancy presumably due to cardiovascular complications and risk of early stroke.28, 30, 31 Studies have also shown an increased prevalence of intracranial aneurysms in patients with BAV and aortic arch abnormalities, and this prevalence is even greater if these anomalies are present together.4, 32 In this study, approximately one-third of patients in both coarctation cohorts remained on anti-hypertensive medications—suggesting that despite repair, vascular changes may lead to increased systemic vascular resistance in some patients. Since the average age at the time of scan was in the teenage years for all cohorts, the longest “follow-up period” from repair was for the patients who underwent repair at a young age. The main repair type in the younger patients included extended end-to-end anastomosis, which can create a focal scar at the site of surgical anastomosis.28 Older patients typically underwent stent or graft placement. Based on recent literature, this can create a rigid and noncompliant region in the aorta and may be associated with less remodeling of left ventricular structure and function.28, 33 The younger patients with surgical repairs had more re-interventions, which may be explained by their need for surgery at a young age and the longer period of time for vascular remodeling to occur and hemodynamic changes to take place. Interestingly, a similar portion of the BAV patients was on anti-hypertensives despite not undergoing repairs. A multicenter pediatric study of 75 patients evaluated for possible associations between treatment modalities (surgical repair, balloon dilation, and stent placement) and major vascular outcomes of global aortic stiffness, endothelial function, and systemic hypertension.28 The aim was to determine whether the treatment modalities could have different effects on vascular integrity of the repaired arterial segments. No statistically significant differences were found.28
This study provides an approach for evaluating hemodynamic changes in coarctation patients. Evaluation of preoperative and postoperative scans in patients who require re-intervention may provide further insight, and prospective long-term follow-up studies are needed to determine which specific patient and treatment factors may predict clinical outcomes. More detailed imaging of head and neck vasculature should be considered in future 4D flow studies in patients with complex aortic arch repairs, with correlation of the findings to potential neurologic morbidities as well.
Although not a primary focus of this study, the elevated peak systolic velocities and flow derangements found in the AAo of the BAV cohorts are supported by previous literature showing AAo flow abnormalities in BAV patients.12-14 Similar to the peak systolic velocity trend in our study (2.3 ± 0.5 m/sec in AAo, 1.6 ± 0.1 m/sec in transverse arch, 1.5 ± 0.1 m/sec in DAo), in Allen et al, the peak systolic velocity in the AAo of BAV patients was 1.3 ± 0.4 m/sec, which was higher than in the transverse arch (1.1 ± 0.3 m/sec) and DAo (1.0 ± 0.2 m/sec).12 In Bissell et al, 76% of BAV patients had helical flow derangement in the AAo (n = 72/95), 13% had “complex flow” in the AAo (n = 12/95), and 36% had helical flow derangement in the DAo (n = 34/95).13 Although we did not see altered flow derangement in the DAo in our BAV cohort, this may be due to the younger age of our patient cohort and thus less time for disease progression.
Limitations
MRI is a valuable tool for surveillance of patients with repaired congenital heart disease, but more longitudinal data are required to determine the effect of hemodynamic alterations on patient outcomes. The main limitation of this study is the inability to follow patients in a planned longitudinal manner due to the retrospective nature. Most children did not undergo MRI evaluation until they were able to tolerate the study without anesthesia unless there were clinical concerns that dictated otherwise. In addition, since patients had varying numbers of scans at different ages and follow-up times, we analyzed only the most recent MRI for each unique patient for consistency. Drawing clinical conclusions regarding the implications of particular surgeries within each cohort was difficult due to the limited number of pediatric patients available for analysis. This limitation is also in part due to differences in management approaches based on surgical era. This study provides detailed observations and demonstrates feasibility of 4D flow analysis that can guide parameters for long-term follow-up to determine clinical correlations.
Conclusions
Coarctation repair alters hemodynamics throughout the aortic arch. Abnormalities are particularly present in the transverse arch and descending aorta with increased flow derangement, wall shear stress, and flow velocities. Despite repair, patients with a history of coarctation continue to have smaller transverse arch segments. The presence of BAV in these patients can alter additional hemodynamic parameters, particularly in the aortic root and ascending aorta. These findings may contribute to vascular remodeling and secondary complications that are well described in the literature (eg, hypertension). 4D flow may be an increasingly valuable tool in the risk stratification, treatment selection and postintervention assessment of cardiovascular patients. Long-term, prospective follow-up studies are warranted to correlate MRI parameters with specific clinical outcomes.
Acknowledgments
Victor Billy Guerra (B.S., CT and MRI Technologist) for second observer arch measurements to ensure reliability. This work is supported by National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number TL1TR001423. National Institutes of Health, Grant Number R01HL115828. National Institutes of Health National Institute of Biomedical Imaging and Bioengineering R21EB024315. American Heart Association, Grant Number 19TPA34850066.
References
- 1.Torok RD, Campbell MJ, Fleming GA, Hill KD. Coarctation of the aorta: Management from infancy to adulthood. World J Cardiol 2015;7: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niaz T, Poterucha JT, Johnson JN, et al. Incidence, morphology, and progression of bicuspid aortic valve in pediatric and young adult subjects with coexisting congenital heart defects. Congenit Heart Dis 2017;129:261–269. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto A, Ota N, Miyakoshi C, et al. Mid to long-term aortic valve related outcomes after conventional repair for patients with interrupted aortic arch or coarctation of the aorta combined with ventricular septal defect: The impact of bicuspid aortic valve. Eur J Cardiothorac Surg 2014;46:952–960. [DOI] [PubMed] [Google Scholar]
- 4.Connolly HM, Huston J, Brown RD Jr, Warnes CA, Ammash NM, Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: A prospective magnetic resonance angiography study of 100 patients. Mayo Clin Proc 2003;78:1491–1499. [DOI] [PubMed] [Google Scholar]
- 5.Brown ML, Burkhart HM, Connolly HM, et al. Coarctation of the aorta: Lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol 2013;10(62):1020–1025. [DOI] [PubMed] [Google Scholar]
- 6.Lee MG, Babu-Narayan SV, Kempny A, et al. Long-term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart 2019;105:1190–1196. [DOI] [PubMed] [Google Scholar]
- 7.Puranik R, Tsang VT, Puranik S, et al. Late magnetic resonance surveillance of repaired coarctation of the aorta. Eur J Cardiothorac Surg 2009;36:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hope MD, Meadows AK, Hope TA, et al. Clinical evaluation of aortic coarctation with 4D flow MR imaging. J Magn Reson Imaging 2010;31: 711–718. [DOI] [PubMed] [Google Scholar]
- 9.Bürk J, Blanke P, Stankovic Z, et al. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J Cardiovasc Magn Reson 2012;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hope TA, Markl M, Wigstrom L, Alley MT, Miller DC, Herfkens RJ. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J Magn Reson Imaging 2007;26:1471–1479. [DOI] [PubMed] [Google Scholar]
- 11.Van Ooij P, Markl M, Collins JD, et al. Aortic valve stenosis alters expression of regional aortic wall shear stress: New insights from a 4-dimensional flow magnetic resonance imaging study of 571 subjects. J Am Heart Assoc 2017;6:e005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen BD, van Ooij P, Barker AJ, et al. Thoracic aorta 3D hemodynamics in pediatric and young adult patients with bicuspid aortic valve. J Magn Reson Imaging 2015;42:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: Flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 2013;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic 3D outflow patterns, wall shear stress and expression of aortopathy. Circulation 2014;129:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-related hemodynamics mediate human bicuspid aortopathy: Insights from wall shear stress mapping. J Am Coll Cardiol 2015;66:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hope MD, Meadows AK, Hope TA, et al. Evaluation of bicuspid aortic valve and aortic coarctation with 3D flow magnetic resonance imaging. Circulation 2008;117:2818–2819. [DOI] [PubMed] [Google Scholar]
- 17.Desai LP, Berhane H, Husain N, Robinson JD, Rigsby CK, Markl M. Altered 4-D magnetic resonance imaging flow characteristics in complex congenital aortic arch repair. 2020;50:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyverfeldt P, Bissell M, Barker AJ, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015; 17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stankovic Z, Allen B, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diag Ther 2014;4:173–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markl M, Harloff A, Bley TA, et al. Time-resolved 3D MR velocity mapping at 3T: Improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25:824–831. [DOI] [PubMed] [Google Scholar]
- 21.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 22.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: Optimized analysis of blood flow and vessel wall parameters. Magn Reson Med 2008;60: 1218–1231. [DOI] [PubMed] [Google Scholar]
- 23.Schnell S, Smith DA, Barker AJ, et al. Altered aortic shape in bicuspid aortic valve relatives influences blood flow patterns. Eur Heart J Cardiovasc Imaging 2016;17:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger J, Markl M, Jung B, et al. 4D-MR flow analysis in patients after repair for tetralogy of Fallot. Eur Radiol 2011;21:1651–1657. [DOI] [PubMed] [Google Scholar]
- 25.Geiger J, Markl M, Herzer L, et al. Aortic flow patterns in patients with Marfan syndrome assessed by flow-sensitive four dimensional MRI. J Magn Reson Imaging 2012;35:594–600. [DOI] [PubMed] [Google Scholar]
- 26.Rose MJ, Jarvis K, Chowdhary V, et al. Efficient method for volumetric assessment of peak blood flow velocity using 4D flow MRI. J Magn Reson Imaging 2016;44:1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potters WV, van Ooij P, Marquering H, van Bavel E, Nederveen AJ. Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. J Magn Reson Imaging 2015;41:505–516. [DOI] [PubMed] [Google Scholar]
- 28.Martins JD, Zachariah J, Tierney ES, et al. Impact of treatment modality on vascular function in coarctation of the aorta: The LOVE-COARCT study. J Am Heart Assoc 2019;8:e011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quail MA, Segers P, Steeden JA, Muthurangu V. The aorta after coarctation repair – Effects of calibre and curvature on arterial haemodynamics. J Cardiovasc Magn Reson 2019;21:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickard SS, Gauvreau K, Gurvitz M, et al. A national population-based study of adults with coronary artery disease and coarctation of the aorta. Am J Cardiol 2018;122:2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickard SS, Gauvreau K, Gurvitz M, et al. Stroke in adults with coarctation of the aorta: A national population-based study. J Am Heart Assoc 2018;7:e009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egbe AC, Padang R, Brown RD, et al. Prevalence and predictors of intracranial aneurysms in patients with bicuspid aortic valve. Heart 2017;103:1508–1514. [DOI] [PubMed] [Google Scholar]
- 33.Egbe AC, Anderson JH, Ammash NM, Taggart NW. Left ventricular remodeling after transcatheter versus surgical therapy in adults with coarctation of aorta. J Am Coll Cardiol Cardiovasc Imaging 2020;13: 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]