Abstract
Objective:
The objective was to compare the resolution of organ dysfunction, 28-day mortality, and biochemical markers in children with thrombocytopenia-associated multiple organ failure who received therapeutic plasma exchange versus no therapeutic plasma exchange.
Design:
Observational longitudinal cohort study.
Setting:
Nine U.S. PICUs.
Patients:
Eighty-one children with sepsis-induced thrombocytopenia-associated multiple organ failure.
Interventions:
Therapeutic plasma exchange.
Measurements and Main Results:
Adjusted relative risk for 28-day mortality was modeled using standard multivariate regression with propensity score weighting to reduce covariate confounding. Change from baseline Pediatric Logistic Organ Dysfunction scores between therapeutic plasma exchange and no therapeutic plasma exchange differed in temporal pattern during the first week (p = 0.009). By day 4, mean Pediatric Logistic Organ Dysfunction score declined by 7.9 points (95% CI, −10.8 to −5.1) in the therapeutic plasma exchange–treated group compared with no change with no therapeutic plasma exchange. Use of therapeutic plasma exchange was associated with reduced 28-day mortality by multivariate analysis (adjusted relative risk, 0.45; 95% CI, 0.23–0.90; p = 0.02) and by propensity score weighting (adjusted relative risk, 0.46; 95% CI, 0.22–0.97; p = 0.04).
Conclusions:
Therapeutic plasma exchange use in thrombocytopenia-associated multiple organ failure was associated with a decrease in organ dysfunction. After accounting for several risk factors, 28-day all-cause mortality was lower in children treated with therapeutic plasma exchange compared with those receiving no therapeutic plasma exchange. A multicenter randomized clinical trial is necessary to determine a causal relationship.
Keywords: multiple organ failure, sepsis, therapeutic plasma exchange, thrombocytopenia, thrombocytopenia-associated multiple organ failure, thrombotic microangiopathy
Severe sepsis in children remains a worldwide dilemma, occurring in over 75,000 cases in the United States annually, with an estimated attributable cost of nearly 5 billion dollars (1). In general, children die of sepsis due to multiple organ dysfunction with occurrence rate of mortality directly proportional to the number of organ failures (2-4). Thrombocytopenia-associated multiple organ failure (TAMOF) is a distinct clinical phenotype of sepsis associated with thrombotic microangiopathy. The clinical syndrome is heralded by 1) thrombocytopenia, 2) microangiopathic hemolysis, 3) hemodynamic failure, and 4) ischemic organ failure that shift TAMOF patients toward a prothrombotic and antifibrinolytic state (5-7). Children meeting TAMOF criteria have higher organ dysfunction, severity of illness scores, and mortality than in other phenotypes of pediatric sepsis (8-10).
TAMOF is among a spectrum of syndromes associated with disseminated microvascular thromboses that include disseminated intravascular coagulopathy (DIC), thrombotic thrombocytopenic purpura (TTP), and hemolytic uremic syndrome (HUS) (5, 7). TTP and HUS are driven by dysregulated platelets due to the release of ultra-large von Willebrand factor (ULVWF) protein clusters by activated endothelium resulting in spontaneous platelet aggregation (11-13). A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) is a metalloprotease that cleaves ULVWF into smaller and less thrombogenic forms (14, 15). Sepsis induces many inflammatory mediators that directly inhibit or inactivate ADAMTS-13, and an ADAMTS-13 deficiency results in disseminated platelet-/VWF-rich microthrombi that are associated with sepsis-induced TAMOF, sometimes in the absence of DIC (16-20). Therapeutic plasma exchange (TPE) has become a standard therapy for patients with TTP/HUS because it replaces ADAMTS-13 and removes ULVWF and ADAMTS-13 inhibitors (11, 21). TPE has been similarly proposed as a biologically plausible treatment in children with TAMOF (11-15).
We created a multicenter TAMOF registry network to perform a prospective observational study with the primary objective of evaluating the response of pediatric patients with TAMOF with and without the use of TPE. We hypothesized that children meeting TAMOF criteria who received TPE would have improved measures of organ dysfunction, improvement of biochemical hemostatic markers compared with children receiving no TPE, and would have a higher likelihood of severity-adjusted 28-day survival.
METHODS
Study Design
Nine quaternary care PICUs in the United States participated in the TAMOF Network Study Group (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E202) led by Emory University/Children’s Healthcare of Atlanta at Egleston. We designed a prospective observational cohort study (ClinicalTrials.gov Identifier: NCT00118664) to evaluate the clinical course, biochemical profile, and outcome of children meeting criteria for TAMOF. Children 1 week to 21 years old were eligible for enrollment if they met the following inclusion criteria within 30 hours of screening: 1) new-onset organ failure in at least three out of five organ systems defined as an Organ Failure Index score greater than or equal to 3 for less than or equal to 30 hours; 2) thrombocytopenia as defined by a platelet count less than or equal to 100,000/μL or a minimum 50% decrease in baseline platelet count if the baseline platelet count was less than or equal to 100,000/μL; and 3) etiology of organ failure was due to systemic infection and shock based on International Pediatric Sepsis Consensus Conference definitions (22). Patients were excluded from enrollment if they had: 1) received TPE within the prior 30 days for any indication; 2) a terminal illness with a life expectancy of less than 60 days; or 3) a limitation on care, such as a decision to withdraw life-sustaining therapies, or orders to allow natural death. Written informed consent was obtained from a parent or legal guardian before study enrollment, and patient enrollment occurred before initiation of TPE. Institutional review board approval was obtained at each site.
Clinical Management
Approach to care and determination of TPE use was made at the discretion of the local treating physician. TPE was initiated using a standard plasma centrifugation technique with citrate as regional anticoagulant. Centers used a standard TPE protocol for TAMOF as described elsewhere (23). Briefly, patient plasma volume used was calculated based on a weight-based nomogram and patient hematocrit value. An initial (day 1) exchange of 1.5 times the calculated plasma volume was performed, followed by a single-volume exchange on days 2–5 (23). After 5 days, ongoing duration of daily exchanges continued at physician discretion. All centers used fresh frozen plasma for TPE.
Clinical Observations and Measurements
Demographic and clinical data were collected, including primary diagnosis resulting in TAMOF, vital signs, laboratory values, blood product administration, vasopressor needs, PICU and hospital length of stay, need for extracorporeal life support/extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT), and 28-day mortality. Organ Failure Index, Pediatric Index of Mortality, and Pediatric Logistic Organ Dysfunction (PELOD) scores were calculated at the time of study entry and consecutively. Platelet counts were obtained consecutively for each patient at the local site. Samples were obtained and processed for ADAMTS-13 activity, VWF antigen, and VWF ristocetin cofactor assays and analyzed at a single central research laboratory (T.N.).
Outcome Measures
The primary study outcome was longitudinal change in PELOD score in patients with or without TPE use. Secondary outcomes included assessment of 28-day all-cause mortality after controlling for baseline differences in severity of illness and organ dysfunction. Comparative changes in biochemical markers were also evaluated. Outcome was stratified by TPE compared with no TPE and by survival.
Study Size and Power Calculation
The study was powered for a mean improvement in PELOD score of 10 points in the TPE group and 5 points in the no TPE group. A sample size of 17 patients per group was determined to provide 80% power to detect a 5-point treatment difference in PELOD score, with an estimated sd for change of 5, using a two-sided two-sample equal variance t test at a 5% significance level.
Statistical Analysis
Demographic and baseline characteristics of TPE and no TPE groups were compared with a two-sample t test for continuous variables. Chi-square test or Fisher exact test for proportions was used for categorical variables. Repeated-measures analyses for total PELOD score, and change from baseline, and biochemical markers were done with a means model (SAS MIXED Procedure version 9.4, mixed linear models; SAS Institute Inc., Cary, NC). A compound symmetric variance-covariance form among the repeated measurements was assumed for each outcome, and robust estimates of sEs of parameters were used to perform statistical tests and construct 95% CIs (24). Specific statistical tests were done within the framework of the mixed-effects linear model. All statistical tests were two sided and unadjusted for multiple comparisons.
Relative risks (RRs) were calculated to measure degree of association between risk factors and mortality by fitting a modified Poisson regression model as described (SAS GENMOD Procedure, version 9.4, generalized linear models [25]). Predictors included in multivariate analysis were limited to main effects for TPE verses no TPE, baseline PELOD, ECMO, and methicillin-resistant Staphylococcus aureus (MRSA) infection. RR and 95% CI were calculated for each risk factor in the presence of others in the final model. Bootstrap bagging was used to identify stable and reliable predictors of mortality. Propensity score modeling was used as a robust approach to reduce the effects of covariate confounding (Supplementary Methods, Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
RESULTS
TAMOF Patient Characteristics
Eighty-one children meeting TAMOF criteria were enrolled in the study between April 2005 and July 2010: 21 children (26%) received standard care and 60 children (74%) received TPE. Demographic characteristics, baseline organ dysfunction, and severity of illness are shown in Table 1. Sepsis was the predominant admitting diagnosis (69/81, 85.2%), and noted in all patients, with MRSA (8/81, 9.9%) or methicillin-sensitive S. aureus (4/81, 4.9%) infection accounting for a substantial portion of bacteremia (12/81, 14.8%), respectively. A detailed list of organisms cultured from patients is shown in Supplementary Table 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
TABLE 1.
Demographic and Clinical Characteristics of Children With Thrombocytopenia-Associated Multiple Organ Failure by Treatment
| Overall (n = 81) |
No TPE (n = 21 [26%]) |
TPE (n = 60 [74%]) |
p a | |
|---|---|---|---|---|
| Characteristics | n/Total (%) | |||
| Male gender | 34/81 (42.0) | 8/21 (38.1) | 26/60 (43.3) | 0.80 |
| Hispanic ethnicity | 7/81 (8.6) | 1/21 (4.8) | 6/60 (10.0) | 0.67 |
| Race | ||||
| White | 53/81 (65.4) | 13/21 (61.9) | 40/60 (66.7) | 0.54 |
| Black | 16/81 (19.8) | 5/21 (23.8) | 11/60 (18.3) | |
| Other | 12/81 (14.8) | 3/21 (14.3) | 9/60 (15.0) | |
| Primary diagnosis | ||||
| Sepsis (Staphylococcus) | 8/81 (9.9) | 1/21 (4.8) | 7/60 (11.7) | 0.63 |
| Sepsis (non-Staphylococcus) | 61/81 (75.3) | 16/21 (76.2) | 45/60 (75.0) | |
| Nonsepsisb | 12/81 (14.8) | 4/21 (19.0) | 8/60 (13.3) | |
| Organism cultured from bloodstream infections | ||||
| Gram-positive | 21/81 (25.9) | 1/21 (4.8) | 20/60 (33.3) | NA |
| Methicillin-resistant Staphylococcus aureus | 8/81 (9.9) | 1/21 (4.8) | 7/60 (11.7) | |
| Methicillin-sensitive S. aureus | 4/81 (4.9) | 0/21 (0.0) | 4/60 (6.7) | |
| Gram-negative | 17/81 (20.9) | 3/21 (14.3) | 14/60 (23.3) | |
| Fungal/yeast | 9/81 (11.1) | 4/22 (19.1) | 6/60 (10.0) | |
| Viral | 3/81 (3.7) | 1/21 (4.8) | 2/60 (3.3) | |
| Influenza | 2/81 (2.5) | 0/21 (0.0) | 2/60 (3.3) | |
| Cytomegalovirus | 1/81 (1.3) | 1/21 (4.8) | 0/60 (0.0) | |
| Indication for plasma exchange (plasma exchange group only) | ||||
| TAMOF with suspected or confirmed sepsis | NA | NA | 58/60 (96.7) | NA |
| TAMOF nonsepsis as primary diagnosis | NA | NA | 2/60 (3.3) | |
| Duration of plasma exchange, median days (range) | ||||
| Overall (n = 60) | NA | NA | 5 (1–14) | NA |
| Survivors (n = 41) | 5 (1–10) | |||
| Ever on extracorporeal membrane oxygenation | 30/81 (37.0) | 4/21 (19.0) | 26/60 (43.3) | 0.07 |
| Ever on continuous renal replacement therapy | 46/81 (56.8) | 8/21 (38.1) | 38/60 (63.3) | 0.07 |
| Length of stay in survivors (d), median (IQR), n | ||||
| ICUc | 14 (11, 28), 53 | 13 (8, 20), 13 | 22 (12, 29), 40 | 0.10 |
| Hospitalc | 23 (17, 45), 53 | 17 (14, 49), 13 | 36 (19, 45), 40 | 0.54 |
| 28-d mortality | 27/81 (33.3) | 8/21 (38) | 19/60 (32) | 0.59 |
| Age (yr) | 8.6 (6.4), 81 | 7.0 (6.4), 21 | 9.1 (6.4), 60 | 0.20 |
| Age range (yr) | 0.1–20.3 | 0.3–19.5 | 0.1–20.3 | NA |
| Weight (kg) | 34.5 (27.5), 80 | 29.2 (24.2), 21 | 36.4 (28.5), 59 | 0.31 |
| Baseline Pediatric Logistic Organ Dysfunction score | 21.3 (11.4), 81 | 17.7 (9.4), 21 | 22.5 (11.9), 60 | 0.09 |
| Baseline Pediatric Index of Mortality score | 18.2 (6.8), 80 | 16.9 (5.7), 21 | 18.7 (7.1), 59 | 0.29 |
| Baseline Organ Failure Index score | 4.5 (1.2), 80 | 4.2 (1.0), 21 | 4.5 (1.2), 59 | 0.24 |
| Baseline platelet count (1,000 cells/μL) | 62.2 (42.1), 79 | 57.2 (35.2), 21 | 64.0 (44.5), 58 | 0.53 |
| Baseline A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13e | 52.9 (27.8), 37 | 63.7 (25.9), 8 | 49.9 (28.0), 29 | 0.22 |
| Baseline von Willebrand factor antigene | 161 (66.3), 37 | 217 (72.8), 8 | 146 (56.4), 29 | 0.005 |
| Baseline ristocetin cofactore | 118 (94.6), 36 | 199 (182), 7 | 98.1 (44.9), 29 | 0.009 |
IQR = interquartile range, NA = not applicable, TAMOF = thrombocytopenia-associated multiple organ failure, TPE = therapeutic plasma exchange.
Fisher exact test (two-tailed).
Other primary admitting diagnoses were as follows: cardiac lesions (3), primary lung disease (3), undefined shock (2), hematologic diseases (2), and systemic lupus erythematosus (2).
Median and IQR for ICU (days) and hospital (days) length of stay for 53 survivors. One patient in the TPE group has missing PICU discharge date and hospital discharge date (sample size 13 for the No TPE group). ICU days and hospital length of stay were compared between the two study groups with the Wilcoxon rank-sum test.
Two-sided two-sample t test.
At least one baseline biomarker value available for 37 patients.
Change in Organ Dysfunction With and Without TPE
Mean baseline PELOD score for TPE patients was not significantly higher than that for non-TPE patients (22.5 [95% CI, 19.5–25.5] vs 17.7 [95% CI, 13.7–21.6]; p = 0.05 by repeated-measures analysis; p = 0.09 by two-sample t test) (Supplementary Table 3, Supplemental Digital Content 1, http://links.lww.com/CCM/E202). However, the change from baseline in PELOD scores in the two treatment groups differed in temporal pattern during the first week on study (p = 0.009, test for interaction between time on study and treatment group) (Fig. 1; and Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E202). By day 4, mean PELOD had declined by 7.9 points (95% CI, −10.8 to −5.1; p = 0.006) with TPE, whereas it did not change in the no TPE group (p = 0.40, test for linear trend) (Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/CCM/E202). PELOD decreased over time but did not differ between survivors and nonsurvivors for the duration of TPE (Supplementary Fig. 2 and Supplementary Table 5, Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
Figure 1.
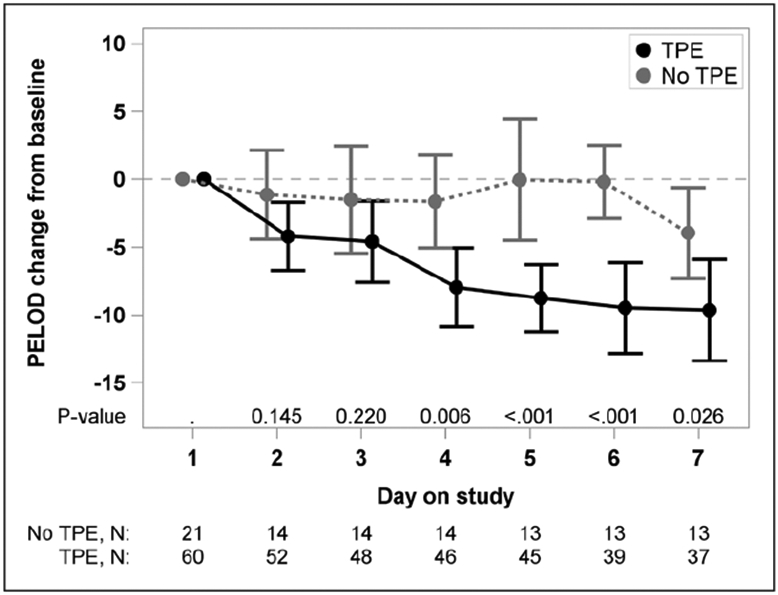
The mean change from baseline in Pediatric Logistic Organ Dysfunction (PELOD) score by treatment group (60 therapeutic plasma exchange [TPE]-treated thrombocytopenia-associated multiple organ failure [TAMOF] patients and 21 standard therapy–treated TAMOF patients). Time trend lines are the model-based means and 95% CIs. The vertical bars are the 95% CIs. Change from baseline in PELOD scores in the two treatment groups differed in temporal pattern during the first week on study (p = 0.009, test for interaction between time on study and treatment group). By day 4, the mean PELOD had declined by 7.9 points (95% CI, −10.8 to −5.1) in the TPE-treated group, but the mean PELOD score did not change over time in the no TPE group (p = 0.40, test for linear trend).
Mortality in TAMOF Patients With and Without TPE
Overall, 28-day all-cause mortality for children receiving TPE was 32% (19/60) and 38% (8/21) for children without TPE (p > 0.05). Regarding risk factors for death (Table 2), an increase of five points in baseline PELOD score was associated with increased RR of mortality (RR, 1.31; 95% CI, 1.19–1.43; p < 0.001). Use of ECMO (RR, 1.83; 95% CI, 1.00–3.35; p = 0.05), CRRT (RR, 2.17; 95% CI, 1.04–4.56; p = 0.04), and MRSA infection (RR, 2.01; 95% CI, 1.10–3.68; p = 0.02) were associated with an increased RR of mortality. Other demographic characteristics and days of TPE treatment were not associated with RR of death.
TABLE 2.
Univariable Analysis of Risk Factors Potentially Associated With 28-Day All-Cause Mortality for 81 Children With Thrombocytopenia-Associated Multiple Organ Failure
| Characteristics | Descriptive Statistics, n (%)/Median (Q1, Q3) |
Regression Coefficient |
se | Relative Risk |
95% CI | p |
|---|---|---|---|---|---|---|
| Male gender | 34 (42.0) | 0.1006 | 0.3151 | 1.11 | 0.60–2.06 | 0.75 |
| Black race (reference: white) | 16 (19.8) | 0.0346 | 0.4256 | 1.04 | 0.45–2.38 | 0.94 |
| Other race (reference: white) | 12 (14.8) | 0.5046 | 0.3563 | 1.66 | 0.82–3.33 | 0.16 |
| Age per 1-yr increase | 7.8 (2, 14.6) | 0.0357 | 0.0240 | 1.04 | 0.99–1.09 | 0.14 |
| Plasma exchange | 60/81 (74.1) | −0.1848 | 0.3367 | 0.83 | 0.43–1.61 | 0.58 |
| Baseline Pediatric Logistic Organ Dysfunction per 5-point increase | 21 (12, 31) | 0.0534 | 0.0095 | 1.31 | 1.19–1.43 | < 0.001 |
| Extracorporeal membrane oxygenation | 30/81 (37.0) | 0.6047 | 0.3089 | 1.83 | 1.00–3.35 | 0.05 |
| Continuous renal replacement therapy | 46/81 (56.8) | 0.7765 | 0.3776 | 2.17 | 1.04–4.56 | 0.04 |
| Methicillin-resistant Staphylococcus aureus infection | 12/81 (14.8) | 0.6994 | 0.3083 | 2.01 | 1.10–3.68 | 0.02 |
| Days on TPE (per 1-d increase; set to 0 for no TPE patients) | 3 (0, 6) | −0.1027 | 0.0722 | 0.90 | 0.78–1.04 | 0.15 |
TPE = therapeutic plasma exchange.
Multivariate analysis was performed with consideration of all univariable characteristics associated with the use of TPE (Table 3). Adjusted RR (ARR) of 28-day all-cause mortality was lower in TAMOF patients treated with TPE compared with those without TPE (ARR, 0.45; 95% CI, 0.23–0.90; p = 0.02). Propensity score weighting analysis was performed to estimate mortality by accounting for covariates that predicted a higher likelihood of receiving TPE using two methods to estimate TPE effect: 1) covariate adjustment using the propensity score; and 2) score inverse probability of treatment weights (IPTWs). Both models demonstrated a decrease in ARR of mortality in TAMOF patients with TPE compared with no TPE (ARR for propensity score, 0.46; 95% CI, 0.22–0.97; p = 0.04; and ARR for IPTW, 0.46; 95% CI, 0.22–0.87; p = 0.02) similar to the multivariate logistic regression model findings (Table 3). Adequacy of weighting of covariates between TPE- and non-TPE–treated TAMOF patients was confirmed (Supplementary Table 6, Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
TABLE 3.
Risk Factors for 28-Day All-Cause Mortality for 81 Children With Thrombocytopenia-Associated Multiple Organ Failure
| Risk Factors | Regression Coefficient |
se | Adjusted Relative Risk |
95% CI | p | % Reliabilitya |
|---|---|---|---|---|---|---|
| Standard multivariable analysis using modified Poisson regression | ||||||
| TPE | −0.7954 | 0.3506 | 0.45 | 0.23–0.90 | 0.02 | 61 |
| Baseline PELODb | 0.0519 | 0.0119 | 1.30 | 1.15–1.46 | < 0.001 | 99 |
| Extracorporeal membrane oxygenation | 0.2407 | 0.3229 | 1.27 | 0.68–2.40 | 0.46 | 17 |
| Continuous renal replacement therapy | 0.4663 | 0.3891 | 1.59 | 0.74–3.42 | 0.23 | 30 |
| Methicillin-resistant Staphylococcus aureus infection | 0.3044 | 0.2916 | 1.36 | 0.77–2.40 | 0.30 | 38 |
| Propensity score adjusted with propensity score specified as a continuous covariate | ||||||
| TPE | −0.7740 | 0.3808 | 0.46 | 0.22–0.97 | 0.04 | – |
| Baseline PELODb | 0.0487 | 0.0130 | 1.28 | 1.12–1.45 | < 0.001 | – |
| Propensity score adjusted with inverse probability of treatment weighting | ||||||
| TPE | −0.7775 | 0.3256 | 0.46 | 0.24–0.87 | 0.02 | – |
| Baseline PELODb | 0.0509 | 0.0142 | 1.29 | 1.12–1.48 | < 0.001 | – |
PELOD = Pediatric Logistic Organ Dysfunction, TPE = therapeutic plasma exchange.
Percentage of time risk factor appears in 1,000 bootstrap models. Risk factors appearing in at least 50% of models are reliable.
Adjusted relative risk and 95% CI reported per 5-point increase in baseline PELOD score. The reference category is no for TPE, extracorporeal membrane oxygenation, CVVH, and methicillin-resistant Staphylococcus aureus infection.
Dashes refer to that fact that the Reliability column applies only to the standard multivariable analysis using modified Poisson regression.
Biochemical Markers in TAMOF Patients With and Without TPE
ADAMTS-13 activity levels were similar at baseline for the two treatment groups (model-based means, 45.6% and 60.7%, respectively, for TPE and no TPE; p = 0.16). Patients with ADAMTS-13 deficiency less than 57% of baseline (23) were more likely to receive TPE (20/22, 91%) than those patients without ADAMTS-13 deficiency (9/15, 60%; p = 0.04). Neither the pattern of change of ADAMTS-13 activity (p = 0.16) nor difference between treatment groups over time (p = 0.71) were significantly different (Supplementary Fig. 3B, Supplemental Digital Content 1, http://links.lww.com/CCM/E202). However, mean ADAMTS-13 levels increased over time in the children receiving TPE (p < 0.001), whereas it did not change over time in the children not receiving TPE (p = 0.14, test for linear trend).
Children receiving TPE had significantly decreased VWF antigen levels at baseline and on days 1, 4, 8, and 15 compared with children receiving no TPE, but levels did not change over the time course compared with baseline levels in either group. Similarly, VWF ristocetin cofactor activity was lower at baseline in the TPE group, but no change was seen in this marker at baseline or after a week of therapy in either of the groups, either relative to baseline or between groups (Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
In children receiving TPE who survived, mean daily platelet count and ADAMTS-13 activity significantly increased over time compared with nonsurvivors (p < 0.001). VWF antigen and VWF ristocetin cofactor activity did not change over time (Supplementary Fig. 4, A-D, Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
Center Differences in Use of TPE
Use of TPE in TAMOF patients and mortality varied considerably (Supplementary Table 7, Supplemental Digital Content 1, http://links.lww.com/CCM/E202). Two centers used TPE in each TAMOF patient, two centers never used TPE, and the remaining five centers variably used TPE for children meeting TAMOF criteria. Median number of days of TPE used was 5 days (range, 1–14 d). Fifty-one percent of survivors received 5 days (range, 4–6) of TPE, whereas only 21% of TPE nonsurvivors (58% of deaths occurring in first 3 d) received that range. No difference was seen in the proportion of survivors and nonsurvivors who received 7–14 days of TPE (Cochran-Armitage test for trend, p = 0.05). No TPE-related serious adverse events were reported. Further biochemical results are reported in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/CCM/E202).
DISCUSSION
In this largest prospective pediatric TAMOF observational cohort published to date, physicians tended to select TPE for TAMOF patients who had marginally higher severity of organ dysfunction (based on PELOD scores) and those receiving ECMO. Children with MRSA-induced sepsis were also more likely to receive TPE, probably reflecting the increased organ failure in children with this drug-resistant organism (26-28). Organ dysfunction, as measured by change from baseline PELOD score, significantly decreased in children receiving TPE. Using both multivariate regression and propensity score weighting, use of TPE was also associated with a 55% lower ARR of 28-day mortality compared with those not receiving TPE.
TPE could potentially have improved sepsis-induced organ dysfunction by removing inflammatory mediators, reducing antifibrinolytic molecules, replenishing anticoagulant proteins, and restoring ADAMTS-13 activity to mitigate the complex and interrelated dysregulated inflammation, coagulation, and fibrinolytic pathways of sepsis in which where traditional targeted biologic therapies have failed (5, 7, 9, 21, 23, 29-35). The most recent American Society of Apheresis guidelines give a level C evidence recommendation for use of TPE in sepsis-induced multiple organ failure, indicating that TPE may be used on an individual basis and acknowledging the need for generation of more evidence (36). Choice of TPE for the treatment of TAMOF varied among our nine study centers which could reflect assessment of severity of illness, predilection for use, or experience with the procedure at individual centers.
Published experience and evidence remain sparse for the efficacy of TPE in sepsis and TAMOF. The largest randomized controlled trial (RCT) of TPE, a single-center study of 106 adult patients with sepsis, demonstrated an absolute mortality risk reduction of 20.5% with a number needed to treat of 4.9 in patients receiving TPE (37). A meta-analysis of two TPE trials found a RR of mortality of 0.63 (95% CI, 0.42–0.96; p = 0.03) for TPE compared with control treatment (38). Three preliminary pediatric proof-of-concept studies have evaluated use of TPE for children meeting TAMOF criteria (23, 34, 39). A 10-patient pilot RCT demonstrated that TPE was associated with reduced organ dysfunction and mortality in children receiving TPE compared with those on no TPE (23). In a small retrospective TAMOF network cohort analysis, “not” receiving TPE was associated with an increased adjusted odds ratio of mortality of 1.81 (95% CI, 0.02–16.29; p = 0.048) (34). Use of TPE in children with TAMOF and secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome was also associated with improved survival (39). The current network findings add additional support for potential efficacy of TPE to reduce organ dysfunction and mortality in TAMOF.
Response to TPE was noted by a significant decline in PELOD score by day 4 of treatment compared with baseline. Biochemical findings in this cohort of TAMOF patients were consistent with previous pediatric TAMOF studies (23, 40). In the current study, TAMOF phenotypic findings, including organ failures and thrombocytopenia, correlated with reduced ADAMTS-13 levels. In addition, our study showed that ADAMTS-13 was lower in TAMOF nonsurvivors and that ADAMTS-13 levels improved over the course of TPE in TAMOF although not significantly increasing in patients not being treated with TPE. More detailed interpretation of biochemical markers is limited by lack of samples for a number of patients, and future studies should focus more directly on marker use to help identify TAMOF patients more likely to benefit from TPE.
This study has several limitations. First, the current definition of TAMOF represents a clinical phenotype. Although rapid determination of ADAMTS-13 activity does not exist for real-time categorization, low levels of ADAMTS-13 activity were subsequently correlated with the TAMOF phenotype. Second, there was an apparent selection bias to use TPE in TAMOF patients with greater organ dysfunction, as also demonstrated by a predilection for greater TPE use in patients with documented ADAMTS-13 deficiency. However, despite the tendency to use TPE in children with greater severity of illness or interventions before initiation of TPE, mortality was not increased in TPE patients, and in fact, risk-adjusted mortality was lower in TPE patients. Third, although the network’s implicit intent was to observe for balanced choice of TPE or standard therapy alone, a tendency toward increased use of TPE occurred at most centers, leading to an imbalance in the number of patients not receiving TPE, and in TPE usage between sites, which could have confounded the positive effect of TPE. Because of the relatively small patient numbers, forcing site identification into our regression model was not possible without creating issues of overfitting. Fourth, although no severe adverse events were noted, the study was not large enough to definitively define safety, nor was cost data obtained to perform cost-benefit analysis. Finally, patients in this study were enrolled approximately 8–13 years before publication. However, this largest reported prospective cohort to date retains the potential to add significantly to published pediatric TAMOF/TPE experience, which remains limited.
The study was not large enough to identify a threshold of organ failure at which TPE could provide benefit in TAMOF. Future trial design may benefit from use of a stratification strategy, such as that proposed by Pediatric Sepsis Biomarker Risk Model (PERSEVERE) II investigators, with use of a biomarker panel to predict mortality risk and response (10). Finally, although patients were screened for meeting TAMOF criteria, patients who met TAMOF criteria but were not enrolled in the study were not documented at each study center.
CONCLUSIONS
In this prospective, multicenter observational cohort of children with sepsis-associated TAMOF, TPE use was associated with improved organ dysfunction. When adjusted for severity of illness, TPE use was associated with a decrease in 28-day all-cause mortality relative to no TPE. Further studies, including well-designed trials stratified for risk of survival, focusing on biochemical marker assessment, and using a broader network of sites with equipoise, could better define the role and cost-effectiveness of TPE in TAMOF patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank the bedside caregivers of the patients involved in this study for their skilled and compassionate care.
Supported, in part, by a grant from The Children’s Miracle Network. Dr. Grunwell is supported by the Atlanta Pediatric Scholars Program grant K12HD072245. Dr. Carcillo received funding through the National Institutes of Health GM108618
Dr. Nguyen’s institution received funding from the National Institutes of Health (NIH). Drs. Nguyen, Grunwell, and Carcillo received support for article research from the NIH. Dr. Grunwell’s institution received funding from the Children’s Miracle Network, and she is supported by the Atlanta Pediatric Scholars Program grant K12HD072245. Dr. Aneja received funding from UptoDate. Drs. Grunwell, Wheeler, and Dalton disclosed off-label product use of therapeutic plasma exchange for sepsis-induced thrombocytopenia–associated multiple organ failure in children. Dr. Hall received funding from Bristol Myers-Squibb (advisory board) and La Jolla Pharmaceuticals (consultant). Dr. Fleming disclosed that he is a member of the American Board of Medical Specialties Vision Commission. Dr. Dalton received funding fromlnnovative ECMO Concepts (consultant). Dr. Dai was paid as a graduate research assistant to work on clinical research studies, but was not paid for her analysis work for the Thrombocytopenia-Associated Multiple Organ Failure study. Dr. Paden received funding from expert witness work, and he disclosed off-label product use of apheresis for sepsis. Dr. Carcillo received funding through the NIH GM108618 and the National Institute of General Medical Sciences. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Drs. Fortenberry, Nguyen, and Carcillo conceived and designed the study. Drs. Fortenberry and Nguyen drafted the initial article. Mr. Easley, Ms. Knezevic, and Dr. Dai conducted statistical analyses, aided with data interpretation, and edited the article. Dr. Grunwell interpreted the data, and drafted and provided critical revision of the article. Drs. Aneja, Wheeler, Hall, Fleming, Tarrago, Buttram, Dalton, Han, and Paden recruited patients, and acquired data. All authors read and provided final approval of the version submitted for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 2.Ruth A, McCracken CE, Fortenberry JD, et al. : Pediatric severe sepsis: Current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15:828–838 [DOI] [PubMed] [Google Scholar]
- 3.Tamburro RF, Jenkins TL: Multiple organ dysfunction syndrome: A challenge for the pediatric critical care community. Pediatr Crit Care Med 2017; 18:S1–S3 [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. : The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003; 167:695–701 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TC, Carcillo JA: Bench-to-bedside review: Thrombocytopenia-associated multiple organ failure–a newly appreciated syndrome in the critically ill. Crit Care 2006; 10:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TC, Carcillo JA: Understanding the role of von Willebrand factor and its cleaving protease ADAM TS13 in the pathophysiology of critical illness. Pediatr Crit Care Med 2007; 8:187–189 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TC, Cruz MA, Carcillo JA: Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carcillo JA, Halstead ES, Hall MW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators: Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatr Crit Care Med 2017; 18:513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carcillo JA, Podd B, Aneja R, et al. : Pathophysiology of pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med 2017; 18:S32–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR, Cvijanovich NZ, Anas N, et al. : Pediatric sepsis biomarker risk model-II: Redefining the pediatric sepsis biomarker risk model with septic shock phenotype. Crit Care Med 2016; 44:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell WR, Braine HG, Ness PM, et al. : Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991; 325:398–403 [DOI] [PubMed] [Google Scholar]
- 12.Noris M, Mescia F, Remuzzi G: STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 2012; 8:622–633 [DOI] [PubMed] [Google Scholar]
- 13.Veyradier A, Obert B, Houllier A, et al. : Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: A study of 111 cases. Blood 2001; 98:1765–1772 [DOI] [PubMed] [Google Scholar]
- 14.Mannucci PM, Capoferri C, Canciani MT: Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol 2004; 126:213–218 [DOI] [PubMed] [Google Scholar]
- 15.Peyvandi F, Ferrari S, Lavoretano S, et al. : Von Willebrand factor cleaving protease (ADAMTS-13) and ADAMTS-13 neutralizing autoantibodies in 100 patients with thrombotic thrombocytopenic purpura. Br J Haematol 2004; 127:433–439 [DOI] [PubMed] [Google Scholar]
- 16.Bernardo A, Ball C, Nolasco L, et al. : Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 2004; 104:100–106 [DOI] [PubMed] [Google Scholar]
- 17.Studt JD, Kremer Hovinga JA, Antoine G, et al. : Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: In vitro inhibition of ADAMTS13 activity by hemoglobin. Blood 2005; 105:542–544 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Fu X, Wang Y, et al. : Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood 2010; 115:706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawley JT, Lam JK, Rance JB, et al. : Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood 2005; 105:1085–1093 [DOI] [PubMed] [Google Scholar]
- 20.Ono T, Mimuro J, Madoiwa S, et al. : Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: Its correlation with development of renal failure. Blood 2006; 107:528–534 [DOI] [PubMed] [Google Scholar]
- 21.Rock GA, Shumak KH, Buskard NA, et al. : Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein B, Giroir B, Randolph A, et al. : International Pediatric Sepsis Consensus Conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TC, Han YY, Kiss JE, et al. : Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med 2008; 36:2878–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diggle PJ, Liang KY, Zeger SL: General linear models for longitudinal data. In: Analysis of Longitudinal Data. Atkinson AC, Copas JB, Pierce DA, et al. (Eds). Oxford, United Kingdom, Clarendon Press, 1994, pp 68–69 [Google Scholar]
- 25.Zou G: A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706 [DOI] [PubMed] [Google Scholar]
- 26.Carpenter SL, Goldman J, Sherman AK, et al. : Clinical variables and Staphylococcus aureus virulence factors associated with venous thromboembolism in children. Thromb Res 2016; 138:69–73 [DOI] [PubMed] [Google Scholar]
- 27.Kravitz GR, Dries DJ, Peterson ML, et al. : Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis 2005; 40:941–947 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen T, Kyle UG, Jaimon N, et al. : Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med 2012; 40:3246–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen T, Hall M, Han Y, et al. : Microvascular thrombosis in pediatric multiple organ failure: Is it a therapeutic target? Pediatr Crit Care Med 2001; 2:187–196 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TC, Carcillo JA: Therapeutic plasma exchange as a strategy to reverse Multiple Organ Dysfunction Syndrome in patients receiving extracorporeal life support. Pediatr Crit Care Med 2015; 16:383–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TC, Kiss JE, Goldman JR, et al. : The role of plasmapheresis in critical illness. Crit Care Clin 2012; 28:453–468, vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TC, Stegmayr B, Busund R, et al. : Plasma therapies in thrombotic syndromes. Int J Artif Organs 2005; 28:459–465 [DOI] [PubMed] [Google Scholar]
- 33.Podd BS, Simon DW, Lopez S, et al. : Rationale for adjunctive therapies for pediatric sepsis induced multiple organ failure. Pediatr Clin North Am 2017; 64:1071–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevketoglu E, Yildizdas D, Horoz OO, et al. : Use of therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure in the Turkish thrombocytopenia-associated multiple organ failure network. Pediatr Crit Care Med 2014; 15:e354–e359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stegmayr BG, Banga R, Berggren L, et al. : Plasma exchange as rescue therapy in multiple organ failure including acute renal failure. Crit Care Med 2003; 31:1730–1736 [DOI] [PubMed] [Google Scholar]
- 36.Schwartz J, Padmanabhan A, Aqui N, et al. : Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The seventh special issue. J Clin Apher 2016; 31:149–162 [DOI] [PubMed] [Google Scholar]
- 37.Busund R, Koukline V, Utrobin U, et al. : Plasmapheresis in severe sepsis and septic shock: A prospective, randomised, controlled trial. Intensive Care Med 2002; 28:1434–1439 [DOI] [PubMed] [Google Scholar]
- 38.Zhou F, Peng Z, Murugan R, et al. : Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit Care Med 2013; 41:2209–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demirkol D, Yildizdas D, Bayrakci B, et al. ; Turkish Secondary HLH/MAS Critical Care Study Group: Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: What is the treatment? Crit Care 2012; 16:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TC, Liu A, Liu L, et al. : Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica 2007; 92:121–124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


