Abstract
Candida dubliniensis is a recently described opportunistic fungal pathogen that is closely related to Candida albicans. Candida dubliniensis readily develops resistance to the azole antifungal agent fluconazole, both in vitro and in infected patients, and this resistance is usually associated with upregulation of the CdMDR1 gene, encoding a multidrug efflux pump of the major facilitator superfamily. To determine the role of CdMDR1 in drug resistance in C. dubliniensis, we constructed an mdr1 null mutant from the fluconazole-resistant clinical isolate CM2, which overexpressed the CdMDR1 gene. Sequential deletion of both CdMDR1 alleles was performed by the MPAR-flipping method, which is based on the repeated use of a dominant mycophenolic acid resistance marker for selection of integrative transformants and its subsequent deletion from the genome by FLP-mediated, site-specific recombination. In comparison with its parental strain, the mdr1 mutant showed decreased resistance to fluconazole but not to the related drug ketoconazole. In addition, we found that CdMDR1 confers resistance to the structurally unrelated drugs 4-nitroquinoline-N-oxide, cerulenin, and brefeldin A, since the enhanced resistance to these compounds of the parent strain CM2 compared with the matched susceptible isolate CM1 was abolished in the mdr1 mutant. In contrast, CdMDR1 inactivation did not cause increased susceptibility to amorolfine, terbinafine, fluphenazine, and benomyl, although overexpression of CdMDR1 in a hypersusceptible Saccharomyces cerevisiae strain had previously been shown to confer resistance to these compounds. The effect of CdMDR1 inactivation was identical to that seen in two similarly constructed C. albicans mdr1 mutants. Therefore, despite species-specific differences in the amino acid sequences of the Mdr1 proteins, overexpression of CaMDR1 and CdMDR1 in clinical C. albicans and C. dubliniensis strains seems to confer the same drug resistance profile in both species.
Candida dubliniensis is a recently described opportunistic fungal pathogen that is closely related to Candida albicans. One interesting feature of this species is its ability to rapidly develop stable resistance to the widely used antifungal agent fluconazole after exposure to the drug in vitro (10, 18). Similar to the case for C. albicans, fluconazole-resistant C. dubliniensis strains have also been isolated from AIDS patients (10, 13), suggesting that drug resistance in C. dubliniensis may be clinically relevant. Fluconazole resistance in both clinical isolates and in vitro-generated fluconazole-resistant C. dubliniensis derivatives has in most cases been associated with the overexpression of the CdMDR1 gene, which encodes a membrane transport protein of the major facilitator superfamily (9). The homologous gene in C. albicans, CaMDR1 (BenR), was originally isolated on the basis of its ability to confer resistance to the tubulin binding drug benomyl upon expression in Saccharomyces cerevisiae (2). It was shown subsequently that CaMDR1 expression in S. cerevisiae can confer resistance to a variety of other, chemically unrelated compounds, including fluconazole (1, 16). Similarly, expression of CdMDR1 in S. cerevisiae also resulted in enhanced resistance to fluconazole and other drugs (9), suggesting that MDR1 is a multidrug resistance gene in both C. albicans and C. dubliniensis.
The C. dubliniensis and C. albicans MDR1 genes are highly homologous, with 96% identity at the deduced amino acid level (9). A notable difference between the two species is the presence of an asparagine-rich region in the N-terminal region of CaMdr1p which is absent from the corresponding C. dubliniensis protein. The role of this region in Mdr1p function is presently unknown, and whether this difference confers any species-specific phenotype has yet to be determined. Overexpression of CdMDR1 in S. cerevisiae confers resistance to the same spectrum of compounds as had been shown before for CaMDR1 (9). However, heterologous expression studies may not necessarily reflect the spectrum of drugs to which MDR1 mediates resistance in C. albicans and C. dubliniensis themselves. For example, although CaMDR1 was originally cloned by its ability to confer benomyl resistance upon S. cerevisiae transformants, a C. albicans mdr1 null mutant did not exhibit hypersusceptibility to benomyl, although disruption of the gene was performed in a strain that expressed high MDR1 mRNA levels (4). Similarly, CaMDR1 inactivation in commonly used C. albicans laboratory strains did not affect susceptibility to fluconazole and several other compounds thought to be Mdr1p substrates as deduced from the S. cerevisiae expression studies (11, 15). These findings may reflect the fact that in these C. albicans strains CaMDR1 expression is undetectable or is barely detectable in vitro. However, when CaMDR1 was inactivated in two different fluconazole-resistant clinical C. albicans isolates that strongly expressed the gene in vitro, fluconazole resistance was diminished, providing direct genetic evidence that CaMDR1 overexpression can cause enhanced fluconazole resistance in C. albicans (21). The same study also confirmed that CaMDR1 overexpression mediates resistance to the unrelated compound 4-nitroquinoline-N-oxide (4-NQO) but not resistance to ketoconazole. All of these findings indicate that the most direct way to determine the drug resistance profile mediated by overexpression of particular genes encoding efflux pumps is to inactivate these genes in the relevant strains and to analyze the effect of the mutation on drug resistance. Such an approach has only recently become possible with the development of a straightforward method to construct homozygous mutants from C. albicans wild-type strains. The MPAR-flipping strategy relies on the repeated use of the dominant marker MPAR for selection of mycophenolic acid (MPA)-resistant integrative transformants from which the marker is subsequently excised again by the site-specific recombinase FLP (21). A DNA cassette that contains the MPAR marker and a C. albicans-adapted FLP gene (caFLP) under the control of the inducible SAP2 promoter and is flanked by direct repeats of the minimal FLP recognition target sequence (FRT) is inserted into one allele of the target gene by homologous recombination. Since the SAP2 promoter controlling caFLP expression is induced in media containing a protein as the sole nitrogen source, such as yeast carbon base-bovine serum albumin, growth of integrative transformants in SAP2-inducing medium results in expression of the FLP recombinase, which, by binding to and recombining its target sequences, ensures efficient excision of the mutagenesis cassette from the genome without the need for negative selection, leaving behind an inactivated copy of the target gene. The procedure can then by repeated to obtain homozygous mutants. This method has also recently been shown to be applicable to gene disruption in C. dubliniensis wild-type strains (17). In the present study, we generated an mdr1 null mutant of a fluconazole-resistant, clinical C. dubliniensis strain to assess the role of CdMDR1 in fluconazole resistance and to compare the drug resistance profiles mediated by MDR1 overexpression in C. albicans and C. dubliniensis.
MATERIALS AND METHODS
Strains and growth media.
The C. dubliniensis and C. albicans strains used in this study are listed in Table 1. The strains were kept as frozen stocks at −80°C and were subcultured on yeast extract-peptone-dextrose (YPD) agar plates (10 g of yeast extract, 20 g of peptone, 20 g of glucose, and 15 g of agar per liter) at 30°C. For routine growth of the strains, YPD liquid medium was used. Cells were grown overnight in yeast carbon base-bovine serum albumin (23.4 g of yeast carbon base and 4 g of bovine serum albumin per liter [pH 4.0]) to induce the SAP2 promoter for excision of the MPAR flipper from MPA-resistant transformants. To screen for MPA-sensitive derivatives, 100 to 200 CFU was plated on minimal agar (6.7 g of yeast nitrogen base without amino acids [BIO 101, Vista, Calif.], 20 g of glucose, 0.77 g of complete supplement medium [BIO 101], and 15 g of agar per liter) containing 1 μg of MPA (Sigma, Taufkirchen, Germany) ml−1. MPAr clones grew as large colonies, while MPAs clones formed much smaller colonies (21).
TABLE 1.
C. dubliniensis and C. albicans strains used in this study
| Species and strain | Parent | Relevant characteristicsa | Reference |
|---|---|---|---|
| C. dubliniensis | |||
| CM1 | Clinical isolate, fluconazole susceptible | 10 | |
| CM2 | Clinical isolate, fluconazole resistant | 10 | |
| CdM1 | CM2 | MDR1/mdr1Δ::MPAR-FLIP | This study |
| CdM2 | CdM1 | MDR1/mdr1Δ::FRT | This study |
| CdM3 | CdM2 | mdr1Δ::MPAR-FLIP/mdr1Δ::FRT | This study |
| CdM4 | CdM3 | mdr1Δ::FRT/mdr1Δ::FRT | This study |
| C. albicans | |||
| F2 | Clinical isolate, fluconazole susceptible | 3 | |
| F5 | Clinical isolate, fluconazole resistant | 3 | |
| F5M432 | F5 | mdr1Δ::FRT/mdr1Δ::FRT | 21 |
| G2 | Clinical isolate, fluconazole susceptible | 3 | |
| G5 | Clinical isolate, fluconazole resistant | 3 | |
| G5M432 | G5 | mdr1Δ::FRT/mdr1Δ::FRT | 21 |
MPAR-FLIP, MPAR flipper cassette.
Sequencing of CdMDR1 flanking regions.
Cloning of the CdMDR1 gene from C. dubliniensis strain CD36 has been described previously (9). An XbaI-SpeI fragment containing 943 bp of upstream and 726 bp of downstream sequences in addition to the CdMDR1 coding region was subcloned from the genomic clone ΦCD1 into the vector pBluescript and completely sequenced.
Construction of a CdMDR1 gene deletion cassette.
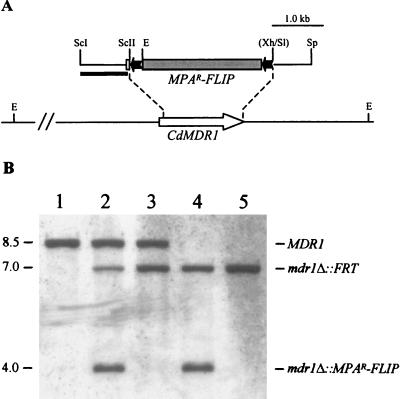
CdMDR1 upstream and downstream fragments were obtained by PCR amplification from C. dubliniensis genomic DNA with the primer pair CdMDR1 (5′-TTGTGAGCTCATCAAAACGTGTTAGAATTGCGC-3′) and CdMDR2 (5′-TTTATCCGCGGACAAATGGTAGACAACTCTACC-3′) and the primer pair CdMDR3 (5′-CTTGAGTCGACCAAAGTTGAGAGCAAGATCG-3′) and CdMDR4 (5′-ACTAGTGGTTAAGCATGCGAATACACACATAG-3′), respectively. CdMDR1 and CdMDR2 amplify CdMDR1 sequences from position −886 to +57, and CdMDR3 and CdMDR4 amplify CdMDR1 sequences from position +1637 to +2382, respectively (positions are with respect to the first base [+1] of the start codon). The PCR products were digested at the introduced SacI/SacII and SalI/SphI restriction endonuclease cleavage sites (underlined) and cloned together with a SacII-XhoI fragment containing the MPAR flipper from pSFI1 (21) into the SacI/SphI-digested vector pBluescript to generate pSFIcdM1 (Fig. 1A). The insert from this plasmid was excised by SacI/SphI digestion and used in transformation experiments.
FIG. 1.
Inactivation of the CdMDR1 gene by MPAR flipping. (A) Structure of the CdMDR1 locus in C. dubliniensis strain CM2 and allelic replacements using the insert from pSFIcdM1. Open arrow, CdMDR1 coding region; solid lines, CdMDR1 upstream and downstream sequences. Only relevant restriction sites are shown: E, EcoRI, ScI, SacI, ScII, SacII, Sl, SalI, Sp, SphI, Xh, XhoI. The sites shown in parentheses were destroyed by the cloning procedure. The 5.6-kb MPAR flipper, details of which have been presented elsewhere (21), is not drawn to scale. Solid bar, DNA fragment used as a probe for verification of the correct allelic replacements by Southern hybridization. (B) Southern hybridization of EcoRI-digested genomic DNA of C. dubliniensis parental isolate CM2 and the mdr1 mutant derivatives using the 5′CdMDR1 fragment from pSFIcdM1 as a probe. The identities of the fragments are shown to the right of the blot, and molecular sizes in kilobases are given on the left. Lane 1, CM2 (MDR1/MDR1); lane 2, CdM1 (MDR1/mdr1Δ::MPAR-FLIP); lane 3, CdM2 (MDR1/mdr1Δ::FRT); lane 4, CdM3 (mdr1Δ::MPAR-FLIP/mdr1Δ::FRT); lane 5, CdM4 (mdr1Δ::FRT/mdr1Δ::FRT).
C. dubliniensis transformation.
C. dubliniensis strains were transformed by electroporation (7) with approximately 1 μg of the gel-purified insert from pSFIcdM1. MPA-resistant transformants were selected on minimal agar plates containing 10 μg of MPA ml−1. Single colonies were picked after 7 days of growth at 30°C and restreaked on the same medium. After confirmation of the correct allelic replacement, the transformants were maintained on YPD agar plates.
Isolation of chromosomal DNA and Southern hybridization.
Genomic DNA from C. dubliniensis strains was isolated as described previously (8). DNA (10 μg) was digested with EcoRI, separated in 1% (wt/vol) agarose gels, and, after ethidium bromide staining, transferred by vacuum blotting onto nylon membranes and fixed by UV cross-linking. Southern hybridization with enhanced chemiluminescence (ECL)-labeled probes was performed with the ECL labeling and detection kit from Amersham (Braunschweig, Germany) according to the instructions of the manufacturer.
DNA fingerprinting.
DNA fingerprinting of C. dubliniensis isolates was performed using the C. dubliniensis-specific DNA fingerprinting probe Cd25, and the fingerprints were compared using the computer program Dendron as described previously (6).
Drug susceptibility tests.
Stock solutions of the drugs were prepared as follows. Fluconazole (1 mg ml−1), amorolfine (0.5 mg ml−1), fluphenazine (20 mg ml−1), and crystal violet (2.5 mg ml−1) were dissolved in water, while ketoconazole (2 mg ml−1), 4-NQO (0.2 mg ml−1), cerulenin (5 mg ml−1), and brefeldin A (5 mg ml−1) were dissolved in dimethyl sulfoxide and terbinafine (10 mg ml−1) was dissolved in ethanol. In the assays, serial twofold dilutions in the assay medium were prepared from the following initial concentrations: fluconazole, 100 μg ml−1; ketoconazole, 5 μg ml−1; 4-NQO, 4 μg ml−1; cerulenin, 50 μg ml−1; brefeldin A, 500 μg ml−1; amorolfine, 25 μg ml−1; terbinafine, 25 μg ml−1; fluphenazine, 1 mg ml−1; and crystal violet, 50 μg ml−1. Susceptibility tests were carried out in high-resolution medium (14.67 g of HR-Medium [Oxoid GmbH, Wesel, Germany], 1 g of NaHCO3, 0.2 M phosphate buffer [pH 7.2]), using a previously described microdilution method (12). Since fluphenazine and terbinafine precipitated in high-resolution medium, the MICs of these drugs were determined using minimal medium instead. Readings were done after 48 h (fluconazole, ketoconazole, cerulenin, brefeldin A, amorolfine, and crystal violet) or 24 h (4-NQO, fluphenazine, and terbinafine) when the later time point was considered to be less reliable because of a slight residual growth, although this did not affect the overall outcome of the test. MICs of selected compounds, including benomyl, were also independently evaluated in RPMI 1640 medium supplemented with 2% (wt/vol) glucose according to the NCCLS protocol for confirmatory purposes, as described by Moran et al. (9).
Nucleotide sequence accession number.
The enlarged sequence of CdMDR1 is accessible in the EMBL nucleotide sequence database under accession no. AJ227752.
RESULTS
Characterization of C. dubliniensis isolates CM1 and CM2.
The C. dubliniensis oral clinical isolates CM1 and CM2 were recovered from the same AIDS patient at two separate clinical evaluations 17 months apart. While these isolates have been shown previously to be the same strain by pulsed-field gel electrophoresis analysis (19), this was confirmed by genomic DNA fingerprinting analysis using the C. dubliniensis-specific fingerprinting probe Cd25 (SAB = 0.98 [data not shown]). CM2 exhibited a strongly reduced susceptibility to fluconazole (MIC of 32 μg ml−1 by the NCCLS method) compared to the initial isolate CM1 (MIC of 0.5 μg ml−1) (10). The fluconazole resistance phenotype of isolate CM2 was correlated with increased levels of expression of the MDR1 gene (9). By sequence analysis the MDR1 coding sequence from strain CM2 was shown to be identical to the previously published MDR1 sequence of the C. dubliniensis type strain CD36 (G. P. Moran, unpublished results); i.e., the deduced Mdr1 protein did not contain the asparagine-rich stretch found in the N-terminal region of Mdr1p of all C. albicans strains analyzed so far (2, 5; J. Morschhäuser, unpublished results).
Construction of a C. dubliniensis mdr1 mutant by targeted gene deletion.
Since the overexpression of the CdMDR1 gene in CM2 has been suggested previously to be responsible for the decreased susceptibility of this isolate to fluconazole compared with isolate CM1 (9), we investigated the contribution of CdMDR1 overexpression to fluconazole resistance by deleting the gene from the resistant isolate CM2. To generate a CdMDR1 deletion cassette, the CdMDR1 coding region from position +58 (with respect to the start codon) to +1636 (36 bp in front of the stop codon) was replaced by the MPAR flipper (Fig. 1A). Isolate CM2 was transformed with the insert from the resulting plasmid pSFIcdM1, and MPA-resistant transformants were analyzed by Southern hybridization. In the parent strain CM2, an EcoRI fragment of about 8.5 kb hybridized with the CdMDR1 probe (Fig. 1B, lane 1). Insertion of the mutagenesis cassette into one of the CdMDR1 alleles in transformant CdM1 generated a new EcoRI fragment of 4.0 kb due to the presence of an EcoRI site in the MPAR flipper (Fig. 1B, lane 2). Deletion of the cassette by FLP-mediated recombination resulted in the derivative CdM2, in which the 4.0-kb EcoRI fragment was replaced by an expected 7.0-kb fragment, which was 1.5 kb smaller than the original wild-type fragment (Fig. 1B, lane 3). The 7.0-kb band was also observed in the parental strain CdM1 (Fig. 1B, lane 2) because some FLP-mediated recombination can occur in SAP2 repressing medium due to a basal activity of the SAP2 promoter fragment controlling caFLP expression in the MPAR flipper (21). The insert from pSFIcdM1 was then used in a second round of mutagenesis to delete the remaining CdMDR1 wild-type allele in the heterozygous mutant, resulting in the homozygous null mutant CdM3 (Fig. 1B, lane 4) from which the MPAR flipper was excised again to produce strain CdM4 (Fig. 1B, lane 5), which, apart from the deleted CdMDR1 alleles, is isogenic to the parental strain CM2.
CdMDR1 deletion affects fluconazole resistance.
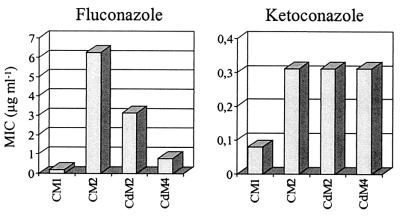
In C. albicans, CaMDR1 overexpression confers resistance to fluconazole but not to the related drug ketoconazole (21). The C. dubliniensis isolate CM2 displayed enhanced resistance to fluconazole (10) and was also less susceptible to ketoconazole (and itraconazole) compared with the matched isolate CM1 (Fig. 2). To assess the contribution of CdMDR1 overexpression in isolate CM2 to the increased drug resistance of this strain, the MICs of fluconazole and ketoconazole for the mdr1 mutants were determined and compared with those for the parent strain CM2. Deletion of both CdMDR1 alleles resulted in increased susceptibility to fluconazole, although the mdr1 null mutant CdM4 was still somewhat less susceptible than isolate CM1 (Fig. 2). The heterozygous mutant CdM2 showed an intermediate phenotype, demonstrating that both CdMDR1 alleles contributed to the enhanced fluconazole resistance of isolate CM2. In contrast, CdMDR1 deletion did not affect susceptibility to ketoconazole. The enhanced resistance of isolate CM2 to ketoconazole must therefore have been caused by another mechanism(s) that may also have contributed to some degree to the increased fluconazole resistance compared with isolate CM1. Similar results were obtained when MICs were determined by an alternative method (i.e., the modified NCCLS method) (data not shown). These results demonstrate that, as previously shown for C. albicans (21), overexpression of MDR1 in a clinical C. dubliniensis isolate conferred resistance to fluconazole but not to the related drug ketoconazole.
FIG. 2.
Susceptibilities to fluconazole and ketoconazole of the clinical C. dubliniensis isolates CM1 and CM2 and the heterozygous (CdM2) and homozygous (CdM4) mdr1 mutants derived from CM2. The MICs of the drugs for the isolates and their derivatives were determined by a broth microdilution method (see Materials and Methods). In this assay strains are defined as fluconazole susceptible if the MIC is <6.25 μg/ml, as intermediately susceptible if the MIC is ≥6.25 μg/ml to <25 μg/ml, and as resistant if the MIC is ≥25 μg/ml (12).
MDR1 overexpression in C. dubliniensis and C. albicans results in reduced susceptibility to a similar spectrum of drugs.
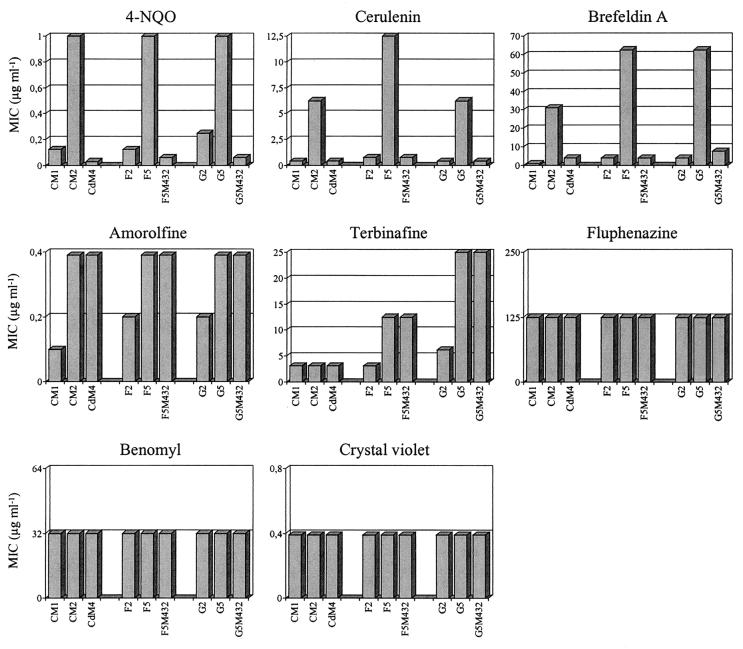
As stated above, the expression of both CaMDR1 and CdMDR1 in S. cerevisiae confers resistance to a similar panel of unrelated drugs in addition to fluconazole, but in C. albicans itself MDR1 does not mediate resistance to some of these drugs. To determine whether MDR1 overexpression in clinical, fluconazole-resistant C. albicans and C. dubliniensis strains also resulted in enhanced resistance to other drugs, the effects of several compounds that inhibit fungal growth by different mechanisms on the homozygous C. dubliniensis mdr1 deletion mutant CdM4, as well as the previously described C. albicans mdr1 mutants F5M432 and G5M432, were analyzed. These were compared with the effects on the corresponding parent strains CM2, F5, and G5, respectively, and the matched fluconazole-susceptible isolates CM1, F2, and G2, respectively.
According to the resistance patterns of the strains, the drugs could be divided into three different groups (Fig. 3). In addition to fluconazole resistance, MDR1 overexpression in both C. albicans and C. dubliniensis was also associated with enhanced resistance to 4-NQO, cerulenin, and brefeldin A, and this resistance was completely or almost completely abolished in the mdr1 null mutants. In contrast, MDR1 overexpression did not result in altered susceptibility to the drugs fluphenazine, benomyl, crystal violet, and (for C. dubliniensis) terbinafine. Correspondingly, MDR1 inactivation had no effect on the susceptibility of the strains to these compounds. The fluconazole-resistant isolates also exhibited increased resistance to amorolfine and (for the C. albicans isolates) terbinafine. However, this enhanced resistance was not caused by MDR1 overexpression, since the mdr1 mutants showed the same level of resistance to these drugs as their parental strains. Similar results were obtained when MICs were determined using the NCCLS method (data not shown). These results demonstrate that MDR1 overexpression in clinical C. albicans and C. dubliniensis isolates confers enhanced resistance to several other drugs in addition to fluconazole and that the drug resistance profile mediated by MDR1 is the same in both species, at least as far as analyzed in these experiments.
FIG. 3.
Susceptibilities of matched fluconazole-susceptible and -resistant clinical C. dubliniensis (CM1-CM2) and C. albicans (F2-F5 and G2-G5) isolate pairs and the mdr1 null mutants derived from the fluconazole-resistant isolates CM2 (CdM4), F5 (F5M432), and G5 (G5M432) to other drugs.
DISCUSSION
Matched pairs of drug-susceptible and drug-resistant isolates are an excellent tool for the analysis of the molecular mechanisms responsible for drug resistance. Fluconazole-resistant isolates that exhibit increased levels of MDR1 expression compared to matched fluconazole-susceptible isolates have been described for both C. albicans and C. dubliniensis. However, the contribution of MDR1 overexpression to the drug resistance phenotype observed in these isolates is difficult to assess, since they may exhibit other changes in addition to MDR1 overexpression. For example, an additional ERG11 mutation or ERG11 overexpression also contributed to fluconazole resistance and probably caused the cross-resistance to ketoconazole in two clinical C. albicans isolates analyzed previously (21). The enhanced ketoconazole resistance of these isolates compared with their matched susceptible isolates was not diminished by MDR1 inactivation, demonstrating that ketoconazole resistance was not due to MDR1 overexpression (21). In the present study, we also observed that the same two C. albicans isolates display reduced susceptibility to amorolfine and terbinafine, two inhibitors that have alternative targets to the azoles in the ergosterol biosynthetic pathway. This enhanced resistance was not affected by MDR1 disruption and could be due to some other alteration in the ergosterol biosynthetic pathway. On the other hand, it was previously shown that the constitutive MDR1 activation in these strains was caused by mutations in trans-regulatory factors (20), which may have resulted in the activation of additional target genes, including other efflux pumps with different substrate specificities. Similar results were obtained for the C. dubliniensis mdr1 mutant constructed in the present study. Compared with the matched isolate CM1, isolate CM2 exhibited enhanced resistance to ketoconazole and also to amorolfine; neither resistance was affected by CdMDR1 deletion, and therefore they must have been caused by other, as-yet-unknown mechanisms. Northern blot analysis has previously shown that isolate CM2 exhibits an approximately twofold-higher level of CdCDR1 expression compared with isolate CM1, which may account for the slightly reduced susceptibility of CM2 to ketoconazole (9).
MDR1 deletion in both C. albicans and C. dubliniensis, however, increased the susceptibility of the mutants to fluconazole, 4-NQO, cerulenin, and brefeldin A, demonstrating that in the case of these compounds, the resistance was mediated by MDR1. Resistance to all of these compounds was also conferred upon S. cerevisiae transformants expressing the MDR1 gene from C. albicans or C. dubliniensis (9, 14, 16). However, MDR1 expression in the heterologous host also resulted in enhanced resistance to a variety of additional chemicals, including amorolfine, terbinafine, fluphenazine, and benomyl, that was not reflected by a corresponding increase in susceptibility of C. albicans and C. dubliniensis mdr1 mutants to these agents. The reasons for this discrepancy are presently unknown. One possible explanation is that heterologous expression of a membrane protein in S. cerevisiae could influence the structure of the cell membrane or induce other cellular alterations, resulting in misleading results about the substrate spectrum of Candida multidrug resistance proteins. Another possibility is that heterologous expression of the Candida genes in other species (e.g., hypersusceptible S. cerevisiae strains) might yield effects that are not seen in Candida strains overexpressing those genes, since C. albicans and C. dubliniensis may have additional mechanisms of resistance to particular drugs (e.g., specific multidrug transporters which could compensate for these mutations). In fact, in some cases enhanced susceptibility of C. albicans mdr1 mutants to certain chemicals was observed only in a specific genetic background, i.e., when additional efflux pumps were inactivated (14, 15). Clearly it is preferable to examine the role of a gene in resistance in its natural genetic background rather than in heterologous expression studies. Investigation of the spectrum of chemicals to which a gene encoding a multidrug efflux pump can confer resistance is therefore best studied in clinical isolates, by inactivating the gene of interest. This approach has now been applied to both C. albicans and C. dubliniensis, demonstrating that MDR1 mediates resistance to the same panel of drugs in both species. The availability of such specifically constructed mutants will also allow the assessment of the potential of C. albicans and C. dubliniensis to develop resistance to new antifungal agents by MDR1 overexpression.
ACKNOWLEDGMENTS
This study was supported by the Bundesministerium für Bildung und Forschung (BMBF grant O1 K1 8906-0) and by the Deutsche Forschungsgemeinschaft (DFG grant MO846/3). Work performed in Dublin was supported by the Irish Health Research Board (grant number 04/99). Joachim Morschhäuser is the recipient of a Heisenberg fellowship from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Ben-Yaacov R, Knoller S, Caldwell G A, Becker J M, Koltin Y. Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob Agents Chemother. 1994;38:648–652. doi: 10.1128/aac.38.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fling M E, Kopf J, Tamarkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 3.Franz R, Kelly S L, Lamb D C, Kelly D E, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldway M, Teff D, Schmidt R, Oppenheim A B, Koltin Y. Multidrug resistance in Candida albicans: disruption of the BENr gene. Antimicrob Agents Chemother. 1995;39:422–426. doi: 10.1128/aac.39.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta V, Kohli A, Krishnamurthy S, Puri N, Aalamgeer S A, Panwar S, Prasad R. Identification of polymorphic mutant alleles of CaMDR1, a major facilitator of Candida albicans which confers multidrug resistance, and its in vitro transcriptional activation. Curr Genet. 1998;34:192–199. doi: 10.1007/s002940050385. [DOI] [PubMed] [Google Scholar]
- 6.Joly S, Pujol C, Rysz M, Vargas K, Soll D R. Development and characterization of complex DNA fingerprinting probes for the infectious yeast Candida dubliniensis. J Clin Microbiol. 1999;37:1035–1044. doi: 10.1128/jcm.37.4.1035-1044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köhler G A, White T C, Agabian N. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol. 1997;179:2331–2338. doi: 10.1128/jb.179.7.2331-2338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, Barale T, Michel-Briand Y. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J Clin Microbiol. 1994;32:1115–1118. doi: 10.1128/jcm.32.4.1115-1118.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran G P, Sanglard D, Donnelly S M, Shanley D B, Sullivan D J, Coleman D C. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morschhäuser J, Michel S, Staib P. Sequential gene disruption in Candida albicans by FLP-mediated site-specific recombination. Mol Microbiol. 1999;32:547–556. doi: 10.1046/j.1365-2958.1999.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruhnke M, Eigler A, Tennagen I, Geiseler B, Engelmann E, Trautmann M. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol. 1994;32:2092–2098. doi: 10.1128/jcm.32.9.2092-2098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruhnke M, Schmidt-Westhausen A, Morschhäuser J. Development of simultaneous resistance to fluconazole in Candida albicans and Candida dubliniensis in a patient with AIDS. J Antimicrob Chemother. 2000;46:291–295. doi: 10.1093/jac/46.2.291. [DOI] [PubMed] [Google Scholar]
- 14.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 15.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staib P, Moran G P, Sullivan D J, Coleman D C, Morschhäuser J. Isogenic strain construction and gene targeting in Candida dubliniensis. J Bacteriol. 2001;183:2859–2865. doi: 10.1128/JB.183.9.2859-2865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 20.Wirsching S, Michel S, Köhler G, Morschhäuser J. Activation of the multiple drug resistance gene MDR1 in fluconazole-resistant, clinical Candida albicans strains is caused by mutations in a trans-regulatory factor. J Bacteriol. 2000;182:400–404. doi: 10.1128/jb.182.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirsching S, Michel S, Morschhäuser J. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol. 2000;36:856–865. doi: 10.1046/j.1365-2958.2000.01899.x. [DOI] [PubMed] [Google Scholar]