Abstract
In 2020, there were an estimated 19.3 million new cancer cases and close to 10 million cancer deaths worldwide. Cancer remains one of the leading causes of death. In recent years, with the continuous improvement of our understanding of tumor immunotherapy, immunotherapeutics, such as immune checkpoint inhibitors, have gradually become a hot spot for tumor treatment. Amongst these, programmed cell death protein 1/programmed cell death protein ligand 1 (PD-1/PD-L1) related inhibitors, such as nivolumab and pembrolizumab, atezolizumab, avelumab and durvalumab have been shown to exhibit a high level of efficacy in several types of tumors. It has been confirmed that these inhibitors play an important role in the anti-tumor process, significantly improving the survival rate of patients and delaying the progress of the underlying cancer. However, its method of therapeutic interference and potential for damaging the immune system has caused concern regarding its suitability. As these adverse effects are caused by an immune response to endogenous tissues, they are designated as immune-related adverse events (irAEs). In this review, the typical irAEs reported in recent years and the management strategies adopted are highlighted, to serve as a reference in assessing the clinical response to these adverse reactions.
Keywords: immune-related adverse events, programmed cell death protein 1, programmed cell death protein ligand 1, cancer, immune-therapy, adverse effects
1. Introduction
In 2020, there were an estimated 19.3 million new cancer cases worldwide (18.1 million excluding non-melanoma skin cancer) and nearly 10 million cancer deaths (9.9 million excluding non-melanoma skin cancer). It is estimated that by 2040, the global cancer burden will reach 28.4 million cases, an increase of 47% over 2020 (1). There is no doubt that cancer is one of the most important risk factors affecting human health in the world today. Surgery remains the most important and effective treatment approach for the majority of tumor types. However, a large number of tumor patients present with treatment difficulties, such as late diagnosis, inability to tolerate surgery, or a cancer which spreads with ease or has recurred (2). Therefore, more effective treatment methods are required.
In recent years, immunotherapy has gradually entered the arena. Immune targeted drugs, such as programmed cell death protein 1/programmed cell death protein ligand 1 (PD-1/PD-L1) have achieved unprecedented results in the field of tumor therapy. Immunotherapy has become a mainstream direction of development of novel tumor therapeutics and the development of immunotherapeutic drugs has also exhibited explosive growth (3-5). The 2019 anti-cancer progress report released by the American Association for Cancer Research (6) listed cancer immunotherapy with surgery, chemotherapy, radiotherapy and targeted therapies as the five pillars of cancer treatment. Compared with 2017, the total number of global immunotherapeutics in 2019 expanded from 2,030 to 3,876, an increase of 91%, the discovery of potential immunotherapeutic targets has increased by 78% and the number of R&D companies focused on immunotherapy have increased by 60% (7). At present, clinical tumor immunotherapy is primarily divided into four categories: Regulatory T lymphocyte immune checkpoints, chimeric antigen receptor T cell immunotherapy, in vitro activation methods and tumor-specific antigen therapy. Among these, the use of antibodies to block cytotoxic T lymphocyte-related antigen 4, PD-1 and its ligand PD-L1 pathways belong to T lymphocyte cellular immune checkpoints (8). The present review primarily focuses on PD1/PD-L1 immune checkpoint inhibitors.
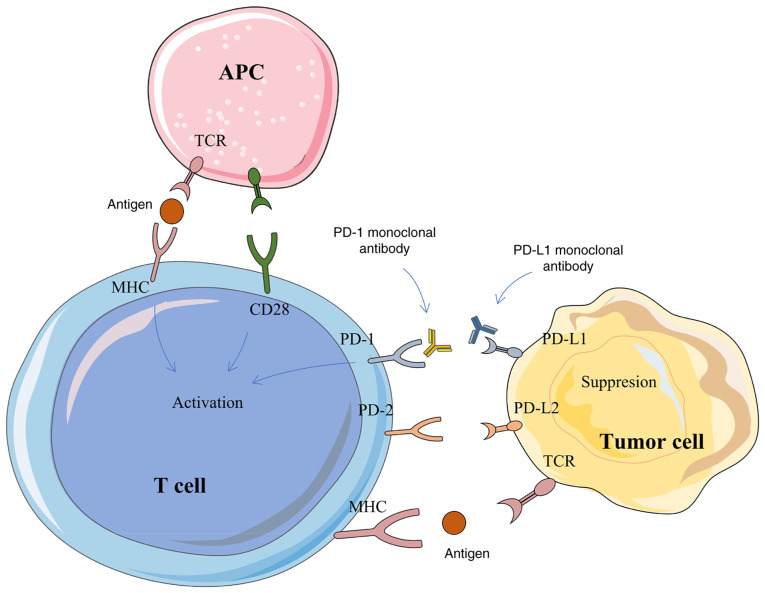
The PD-1 protein is primarily expressed in activated T/B cells, monocytes, dendritic cells, regulatory T cells and natural killer T cells (9), while the PD-L1 protein is widely expressed in antigen presenting cells, activated T/B cells, macrophages and some non-immune cells, such as placental trophoblasts, myocardial endothelium cells and thymic cortical epithelial cells (10). The expression of PD-L1 protein is detectable in several human tumor tissues (11). In a healthy individual, PD-1 binds to the PD-L1 receptor on the surface of T cells, thereby inhibiting the proliferation and activation of T cells, blocking their immune functions and preventing the body from autoimmune diseases (12). However, the tumor tissue also cunningly evades the immune system's attack through the characteristic action of PD-1/PD-L1 (13). Due to the lack of an effective immune response in tumor patients, tumor cells proliferate in large quantities and the PD-L1 receptor protein on the surface can bind to the PD-1 protein on the surface of T cells, leading to the recruitment of tyrosine phosphatase-2 in the src homologous region and then lead to phosphorylation of downstream protein spleen tyrosine kinase and phosphoinositide-3 kinase, inhibit downstream signal transduction, T cell proliferation, cytokine secretion and cytotoxicity (14). Ultimately, this leads to substantial depletion of T cells. PD-1/PD-L1 monoclonal antibody (mAb)-based therapeutics can block the binding between the receptor and its ligand, reactivate T cells and re-initiate the killing of tumorigenic cells (14). At present, there are >10 approved PD-1/PD-L1 mAbs worldwide. In addition, dozens of drugs have been or are about to enter the clinical trial stage. The exploration of their regulatory mechanisms are still the key for improving the development of novel targets, such as FBXO38, a key enzyme for PD-1 ubiquitination and degradation and CMTM6, a key molecule for PD-L1 expression regulation (15-17). In addition, small molecule peptides, treatment-related biomarkers and treatment of drug resistance remain the focus of research (18,19). The mechanism of PD-1/PD-L1 based therapeutics is briefly described in Fig. 1.
Figure 1.
The mechanism of PD-1/PD-L1 in tumorigenesis and development. PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; APC, antigen-presenting cell; TCR, T-cell receptor; MHC, major histocompatibility complex CD, cluster of differentiation.
According to a study in 2020, the PD-L1 mAb atezizumab combined with bevacizumab in the treatment of advanced liver cancer was significantly improved on traditional sorafenib treatment in terms of overall survival and progression-free survival (20). A clinical study in 2018 reported that the preoperative use of neoadjuvant PD-1 related immunotherapy achieved favorable surgical results in patients with lung cancer (21). The combination of immunization and targeted neoadjuvant therapy-PD-1 mAb combined with TKI also achieved curative effects in liver cancer in preliminary studies (22-24). However, as with almost all therapeutics, PD-1/PD-L1 immunotherapy may inevitably cause patients to exhibit varying degrees of immune-related damage. A phase II clinical trial calculated that the probability of treatment-related adverse events in the treatment of advanced hepatocellular carcinoma with carrelizumab combined with apatinib was 77%; 29% of patients experienced more serious adverse effects including liver damage and two patients died due to treatment (25). According to the statistics of reported adverse reactions, skin injuries including pruritus, psoriasis and nodular dermatitis accounted for 46-62% of adverse events, autoimmune colitis accounted for 22-48% of adverse events and autoimmune hepatitis accounted for 7-33% of adverse events. Endocrine diseases such as thyroiditis, hypophysitis, adrenalitis and diabetes accounted for 12-34% of adverse events. In addition, there are other rare adverse effects including pneumonia (3-8%), nephritis (1-7%), cardiac adverse effects including myocarditis (5%) and nervous system adverse effects (1-5%) (26,27). As these adverse effects result from the immune response to endogenous tissues, they are defined as immune-related adverse events (irAEs) (28).
The present review appraises the typical adverse reactions caused by the use of PD-1/PD-L1 related inhibitors and the management strategies developed in recent years, with the aim of providing an up-to-date reference for clinical response to these adverse reactions in the future.
2. Neuromuscular system
The manifestations of neuromuscular system-related adverse events primarily include symptoms such as tremor, visual disturbances, dysarthria, ataxia, paresthesia and seizures; however, symptoms may also be unspecific, such as headaches, dizziness, fatigue and drowsiness. The most common neuromuscular system-related side effect is myasthenia gravis (29). In addition to cancer, immune checkpoint inhibitors are often used to treat neurological diseases, such as ipilimumab for aseptic meningitis (30,31), Guillain-Barre syndrome (32), transversal Myelitis (33) and enteric neuropathy (34), it is therefore of interest to discuss the nervous system-related side effects of these drugs. A case in China reported a patient with melanoma who developed exertional dyspnea and diplopia after 20 days of nivolumab treatment. Laboratory tests revealed myositis with myocarditis and rhabdomyolysis. Following diagnosis, patients should be administered a course of intravenous immunoglobulin (IVIG). During IVIG, weekly subcutaneous methotrexate and methylprednisolone were administered and discontinued slowly. This coping strategy was clearly beneficial to the patient, who gradually exhibited clinical improvements (35). A case report described an 85-year-old woman with metastatic melanoma who developed diplopia after a second cycle of pembrolizumab monotherapy, followed by asymmetric bilateral ptosis; Myasthenia gravis was highly suspected clinically. After a diagnosis was made, the primary treatment options were intravenous immunoglobulin, prednisone and pyridamide. This protocol elicited a rapid clinical response and completely resolved the problems of bilateral ptosis and diplopia. Subsequent treatment included monthly IVIG and daily oral pyridostigmine without any further recurrence of symptoms (36). In addition to the aforementioned reports, there are also reports documenting the adverse effects of myasthenia gravis after the use of pembrolizumab in undifferentiated cholangiopancreatic carcinoma (37-40). The management strategy is administration of pyridostigmine and cessation of pembrolizumab (41). Another article reported on a patient with melanoma who received dacarbazine and ipilimumab. On the fifth treatment cycle, he developed progressive ataxia and dizziness, with intermittent numbness in his left arm. On the seventh cycle, his left arm began to twitch. The final diagnosis was persistent seizures. He was treated with oxcarbazepine plus oxcarbazepine and levetiracetam. After being discharged from the hospital, his seizures continued for three weeks. The management strategy was addition of phenobarbital to oxcarbazepine and levetiracetam. The motor seizures gradually improved after treatment (42). Additional neuromuscular related adverse events and their management strategies are described in Table SI.
3. Respiratory system
Respiratory adverse events are relatively common irAEs. Several life-threatening respiratory events have been reported following the use of anti-CTLA-4 blockers, including tissue inflammatory pneumonitis, sarcoidosis and pulmonary granulomatosis (43-46). The incidence of respiratory-related adverse events in patients receiving anti-PD-1/PD-L1 therapy cannot be ignored. In total, 18-38% of patients most frequently develop a cough and dyspnea. In this subset of patients, 2-9% had a severe grade 3-4 cough and 1-2% had life-threatening grade 3-4 dyspnea (47-49). Below, some of the more serious clinical adverse effects and their management strategies are described.
There is a case report of a patient with poorly differentiated squamous cell lung cancer who was diagnosed with grade 3 immune checkpoint inhibitor-associated pneumonitis (Pneumonia Severity Index classification) after receiving second-line single-agent nivolumab (50). After diagnosis, the management strategy was high-dose glucocorticoid pulse therapy, following which the patient's clinical symptoms gradually eased. Subsequent treatment included oral pirfenidone for 11 months. During pirfenidone treatment, the CT images and clinical symptoms of the patients showed significant improvements (50). Donato and Krol (51) report a case of allergic bronchopulmonary aspergillosis after four months of treatment with the PD-1 inhibitor pembrolizumab. The final diagnosis was pembrolizumab-induced allergic bronchopulmonary aspergillosis. The management strategy for the patient was administration of corticosteroids and voriconazole. The patient responded to treatment, showing improvement and was able to resume pembrolizumab with a good clinical response. Fragkou et al (52) report a lower respiratory tract infection affecting all lobes of a patient with metastatic melanoma following second-line pembrolizumab immunotherapy. Following confirmation of the diagnosis, the management strategy for this side effect was administration of the corticosteroid prednisolone (50 mg/day intravenously). The patient was sensitive to this treatment and his clinical symptoms and radiological results improved rapidly. Unfortunately, three months later, the patient died of advanced metastatic disease in the brain. Additional respiratory related adverse events and their management strategies are described in Table SI.
4. Circulatory system
To date, there have been numerous reports of circulatory system-related adverse events in patients with cancer receiving anti-PD-1/PD-L1 treatment. A case of third-degree atrioventricular block was reported in a patient with metastatic non-small cell lung cancer receiving ipilimumab-nivolumab combination therapy. The patient first developed symptoms of lower extremity swelling after 15 days of ipilimumab and nivolumab treatment and was subsequently diagnosed with left bundle branch block, progressive PR interval prolongation, neutropenia and normocytic anemia. Due to metastatic disease and comorbidities, the patient and medical team chose not to undergo emergency pacemaker placement and the patient was instead scheduled for outpatient event monitoring. Unfortunately, during the hospital stay, the patient was found to have suffered cardiac arrest and eventually succumbed (53). Läubli et al (54) report a case of a melanoma patient who developed myocarditis following pembrolizumab treatment. Echocardiography of the patient revealed severely impaired left ventricular function with dyssynchrony and histological analysis of myocardial biopsy showed lymphocytic infiltration, predominance of CD8+ cells and a decrease in FOXP3+ regulatory T cells. The management strategy employed resulted in rapid improvement of symptoms and recovery of left ventricular function and included initiation of corticosteroids and heart failure treatment according to relevant guidelines. Bukamur et al (55) document the case of a patient on statins with a history of hypertension and hyperlipidemia who developed muscle mass pain after completing two cycles of nivolumab (240 mg every two weeks). The management strategy for her condition after admission was cessation of statin use and administration of high-dose pulsed steroids. Sinus bradycardia developed and progressed to complete atrioventricular block. After consultation with an electrophysiologist, the patient was implanted with a temporary transvenous pacemaker and then a permanent pacemaker. The overall condition of the patient with this management strategy improved.
5. Digestive system
The probability of digestive system-related AEs in patients with cancer treated with immune checkpoint inhibitors varies with the specific medications administered. The incidence of gastrointestinal reactions in patients treated with anti-CTLA-4/anti-PD-1 combination therapy is 44%, 23-33% in patients treated with CTLA-4 alone and <20% in patients treated with anti-PD-1/PD-L1 alone (56). Gastrointestinal-related irAEs in patients treated with anti-PD-1/PD-L1 primarily include diarrhea, abdominal pain and occasionally fever and some of these will be severe enough to cause substantial damage to the gastrointestinal system (57). Immune-related liver injury is a relatively common irAE. Immune-related hepatitis is the most common liver-related adverse event, affecting ~5% of patients receiving anti-PD-1 therapy, 5-15% of patients receiving ipilimumab monotherapy and one-third of patients receiving combination therapy (57). The following are a few typical digestive system-related adverse events and the associated clinical response strategies adopted. A case report by Tso et al (58) documents a patient with metastatic non-small cell lung cancer who presented with acute abdominal pain following long-term treatment with nivolumab and a CT scan showed small dilatation of the proximal ileum, thickening of the vessel wall and perforation near the transition point. The management strategy was a laparotomy and the patient eventually recovered. There is also a report of hepatitis in a woman treated for recurrent renal cell carcinoma. The physicians eventually attributed the hepatitis to the use of nivolumab. The management strategy for this side effect was administration of steroids and the patient began to exhibit improvements in liver function. However, she later developed substantial upper gastrointestinal bleeding secondary to a gastroduodenal ulcer and then developed acute tubular necrosis, ultimately succumbing to the complications (59). Lankes et al (60) reported severe diarrhea with ≤18 watery bowel movements per day in a patient with metastatic melanoma treated with ipilimumab. The management strategy was immunosuppression (high-dose steroids and infliximab) combined with parenteral therapy. After nutritional therapy, his condition initially improved, but subsequently worsened. The patient's symptoms improved by changing the treatment strategy to antiviral drugs whilst reducing the application of glucocorticoids. In addition to the aforementioned more common and severe digestive system-related adverse events, additional digestive related adverse events and their management strategies are described in Table SI.
6. Endocrine system
Endocrine-related AEs caused by the use of immune checkpoint inhibitors are more common when treated with anti-CTLA-4 antibodies, whereas a relatively lower incidence of events is recorded in patients treated with anti-PD-1/PD-L1 treatment. Major AEs include hypophysitis, abnormal thyroid function and other less common endocrine diseases such as diabetes and hypercalcemia. In total, ~1% of patients treated with anti-PD-1/PD-L1 develop hypophysitis and 4% of patients develop abnormal thyroid function. Most of these adverse effects are irreversible and require lifelong hormone replacement therapy (56). The next is a case of a more typical anti-PD-1/PD-L1 treatment-related immune adverse event and the clinical management strategy employed. A 77-year-old woman with stage IV left sigmoid colon cancer developed somnolence and fatigue after receiving second-line pembrolizumab monotherapy and progressively developed polydipsia, nausea and vomiting every day, with progressively more severe symptoms. Diabetic ketoacidosis was diagnosed based on laboratory tests and the management strategy for the patient included fluid replacement, insulin therapy, dose adjustment and electrolyte management. Eventually, the patient recovered and was discharged home for basal and dietary insulin therapy (61).
7. Skin lesions
Immunotherapy-related skin damage is the most common irAE and is very common in patients with cancer treated with anti-CTLA-4 and anti-PD-1/PD-L1. In total, ~50% of patients treated with anti-CTLA-4 exhibit some form of skin damage. The incidence of patients treated with anti-PD-1/PD-L1 who exhibit skin damage is slightly lower at <40% (62). The most common skin-related irAE is skin rashes; most patients report itchy skin. Skin biopsies show large quantities of infiltrated T cells (63,64). The primary treatment measures include topical steroids. Next, a few examples of typical clinical cases reported in recent years are described. Mullangi et al (65) reported a patient with renal cell carcinoma who developed psoriasis with nivolumab and showed involvement of the palms and soles. After a diagnosis of palmoplantar psoriasis, he was started on a regimen of topical steroids with triamcinolone acetonide. This did not help his symptoms. Thus, he was instead administered apremilast and retinoic acid and continued nivolumab. After three months, he developed severe diarrhea requiring systemic steroids and infliximab, which improved his condition. No recurrence of symptoms in the last two years of follow-up after discontinuing nivolumab were reported. Acar et al (66) also reported localized plaques and hard plaques, but no systemic involvement in a melanoma patient treated with nivolumab. The patient was treated with topical corticosteroids and calcipotriol. Following treatment, the patient's lesions responded well and the patient's condition was ultimately relieved. Mobini et al (67) report a patient with renal cell carcinoma who received nivolumab and ipilimumab after developing lung metastases. A total of one month following the first round of treatment, the patient developed large, nontender, firm subcutaneous nodules and plaques on the left forearm and elbow. These nodules and plaques were visible to the naked eye. Skin biopsy showed granulomatous inflammation of the dermis and subcutaneous tissue. Dermatitis nodosa and panniculitis are thought to be secondary to combination therapy with nivolumab and ipilimumab (67). Following consultation with the oncologist, the attending physician decided to discontinue checkpoint inhibitor therapy after the third round. Over the next three weeks of follow-up, the patient reported that the size and stiffness of the lesions were decreasing. There is also a case report of a melanoma patient with a history of psoriasis that worsened during treatment with nivolumab (anti-PD-1). The patient was treated with topical steroids with good results (68). More skin lesion-related irAEs are described in the Table SI.
8. Urinary system
Urinary system-related irAEs rarely occur. In total, ~2% of patients using anti-CTLA-4 will develop urinary system-related irAEs, such as renal injury nephritis and only sporadic adverse effects have been reported in patients treated with anti-PD-1/PD-L1 (69). One of the most serious reports describes a patient treated with anti-PD-1 who exhibited immune rejection following kidney transplantation (70). Next, some of the more common urinary system-related irAEs after the use of anti-PD-1/PD-L1 are described. Schneider et al (71) report a patient with melanoma who developed aseptic cystitis during combination therapy with nivolumab and ipilimumab, with diarrhea, frequent urination, severe bladder pain and urgency. The final diagnosis was aseptic cystitis. Treatment with oral steroids was the most effective treatment option. Thummalapalli et al (72) report on a patient with BRAF-mutant melanoma who received anti-PD-1 therapy while taking a RAF/MEK inhibitor and experienced severe acute kidney injury at the start of therapy. This process was quickly reversed after symptomatic treatment with corticosteroids. Uchida et al (73) report on a patient with lung adenocarcinoma who gradually developed complications of acute tubulointerstitial nephritis following nivolumab treatment. Kidney biopsy showed massive proliferation of CD38+ and IgG+ plasma cells and massive infiltration of FoxP3+ regulatory T cells. Following the onset of symptoms, the management strategy was discontinuation of nivolumab and initiation of oral prednisolone, which was tapered off gradually. The patient eventually recovered from nivolumab-induced tubulointerstitial nephritis without any treatment for lung cancer. More urinary system-related irAEs are described in the Table SI.
9. Hematological system
Compared with conventional tumor chemotherapy methods that often cause adverse effects of the blood system, tumor patients treated with immune checkpoint inhibitors rarely exhibit related adverse effects, especially for patients treated with anti-PD-1/PD-L1 based therapy. Of those reported, adverse effects primarily included aplastic anemia (bone marrow) and autoimmune hemolytic anemia, which often occurred in the twelfth week of treatment (74-76). Jotatsu et al (77) report on a patient with non-small cell lung cancer who developed nivolumab-induced immune thrombocytopenia after nivolumab treatment. The day after the first nivolumab infusion, the patient presented with fever and elevated C-reactive protein levels. Computed tomography of the chest showed no interstitial lung disease or pneumonia. The fever subsided on day 9 and has not recurred since. On day 15 after the first infusion of nivolumab, severe thrombocytopenia developed suddenly and was diagnosed as nivolumab-induced immune thrombocytopenia. This was managed with 60 mg prednisolone per day, which restored the patient's platelet counts and platelet-associated IgG levels, with the patient eventually achieving remission. Another lung cancer patient developed immune-mediated thrombocytopenia and hypothyroidism after receiving nivolumab treatment. The specific manifestation was detection of IgG in the red blood cells of the patient, consistent with the warm autoimmune hemolytic anemia. The patient recovered after receiving steroid treatment (77). Additional hematological AEs and their management strategies are described in Table SI.
10. Ocular complications
The eyes are not typically regulated by the immune-system and the probability of irAEs there is very low, ~1%. A few sporadic cases report vision-related adverse reactions in patients with cancer receiving anti-PD-1/PD-L1 therapy (62,78). Next, several typical adverse effects of tumor patients treated with anti-PD-1/PD-L1 are described. Obata et al (79) report bilateral vision loss in a 63-year-old woman with metastatic cutaneous malignant melanoma 10 days after the second nivolumab injection. Following the onset of symptoms, the patient was started on topical glucocorticoid therapy. This management strategy proved effective and after 3 weeks, the patient's ante-rior chamber inflammation disappeared. Theillac et al (80) report on a man treated with nivolumab for a melanoma of the leg with duodenal and lymph node metastases who suddenly developed bilateral visual impairment and bilateral painless-ness after the third infusion of the drug. The patient was eventually treated with oral corticosteroids and his symptoms improved. Additional ocular-related adverse events and their management strategies are described in Table SI.
11. Joint damage
Joint-related irAEs occur in ~15% of tumor patients treated with anti-PD-1/PD-L1 and is considerably higher compared with that in tumor patients treated with anti-CTLA-4 (~1%). These adverse effects often manifest as joint swelling, stiffness, tenderness and erythema, which can last for several years and persist after immunotherapy is discontinued, and joint-related irAEs often occur in patients who have had at least one organ irAE (81-84). There have been case reports of arthralgias in some patients when pembrolizumab or nivolumab have been administered in combination with ipilimumab for treatment of metastatic cutaneous malignancies (85-87). Most patients with this complication receive nonsteroidal anti-inflammatory drugs (NSAIDs), 23.1% require additional low-dose corticosteroids and only 7.6% receive further immunosuppressive therapy. Arthralgia patients recovered following these treatments and exhibited improved PFS and OS (88).
12. Granulomatous venereal disease
There are also some published case reports documenting granulomatous lesions in patients following use of PD-1/PD-L1. Al-Dliw et al (89) report on a 65-year-old Caucasian woman with superficial melanoma of the left hip who, 1 year after pembrolizumab treatment, had a biopsy showing chronic granulomatous inflammation in histiocytes. The patient was started on a high-dose of intravenous steroids and showed significant clinical improvement. Noguchi et al (90) report on a case of a patient with cT1aN2M1b stage IV left upper lobe pleomorphic carcinoma who received nivolumab as a second treatment and had reduced swelling of the left supraclavicular lymph nodes and left adrenal gland but increased tumor shad-owing in the right upper lobe. Bronchoscopy biopsy revealed a granuloma that resembled a sarcomatoid reaction. The patient was not administered a specific targeted therapy and following the withdrawal of nivolumab, the granuloma disappeared. Additional granulomatous-related adverse events and their management strategies are described in Table SI.
13. Other AEs
In addition to the irAEs of the various systems aforementioned, patients using anti-PD-1/PD-L1 have also been recorded to have lymphatic system, oral cavity and other idiopathic irAEs, but the probability of these is very small. In the lymphatic system, a patient with malignant melanoma received pembrolizumab for 3 months. Although there was a partial response to skin metastasis and tumor progression was abated, the patient developed mediastinal lymphadenopathy. The patient under-went selective lymph node resection. The histopathological results were consistent with the nodule response. The patient's pembrolizumab treatment was interrupted and systemic steroid pulse therapy was used, which significantly relieved the lymphadenopathy (91). Lederhandler et al (92) report an oral-related irAE in a patient with grade 3 ulcerative oral mucositis in a 78-year-old woman with lung adenocarcinoma 13 months after starting treatment with the PD-1 inhibitor pembrolizumab. The condition was successfully relieved after treatment with prednisone. Additionally, it was reported that patients with metastatic melanoma developed delayed autoimmunity 8 months after stopping the anti-PD-1 antibody nivolumab treatment (93). Therefore, even after the treatment is discontinued, patients receiving immune checkpoint inhibitor therapy need continuous monitoring, especially as the propor-tion of individuals who have ended treatment after achieving a lasting response increases. Other AEs and the management strategies are listed in Table SI.
14. General management strategies
The irAEs of each system are summarized in the aforementioned sections and Table SI and what is evident is that the treatment strategies for these irAEs vary by patient. However, the common primary management strategies include symptomatic treatment and/or discontinuation of PD-1/PD-L1 mAb. Following the use of PD-1/PD-L1 mAb in patients with cancer who develop nervous system-related irAEs, the earliest symptoms are predominantly a headache, dizziness, fatigue and lethargy. Others present initially with tremors, visual disturbances, dysarthria, ataxia, paresthesia and seizures. Effective coping strategies primarily include intravenous infusion of immune globulin and hormone shock therapy, and it also includes certain targeted symptom-atic treatments (32-36,41,42). Respiratory-related irAEs (e.g., post-medication pneumonia, pulmonary aspergillosis and respiratory tract infection) are the earliest manifestations of cough and dyspnea. After these symptoms appear, further laboratory tests can be used confirm the diagnosis (47-49). The primary management strategies for these types of irAEs are drug withdrawal, administration of corticosteroids and targeted anti-inflammatory and antibacterial drugs. For critically ill patients, high-dose hormonal shock therapy should be considered. In the majority of cases, these targeted treatments improve the patient's symptoms (47-52). When a patient develops symptoms such as increased lower limb swelling, muscle pain and precordial pain after using PD-1/PD-L1-related drugs, it is necessary to consider whether it is a circulatory system-related irAEs; thus an electro-cardiogram is a necessary test. If irAEs related to the circulatory system (such as atrioventricular block, myocarditis, etc.) are diagnosed, symptomatic treatment should be performed according to the patient's condition, including the application of hormones and pacemaker implantation (53-55). Gastrointestinal-related irAEs in patients treated with anti-PD-1/PD-L1 primarily manifest as diarrhea, abdominal pain and occasionally fever, with a subset of patients exhibiting significant gastrointestinal damage (57). In addition to conventional symptomatic treatment, patients with surgical indications should undergo timely surgical treatment (58-60). Of patients receiving anti-PD-1/PD-L1 therapy, ~1% develop hypophysitis, 4% develop thyroid dysfunction and some patients develop post-medication diabetes (56). Most of these side effects are irreversible and require lifelong hormone replacement therapy (25,61). The most common irAEs in patients receiving anti-PD-1/PD-L1 therapy are skin lesions, which primarily manifest as rashes, skin pruritus, skin plaques and hard plaques, subcutaneous nodules and plaques and psoriasis (63,64). The majority of patients with skin-related irAEs receive topical hormonal therapy with topical corticosteroids and this proves efficacious. However, for patients with severe symptoms, systemic medication should be considered (65-68). Urinary system-related irAEs including nephritis and cystitis are rare. Further laboratory tests should be considered in patients with diarrhea, frequent urination, severe bladder pain and urgency to determine whether urinary-related irAEs have occurred (69,70). Once the diagnosis is confirmed, the management strategies should primarily include oral hormones and symptomatic treatment and whether anti-PD-1/PD-L1 therapy should be discontinued should be evaluated according to the patient's specific situation (71-73). Hematologic-related irAEs have rarely been reported in patients receiving anti-PD-1/PD-L1 therapy. If the patient exhibits changes in blood indicators. such as fever or abnormal C-reactive protein levels during medication, it is necessary to consider whether there are blood system-related irAEs. The primary management strategy is hormone therapy (74-77). Of patients with cancer treated with anti-PD-1/PD-L1, ~15% developed joint-related irAEs, usually characterized by joint swelling, stiffness, tenderness and erythema, which can persist for years after immunotherapy is discontinued (81-84). Most patients receive NSAIDs, 23.1% require additional low-dose corticosteroids and only 7.6% receive further immunosuppressive therapy (88). In summary, targeted therapy for most patients with side effects includes hormone therapy such as corticosteroids and symptomatic therapy, which has proven to be effective in the vast majority of cases.
15. Conclusions
As the number of PD-1/PD-L1-related inhibitors developed increases, an growing number of patients with cancer will benefit from them. However, unfortunately this also means there will be an increase in the number of irAEs and this danger should be taken into consideration when administering these drugs and patients should be carefully monitored throughout the treatment course and even after treatment is discontinued.
A phase II clinical trial calculated a 77% probability of treatment-related adverse events for camrelizumab in combination with apatinib in advanced hepatocellular carcinoma. It was shown that 29% of patients experienced more serious adverse reactions, including liver damage and two patients died as a result of the treatment (25). According to the reported statistics on adverse reactions, skin lesions including pruritus, psoriasis and nodular dermatitis account for 46-62% of reports, autoimmune colitis accounted for 22-48% of reports, autoimmune hepatitis accounted for 7-33% of reports and endocrine diseases such as thyroiditis, hypophysitis, adrenalitis and diabetes account for 12-34% of reports. In addition, there were other rarer adverse reactions including pneumonia (3-8%), nephritis (1-7%), cardiac adverse reactions including myocarditis (5%) and neurological adverse reactions (1-5%). The types and severity of irAEs differ for patients with different constitutions. In severe cases, it will cause irreversible damage to the patient and potentially even life-threatening complications. As with all treatments, the risks from immunotherapy should be minimized through a careful combination of monitoring, management of side effects and improvements in the therapeutic regimens including the choice of drugs available. Additionally, methods for detecting and diagnosing irAEs at an early stage are of paramount importance.
However, at present, there is no perfect system for detecting and diagnosing these side effects in a timely manner and most physicians just follow a simple 'discovery-symptom treatment' model. This will undoubtedly increase the risk of missed diagnoses and misdiagnoses. Therefore, further exploration of relevant markers and how to deal with these treatment-related immune side effects are increasing becoming an important part of broadening the efficacy of immunotherapy.
The present review summarized the different adverse reactions reported by patients with various types of cancer after treatment with PD-1/PD-L1-related inhibitors and summarized the management options adopted by the attending physician as well as the outcomes of the patients. These adverse reactions include damage to the hematological system, circulatory system, digestive system, urinary system, lymphatic system, neuromuscular system, vision and oral cavity, among others. The purpose of the present review was to highlight the need for improvement of the knowledge of the physicians to these side effects, to improve early detection, early diagnosis and early treatment. In Fig. 2 some of the adverse effects on the various systems listed in this review are summarized for a more intuitive understanding.
Figure 2.
Schematic diagram of irAEs of various systems in the human body after using PD-1/PD-L1 related inhibitors. PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; irAEs, immune-related adverse events.
In conclusion, immune checkpoint inhibitor therapy has exhibited significant potential. However, such drugs will inevitably cause adverse reactions in clinical applications and the existing case reports and the management strategies used can aid clinicians awareness and guide their response in dealing with these adverse reactions, ultimately improving the health and quality of care for the patients.
Supplementary Data
Acknowledgments
Not applicable.
Funding Statement
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.
Authors' contributions
GS, XC, HL and GS were responsible for gathering the related research and designing the review. WY, XK and ZZ were responsible for creating the figures. HC, XS, GS and WT contributed to study design, interpretation of the research articles, editing of the manuscript and critical revision of the manuscript. PT and GS contributed to respond to reviewer comments and make revisions. PT contributed to the language editing. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5; doi: 10.1002/ijc.33588. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Xia YX, Zhang F, Li XC, Kong LB, Zhang H, Li DH, Cheng F, Pu LY, Zhang CY, Qian XF, et al. Surgical treatment of primary liver cancer:A report of 10 966 cases. Zhonghua Wai Ke Za Zhi. 2021;59:6–17. doi: 10.3760/cma.j.cn112139-20201110-00791. In Chinese. [DOI] [PubMed] [Google Scholar]
- 3.Cordova-Bahena L, Velasco-Velazquez MA. Anti-PD-1 And Anti-PD-L1 antibodies as immunotherapy against cancer: A structural perspective. Rev Invest Clin. 2020;73:008–016. doi: 10.24875/RIC.20000341. [DOI] [PubMed] [Google Scholar]
- 4.Kuol N, Stojanovska L, Nurgali K, Apostolopoulos V. PD-1/PD-L1 in disease. Immunotherapy. 2018;10:149–160. doi: 10.2217/imt-2017-0120. [DOI] [PubMed] [Google Scholar]
- 5.Emens LA. Breast cancer immunotherapy: Facts and hopes. Clin Cancer Res. 2018;24:511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta R, Honey K. AACR cancer progress report 2019: Transforming lives through innovative cancer science. Clin Cancer Res. 2019;25:5431. doi: 10.1158/1078-0432.CCR-19-2655. [DOI] [PubMed] [Google Scholar]
- 7.Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18:899–900. doi: 10.1038/d41573-019-00167-9. [DOI] [PubMed] [Google Scholar]
- 8.Ho YJ, Li JP, Fan CH, Liu HL, Yeh CK. Ultrasound in tumor immunotherapy: Current status and future developments. J Control Release. 2020;323:12–23. doi: 10.1016/j.jconrel.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 11.Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. Pd-L1. J Clin Pathol. 2018;71:189–194. doi: 10.1136/jclinpath-2017-204853. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12:2735–2746. doi: 10.7150/jca.57334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: Signaling, cancer, and beyond. Adv Exp Med Biol. 2020;1248:33–59. doi: 10.1007/978-981-15-3266-5_3. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111–1122. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, Liu H, Bai Y, Xue M, Hu R, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564:130–135. doi: 10.1038/s41586-018-0756-0. [DOI] [PubMed] [Google Scholar]
- 16.Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, Lam EYN, Henderson MA, Bell CC, Stolzenburg S, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549:101–105. doi: 10.1038/nature23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regula-tors. Nature. 2017;549:106–110. doi: 10.1038/nature23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 19.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52:39–52. doi: 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 21.Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;379:e14. doi: 10.1056/NEJMc1808251. [DOI] [PubMed] [Google Scholar]
- 22.Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, Chen L, Shi WK, Li ML, Zhu JJ, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10:320–329. doi: 10.1159/000514313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu ZN, Huang JJ, Zhou J, Huang WS, Guo YJ, Cai MY, Zhou JW, Lin LT, Liang LC, Zhu KS. Efficacy and safety of anti-PD-1 monoclonal antibody in advanced hepatocellular carcinoma after TACE combined with TKI therapy. Zhonghua Nei Ke Za Zhi. 2021;60:630–636. doi: 10.3760/cma.j.cn112138-20200928-00841. In Chinese. [DOI] [PubMed] [Google Scholar]
- 24.Xie D, Sun Q, Wang X, Zhou J, Fan J, Ren Z, Gao Q. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. 2021;9:652. doi: 10.21037/atm-20-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, Zhang Y, Xu L, Yin T, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 26.Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, Fernandes R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors-A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 27.Heinzerling L, de Toni EN, Schett G, Hundorfean G, Zimmer L. Checkpoint Inhibitors. Dtsch Arztebl Int. 2019;116:119–126. doi: 10.3238/arztebl.2019.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, et al. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol. 2011;22:991–993. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 33.Bot I, Blank CU, Boogerd W, Brandsma D. Neurological immune-related adverse events of ipilimumab. Pract Neurol. 2013;13:278–280. doi: 10.1136/practneurol-2012-000447. [DOI] [PubMed] [Google Scholar]
- 34.De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, Moses PL, Sharkey KA, Mawe GM. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872–1883. doi: 10.1053/j.gastro.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Liu WK, Naban N, Kaul A, Patel N, Fusi A. Life-threatening polymyositis with spontaneous hematoma induced by nivolumab in a patient with previously resected melanoma. Melanoma Res. 2021;31:85–87. doi: 10.1097/CMR.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 36.Makarious D, Horwood K, Coward JIG. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur J Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa N, Kikuchi E, Suzuki S, Oya M. Myasthenia gravis with myositis induced by pembrolizumab therapy in a patient with metastatic urothelial carcinoma. Int Cancer Conf J. 2020;9:123–126. doi: 10.1007/s13691-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safa H, Johnson DH, Trinh VA, Rodgers TE, Lin H, Suarez-Almazor ME, Fa'ak F, Saberian C, Yee C, Davies MA, et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. J Immunother Cancer. 2019;7:319. doi: 10.1186/s40425-019-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pottier C, El Habnouni C, Kervarrec T, Beltran S, Samimi M. Myasthenia gravis induced by pembrolizumab in a patient with metastatic Merkel cell carcinoma. Ann Dermatol Venereol. 2022 Feb 18; doi: 10.1016/j.annder.2022.01.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Tian CY, Ou YH, Chang SL, Lin CM. Pembrolizumab-induced myasthenia gravis-like disorder, ocular myositis, and hepatitis: A case report. J Med Case Rep. 2021;15:244. doi: 10.1186/s13256-021-02722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heleno CT, Mustafa A, Gotera NA, Tesar A. Myasthenia gravis as an immune-mediated side effect of checkpoint inhibitors. Cureus. 2021;13:e16316. doi: 10.7759/cureus.16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel WV, Guislain A, Kvistborg P, Schumacher TN, Haanen JB, Blank CU. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol. 2012;30:e7–e10. doi: 10.1200/JCO.2011.37.9693. [DOI] [PubMed] [Google Scholar]
- 43.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218:69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 44.Wilgenhof S, Morlion V, Seghers AC, Du Four S, Vanderlinden E, Hanon S, Vandenbroucke F, Everaert H, Neyns B. Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitor. Anticancer Res. 2012;32:1355–1359. [PubMed] [Google Scholar]
- 45.Mandel JJ, Olar A, Aldape KD, Tremont-Lukats IW. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci. 2014;344:229–231. doi: 10.1016/j.jns.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barjaktarevic IZ, Qadir N, Suri A, Santamauro JT, Stover D. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest. 2013;143:858–861. doi: 10.1378/chest.12-1467. [DOI] [PubMed] [Google Scholar]
- 47.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al. Activity and safety of nivolumab, an anti-PD-1 immune check-point inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H, Li J, Yu L, Cheng X, Han X, Zhang X. A case report of checkpoint inhibitor pneumonitis caused by PD-1Antibody-safety and effectiveness of pirfenidone. Zhongguo Fei Ai Za Zhi. 2021;24:519–525. doi: 10.3779/j.issn.1009-3419.2021.103.08. In Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donato AA, Krol R. Allergic bronchopulmonary aspergillosis presumably unmasked by PD-1 inhibition. BMJ Case Rep. 2019;12:e227814. doi: 10.1136/bcr-2018-227814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S, Koumarianou A. A case of organizing pneumonia (OP) associated with pembrolizumab. Drug Target Insights. 2016;10:9–12. doi: 10.33393/dti.2016.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vartanov A, Kalotra A, Varughese J, Gautam S, Kandel S, Hosmer W. Immunotherapy-associated complete heart block in a patient with NSCLC: A case report and literature review. Respir Med Case Rep. 2021;33:101390. doi: 10.1016/j.rmcr.2021.101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. doi: 10.1186/s40425-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bukamur HS, Mezughi H, Karem E, Shahoub I, Shweihat Y. Nivolumab-induced third degree atrioventricular block in a patient with stage IV squamous cell lung carcinoma. Cureus. 2019;11:e4869. doi: 10.7759/cureus.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cousin S, Seneschal J, Italiano A. Toxicity profiles of immunotherapy. Pharmacol Ther. 2018;181:91–100. doi: 10.1016/j.pharmthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Puzanov I, Diab A, Abdallah K, Bingham CO, III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tso DK, Avery LL, Lev MH, Kamalian S. Nivolumab-induced small bowel obstruction and perforation: A rare but life-threatening side effect of immunotherapy. Emerg Radiol. 2020;27:107–110. doi: 10.1007/s10140-019-01731-x. [DOI] [PubMed] [Google Scholar]
- 59.Mathew Thomas V, Bindal P, Ann Alexander S, McDonald K. Nivolumab-induced hepatitis: A rare side effect of an immune check point inhibitor. J Oncol Pharm Pract. 2020;26:459–461. doi: 10.1177/1078155219837342. [DOI] [PubMed] [Google Scholar]
- 60.Lankes K, Hundorfean G, Harrer T, Pommer AJ, Agaimy A, Angelovska I, Tajmir-Riahi A, Gohl J, Schuler G, Neurath MF, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: Possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5:e1128611. doi: 10.1080/2162402X.2015.1128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kichloo A, Albosta MS, McMahon S, Movsesian K, Wani F, Jamal SM, Aljadah M, Singh J. Pembrolizumab-induced diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic colonic adenocarcinoma. J Investig Med High Impact Case Rep. 2020;8:2324709620951339. doi: 10.1177/2324709620951339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, Lacouture ME. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362. doi: 10.1093/annonc/mdw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mullangi S, Ponnam S, Lekkala MR, Koya S. A case of de novo psoriasis secondary to nivolumab in a patient with meta-static renal cell carcinoma. Cureus. 2021;13:e15703. doi: 10.7759/cureus.15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acar A, Oraloglu G, Yaman B, Karaarslan I. Nivolumab-induced plaque morphea in a malign melanoma patient. J Cosmet Dermatol. 2021;20:2645–2647. doi: 10.1111/jocd.13914. [DOI] [PubMed] [Google Scholar]
- 67.Mobini N, Dhillon R, Dickey J, Spoon J, Sadrolashrafi K. Exclusive cutaneous and subcutaneous sarcoidal granulomatous inflammation due to immune checkpoint inhibitors: Report of two cases with unusual manifestations and review of the literature. Case Rep Dermatol Med. 2019;2019:6702870. doi: 10.1155/2019/6702870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Bock M, Hulstaert E, Kruse V, Brochez L. Psoriasis vulgaris exacerbation during treatment with a PD-1 check-point inhibitor: Case report and literature review. Case Rep Dermatol. 2018;10:190–197. doi: 10.1159/000491572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Izzedine H, Gueutin V, Gharbi C, Mateus C, Robert C, Routier E, Thomas M, Baumelou A, Rouvier P. Kidney injuries related to ipilimumab. Invest New Drugs. 2014;32:769–773. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 70.Lefebvre J, Glezerman IG. Kidney toxicities associated with novel cancer therapies. Adv Chronic Kidney Dis. 2017;24:233–240. doi: 10.1053/j.ackd.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Schneider S, Alezra E, Yacoub M, Ducharme O, Gerard E, Dutriaux C, Prey S. Aseptic cystitis induced by nivolumab and ipilimumab combination for metastatic melanoma. Melanoma Res. 2021;31:487–489. doi: 10.1097/CMR.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 72.Thummalapalli R, Melms JC, Mier J, Izar B. Rapid evolution of acute kidney injury after initial infusion of pembrolizumab in a melanoma patient concurrently treated with RAF/MEK inhibitors. Melanoma Res. 2020;30:219–222. doi: 10.1097/CMR.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uchida A, Watanabe M, Nawata A, Ikari Y, Sasaki M, Shigemoto K, Hisano S, Nakashima H. Tubulointerstitial nephritis as adverse effect of programmed cell death 1 inhibitor, nivolumab, showed distinct histological findings. CEN Case Rep. 2017;6:169–174. doi: 10.1007/s13730-017-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atwal D, Joshi KP, Ravilla R, Mahmoud F. Pembrolizumab-induced pancytopenia: A case report. Perm J. 2017;21:17–004. doi: 10.7812/TPP/17-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michot JM, Vargaftig J, Leduc C, Quere G, Burroni B, Lazarovici J, Champiat S, Ribrag V, Lambotte O. Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer. 2017;80:1–4. doi: 10.1016/j.ejca.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Comito RR, Badu LA, Forcello N. Nivolumab-induced aplastic anemia: A case report and literature review. J Oncol Pharm Pract. 2019;25:221–225. doi: 10.1177/1078155217726159. [DOI] [PubMed] [Google Scholar]
- 77.Jotatsu T, Oda K, Yamaguchi Y, Noguchi S, Kawanami T, Kido T, Satoh M, Yatera K. Immune-mediated thrombocytopenia and hypothyroidism in a lung cancer patient treated with nivolumab. Immunotherapy. 2018;10:85–91. doi: 10.2217/imt-2017-0100. [DOI] [PubMed] [Google Scholar]
- 78.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Corrigendum: Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:311. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Obata S, Saishin Y, Teramura K, Ohji M. Vogt-koyanagiharada disease-like uveitis during nivolumab (Anti-PD-1 Antibody) treatment for metastatic cutaneous malignant melanoma. Case Rep Ophthalmol. 2019;10:67–74. doi: 10.1159/000496682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theillac C, Straub M, Breton AL, Thomas L, Dalle S. Bilateral uveitis and macular edema induced by Nivolumab: A case report. BMC Ophthalmol. 2017;17:227. doi: 10.1186/s12886-017-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lidar M, Giat E, Garelick D, Horowitz Y, Amital H, Steinberg-Silman Y, Schachter J, Shapira-Frommer R, Markel G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:284–289. doi: 10.1016/j.autrev.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, Lipson EJ, Bleich KB, Shah AA, Naidoo J, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76:43–50. doi: 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belkhir R, Burel SL, Dunogeant L, Marabelle A, Hollebecque A, Besse B, Leary A, Voisin AL, Pontoizeau C, Coutte L, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. 2017;76:1747–1750. doi: 10.1136/annrheumdis-2017-211216. [DOI] [PubMed] [Google Scholar]
- 85.Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with mono-therapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front Oncol. 2020;10:91. doi: 10.3389/fonc.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, Zhou GQ, Li WF, Mao YP, Hsu C, et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J, Graham A, Sion A. Evaluation of arthralgias in adult oncology patients receiving immune checkpoint inhibitors. J Oncol Pharm Pract. 2019;25:1867–1872. doi: 10.1177/1078155218822707. [DOI] [PubMed] [Google Scholar]
- 88.Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, Enk A, Lorenz HM, Hassel JC. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother. 2018;67:175–182. doi: 10.1007/s00262-017-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Dliw M, Megri M, Shahoub I, Sahay G, Limjoco TI, Shweihat Y. Pembrolizumab reactivates pulmonary granulomatosis. Respir Med Case Rep. 2017;22:126–129. doi: 10.1016/j.rmcr.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noguchi S, Kawachi H, Yoshida H, Fukao A, Terashita S, Ikeue T, Horikawa S, Sugita T. Sarcoid-Like granulomatosis induced by nivolumab treatment in a lung cancer patient. Case Rep Oncol. 2018;11:562–566. doi: 10.1159/000492383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nikolakis G, Brunner M, Boye H, Arndt N, Altenburg A, Vaiopoulos A, Zouboulis CC. Enlarged mediastinal lymph nodes of a patient with malignant melanoma stage IV under pembrolizumab treatment. Hautarzt. 2019;70:443–446. doi: 10.1007/s00105-019-4392-x. In German. [DOI] [PubMed] [Google Scholar]
- 92.Lederhandler MH, Ho A, Brinster N, Ho RS, Liebman TN, Lo Sicco K. Severe oral mucositis: A rare adverse event of pembrolizumab. J Drugs Dermatol. 2018;17:807–809. [PubMed] [Google Scholar]
- 93.Parakh S, Cebon J, Klein O. Delayed autoimmune toxicity occurring several months after cessation of anti-PD-1 therapy. Oncologist. 2018;23:849–851. doi: 10.1634/theoncologist.2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreira A, Loquai C, Pfohler C, Kahler KC, Knauss S, Heppt MV, Gutzmer R, Dimitriou F, Meier F, Mitzel-Rink H, et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:12–23. doi: 10.1016/j.ejca.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 95.Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, Hariharan S, Hammad TA, Lindenfeld J, Murphy MJ, et al. Myocarditis associated with immune checkpoint inhibitors: An expert consensus on data Gaps and a call to action. Oncologist. 2018;23:874–878. doi: 10.1634/theoncologist.2018-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alnabulsi R, Hussain A, DeAngelis D. Complete ophthal-moplegia in Ipilmumab and Nivolumab combination treatment for metastatic melanoma. Orbit. 2018;37:381–384. doi: 10.1080/01676830.2017.1423349. [DOI] [PubMed] [Google Scholar]
- 97.Kurokawa M, Kurokawa R, Hagiwara A, Gonoi W, Harayama S, Koizumi K, Yoshino K, Hishima T, Baba A, Ota Y, et al. CT imaging findings of anti-PD-1 inhibitor-related enterocolitis. Abdom Radiol (NY) 2021;46:3033–3043. doi: 10.1007/s00261-021-02986-0. [DOI] [PubMed] [Google Scholar]
- 98.Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell. 2020;182:655–671.e22. doi: 10.1016/j.cell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao L, Yu J, Wang J, Li H, Che J, Cao B. Risk of immune-related diarrhea with PD-1/PD-L1 inhibitors in different cancer types and treatment regimens. J Cancer. 2020;11:41–50. doi: 10.7150/jca.32724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shivaji UN, Jeffery L, Gui X, Smith SCL, Ahmad OF, Akbar A, Ghosh S, Iacucci M. Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their manage-ment. Therap Adv Gastroenterol. 2019 Nov 5; doi: 10.1177/1756284819884196. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alvarez M, Otano I, Minute L, Ochoa MC, Perez-Ruiz E, Melero I, Berraondo P. Impact of prophylactic TNF blockade in the dual PD-1 and CTLA-4 immunotherapy efficacy and toxicity. Cell Stress. 2019;3:236–239. doi: 10.15698/cst2019.07.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nadeau BA, Fecher LA, Owens SR, Razumilava N. Liver toxicity with cancer checkpoint inhibitor therapy. Semin Liver Dis. 2018;38:366–378. doi: 10.1055/s-0038-1667358. [DOI] [PubMed] [Google Scholar]
- 103.Straub BK, Ridder DA, Schad A, Loquai C, Schattenberg JM. Liver injury induced by immune checkpoint inhibitor-therapy: Example of an immune-mediated drug side effect. Pathologe. 2018;39:556–562. doi: 10.1007/s00292-018-0519-6. In German. [DOI] [PubMed] [Google Scholar]
- 104.Rahman W, Conley A, Silver KD. Atezolizumab-induced type 1 diabetes mellitus in a patient with metastatic renal cell carcinoma. BMJ Case Rep. 2020;13:e233842. doi: 10.1136/bcr-2019-233842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: A novel form of autoimmune diabetes. Clin Exp Immunol. 2020;200:131–140. doi: 10.1111/cei.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agrawal L, Bacal A, Jain S, Singh V, Emanuele N, Emanuele M, Meah F. Immune checkpoint inhibitors and endocrine side effects, a narrative review. Postgrad Med. 2020;132:206–214. doi: 10.1080/00325481.2019.1709344. [DOI] [PubMed] [Google Scholar]
- 107.Bailly C. Potential use of edaravone to reduce specific side effects of chemo-, radio-and immuno-therapy of cancers. Int Immunopharmacol. 2019;77:105967. doi: 10.1016/j.intimp.2019.105967. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Y, Wu HH, Wang W. A case of small-cell lung cancer with adrenocorticotropic hormone deficiency induced by nivolumab. Onco Targets Ther. 2019;12:2181–2186. doi: 10.2147/OTT.S194094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S, Zhang Y, Sun Z, Hu J, Fang C. Anti-PD-1 pembrolizumab induced autoimmune diabetes in Chinese patient: A case report. Medicine (Baltimore) 2018;97:e12907. doi: 10.1097/MD.0000000000012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Solinas C, Porcu M, De Silva P, Musi M, Aspeslagh S, Scartozzi M, Willard-Gallo K, Mariotti S, Saba L. Cancer immunotherapy-associated hypophysitis. Semin Oncol. 2018;45:181–186. doi: 10.1053/j.seminoncol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Briet C, Albarel F, Kuhn E, Merlen E, Chanson P, Cortet C. Expert opinion on pituitary complications in immunotherapy. Ann Endocrinol (Paris) 2018;79:562–568. doi: 10.1016/j.ando.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 112.Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:3144–3154. doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]
- 113.Sagiv O, Kandl TJ, Thakar SD, Thuro BA, Busaidy NL, Cabanillas M, Jimenez C, Dadu R, Graham PH, Debnam JM, Esmaeli B. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast Reconstr Surg. 2019;35:50–52. doi: 10.1097/IOP.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 114.Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L, Madelaine-Chambrin I, Bagot M, Basset-Seguin N, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: A case report and literature review. Cancer Immunol Immunother. 2017;66:1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rossi E, Sgambato A, De Chiara G, Casaluce F, Losanno T, Sacco PC, Santabarbara G, Gridelli C. Endocrinopathies induced by immune-checkpoint inhibitors in advanced non-small cell lung cancer. Expert Rev Clin Pharmacol. 2016;9:419–428. doi: 10.1586/17512433.2016.1133289. [DOI] [PubMed] [Google Scholar]
- 117.Kim KH, Sim WY, Lew BL. Nivolumab-induced alopecia areata: A case report and literature review. Ann Dermatol. 2021;33:284–288. doi: 10.5021/ad.2021.33.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Navarro-Fernandez I, Gonzalez-Vela C, Gomez-Fernandez C, Duran-Vian C, Reguero L, Gonzalez-Lopez M. Vitiligo-like depigmentation in a patient undergoing treatment with nivolumab for advanced renal-cell carcinoma. Acta Dermatovenerol Croat. 2021;291:54–55. [PubMed] [Google Scholar]
- 119.Gracia-Cazana T, Padgett E, Hernandez-Garcia A, Sanchez-Salas MP. Vitiligo-like lesions located over In-transit metastases of malignant melanoma as a clinical marker of complete response to pembrolizumab. Dermatol Online J. 2019;25:13030/qt8d3818j5. doi: 10.5070/D32512046728. [DOI] [PubMed] [Google Scholar]
- 120.Cardis MA, Jiang H, Strauss J, Gulley JL, Brownell I. Diffuse lichen planus-like keratoses and clinical pseudo-progression associated with avelumab treatment for Merkel cell carcinoma, a case report. BMC Cancer. 2019;19:539. doi: 10.1186/s12885-019-5759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rambhia PH, Honda K, Arbesman J. Nivolumab induced inflammation of seborrheic keratoses: A novel cutaneous manifestation in a metastatic melanoma patient. Melanoma Res. 2018;28:475–477. doi: 10.1097/CMR.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664–669. doi: 10.1111/ijd.13984. [DOI] [PubMed] [Google Scholar]
- 123.Obara K, Masuzawa M, Amoh Y. Oral lichenoid reaction showing multiple ulcers associated with anti-programmed death cell receptor-1 treatment: A report of two cases and published work review. J Dermatol. 2018;45:587–591. doi: 10.1111/1346-8138.14205. [DOI] [PubMed] [Google Scholar]
- 124.Seethapathy H, Zhao S, Strohbehn IA, Lee M, Chute DF, Bates H, Molina GE, Zubiri L, Gupta S, Motwani S, et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int Rep. 2020;5:1700–1705. doi: 10.1016/j.ekir.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bickel A, Koneth I, Enzler-Tschudy A, Neuweiler J, Flatz L, Fruh M. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer. 2016;16:656. doi: 10.1186/s12885-016-2718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boegeholz J, Brueggen CS, Pauli C, Dimitriou F, Haralambieva E, Dummer R, Manz MG, Widmer CC. Challenges in diagnosis and management of neutropenia upon exposure to immune-checkpoint inhibitors: Meta-analysis of a rare immune-related adverse side effect. BMC Cancer. 2020;20:300. doi: 10.1186/s12885-020-06763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang W, Lam WC, Chen L. Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2019;68:85–95. doi: 10.1007/s00262-018-2260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yatim N, Mateus C, Charles P. Sarcoidosis post-anti-PD-1 therapy, mimicking relapse of metastatic melanoma in a patient undergoing complete remission. Rev Med Interne. 2018;39:130–133. doi: 10.1016/j.revmed.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 129.Zheng Y, Meng X, Zweigenbaum P, Chen L, Xia J. Hybrid phenotype mining method for investigating off-target protein and underlying side effects of anti-tumor immunotherapy. BMC Med Inform Decis Mak. 2020;20(Suppl 3):S133. doi: 10.1186/s12911-020-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gauci ML, Baroudjian B, Laly P, Madelaine I, Da Meda L, Vercellino L, Bagot M, Liote F, Pages C, Lebbe C. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab. Semin Arthritis Rheum. 2017;47:281–287. doi: 10.1016/j.semarthrit.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Uemura M, Fa'ak F, Haymaker C, McQuail N, Sirmans E, Hudgens CW, Barbara L, Bernatchez C, Curry JL, Hwu P, et al. Erratum to: A case report of Grover's disease from immunotherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J Immunother Cancer. 2017;5:7. doi: 10.1186/s40425-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.