Abstract
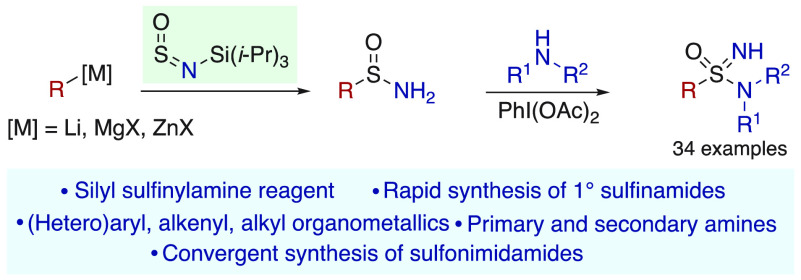
A new N-silyl sulfinylamine reagent allows the rapid preparation of a broad range of (hetero)aryl, alkenyl, and alkyl primary sulfinamides, using Grignard, organolithium, or organozinc reagents to introduce the carbon fragment. Treatment of these primary sulfinamides with an amine in the presence of a hypervalent iodine reagent leads directly to NH-sulfonimidamides. This two-step sequence is straightforward to perform and provides a modular approach to sulfonimidamides, allowing ready variation of both reaction components, including primary and secondary amines.
Sulfonimidamides1 are becoming established as valuable motifs in medicinal chemistry2 and feature in molecules used in an increasing range of therapeutic areas.3 The growth in use of sulfonimidamides has been mirrored by recent innovations4 in their synthesis.5 Approaches that employ sulfonimidoyl halides,6 or sulfonimidates,7 have been used extensively; however, access to these substrates can be challenging. The imination, or imination/oxidation, of lower oxidation-state precursors have emerged as useful methods to access sulfonimidamides. In this context, Bull has shown that an iodosobenzene/ammonium carbamate combination can be used to convert tertiary sulfenamides directly to sulfonimidamides,8 and Stockman has employed related reagents with tertiary sulfinamide substrates (Scheme 1a,b).9 Both of these methods are efficient, and both show commendable scope. However, both approaches are essentially linear; the last step in each is installation of an imidic NH group to a functionalized precursor, where the key S–N bond, linking the S-fragment and the N-fragment, has been established earlier in the reaction sequence. To provide more convergency, and to enable analogue synthesis, we conceived of an approach to NH-sulfonimidamides in which primary sulfinamides are combined with diversely substituted amines, using a hypervalent iodine reagent, as the final step of the synthesis (Scheme 1d). Our confidence in the success of this final step was due to in part to the chemistry from Bull, and Stockman, but also to the pioneering work from Malacria and Fensterbank, who converted primary sulfinamides into sulfonimidates using iodosobenzene with alcoholic solvents (Scheme 1c).10
Scheme 1. (a–c) Hypervalent-Iodine Mediated Synthesis of Sulfonimidamides and Sulfonimidates; (d) This Work: A Modular Route to Sulfonimidamides.
To deliver a flexible, fully modular sulfonimidamide synthesis, our approach would also require a straightforward method to access primary sulfinamides. Although a number of primary sulfinamide syntheses are known, most require several steps,11 or the need to use thiol substrates;12 we wished to avoid both of these constraints. To address this, we proposed the combination of a suitable sulfinylamine reagent with an organometallic nucleophile, which should directly provide the required primary sulfinamides (Scheme 1d). Herein, we report the successful realization of this plan.
Although sulfinylamines (R-NSO) have been known for over 140 years,13 there has only recently been a flurry of activity using these reagents.14,5d,6a,7a,15 For our proposed synthesis we required a sulfinylamine reagent with an N-substituent that would be easily removed using mild conditions, ideally avoiding strongly acidic or basic media. To meet these requirements, we settled on a N-silyl substituted reagent, and initially considered the N-triphenylsilyl derivative, originally prepared by Ismail and co-workers.16 However, it was soon apparent that the N-triphenylsilyl sulfinylamine was prone to hydrolysis, and its use after storage was challenging. To achieve the desired balance between stability and reactivity, we turned to the N-triisopropylsilyl derivative 1. This novel sulfinylamine could be prepared on a multigram scale, in high yield, in two steps starting from triisopropylsilyl chloride and ammonia (Scheme 2). Reagent 1 is a light-yellow colored liquid that is stable to refrigerated storage for at least one month.17
Scheme 2. Preparation of Triisopropylsilyl Sulfinylamine 1.
Sulfinylamine 1 (TIPS-NSO) showed good reactivity with a broad range of Grignard, organolithium, and organozinc reagents (Scheme 3). Although the intermediate N-silyl sulfinamides could be isolated, it was more convenient to treat the reaction mixtures directly with TBAF to form the desired primary sulfinamides. In this way, aryl organometallics of varied steric (2a–2c) and electronic character (2d–2f) could be smoothly converted to the corresponding primary sulfinamides in excellent yields. A gram-scale reaction provided p-fluoro-derivative 2d in 89% yield. Heteroaryl organometallics derived from pyridine, thiophene, and benzofuran could be employed (2g–2j). Alkyl sulfinamides could also be prepared, with representative primary, benzylic, secondary, and tertiary Grignard reagents being used (2k–2n). An alkenyl-Grignard reagent was also successful (2o). Finally, the aryl core of the COX-2 inhibitor Celecoxib was incorporated (2p).
Scheme 3. Synthesis of Primary Sulfinamides 2.
Reaction conditions: TIPS-NSO (1.00 equiv), R-[M] (1.20 equiv), THF (0.1 M), 0 °C, 5 min; then TBAF (2.00 equiv), 0 °C to rt, 10 min. Isolated yields.
Commercial Grignard reagent.
Organolithium reagent, generated using lithium-halogen exchange (n-BuLi).
Grignard reagent, generated from aryl bromide using i-Pr-MgCl.LiCl.
Organolithium reagent, generated by deprotonation, using n-BuLi.
Using Ar-ZnCl, generated from Grignard reagent and ZnCl2.
With a selection of primary sulfinamides readily available, our attention turned to their conversion into sulfonimidamides. An efficient procedure was established, involving treating the sulfinamide with the desired amine in the presence of 1.5 equiv of PhI(OAc)2, using triethylamine as base (Scheme 4). Applying this procedure, using morpholine as the amine component, allowed all of the primary sulfinamides shown in Scheme 3 to be smoothly converted into the corresponding sulfonimidamides. The reactions were performed at ambient temperature, for between 1.5 and 23 h. The para-fluoro example (3d) was prepared on a gram scale (2.3 g) in an identical yield (92%) to that achieved in the smaller-scale scoping experiments. The para-fluorophenyl sulfinamide (2d) was then used to explore the range of secondary amines that could be used. Cyclic examples substituted with cyano and ketone groups performed well (3q, 3r). Acyclic amines featuring pyridyl and cyclohexylmethyl groups were also included (3s, 3t). The remainder of the amines used were selected as they feature in marketed pharmaceuticals (Clopidogrel 3u, Perospirone 3v, Buspirone 3w, Amoxapine 3x), and as can be seen, these more complex, heterocylic scaffolds provided the desired sulfonimidamides in generally excellent yields.
Scheme 4. Conversion of Primary Sulfinamides into Sulfonimidamides, Using Secondary Amine Nucleophiles.
Reaction conditions: sulfinamide 2a–p (1.0 equiv), amine (1.5 equiv), PhI(OAc)2 (1.5 equiv), Et3N (3.0 equiv), toluene (0.1 M), rt. Isolated yields.
All of the amines used in Scheme 4 are secondary amines; primary amines were poor substrates using the original reaction conditions, generally providing only 5–10% of product. After optimization (see Supporting Information), we were able to identify suitable conditions for primary amine substrates. The new conditions required the use of the more robust hypervalent iodine reagent PhI(OC(O)t-Bu)2,18 a greater excess of triethylamine, and an increased reaction temperature of 60 °C. These modified conditions were successfully applied to a range of primary amines, including primary alkyl (4a–c) and secondary alkyl (4d, 4e), using the para-fluorophenyl sulfinamide (2d) as the substrate. Amines featuring alkyne (4f, 4g) and alkene (4h) groups could be used (Scheme 5). The final example establishes that these new conditions could also be extended to a heterocyclic sulfinamide substrate (4i). The successful use of primary amines to prepare the corresponding sulfonimidamides is notable, as neither the Bull nor Stockman approaches shown in Scheme 1 could accommodate this class of amine.8,9
Scheme 5. Conversion of Primary Sulfinamides into Sulfonimidamides, Using Primary Amine Nucleophiles.
Reaction conditions: sulfinamide (1.0 equiv), amine (2.0 equiv), PhI(OC(O)t-Bu)2 (2.5 equiv), Et3N (12.0 equiv), MeCN (0.1 M), 60 °C, 16 h. Isolated yields.
As a final demonstration of the utility of the developed method, we targeted the preparation of a more complex sulfonimidamide derivative (Scheme 6). Pyrimidinone-substituted aryl bromide 5 contains the aryl core of the marketed PDE5-inhibitor Sildenafil. Lithiation of bromide 5 using a combination of MeLi and n-BuLi generated an aryl lithium reagent that underwent smooth addition into TIPS-NSO. In situ treatment with TBAF then provided complex primary sulfinamide 6 in an excellent 89% yield. The coupling of sulfinamide 6 with N-methyl piperazine required the use of DBU in place of triethylamine and MeCN as solvent; with these modifications, sulfonimidamide 7,7a the monoaza analogue of Sildenafil, was isolated in 64% yield.
Scheme 6. Synthesis of Sildenafil Analogue 7.
In summary, we have developed a modular, two-step synthesis of sulfonimidamides, with organometallics such as Grignard, organolithium, or organozinc reagents, and amines, being the key building blocks. This strategy alleviates the necessity of thiol starting materials. A new N-silyl sulfinylamine reagent is introduced that allows ready preparation of a broad range of primary sulfinamides. The convergent nature of this approach should be attractive to medicinal chemists preparing collections of sulfonimidamides or sulfinamides.
Acknowledgments
T.Q.D. is grateful to the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for a studentship, generously supported by GlaxoSmithKline, Vertex, AstraZeneca, Diamond Light Source, Defense Science and Technology Laboratory, Evotec, Janssen, Novartis, Pfizer, Syngenta, Takeda, and UCB. Z.Z. is grateful to the China Scholarship Council for fellowship support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c00347.
Experimental procedures and supporting characterization data and spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Clarke S. G.; Kenyon J.; Phillips H. CLVI.—The preparation of compounds analogous in structure to sulphinic acids but containing p-toluenesulphonimido-groups in place of oxygen atoms. Phenyl- and methyl-p-toluenesulphonimido-sulphine-p-toluenesulphonylimines. J. Chem. Soc. 1930, 0, 1225–1232. 10.1039/JR9300001225. [DOI] [Google Scholar]
- a Chinthakindi P. K.; Naicker T.; Thota N.; Govender T.; Kruger H. G.; Arvidsson P. I. Sulfonimidamides in Medicinal and Agricultural Chemistry. Angew. Chem., Int. Ed. 2017, 56, 4100–4109. 10.1002/anie.201610456. [DOI] [PubMed] [Google Scholar]; b Tilby M. J.; Willis M. C. How do we address neglected sulfur pharmacophores in drug discovery?. Expert Opin Drug Discov 2021, 16, 1227–1231. 10.1080/17460441.2021.1948008. [DOI] [PubMed] [Google Scholar]; c Lücking U. Neglected sulfur(vi) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 2019, 6, 1319–1324. 10.1039/C8QO01233D. [DOI] [Google Scholar]; d Izzo F.; Schafer M.; Lienau P.; Ganzer U.; Stockman R.; Lucking U. Exploration of Novel Chemical Space: Synthesis and in vitro Evaluation of N-Functionalized Tertiary Sulfonimidamides. Chem.—Eur. J. 2018, 24, 9295–9304. 10.1002/chem.201801557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sehgelmeble F.; Janson J.; Ray C.; Rosqvist S.; Gustavsson S.; Nilsson L. I.; Minidis A.; Holenz J.; Rotticci D.; Lundkvist J.; Arvidsson P. I. Sulfonimidamides as Sulfonamides Bioisosteres: Rational Evaluation through Synthetic, in Vitro, and in Vivo Studies with γ-Secretase Inhibitors. ChemMedChem. 2012, 7, 396–399. 10.1002/cmdc.201200014. [DOI] [PubMed] [Google Scholar]; b Gege C.; Bravo F. J.; Uhlig N.; Hagmaier T.; Schmachtenberg R.; Elis J.; Burger-Kentischer A.; Finkelmeier D.; Hamprecht K.; Grunwald T.; Bernstein D. I.; Kleymann G. A helicase-primase drug candidate with sufficient target tissue exposure affects latent neural herpes simplex virus infections. Sci. Transl. Med. 2021, 13, eabf8668 10.1126/scitranslmed.abf8668. [DOI] [PubMed] [Google Scholar]; c Benediktsdottir A.; Lu L.; Cao S.; Zamaratski E.; Karlen A.; Mowbray S. L.; Hughes D.; Sandstrom A. Antibacterial sulfonimidamide-based oligopeptides as type I signal peptidase inhibitors: Synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 224, 113699. 10.1016/j.ejmech.2021.113699. [DOI] [PubMed] [Google Scholar]; d Toth J. E.; Ray J.; Deeter J. Synthesis and resolution of sulfonimidamide analogs of sulfonylureas. J. Org. Chem. 1993, 58, 3469–3472. 10.1021/jo00064a044. [DOI] [Google Scholar]
- Nandi G. C.; Arvidsson P. I. Sulfonimidamides: Synthesis and Applications in Preparative Organic Chemistry. Adv. Synth. Catal. 2018, 360, 2976–3001. 10.1002/adsc.201800273. [DOI] [Google Scholar]
- a Wen J.; Cheng H.; Dong S.; Bolm C. Copper-Catalyzed S-C/S-N Bond Interconversions. Chem.—Eur. J. 2016, 22, 5547–5550. 10.1002/chem.201600661. [DOI] [PubMed] [Google Scholar]; b Yu H.; Li Z.; Bolm C. Copper-Catalyzed Transsulfinamidation of Sulfinamides as a Key Step in the Preparation of Sulfonamides and Sulfonimidamides. Angew. Chem., Int. Ed. 2018, 57, 15602–15605. 10.1002/anie.201810548. [DOI] [PubMed] [Google Scholar]; c Zasukha S. V.; Timoshenko V. M.; Tolmachev A. A.; Pivnytska V. O.; Gavrylenko O.; Zhersh S.; Shermolovich Y.; Grygorenko O. O. Sulfonimidamides and Imidosulfuric Diamides: Compounds from an Underexplored Part of Biologically Relevant Chemical Space. Chem.—Eur. J. 2019, 25, 6928–6940. 10.1002/chem.201900440. [DOI] [PubMed] [Google Scholar]; d Davies T. Q.; Tilby M. J.; Ren J.; Parker N. A.; Skolc D.; Hall A.; Duarte F.; Willis M. C. Harnessing Sulfinyl Nitrenes: A Unified One-Pot Synthesis of Sulfoximines and Sulfonimidamides. J. Am. Chem. Soc. 2020, 142, 15445–15453. 10.1021/jacs.0c06986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Davies T. Q.; Hall A.; Willis M. C. One-Pot, Three-Component Sulfonimidamide Synthesis Exploiting the Sulfinylamine Reagent N-Sulfinyltritylamine. TrNSO. Angew. Chem. Int. Ed. 2017, 56, 14937–14941. 10.1002/anie.201708590. [DOI] [PubMed] [Google Scholar]; b Gao B.; Li S.; Wu P.; Moses J. E.; Sharpless K. B. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem., Int. Ed. 2018, 57, 1939–1943. 10.1002/anie.201712145. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Greed S.; Briggs E. L.; Idiris F. I. M.; White A. J. P.; Lucking U.; Bull J. A. Synthesis of Highly Enantioenriched Sulfonimidoyl Fluorides and Sulfonimidamides by Stereospecific Sulfur-Fluorine Exchange (SuFEx) Reaction. Chem.—Eur. J. 2020, 26, 12533–12538. 10.1002/chem.202002265. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lo P. K. T.; Willis M. C. Nickel(II)-Catalyzed Addition of Aryl and Heteroaryl Boroxines to the Sulfinylamine Reagent TrNSO: The Catalytic Synthesis of Sulfinamides, Sulfonimidamides, and Primary Sulfonamides. J. Am. Chem. Soc. 2021, 143, 15576–15581. 10.1021/jacs.1c08052. [DOI] [PubMed] [Google Scholar]; e Mancheno O. G.; Bolm C. Synthesis of sulfonimidamides from sulfinamides by oxidation with N-chlorosuccinimide. Beilstein J. Org. Chem. 2007, 3, 25. 10.1186/1860-5397-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Johnson C. R.; Jonsson E. U.; Bacon C. C. Preparation and reactions of sulfonimidoyl chlorides. J. Org. Chem. 1979, 44, 2055–2061. 10.1021/jo01327a001. [DOI] [Google Scholar]; g Chen Y.; Gibson J. A convenient synthetic route to sulfonimidamides from sulfonamides. RSC Adv. 2015, 5, 4171–4174. 10.1039/C4RA14056G. [DOI] [Google Scholar]; h Richards-Taylor C. S.; Martinez-Lamenca C.; Leenaerts J. E.; Trabanco A. A.; Oehlrich D. The Synthesis of Trifluoromethyl-sulfonimidamides from Sulfinamides. J. Org. Chem. 2017, 82, 9898–9904. 10.1021/acs.joc.7b01628. [DOI] [PubMed] [Google Scholar]
- a Bremerich M.; Conrads C. M.; Langletz T.; Bolm C. Additions to N-Sulfinylamines as an Approach for the Metal-free Synthesis of Sulfonimidamides: O-Benzotriazolyl Sulfonimidates as Activated Intermediates. Angew. Chem., Int. Ed. 2019, 58, 19014–19020. 10.1002/anie.201911075. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Matos P. M.; Stockman R. A. Synthetic approaches and applications of sulfonimidates. Org. Biomol. Chem. 2020, 18, 6429–6442. 10.1039/D0OB01191F. [DOI] [PubMed] [Google Scholar]; c Wright M.; Martinez-Lamenca C.; Leenaerts J. E.; Brennan P. E.; Trabanco A. A.; Oehlrich D. Bench-Stable Transfer Reagent Facilitates the Generation of Trifluoromethyl-sulfonimidamides. J. Org. Chem. 2018, 83, 9510–9516. 10.1021/acs.joc.8b01244. [DOI] [PubMed] [Google Scholar]
- Briggs E. L.; Tota A.; Colella M.; Degennaro L.; Luisi R.; Bull J. A. Synthesis of Sulfonimidamides from Sulfenamides via an Alkoxy-amino-λ6-sulfanenitrile Intermediate. Angew. Chem., Int. Ed. 2019, 58, 14303–14310. 10.1002/anie.201906001. [DOI] [PubMed] [Google Scholar]
- Izzo F.; Schafer M.; Stockman R.; Lucking U. A New, Practical One-Pot Synthesis of Unprotected Sulfonimidamides by Transfer of Electrophilic NH to Sulfinamides. Chem.—Eur. J. 2017, 23, 15189–15193. 10.1002/chem.201703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Leca D.; Fensterbank L.; Lacôte E.; Malacria M. A New Practical One-Pot Access to Sulfonimidates. Org. Lett. 2002, 4, 4093–4095. 10.1021/ol026837b. [DOI] [PubMed] [Google Scholar]; b Leca D.; Song K.; Amatore M.; Fensterbank L.; Lacôte E.; Malacria M. Iodine(III)-Mediated Preparations of Nitrogen-Containing Sulfur Derivatives: Dramatic Influence of the Sulfur Oxidation State. Chem.—Eur. J. 2004, 10, 906–916. 10.1002/chem.200305525. [DOI] [PubMed] [Google Scholar]; c Felim A.; Toussaint A.; Phillips C. R.; Leca D.; Vagstad A.; Fensterbank L.; Lacôte E.; Malacria M. Improved Method for the Iodine(III)-Mediated Preparation of Aryl Sulfonimidates. Org. Lett. 2006, 8, 337–339. 10.1021/ol052790t. [DOI] [PubMed] [Google Scholar]
- a Robak M. T.; Herbage M. A.; Ellman J. A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]; b Savile C. K.; Kazlauskas R. J. The 3-(3-Pyridine)propionyl Anchor Group for Protease-Catalyzed Resolutions:p-Toluenesulfinamide and Sterically Hindered Secondary Alcohols. Adv. Synth. Catal. 2006, 348, 1183–1192. 10.1002/adsc.200606040. [DOI] [Google Scholar]; c Kawȩcki R. Facile synthesis of homochiral derivatives of 10-bornane sulfinates, sulfinamides and sulfinimines. Tetrahedron: Asymmetry 1999, 10, 4183–4190. 10.1016/S0957-4166(99)00433-4. [DOI] [Google Scholar]; d Lo P. K. T.; Oliver G. A.; Willis M. C. Sulfinamide Synthesis Using Organometallic Reagents, DABSO, and Amines. J. Org. Chem. 2020, 85, 5753–5760. 10.1021/acs.joc.0c00334. [DOI] [PMC free article] [PubMed] [Google Scholar]; e García Ruano J. L.; Parra A.; Yuste F.; Mastranzo V. M. Mild and General Method for the Synthesis of Sulfonamides. Synthesis 2008, 2008, 311–319. 10.1055/s-2007-1000850. [DOI] [Google Scholar]
- a Chatterjee S.; Makai S.; Morandi B. Hydroxylamine-Derived Reagent as a Dual Oxidant and Amino Group Donor for the Iron-Catalyzed Preparation of Unprotected Sulfinamides from Thiols. Angew. Chem., Int. Ed. 2021, 60, 758–765. 10.1002/anie.202011138. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sudarikov D. V.; Krymskaya Y. V.; Il’chenko N. O.; Slepukhin P. A.; Rubtsova S. A.; Kutchin A. V. Synthesis and biological activity of fluorine-containing amino derivatives based on 4-caranethiol. Russ. Chem. Bull. 2018, 67, 731–742. 10.1007/s11172-018-2130-7. [DOI] [Google Scholar]; c Davis F. A.; Reddy R. E.; Szewczyk J. M.; Reddy G. V.; Portonovo P. S.; Zhang H.; Fanelli D.; Zhou P.; Carroll P. J. Asymmetric Synthesis and Properties of Sulfinimines (Thiooxime S-Oxides). J. Org. Chem. 1997, 62, 2555–2563. 10.1021/jo970077e. [DOI] [PubMed] [Google Scholar]
- Böttinger C. Einwirkung von Thionylchlorid auf Anilin. Chem. Ber. 1878, 11, 1407–1408. 10.1002/cber.18780110235. [DOI] [Google Scholar]
- a Davies T. Q.; Willis M. C. Rediscovering Sulfinylamines as Reagents for Organic Synthesis. Chem.—Eur. J. 2021, 27, 8918–8927. 10.1002/chem.202100321. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kresze G.; Maschke A.; Albrecht R.; Bederke K.; Patzschke H. P.; Smalla H.; Trede A. Organic N-Sulfinyl Compounds. Angew. Chem., Int. Ed. 1962, 1, 89–98. 10.1002/anie.196200891. [DOI] [Google Scholar]
- a Zhang Z. X.; Davies T. Q.; Willis M. C. Modular Sulfondiimine Synthesis Using a Stable Sulfinylamine Reagent. J. Am. Chem. Soc. 2019, 141, 13022–13027. 10.1021/jacs.9b06831. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Davies T. Q.; Tilby M. J.; Skolc D.; Hall A.; Willis M. C. Primary Sulfonamide Synthesis Using the Sulfinylamine Reagent N-Sulfinyl-O-(tert-butyl)hydroxylamine, t-BuONSO. Org. Lett. 2020, 22, 9495–9499. 10.1021/acs.orglett.0c03505. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bayeh L.; Le P. Q.; Tambar U. K. Catalytic allylic oxidation of internal alkenes to a multifunctional chiral building block. Nature 2017, 547, 196–200. 10.1038/nature22805. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Oliver G. A.; Loch M. N.; Augustin A. U.; Steinbach P.; Sharique M.; Tambar U. K.; Jones P. G.; Bannwarth C.; Werz D. B. Cycloadditions of Donor-Acceptor Cyclopropanes and -butanes using S=N-Containing Reagents: Access to Cyclic Sulfinamides, Sulfonamides, and Sulfinamidines. Angew. Chem., Int. Ed. 2021, 60, 25825–25831. 10.1002/anie.202106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed G. A.; Ismail N. A.; Yassin F. A. Synthesis and Reactions of Some New Organometallic Compounds: Synthesis of Triphenylsilylsulfinylamine. Phosphorus Sulfur Silicon Relat. Elem. 1992, 69, 253–255. 10.1080/10426509208040643. [DOI] [Google Scholar]
- TIPS-NSO is available from Cortex Organics (www.cortexorganics.com).
- Mocci F.; Uccheddu G.; Frongia A.; Cerioni G. Solution Structure of Some λ3 Iodanes: An 17O NMR and DFT Study. J. Org. Chem. 2007, 72, 4163–4168. 10.1021/jo070111h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.