Abstract
Epigenetics is the study of heritable molecular determinants that are independent of phenotypic features. The epigenetic features include DNA methylation, histone modifications, non-coding RNAs, and chromatin remodeling. In multicellular organisms, the epigenetic state of a cell is critical in determining its differentiation status and its ability to perform its proper function. These processes are now well recognized as being a substantial factor in tumor progression and metastasis. The process through which epithelial cells acquire mesenchymal features is known as epithelial-mesenchymal transition (EMT). EMT is associated with tumorigenesis, invasion, metastasis, and resistance to therapy in cancer. In the present review, we examine the recent studies that demonstrate the biological role of epigenetics, in particular, DNA methylation, histone modifications, non-coding RNAs, and chromatin remodeling in tumor progression and metastasis by regulating EMT status, and we provide an overview of the current state of knowledge regarding the epigenetics involvement in tumor progression and metastasis. Because epigenetic changes can be reversed, learning more about their biological roles in EMT will not only help us better understand how cancer progresses and spreads, but it will also help us identify new ways to diagnose and treat human malignancy which is currently lacking in the clinical setting.
Keywords: methylation, acetylation, glycosylation, non-coding RNA, EMT, metastasis
1. Introduction
Metastasis remains the leading cause of mortality in cancer patients, despite recent advancements in the diagnosis and treatment of a variety of cancers. It becomes more and more heterogeneous as cancer progresses, resulting in the production of aggressive subsets of cancer cells that invade local tissues, lymph vessels, and circulatory systems before spreading throughout the body. This aggressive behavior of the original tumor eventually results in the extensive spread and metastasis of the primary tumor. Therefore, understanding the mechanism of cancer metastasis is an important step in determining therapeutic targets that can be used to slow or stop cancer growth and progression (1). The process of cell transformation and cancer progression includes gene mutations and epigenetic changes and the rewiring of cell signals, and the reprogramming of metabolic pathways. Furthermore, a vast body of literature published over the past few decades has shown that epigenetic modifications are implicated in the formation and progression of the tumor. It has also been said that genetic and epigenetic changes are closely linked during the development of tumors (2).
Epigenetic mechanisms of tumor cell growth and gene expression regulation include DNA methylation, covalent histone modifications, glycosylation, and ubiquitination. These epigenetic modifications exhibit unique features and distribution patterns in different tumor cells. The unique pattern of combinations of these modifications, collectively referred to as the epigenome, is a critical determinant of cell fate and gene activity (3). Collectively, the epigenetic state within cells is tightly controlled to maintain an appropriate state of differentiation. In cancer, this fine-tuned genomic programming is disrupted, resulting in unregulated cell proliferation, defective differentiation, and resistance to apoptosis (4). Epigenetic events, most notable stability of gene expression and genetic modifications, are not attributed to any changes in the primary DNA sequence. Here, we examine the current findings into the contribution of epigenetics to metastasis, which may guide cell dissemination from the primary tumor or eventual growth and colonization at distant sites. This information will allow us to uncover the functional importance of these epigenetic phenomena and provide informative therapeutic value for cancers targeting epigenetic changes in metastatic cells.
2. Process of cancer metastasis
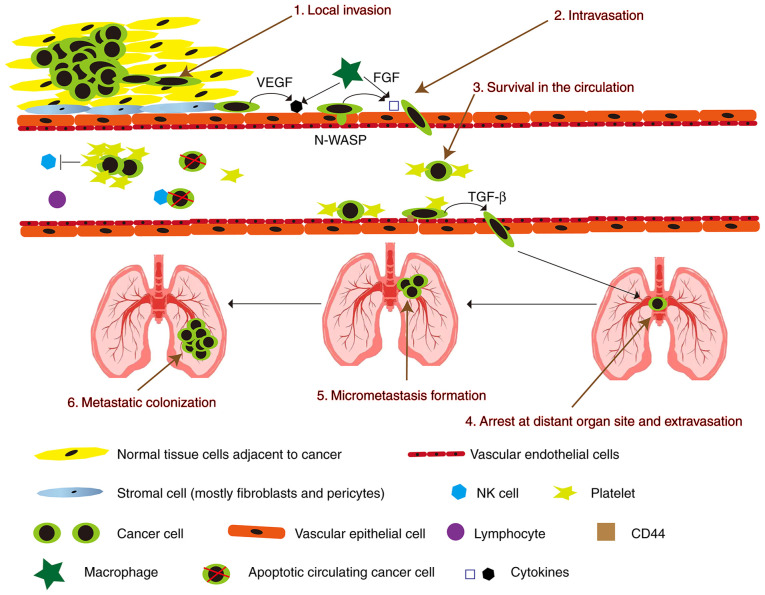
Metastasis represents a major obstacle in cancer treatment and is the leading cause of cancer-related deaths. The process from primary local tumor to distant metastasis is divided into 6 steps: i) local invasion, ii) intravasation, iii) survival in the circulation, iv) arrest at the distant organ site and extravasation, v) micrometastasis formation, and vi) metastatic colonization (Fig. 1) (5). In order to pass these steps, different molecular pathways have been shown to be important, including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR), hepatocyte growth factor (HGF)/mesenchymal-epithelial transition tyrosine kinase receptor (Met), Wnt/β-catenin, and vascular endothelial growth factor (VEGF) signal transduction (6). Furthermore, some cytokines secreted by macrophages can lead to the loss of vascular connections and increase the permeability of blood vessels when tumor cells invade blood vessels. Various cellular components in blood vessels will kill circulating tumor cells, whereas circulating tumor cells can recruit platelets to resist killing (7).
Figure 1.
Schematic diagram showing the invasion-metastasis cascade. VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; N-WASP, neural Wiskott-Aldrich syndeome protein; TGF-β, transforming growth factor-β; NK, natural killer.
Cancer cells break away from the original site and infiltrate the surrounding normal tissues. Morphologically, the cancer cell transitions from a highly differentiated to an undifferentiated state (5). Cancer cells undergo mesenchymal-epithelial transition (EMT) during migration and invasion. EMT is a cellular process in which cells lose their epithelial features and acquire mesenchymal ones (8). The loss of E-cadherin and its inhibition or attenuation of cell adhesion during EMT are considered to be a critical step. E-cadherin is a type of Ca2+-dependent intercellular adhesion molecule in epithelial tissues. E-cadherin is a single-channel transmembrane glycoprotein containing five extracellular repeats that mediate Ca2+-dependent interactions with opposing molecules on adjacent cells (9). Expression or the cell surface localization of E-cadherin is frequently lost in advanced tumors and is associated, at least in some cases, with the incidence of metastasis and tumor recurrence (10). Loss of E-cadherin expression in human tumors is most commonly caused by methylation of its promoter, or upregulation of the transcriptional repressors SNAIL, SIP1 and zinc finger E-box-binding homeobox 1 (ZEB1), which target the E-cadherin promoter (11). Loss of E-cadherin function is a cause of promotion of invasion and metastasis, primarily through the transformation of epithelial tumor cells into highly migratory and invasive cells (12). It can combine with β-catenin in the cytoplasm to form a complex. This complex can be directly connected to the actin cytoskeleton to maintain the stability of cell adhesion and cell polarity (13). If the expression of E-cadherin is blocked, tumor growth factor (TGF)-β, Wnt/β-catenin, Hedgehog, Notch, and tumor necrosis factor (TNF) signals will induce cancer cell EMT via Twist/Snail/Slug/ZEB1, causing cancer cells to invade and metastasize (8,14).
For intravasation, the migration of cancer cells to blood vessels is caused by factors involved in local invasion-the secretion of proteases such as matrix metalloproteinase (MMP)-1, -2, and -9 and the activation of the urokinase-type plasminogen activator (uPA)/uPA receptor (uPAR) (15). Cancer cells mainly invade capillaries and venules, and large blood vessels are resistant to tumor cell invasion. Tissue fibrosis is also resistant to cancer cell invasion. Cirrhotic organs are not prone to metastasis, and myofibroblasts from cirrhosis can secrete MMP inhibitors (TIMP). In addition, the heart and skeletal muscles are also resistant to metastasis (16).
After cancer cells enter the circulation, they are prone to anoikis due to their lack of support from the extracellular matrix (ECM) (17). It is estimated that less than 1% of circulating tumor cells (CTCs) that circulate in the blood on a daily basis would survive and have a possibility of spreading to produce distant metastases (18). When cancer cells are in circulation, they are attacked by immune cells, which in turn are assaulted by cancer cells. In order to prevent immune cells from attacking cancer cells, fibrin and platelets adhere to the surface of cancer cells (19). Less than 1% of cancer cells found in the blood have a chance of surviving, and some cancer cells are in a dormant state, and less than 0.01% of cells with high metastatic potential can emerge from blood vessels to form metastases (20).
The following are the leading causes of cancer cells to stop growing. i) Cancer cells are prevented from spreading by capillary stenosis. Then the cancer cells extravasate through the endothelial cells and exit the blood vessels to establish metastases. ii) The cancer cell surface protein interacts with the microvascular endothelial surface (21). The detailed mechanism of cancer cell metastasis to the organs is currently unclear, which may be related to organ microvascular endothelial cells. Studies have shown that cancer cells only adhere to microvascular endothelial cells, not large vascular endothelial cells (22). There are some specific molecules in organ micro-vascular endothelial cells. These molecules include adhesion molecules, intercellular adhesion molecule-1 (ICAM-1), selectin and integrin (23), and endothelial cells secrete chemo-attractants [CXC chemokine receptor 4 (CXCR4), CXC chemokine receptor 12 (CXCR12), and chemokine (C-C motif) receptor 1 (CCR1)] (24). Therefore, the specific molecules may be the main reason for cancer cell arrest. The specificity of metastatic sites for each tumor entity is also called tissue tropism (25). Tissue tropism helps predict the future metastatic site through these specific molecules and may become the target of future anti-metastatic drug therapy (26).
After cell extravasation, CTCs need to create specific conditions to reach the target organs (27). The steps of cancer cells exiting the vessel wall are opposite to the direction of their entering the vessel wall (28). Cancer cells removed from blood vessels are in an unfavorable environment, and they have to overcome various difficulties to establish metastases (29). Primary cancer cells release growth factors into the circulation (30). These growth factors lead to an upregulation of vascular endothelial growth factor (VEGF)-A, placental growth factor (PlGF), transforming growth factor-β (TGF-β), and inflammatory proteins S100A8/9 at the future metastatic sites (31). From the results, the cells that can establish metastases should have the properties of cancer stem cells (32).
As the final step of the metastatic cascade, micro-metastases have now spread to distant organs. In specific niches or in undefined spots, a plethora of genes and signals support metastatic cell growth and survival (33). Many of these pro-metastatic stromal mediators ultimately activate stem cell support pathways (Wnt, TGF-β, BMP, Notch, Stat3), positional and mechanical pathways (Hedgehog, Hippo), pathways that integrate cell metabolism and survival (PI3K/AKT, MAPK, HIF), and inflammatory pathways (NF-κB, Stat1) (34). Cancer kills the most individuals when it spreads to other parts of the body. Preventing the formation of new niches may be a new way to treat metastatic diseases.
3. Epigenetic in tumor progression and metastasis
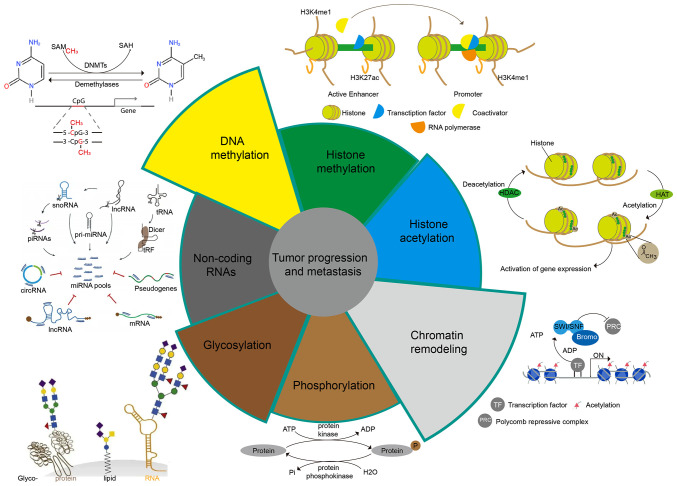
Epigenetic research aims to reveal how the environment, social status, psychosocial factors, and nutrition influence the expression of an individual's genetic information (35). Epigenetics is responsible for initiating and maintaining epigenetic silencing and regulating gene expression profiles and is the cornerstone of a range of cellular processes, including cell differentiation, gene expression, X chromosome inactivation, embryogenesis, and genomic imprinting (36). In addition, epigenetics also plays a significant role in the regulation of tumor metastasis. We will illustrate the impact of epigenetics on tumor metastasis from the following aspects (Fig. 2).
Figure 2.
Epigenetic types of tumor metastasis. DNMTs, DNA methyltransferases; snoRNA, small nucleolar RNA; lncRNA, long non-coding RNA; piRNAs, piwi-interacting RNAs; circRNA, circular RNA; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SWI/SNF, switching defective/sucrose non-fermenting; Bromo, bromodomain.
Metastasis is regulated by DNA methylation
DNA methylation is an epigenetic modification first discovered in humans in the early 1980's and the most intensely studied in epigenetic regulatory mechanisms (37,38). In a broad sense, DNA methylation refers to the chemical modification process in which a specific base in the DNA sequence obtains a methyl group by covalent bonding with S-adenosyl methionine (SAM) as a methyl donor under the catalysis of DNA methyltransferase (DNMT). This DNA methylation modification can occur at the C-5 position of cytosine, the N-6 position of adenine, and the G-7 position of guanine (39). In general, DNA methylation mainly refers to the methylation process that occurs at the 5th carbon atom on cytosine in CpG dinucleotides, and the product is called 5-methylcytosine (5-mC), which is the main form of DNA methylation in eukaryotes such as plants and animals, and the only form of DNA methylation in mammals found (40). DNA methylation plays an important role in regulating individual growth, development, gene expression patterns, and genome stability without changing the DNA sequence, and this modification is stable during development and cell proliferation. A large number of studies in recent years have shown that DNA aberrant methylation is closely related to the occurrence, development, and carcinogenesis of tumors (41,42).
The DNA methyltransferase (DNMT) family consists of a group of conserved DNA-modifying enzymes that play central roles in epigenetic regulation. Five DNMT family members have been found in mammals: DNMT1, DNMT2, DNMT3a, DNMT3b, and DNMT3L (43). However, only DNMT1, DNMT3a, and DNMT3b interact to generate the overall cytosine methylation pattern. These independently encoded proteins are divided into generating DNA methylases (DNMT3a and DNMT3b) and maintaining DNA methylases (DNMT1). DNMT2 and DNMT3L are not considered cytosine methyltransferases (44). DNMT3L, on the other hand, has been shown to stimulate de novo DNA methylation via DNMT3a and to mediate transcriptional repression via interaction with histone deacetylase 1 (45).
The role of DNA methylation in tumors is mainly manifested in the following aspects. First, cytosine in methylated CpG island dinucleotides is deaminated to thymine at a high frequency, causing gene mutation (46). Second, tumor-suppressor genes and DNA repair genes are silenced due to hypermethylation (47). Third, oncogene methylation levels are reduced and activated (48); and fourth, the overall reduction in methylation levels of the genome causes transposons and repetitive sequences to activate, resulting in decreased chromosomal stability (49). These factors are important reasons for the development, metastasis, and progression of tumors, which eventually lead to the death of patients. The overall DNA methylation level (i.e., methylation profile) and changes in the degree of methylation of specific genes can be used as tumor diagnostic indicators (50).
In normal cells, heterochromatin is hypermethylated around the central point; however, in many tumors, this mechanism is disrupted, and DNA methylation in normally inactive regions is lost. Transposable elements are subsequently reactivated and can integrate at arbitrary sites in the genome, leading to mutation and genomic instability (51). Therefore, DNA methylation plays a vital role in tumor progression and metastasis. DNMT1 is a maintenance methyltransferase that methylates cytosines in hemimethylated CpG dinucleotides (52). DNMT1 is required for the maintenance of trimethylation of lysine 9 at histone H3 in pericentromeric regions (53), and DNMT1 can bind to H3K9me3 and H3K27me3 at the promoter sites of ZEB2 [0.4 kb (3502) site] or Kruppel-like factor 4 (KLF4) [0.4 kb (3110) site] or Snail to inhibit EMT of prostate cancer (PCa) and hepatocellular carcinoma (HCC) (54,55). miR-185 and miR-148a can directly target DNMT1 so that the expression of breast cancer gene 1 (BRCA1) can be increased to stop the spread of breast cancer and gastric cancer (GC) (56).
In addition to the inhibitory effect of DNMT1, DNMT1 can also regulate the expression of some genes to promote tumor metastasis. For example, DNMT1 can bind to the nuclear transcriptional repressor CpG region of RAD9, which will promote the transcriptional expression of RAD9 (a gene that maintains genome integrity, DNA repair, cell cycle checkpoints, apoptosis, and transcriptional transactivation of specific target genes) to promote metastasis of PCa cells (57). Osteopontin increases the expression of DNMT1 to increase the methylation of tumor-suppressor genes, Ras-associated domain family 1A (RASSF1A), GATA binding protein 4 (GATA4), cyclin-dependent kinase-like 2 (CDKL2), and death-associated protein kinase (DAPK) to induce metastasis of liver cancer and esophageal squamous cell carcinoma (ESCC) (58,59). Moreover, DNMT1 also reduces the expression of lncRNA-SPRY4-IT1 to promote gastric cancer cell migration and invasion (60). However, lncRNA-HNF1A-AS1 can bind to DNMT1 and inhibit its activation from promoting EMT of lung adenocarcinoma (61). SET and MYND domain-containing protein 2 (SMYD2) can increase adenomatous polyposis coli 2 (APC2) methylation by DNMT1 to activate the Wnt/β-catenin pathway, which promotes colorectal cancer (CRC) EMT (62).
De novo methylation and mammalian development require DNMT3a and DNMT3b (63). The target recognition domain (Trd) (residues R 831-f848), the catalytic loop (residues G 707-k721), and the homodimeric interface of DNMT3a mediate DNMT3a binding to DNA, and these loops come together to form a continuous DNA binding surface (64). Recognizing of CpG dinucleotides by DNMT3a is mediated by catalytic and TRD cycles (65). DNMT3b-mediated transcriptional repression occurs at CpG island (CGI)-associated promoters and repeats. The activity of DNMT3b is mainly to promote long-term gene silencing, which needs to be preserved in certain tissues to maintain the body's life (66). In addition to centromeric, pericentromeric, and subtelomeric repeats, germline genes are also known genomic targets of DNMT3b. Notably, the maintenance of CGI methylation of certain germ cell-specific genes in somatic cells depends entirely on DNMT3b activity (67). DNMT3a and DNMT3b also play an important role in tumor metastasis. For example, DNMT3a mutations are more common in acute myeloid leukemia (AML), and DNMT3A mutations can promote extra-medullary infiltration (EMI) by upregulating the expression of TWIST1 (68). In addition, DNMT3a also regulates the expression of Snail and E-cadherin to affect the metastasis of GC (69). Metastasis-associated protein 1 (Mta 1) is one of the important chromatin remodeling factors in eukaryotic cells; it is one of the most upregulated oncogenes in human tumors and is involved in tumor progression and metastasis. Mta1 can inhibit the transcription of DNMT3a from increasing the expression of insulin-like growth factor binding protein-3 (IGFBP-3), which promotes the metastasis of breast cancer (70). Smad4 and mastermind-like transcriptional coactivator 1 (MAML1) are novel direct targets of miR-34a and miR-133a-3p, but lncRNA-34a can bind to DNMT3a and inhibit the activity of miR-34a and miR-133a-3p, which increases Smad4 and MAML1 expression in HCC metastasis (71,72). In addition, DNMT3b also binds to the promoter region of miR-34, which enhances the expression levels of hepatocyte nuclear factor 4 γ (HNF4G) and Notch1, which are downstream targets of miR-34a to promote migration and invasion of bladder cancer (73). Furthermore, lncRNA-H19 can directly bind to miR-29b-3p (miR-29b) and inhibit the expression of DNMT3b in bladder cancer cells. More importantly, upregulation of lncRNA-H19 was found to antagonize miR-29b-3p-mediated inhibition of breast cancer cell proliferation, migration and EMT. On the contrary, lncRNA-H19 knockdown partially reversed the effect of the miR-29b-3p inhibitor on Dnmt3b and promoted miR-29b-3p-induced MET (74). DNMT3b also can bind to the nuclear transcriptional repressor CpG region of RAD9 to promote metastasis of PCa cells (57).
Demethylation is one method of tumor prevention and treatment that involves restoring the activity of some key tumor suppressor or DNA repair genes. At present, DNMT inhibitors are the most studied, as they can reverse abnormal DNA methylation by inhibiting DNMT activity (75). The first phenotype-modifying drug, 5-azacytidine and its analog 5-aza-2′-deoxycytidine (5-aza-CdR), have been approved by the US FDA to treat preleukemic myelodysplastic syndromes (76). 5-Aza-CdR is an analog of cytosine, which can be incorporated into the DNA chain during DNA replication. On the one hand, it can reduce the ability of DNA to receive methyl groups, and on the other hand, it inhibits DNMT activity, resulting in the reduction in the DNA methylation level (77). It has been clinically shown that 5-aza-CdR can improve the survival rate of some patients with stage IV small cell lung cancer. However, the drug also has toxic and side effects that cannot be ignored (for example, the specificity is not strong, and it cannot be targeted for a specific tumor-suppressor gene; targeted therapy; high doses of 5-aza-CdR may induce tumor metastasis), so its clinical application is greatly limited (78). However, MCF-7 breast cancer cells were treated with the DNMT inhibitor 5-aza-cytidine to maintain a hypomethylated state. The results showed that the expression levels of pro-invasive EMT-associated genes related to the EMT process were upregulated, and the invasive and metastatic abilities of the cells were enhanced (79). The results of this study are worth serious deliberation. Although the use of DNMT inhibitors to treat tumors may inhibit the expression of proto-oncogenes, it may also increase the risk of tumor cell metastasis and dissemination. Therefore, we should use these drugs with caution in clinical practice.
Metastasis regulated by histone modifications
Histone methylation and demethylation
Histones protect genetic information, maintain DNA structure, and regulate gene expression. Histone amino-terminal (N-terminal) domains protrude from the nucleosome and can interact with other regulatory proteins and DNA. Histone modifications include methylation, phosphorylation, acetylation, crotonylation, ubiquitination, glycosylation, and ADP ribosylation. Imbalances in histone modifications can lead to tumorigenesis, and loss of methylation, and acetylation of histone H3 and H4 residues has been shown to be a marker of tumor cells (80). Histone methylation and demethylation are usually carried out by histone methyltransferases (HMTs) and histone demethyltransferase (HDMs). The methylation of histones is a covalent modification of arginine and lysine. Arginines can be mono-or di-methylated, while lysines can be mono-, di-, or tri-methylated (81). There are three types of enzymes responsible for histone methylation, lysine-specific SET domain histone methylase, methylase of amino acid, and methylase of lysine (82). In general, the methylation of different sites of histone H3 and H4 and the amount of methylation have great significance for the transcriptional regulation of genes. Among them, H3K9me3, H3K27me3, and H4K20me2/3 mediate transcriptional repression, while H3K4me1/2/3, H3K9me1, H3K27me1, H3K36me1/2/3, and H3k79me1/2/3 mediate transcriptional activation (83).
According to the different amino acids catalyzed by histone transferases, HMTs can be divided into two families: protein lysine methyltransferases (PKMTs) and protein arginine methyltransferases (PRMTs). The KMT family is further divided into enzymes that contain SET domains, such as G9a, EZH2, DOT1L, SUV39H1, CLL8, MLL1, SET8, SETDB1, GLP, and SETD2; the PRMT family includes PRMT1-9 and CARM1 in mammals. HMTs have a direct regulatory effect on tumor development and metastasis (84). The enhancer of zeste homolog 2 (EZH2) gene can promote tumor development and is the catalytic component of the polycomb repressive complex 2 (PRC2). EZH2 utilizes its HMT activity to catalyze the trimethylation of histone H3 lysine 27, inhibiting the downstream tumor-suppressor genes such as E-cadherin, transforming growth factor-β (TGF-β) receptor 2 (TGFBR2), P57, and PSP94 (85). However, EZH2 itself lacks enzymatic activity, and its activity requires the assistance of the zinc-finger-containing protein (SUZ12) and the WD40-repeat protein (EED) to maintain the integrity of the PRC2 complex and the methyltransferase activity of EZH2 (86). EZH2 binds phosphorylated p38 (p-p38) in breast cancer cells to other core members of PRC2, EED, and SUZ12. EZH2 overexpression leads to the expression of p-p38 and activates downstream pathway proteins, which promote breast cancer motility and metastasis (87). EZH2 overexpression inhibits the expression of metalloproteinase 2 (MMP-2) and promotes the proteolytic activity of MMP-2 and -9, which also promotes ovarian cancer invasion and migration (88). In addition, numerous studies have shown that EZH2 overexpression promotes the proliferation, migration, and invasion of cancer cells in lung, bladder, melanoma, and colorectal cancers (89,90). In addition to the high expression of EZH2, the low expression of some members of this family promotes tumor metastasis. For example, loss of SETD2 leads to persistent activation of AKT through extracellular matrix (ECM) production, thereby facilitating metastasis of pancreatic ductal adenocarcinoma (PDAC) (91). More importantly, loss of SETD2 can activate EZH2 signaling and AMPK signaling to promote PCa metastasis (92). KMT1E (also known as SETDB 1) is involved in the epigenetic silencing of oncogenes and tumor-suppressor genes in cancer cells. KMT1E, a metastasis suppressor, is strongly downregulated in highly metastatic lung cancer cells (93). In addition, SET8 (94), G9a (95), GLP (95), DOT1L (95), and MLL1 (96) have also been reported to be closely associated with tumor metastasis. The PRMT family also has members involved in tumor metastasis. PRMT1 can directly target the leukocyte adhesion molecule (ALCAM) to promote the growth and metastasis of melanoma (97). PRMT1, PRMT5, PRMT6, and PRMT7 can promote EMT of head and neck cancer, colorectal cancer, breast cancer, ovarian cancer, lung adenocarcinoma, and oral cancer cells (98–102).
HDMs also have a variety of domains, including Jumonji (N/C terminal domains) (83), PHD-finger, Zinc-finger, SWIRM1 (Swi3, Rsc, and Moira domains), and the amine oxidase domain (103). Lysine-specific demethylases (KDMs) work in concert with histone lysine methylases to maintain global histone methylation patterns. The histone demethylases characterized to date belong to the amine oxidase and oxygenase superfamilies. The amino oxidase family members degrade histones through flavin adenine dinucleotide (FAD)-dependent amine oxidase reaction substrate demethylation (104). The JmjC protein belongs to the family of oxygenases and demethylates histones in an α-ketoglutarate and Fe(II) ion-dependent manner (105). Furthermore, they can also be divided into several families: UTY, KDM1A, KDM1B, KDM2A, KDM2B, KDM3A, KDM3B, JMJD1C, KDM4A, KDM4B, KDM4C, KDM4D, KDM5A, KDM5B, KDM5C, KDM5D, KDM6A, and KDM6B (45). Although not extensively studied as histone methylases, KDMs are also associated with tumor progression and metastasis. For example, KDM1A (also known as LSD1)-mediated stabilization of SEPT6 can activate the TGF-β1/SMAD pathway and VEGF-C/PI3K/AKT signaling pathway, to stability promote the metastasis of GC, non-small cell lung cancer (NSCLC), and breast cancer (106–108). At the same time, KDM2A and KDM2B can downregulate the expression of programmed cell death 4 (PDCD4) and active the TGF-β1/SMAD and PI3K/AKT/mTOR pathways to stabilize and promote the metastasis of GC, lung cancer, pancreatic cancer, and ovarian cancer (109–111). KDM3A and KDM3B can activate the Wnt/β-catenin pathway and upregulate c-MYC, lncRNA-MALAT1, and melanoma cell adhesion molecule (MCAM), and MCAM to promote CRC, Ewing sarcoma, and neuroblastoma migration and invasion (112,113). In addition to this, lysine-specific demethylases KDM4A (114) and KDM4B (115) are also closely related to tumor progression and metastasis.
In the past few years, specific inhibitors of a large number of different HMTs and HDMs have been developed. EZH2, DOT1L, PRMT1, PRMT5, LSD1, KDM2B, KDM4D inhibitors have entered the clinical trial stage. These results demonstrate that HMTs and HDMs play a critical function in tumor metastasis. Furthermore, we believe that more targeted drugs for HMTs and HDMs will enter the clinic in the future, bringing good news to patients.
Histone acetylation and deacetylation
Protein acetylation refers to the process of adding acetyl groups to protein lysine residues under the action of an acetyltransferase. Histone acetylation is a post-translational modification that mostly occurs at specific lysine residues in the basic amino acid concentration region at the N-terminal of core histones and transfers the acetyl group of acetyl-CoA to sNH3+ of lysine to neutralize a positive charge (116). Histone acetylation and deacetylation are usually carried out by histone acetyltransferases (HATs) and histone deacetylases (HDACs).
HATs can be divided into two families according to the properties of their substrates, the GNAT family (GCN5-related N-acetyltransferase family) and the MYST family (MOZ, Ybf2/Sas3, Sas2, and Tip60). Although both contain acetyl-CoA homologous sequences, there are differences in their core regions (117). Functionally, the GNAT family is mainly responsible for the acetylation of lysine sites on histone H3. In contrast, the MYST family is related to the acetylation of lysine sites (such as H4K16) on histone H4. According to the different domains contained, the MYST family can be divided into the following three types: the subgroup containing the plant homology domain (including MOZ and MORF), and the subgroup containing the chromatin domain (including Esa1, dMOF, and Tip60), and a subgroup containing zinc fingers (HBO1) (118). HATs facilitate the dissociation of DNA and histone octamers and the relaxation of nucleosome structure so that various transcription factors and co-transcription factors can specifically bind to DNA binding sites and activate gene transcription. Studies have shown that HATs are involved in tumor growth and metastasis. For example, lysine acetyltransferase 6A (KAT6A) can activate the Wnt/β-catenin pathway to promote the growth and metastasis of ovarian cancer (119). Histone acetyltransferase 1 (HAT1) and HBO1 can regulate the expression of TP53 to affect tumor metastasis (120).
HDACs deacetylate histones, bind tightly to negatively charged DNA, and make chromatin dense and coiled, repressing gene transcription (121). HDACs have been identified and grouped into four classes, including class I consisting of HDAC1, HDAC2, HDAC3, HDAC8; class II consisting of HDAC4, HDAC5, HDAC6, HDAC7, HDAC7, HDAC10; class III consisting of SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, SIRT7; and class IV consisting of HDAC11 (122). Although HDACs can promote tumor metastasis, sometimes, some HDACs can also inhibit tumor metastasis. For example, HDAC1, HDAC2, HDAC4, HDAC5, and HDAC6 are required for both proliferation and metastasis of melanoma, pancreatic cancer, nasopharyngeal carcinoma, and hepatocellular carcinoma by downregulating E-cadherin (123–126). IRT7 is transcriptionally repressed by HDAC8, a novel cofactor of the SMAD3/4 complex, through local chromatin remodeling, further activating TGF-β signaling and causing lung cancer metastasis (127). SIRT1 can promote the expression of ZEB1 to induce EMT of osteosarcoma (128). Overexpression of SIRT6 not only enhances the phosphorylation of extracellular signal-regulated kinase 1/2 (p-ERK 1/2) but also activates MMP 9 to promote tumor cell migration and invasion (129). SIRT6 also suppresses cell metastasis of pancreatic ductal adenocarcinoma (PDAC) by regulating the expression of HMGA 2, IGF2BP1, and IGF2BP3 (130). In addition, SIRT3 can activate FOXO3a and inhibit the wnt/β-catenin pathway, thereby inhibiting EMT of prostate cancer cells (131). SIRT4 inhibits Drp1 phosphorylation through interaction with fis-1, and suppresses MEK/ERK activity to inhibit NSCLC cell invasion and migration (132). SIRT7 is a key regulator of TGF-β signaling and a suppressor of breast cancer metastasis, and its deletion promotes the metastasis of breast cancer cells (133).
Several HDAC inhibitors (HDACi) with different chemical structures have been purified from natural extracts or chemical synthesis. To date, there are at least nine different HDACi, including Saha, LAQ 824, FK 228, MS-275, CI-994, PXD 101, valproic acid, pyroxamine, and sodium butyrate, currently in use alone or in combination with therapy for blood disorders and solid tumors (134,135). HDACi selectively kills tumor cells and has little toxicity to normal cells. The basis for the selective toxicity of HDACi is unclear but may be related to the HDAC overexpression observed in cancer cells (2). HDACi has the ability to effectively activate multiple molecular pathways and mediate its antitumor effect (136,137). The antitumor effect of HDACi apparently depends on inhibiting the proliferation, survival, migration, invasion, metastasis, and angiogenesis of cancer cells by regulating gene transcription (138). In conclusion, the molecular basis for the tumor-selective cytotoxicity of HDACi has not been extensively studied; therefore, the molecular basis requires further in-depth validation.
Metastasis regulated by ubiquitination and deubiquitination
Ubiquitination refers to the process in which the ubiquitin (a small 76-residue regulatory protein ubiquitously expressed in eukaryotes) molecule classifies proteins in cells under the action of a series of special enzymes, selects target protein molecules from them, and modifies explicitly the target protein (139). These special enzymes include ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), ubiquitin-protein ligase (E3), and degrading enzymes (140). Substrates can be modified with single ubiquitin (monoubiquitination) or polymeric Ub chains. Depending on which internal lysine (K6, K11, K27, K29, K33, K48, K63), or the N-terminal methionine residue of the Ub (m1, linear or head-to-tail) can be used to link to the far Ub chain type of end (141). Deubiquitinating enzymes (Dubs) balance ubiquitin chain growth by removing ubiquitin. The synergistic effect of Dubs recognition and Ub hydrolysis creates a dynamic network that controls the distribution of different ubiquitin signals, which in turn regulates numerous biological processes within the cancer cell (142). Approximately 103 Dubs have been recognized in the human genome, and they can be divided into six families according to their sequence and the sequence of conserved regions: USPs (ubiquitin-specific proteases) such as USP2, USP6, USP7, USP8, USP11, USP15, USP16, USP21, USP 28, USP35; UCHs (ubiquitin C-terminal hydrolysis enzymes) such as UCH-L1, UCH-L3, UCH-L5; MJDs (Machado-Joshphin domain-containing proteases) such as JosD1, JosD1; OUTs (ovarian cancer proteases) such as A20, OTUB2, TRABIO; SENPs (motif-interacting with ubiquitin-containing novel DUB family), such as SENP1, SENP2, SENP8; JAMMs (JAB1, MPN, MOV34 family) such as AMSH (143).
Ubiquitination and deubiquitination play an essential role in protein localization, metabolism, function, regulation, and degradation. At the same time, it regulates nearly all life activities, including cell cycle, proliferation, apoptosis, differentiation, metastasis, gene expression, transcriptional regulation, signal transmission, damage repair, inflammation, and immunity (144). For example, E3 ubiquitin ligase RNF126 specifically regulates PTEN stability and then induces cell proliferation and metastasis of bladder cancer by upregulating the EGFR/PI3K/AKT signaling pathway (145). STAMBP is a deubiquitinase (Dubs) family member in the Jab1/MPN family of metalloenzymes that specifically cleaves K63-linked polyubiquitinated chains from substrates to promote the stabilization of EGFR and the active AMPK signaling pathway to induce cell metastasis of lung adenocarcinoma (146). RING finger-domain E3 ubiquitin ligase RBBP6-mediated ubiquitination and degradation of IκBα enhances p65 nuclear translocation, triggering activation of the NF-κB pathway, which in turn induces EMT of colorectal cancer (147). In addition, some deubiquitinating enzymes can also play a role in promoting tumor metastasis. USP5 can induce EMT of non-small cell lung cancer (NSCLC) by activating the Wnt/β-catenin pathway (148). USP10 directly interacts with SMAD4 and stabilizes it to promote HCC proliferation and metastasis (149). USP21 can deubiquitinate and stabilize EZH2 to induce cell proliferation and metastasis of bladder cancer (150). USP11 promotes colorectal cancer growth and metastasis by stabilizing PPP1CA, activating the ERK/MAPK pathway, and insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) signaling (151), and interacting with nuclear factor 90 (NF90) to promote HCC proliferation and metastasis (152). OTU domain-containing protein 3 (OTUD3), a deubiquitinating enzyme, also promotes HCC proliferation and metastasis by regulating α-actinin 4 (ACTN4) (153). The deubiquitinating enzyme PSMD14 directly interacts with Snail and stabilizes it to promote cell migration and tumor metastasis of esophageal squamous cell carcinoma (154). In addition to their promoting effects, ubiquitinases and deubiquitinases can also inhibit tumor metastasis. For instance, E3 ligase zinc finger protein 91 (ZFP91) can regulate PKM splicing to inhibit HCC metastasis (155). Ubiquitin-conjugating enzyme variant proteins (Ube2v) and E3 ubiquitin ligase SMURF2 can degrade the protein level of SIRT1, thereby suppressing cell proliferation and metastasis of CRC (156,157). BTRC, an E3 ubiquitin ligase, can degrade the protein level of Twist1 to suppress cell proliferation and metastasis of GC (158).
The drug development of E3 ligase has been a very challenging research hotspot in recent years. At present, antitumor drugs targeting E3 ligase are mainly divided into four categories according to their mechanism of action: E3 ligase targeted inhibitors, E3 ligase targeted agonists, proteolytic targeting chimeras (PROTACs), and molecular glues (159). Considering the complex and extensive life activities regulated by ubiquitination, blocking or activating E3 ligase to treat tumors may have adverse effects on other everyday life metabolic activities. Therefore, exploring and solving this problem remains a considerable challenge. Moreover, most ubiquitination inhibitors found to be beneficial in preclinical studies have shown poor results in clinical trials. This discrepancy may be because we do not know enough about the target protein's structural analysis and medicinal chemistry, which requires technological advances.
Similarly, a large number of Dubs play an important role in the development of several stages of cancer development. The potential to affect processes such as signal transduction, proliferation, and apoptosis by affecting ubiquitination and proteasomal degradation of key regulators is promising and exciting. Dubs are more likely candidates than E3 ligases, which lack well-defined catalytic residues (160). For example, the first highly selective proteasome-bound USP14 inhibitor uu1(ubiquitin-7-amino-4-methylcoumarin, ub-amc) was identified by high-throughput screening of Ub-amc (161). P5091 (1-[5-[(2,3-dichlorophenyl)thio]-4-nitro-2-thienyl]-ethanone) was discovered to be a specific inhibitor of USP7 for the first time. This compound inhibits the stabilizing effect of USP7 on Hdm2 and exerts a pro-apoptotic effect by stabilizing p21 and p53 (162). Betulinic acid (BA) [(3β)-3-hydroxy-lup-20(29)-en-28-oc acid)] is a naturally occurring plant-derived compound with proapoptotic and highly specific effects on cancer cells. BA was identified as a broad inhibitor of deubiquitination that induces apoptosis by releasing mitochondrial proteins and leads to the downregulation of angiogenic markers (163). Overall, USPs are a complex system, and not all components have been adequately evaluated. It is well known that the UPSs are involved in almost all tumor cell processes. There are many important clinical implications to new ways to target UPS in cancer therapy, and we expect that UPS-based therapy will play a significant role on a clinical level in the near future.
Metastasis regulated by glycosylation
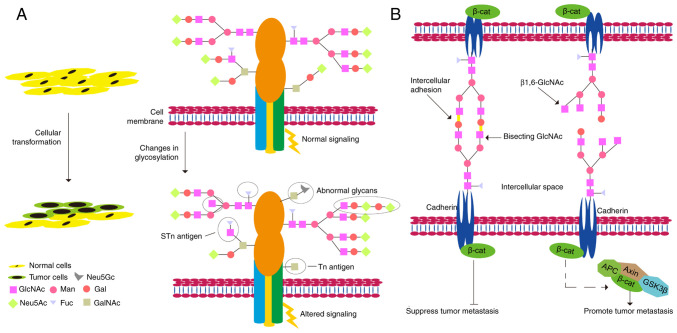
Abnormal glycosylation plays a vital role in the key pathological processes of tumorigenesis and development (Fig. 3) (164). Glycans play important roles in tumor cell signaling, tumor cell separation and invasion, cell-matrix interaction, angiogenesis, metastasis, and immune regulation, and abnormal glycosylation is often referred to as a 'signature of cancer' (165). The synthesis of N-linked sugar chains starts in the endoplasmic reticulum and is completed in the Golgi apparatus. Most of the mannose in the original sugar chain is excised, but various glycosyltransferases add different types of sugar molecules, in turn, to form oligosaccharide chains with different structures (166). The spatial structure of a glycoprotein determines which glycosyltransferase it can bind to undergo specific glycosylation modifications (167). Many glycoproteins have both N-linked sugar chains and O-linked sugar chains (168). The O-linked glycosylation is carried out in the Golgi apparatus; usually, the first linked sugar unit is N-acetylgalactose, and the linked sites are the hydroxyl groups of Ser, Thr, and Hyp, and then the sugar groups are successively transferred to it to form an oligosaccharide chain; the donor of sugar is also a nucleoside sugar, such as UDP-galactose (169). As a result of glycosylation, different proteins are marked differently, changing the polypeptide's conformation and increasing the protein's stability (170). Metastatic tumor cells must undergo a series of important events, including EMT, detachment from the primary tumor mass, adherence to ECM proteins, migration and degradation of ECM proteins, invasion of adjacent tissues, penetration of lymphatic or blood vessels, spread to different parts of the body, and outflow from blood vessels to form metastatic tumors (171). In humans, there are more than 2,000 proteins that contain an amino acid motif suitable for N-glycosylation. They are either membrane-bound or secreted but by no means cytoplasmic or nuclear. Therefore, glycoproteins are critical for tumor metastasis (172). Examples of N-glycans are secreted proteinases such as kallikreins, carboxypeptidase E, cathepsins, and others; adhesion proteins including members of the immunoglobulin superfamily (ALCAM, ICAM1, BCAM, and others), cadherins; Wnt family members; c-Kit, TIMP1, tetraspanins, clusterin, and others; ECM molecules such as fibronectin, laminin, and others (173). N-glycosylated cadherins have substantial effects on their functions as adhesion molecules, signaling proteins, and tumor suppressors (174). Human E-cadherin displays four potential N-glycosylation sites (175). Moreover, alterations in the expression profiles of E-cadherin-linked N-glycans are associated with malignant and invasive phenotypes and poor survival in cancer patients (176). Another important part of N-glycosylation is that it helps keep N-cadherin stable. It also plays a role in preventing cell-cell adhesion and promoting GBM cell migration (177).
Figure 3.
Glycosylation and tumor metastasis. (A) The effect of glycosylation on the transformation of normal cells into tumor cells. (B) Effects of glycosylation on the transformation of tumor cells into metastatic tumor cells. β-cat, β-catenin; APC, adenomatous polyposis coli; GSK3β, glycogen synthase kinase 3β.
The ECM is the acellular part of tissue composed of collagen, glycoproteins, proteoglycans, and crevices. Dynamic changes in cell-ECM interactions, including those coordinated by glycans, are critical for tumor cells to acquire migratory and invasive capabilities (178). The integrin family encompasses the primary surface receptors involved in the adhesion of cells to the ECM elements. N-linked glycans modulate integrin function regulating the migration capacity of tumor cells (179). ST6GAL1 (ST6 β-galactoside α-2,6-sialyltransferase 1), which catalyzes the transfer of sialic acids to terminal galactose residues of N-glycans, is upregulated, which can increase the migration and invasion of human CRC cells (180).
Similarly, ST6GAL1 can increase adhesion to ECM structures and increase the invasiveness of breast cancer (181). N-glycosylation of N-acetylglucosaminyltransferase V enhances CD 147/basgin interaction with integrin β1 to promote liver cancer metastasis (182). Cluster of differentiation 147 (CD147) is an extracellular matrix metalloproteinase inducer, and modification of N-glycosylation of Asn152 on CD147 strongly promotes invasion and migration of HCC (183). Of course, in addition to the promoting effect, N-glycosylation also has an inhibitory effect on the metastasis of tumor cells. For example, glycosylation of Asn-144 of Golgi protein 73 (GP73) inhibits HCC metastasis (184).
O-glycosylation has the same effect on tumor cell metastasis as N-glycosylation. For instance, GalNAc-type O-glycosylation can modify TGF-β to facilitate breast cancer cell migration and invasion via the EMT process (184). N-ac etylgalactosaminyltransferase 6 (GALNT6), an enzyme that mediates the initial step of mucin-type O-glycosylation, enhances O-glycosylation of α2-macroglobulin (α2M) and activates the downstream PI3K/Akt signaling pathway to promote migration and invasion of breast cancer and ovarian cancer (185,186). However, N-acetyl-galactosaminotransfe rase 2 (GALNT2), an enzyme that initiates O-glycosylation of mucins, inhibits metastasis of gastric adenocarcinoma by reducing EGFR-AKT signaling (187). Osteopontin (OPN) is a multiphosphorylated extracellular glycoprotein. O-terminal glycosylation of OPN can increase cell adhesion, thereby inhibiting tumor cell metastasis (188).
As glycosylation is implicated in every link from tumor occurrence to metastasis, antitumor medication development for glycosylation, such as cancer-related glycoforms and glycosyltransferases related to synthetic sugars, which are all potential therapeutic targets is urgently needed (189). Glycolectin interactions are central axes of multiple aspects of cancer progression, such as immune evasion, cell proliferation, invasion, and extravasation. Blocking this interaction is a promising new strategy for single-agent and combination therapy (190). Finally, we emphasize that in addition to the need to develop new cancer drug strategies targeting glycosylation, it is worth exploring efficient cancer delivery systems to avoid side effects. We believe that exploring specific glycosylation targets may be the beginning of a new era in cancer therapy.
Metastasis regulated by phosphorylation and dephosphorylation
Phosphorylation and dephosphorylation are some of the most prevalent chemical modifications in cells and are catalyzed by phosphorylase and phosphatase, respectively (191). Protein phosphorylation is a ubiquitous regulatory mechanism in organisms. It plays an important role in various aspects of every organism, such as gene transcription, expression, cell proliferation, differentiation, apoptosis, signal transduction, immune regulation, tumor occurrence, and transfer (192). Phosphorylation refers to the addition of a phosphate (PO4) group to a protein or other types of molecules, which can also be defined as 'introducing a phosphate group into an organic molecule' (193). Protein phosphorylation can be divided into four categories according to the amino acid residues that are phosphorylated: phosphorylation of serine, threonine, and tyrosine constitutes O-phosphorylation; phosphorylation of histidine, arginine, and lysine constitutes N-phosphorylation; phosphorylation of aspartate and glutamate constitutes S-phosphorylation; phosphorylation of cysteine constitutes acyl phosphorylation (192). More than 90% of the proteins encoded by the human genome are phosphorylated. Therefore, phosphorylated or dephosphorylated proteins can regulate tumor growth and metastasis.
For example, phosphorylation of cyclase-associated protein 1 (CAP1) can induce EMT of lung cancer (194). Longevity assurance homolog 2 of yeast LAG1 (LASS2), a novel tumor-suppressor gene, is thought to be a ceramide synthase that synthesizes very long acyl-chain ceramides. Therefore, phosphorylated LASS2 inhibits Wnt/β-catenin signaling to reduce prostate cancer growth and metastasis (195). Protein phosphatase 2A (PP2A)-induced dephosphorylation of girdin is involved in inhibiting breast cancer cell migration (196).
Signaling pathways regulated by protein kinases are involved in the occurrence and development of almost all types of cancer. Therefore, the study of kinase-mediated signaling pathways and the possibility of blocking them with targeted therapy may have important clinical therapeutic implications, especially since many of these proteins are oncogenes (197). The signaling networks through which protein kinases operate are very complex, but we believe that understanding the regulatory functions of kinases may be an effective means of identifying more effective cancer treatments (198). Many drug kinase inhibitors, such as imatinib mesylate (199), crizotinib (200), vemurafenib, and cobimetinib (201), are already on the market; nevertheless, their efficacy is often reduced due to the development of complex resistance mechanisms (202). In short, protein phosphatase may be a new drug target for treating malignant tumors in the future.
Metastasis regulated by chromatin remodeling
Chromatin remodeling refers to the molecular mechanism of changes in the packaging state of chromatin, histones in nucleosomes, and corresponding DNA molecules during the replication and recombination of gene expression (203). Chromatin remodeling can lead to changes in the position and structure of nucleosomes, causing chromatin changes. ATP-dependent chromatin remodelers reposition nucleosomes, alter nucleosome structure, and covalently modify histones (204). In the process of nucleosome remodeling, the role of the remodeling factor complex (ATPase activity) is crucial, including the SWI/SNF family, ISWI family, CHD family, and INO80 family (205). These shared properties enable nucleosome engagement, selection, and remodeling. Each ATPase family catalyzes a different remodeling activity which can include incremental nucleosome sliding on DNA in cis-ribosomes; DNA loops generated on the surface of nucleosomes; histone H2A/H2B dimers cleared; histone VIII aggregates are cleared, or histone octapeptide subunits are exchanged within the nucleosome to alter their composition (206). Each of these activities alters the accessibility of DNA in chromatin to DNA-binding factors, which in turn regulate fundamental nuclear processes such as transcription, DNA replication, and DNA repair.
The chromatin remodeling activity of ATPases usually occurs in a large multi-subunit complex, but they are called single ATPase subunits in rare cases. Complexes in the SWI/SNF family include the large multi-subunit BAF, PBAF, and WINAC complexes, which are co-regulators of transcription and which also contribute to DNA damage repair (207). Like SWI/SNF, members of the INO 80 family are large multi-subunit complexes that regulate transcription, but their roles in DNA damage repair are more pronounced (208). These complexes are unique among ATP-dependent remodeling complexes in that they catalyze the exchange of histones from the nucleosomal structure. Complexes with optimal function include SRCAP and Tip 60/P 400, which exchange H2A/H2B histone dimers found in standard nucleosomes for variant H2A.Z/H2B dimers (209,210). The CHD family contains nine different ATPases and is the largest in the remodeling family. The most characteristic complex in this family is NURD (211). NURD contains both ATP-dependent chromatin remodeling and HDAC activities (211). MBD2 is a unique subunit of the NURD complexes and can bind 5mC DNA (212). Once recruited by 5mC-enriched DNA, the MBD2-NURD complex inhibits gene expression through its remodeling and histone deacetylase activity. MBD3 can replace the MBD2 subunit, dissociating NURD from 5mC and promoting its function as a transcriptional activator (213). Most imitation switch (ISWI) complexes are relatively small, consisting of only two or three subunits. Each of these complexes contains a large subunit with several histone-binding domains (including PHD and bromodomains) and an ISWI family of ATPases (214). These complexes have multiple functions, including spacing of nucleosomes after DNA replication (CHRAC, ACF), RNA polymerase elongation (RSF), as co-regulators of transcription (CERF, NURF, NoRC, b-WICH) and regulation of DNA damage repair (WICH) (215).
All proteins involved in chromatin remodeling also share five basic properties: a) domains that recognize covalent histone modifications; b) an affinity for the nucleosome, beyond DNA itself; c) domains and/or proteins that regulate the ATPase domain; d) a similar DNA-dependent ATPase domain, required for remodeling and serving as a DNA-translocating motor to break histone-DNA contacts; and e) domains and/or proteins for interaction with other chromatin or transcription factors (205).
The role of chromatin remodeling in cancer metastasis is well-studied (216). For example, chromatin remodeling protein BRG1, a core component of the mammalian chromatin remodeling complex, regulates the transcription of long-chain fatty acid elongase 3 (Elovl3), which promotes cell metastasis of PCa (217). MORC family CW-type zinc finger 2 (MORC2) is a newly discovered chromatin remodeling protein that promotes breast cancer invasion and metastasis through interacting with catenin delta 1 (CTNND1, also known as p120-catenin, was originally identified as a substrate of the oncogenic tyrosine kinase Src and subsequently defined as a component of the adherens junction complex that includes E-cadherin and α-, β-, γ-catenins) (218). However, chromatin remodeling factor ARID2 is one subunit of the chromatin-remodeling SWI/SNF complex and inhibits EMT of HCC cells by recruiting DNMT1 to Snail promoter, which increases promoter methylation and inhibits Snail transcription [55]. ARID1A, a SWI/SNF chromatin remodeling complex subunit, inhibits cancer cell migration, invasion, and metastasis by downregulating β-catenin signaling (219).
Signaling pathways regulated by chromatin remodeling proteins are involved in the occurrence and development of almost all types of cancer. Changes in chromatin structure have profound effects on gene expression during normal cellular homeostasis and malignant transformation. Therefore, screening small molecules targeting recombinant chromatin proteins has important clinical implications.
Metastasis regulated by non-coding RNAs
Non-coding RNAs (ncRNAs) refer to RNAs that do not encode proteins. These include RNAs with known functions such as rRNA, tRNA, circular RNA, snRNA, snoRNA, and microRNA and RNAs with unknown functions (220). The common feature of these RNAs is that they can all be transcribed from the genome but not translated into proteins to perform their respective biological functions at the RNA level. Non-coding RNAs can be divided into three categories in terms of length: less than 50 nt, including microRNAs, siRNAs, piRNAs; 50–500 nt, including rRNAs, tRNAs, snRNAs, snoRNAs, siRNAs, srpRNAs; greater than 500 nt, including long non-coding RNAs, long non-coding RNAs without polyA tails (221).
MicroRNA is a class of 21–23 nt small RNAs, its precursor is approximately 70–100 nt, forming a standard stem structure, and after processing, it becomes a 21–23 nt single-stranded RNA. The mechanism of action of a microRNA is to complement mRNA, silencing or degrading mRNA (222). snRNA is short for small nuclear RNA. Its function is to combine with protein factors to form small nuclear ribonucleoprotein particles (snRNPs) and perform the function of splicing mRNA. There are mainly five types of snRNAs: U1, U2, U4, U5, and U6 (223). snoRNAs are the first small RNAs discovered in the nucleolus, called small nucleolar RNAs, and their biological functions were initially discovered to modify rRNA. Most small nucleolar RNAs can be divided into two categories. One is C Dbox snoRNA, which is methylated on RNA bases. It contains 4 Boxes: Box C, Box D, BoxC' and BoxD'. The other type is the H/ACA box. This type of snoRNA carries out methyluracilylation modification to the base of RNA. Its characteristic is to form a double stem and add a loop area in the middle, among which boxH in the middle loop area (224). Long non-coding RNAs (lncRNAs) are longer than 200 bp and are transcribed from independent promoters by RNA Pol II (225). Its sequence conservation is not high, and its expression abundance is low, showing strong specificity in tissues and cells. lncRNAs are involved in various critical regulatory processes such as X chromosome silencing, genomic imprinting, chromatin modification, transcriptional activation, transcriptional interference, and intranuclear transport (226). Unlike known linear RNAs, circular RNAs (circRNAs) form a covalently closed continuous loop. In circRNAs, the 3' and 5'ends of the RNA molecule are usually joined together. This property confers several properties on circRNAs, many of which have only recently been identified. Many circRNAs are derived from protein-coding genes, but some studies have shown that some circRNAs can encode proteins (227). Because circRNAs were previously found to have no protein-coding function, they were classified as non-coding RNAs. Recently, some circRNAs have shown potential as gene regulators (228).
There are many studies showing that non-coding RNAs can regulate tumor growth and metastasis. Over the past decade, the regulatory roles of lncRNAs and miRNAs in various biological processes have emerged. The regulatory mechanisms of lncRNAs and miRNAs are diverse, relying primarily on their localization and interacting proteins or RNAs (229,230). For many metastasis-related miRNAs, target genes with established roles in tumor cell invasion, migration, and metastasis have been identified, such as MMPs, HER receptors, BMPs, PTEN, ZEB1, ZEB2, or E-cadherin (231). Some metastasis-related genes are directly or indirectly regulated by multiple miRNAs simultaneously. For example, members of the miR-200 family (miR-141, miR-200a, miR-200b, miR-200c, miR-429) have been shown to regulate the epithelial properties of cells by silencing Zeb proteins (232), and ZEB1/ZEB2 are also affected by regulation of miR-205 and or miR-192 (233).
lncRNAs are abnormally regulated in many cancers and are associated with tumor metastasis. lncRNAs can be used as new biomarkers for tumor diagnosis and treatment. Using lncRNA arrays or RNA-seq analysis, many lncRNAs have been found to be markers of tumor prognosis (234). More and more studies have shown that lncRNAs are potent regulators of EMT during tumor metastasis by controlling key molecules in several intracellular signaling pathways, including TGF-β/SMAD, Wnt/β-catenin, Notch, MEK/ERK, PI3K/AKT, and JAK/STAT, notably Snail, ZEB and TWIST, which inhibit the expression of epithelial state-associated genes while simultaneously activating the expression of mesenchymal state-associated genes and concomitantly (235–238).
Since miRNAs play critical roles in tumorigenesis and metastasis, they are likely to be tumor-suppressor targets or therapeutic drugs. In the case of abnormal upregulation, miRNAs can be silenced by directly targeting miRNAs involved in tumor pathogenesis by 'anti-miRNA' molecules. Conversely, when miRNAs aberrantly downregulate mRNAs that directly target genes involved in tumor pathogenesis, so-called miRNA mimics or similar drugs can be used as drugs to replace pathologically downregulated miRNAs (231). Furthermore, to date, research on lncRNAs and metastasis has mainly focused on different organ-specific metastases. However, the reality is that many patients suffer from or are at risk for multi-organ metastases. In addition, the function of lncRNAs can be altered through epigenetic modification and microenvironmental shift (239). This complexity makes it difficult to fully understand the specific molecular mechanisms of tumor metastasis mediated by lncRNAs (240). Because lncRNAs are mediators of tumor metastasis, targeting them as a common therapeutic target in different types of tumors may be important for future research.
4. Conclusion
Epigenetic regulation and metastasis play unique roles in the occurrence and development of tumors, and related enzymes and regulatory factors are potential drug targets for cancer treatment. Although no single epigenetic regulator is mutated in tumor metastasis, there is growing evidence that metastatic tissue has a distinct epigenetic status compared to the primary tumor tissue for use in cancer therapy and diagnosis (241). Due to the nature of epigenetic modifications, modulating these changes and understanding the role of epigenetics in EMT and metastasis will provide new insights into our understanding of tumor progression and metastasis. Because epigenetic modifications are reversible, a thorough understanding of their function in EMT provides us with new insights into tumor progression and metastasis. In addition, it can further facilitate the development of human cancer diagnostic and therapeutic strategies. Epigenetic alterations associated with EMT are considered clinically as a potential biomarker. Given the complexity of epigenetic transformation, we also need to pay special attention to the epigenetic characteristics of the target protein or RNA when using targeted drugs, which means that our drugs need to be more cautious. Nevertheless, we believe that a detailed understanding of the epigenetic aspects of EMT regulation and metastasis will undoubtedly be an excellent way to develop prognostic cancer biomarkers. Moreover, the development of specific inhibitors of enzymatic proteins designed by epigenetic modification will also be new hope for treating tumor metastasis.
Acknowledgements
Not applicable.
Funding Statement
This research was supported by the Natural Science Foundation of Chongqing (cstc2019jcyjzdxmX0033), and the Graduate Research and Innovation Project of Chongqing of China (no. 2019CYB19117).
Availability of data and materials
All information provided in this review is documented by relevant references. All references are open-ended articles.
Authors' contributions
HC and YC contributed to the conception and design of the review. TT and PS organized the database and wrote the first draft of the manuscript. MNA, YW and JX wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
References
- 1.Rodenhiser DI. Epigenetic contributions to cancer metastasis. Clin Exp Metastasis. 2009;26:5–18. doi: 10.1007/s10585-008-9166-2. [DOI] [PubMed] [Google Scholar]
- 2.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dario LS, Rosa MA, Mariela E, Roberto G, Caterina C. Chromatin remodeling agents for cancer therapy. Rev Recent Clin Trials. 2008;3:192–203. doi: 10.2174/157488708785700320. [DOI] [PubMed] [Google Scholar]
- 4.Werner RJ, Kelly A, DIssa JJ. Epigenetics and precision oncology. Cancer J. 2017;23:262–269. doi: 10.1097/PPO.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan X. Cancer metastases: Challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachmayr E, Treese C, Stein U. Underlying mechanisms for distant metastasis-molecular biology. Visc Med. 2017;33:11–20. doi: 10.1159/000454696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017;31:1827–1840. doi: 10.1101/gad.305805.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 9.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birchmeier W, Behrens J. Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 11.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, et al. Somatic inactivation of E-cadherin and p53 in mice leads to meta-static lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Odero-Marah V, Hawsawi O, Henderson V, Sweeney J. Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv Exp Med Biol. 2018;1095:101–110. doi: 10.1007/978-3-319-95693-0_6. [DOI] [PubMed] [Google Scholar]
- 15.Chiang SP, Cabrera RM, Segall JE. Tumor cell intravasation. Am J Physiol Cell Physiol. 2016;311:C1–C14. doi: 10.1152/ajpcell.00238.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton G, Rath B. Mesenchymal-epithelial transition and circulating tumor cells in small cell lung cancer. Adv Exp Med Biol. 2017;994:229–245. doi: 10.1007/978-3-319-55947-6_12. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016;35:1216–1224. doi: 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 19.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paoletti C, Hayes DF. Circulating tumor cells. Adv Exp Med Biol. 2016;882:235–258. doi: 10.1007/978-3-319-22909-6_10. [DOI] [PubMed] [Google Scholar]
- 21.Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: Challenges and opportunities. Cancer Cell. 2018;33:965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu BM. Tumor metastasis in the microcirculation. Adv Exp Med Biol. 2018;1097:201–218. doi: 10.1007/978-3-319-96445-4_11. [DOI] [PubMed] [Google Scholar]
- 23.Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mielgo A, Schmid MC. Liver Tropism in Cancer: The hepatic metastatic niche. Cold Spring Harb Perspect Med. 2020;10:a037259. doi: 10.1101/cshperspect.a037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pramani KA, Jones S, Gao Y, Sweet C, Vangara A, Begum S, Ray PC. Multifunctional hybrid graphene oxide for circulating tumor cell isolation and analysis. Adv Drug Deliv Rev. 2018;125:21–35. doi: 10.1016/j.addr.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Dabagh M, Randles A. Role of deformable cancer cells on wall shear stress-associated-VEGF secretion by endothelium in microvasculature. PLoS One. 2019;14:e0211418. doi: 10.1371/journal.pone.0211418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu SK, Chiu CC, Dahms HU, Chou CK, Cheng CM, Chang WT, Cheng KC, Wang HD, Lin IL. Unfolded protein response (UPR) in survival, dormancy, immunosuppression, metastasis, and treatments of cancer cells. Int J Mol Sci. 2019;20:2518. doi: 10.3390/ijms20102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Zang X, Lv Y. Detection of circulating tumor cells: Advances and critical concerns. Oncol Lett. 2021;21:422. doi: 10.3892/ol.2021.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, Alonso-Basanta M, Zhang Z, O'Rourke DM, Zhang L, et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 2018;78:6632–6642. doi: 10.1158/0008-5472.CAN-18-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 34.Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41:240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 36.Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14:9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 37.Cooper DN. Eukaryotic DNA methylation. Human Genet. 1983;64:315–333. doi: 10.1007/BF00292363. [DOI] [PubMed] [Google Scholar]
- 38.Compere SJ, Palmiter RD. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981;25:233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- 39.Dong Z, Pu L, Cui H. Mitoepigenetics and its emerging roles in cancer. Front Cell Dev Biol. 2020;8:4. doi: 10.3389/fcell.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan AE, Davies TJ, Mc Auley MT. The role of DNA methylation in ageing and cancer. Proc Nutr Soc. 2018;77:412–422. doi: 10.1017/S0029665118000150. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, Yang L, Cui H. SIRT1 regulates autophagy and diploidization in parthenogenetic haploid embryonic stem cells. Biochem Biophys Res Commun. 2015;464:1163–1170. doi: 10.1016/j.bbrc.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 43.Lyko F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19:81–92. doi: 10.1038/nrg.2017.80. [DOI] [PubMed] [Google Scholar]
- 44.Kausar S, Abbas MN, Cui H. A review on the DNA methyltransferase family of insects: Aspect and prospects. Int J Biol Macromol. 2021;186:289–302. doi: 10.1016/j.ijbiomac.2021.06.205. [DOI] [PubMed] [Google Scholar]
- 45.Dong Z, Cui H. Epigenetic modulation of metabolism in glioblastoma. Semin Cancer Biol. 2019;57:45–51. doi: 10.1016/j.semcancer.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Anteneh H, Fang J, Song J. Structural basis for impairment of DNA methylation by the DNMT3A R882H mutation. Nat Commu. 2020;11:2294. doi: 10.1038/s41467-020-16213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Hishikawa A, Itoh H. DNA damage repair and DNA methylation in the kidney. Am J Nephrol. 2019;50:81–91. doi: 10.1159/000501356. [DOI] [PubMed] [Google Scholar]
- 48.de Araújo ÉS, Pramio DT, Kashiwabara AY, Pennacchi PC, Maria-Engler SS, Achatz MI, Campos AH, Duprat JP, Rosenberg C, Carraro DM, Krepischi AC. DNA methylation levels of melanoma risk genes are associated with clinical characteristics of melanoma patients. Biomed Res Int. 2015;2015:376423. doi: 10.1155/2015/376423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farhadova S, Gomez-Velazquez M, Feil R. Stability and lability of parental methylation imprints in development and disease. Genes (Basel) 2019;10:999. doi: 10.3390/genes10120999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 51.Wu A, Cremaschi P, Wetterskog D, Conteduca V, Franceschini GM, Kleftogiannis D, Jayaram A, Sandhu S, Wong SQ, Benelli M, et al. Genome-wide plasma DNA methylation features of metastatic prostate cancer. J Clin Invest. 2020;130:1991–2000. doi: 10.1172/JCI130887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermann A, Goyal R, Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 53.Espada J, Ballestar E, Fraga MF, Villar-Garea A, Juarranz A, Stockert JC, Robertson KD, Fuks F, Esteller M. Human DNA methyltransferase 1 is required for maintenance of the histone H3 modification pattern. J Biol Chem. 2004;279:37175–37184. doi: 10.1074/jbc.M404842200. [DOI] [PubMed] [Google Scholar]
- 54.Lee E, Wang J, Yumoto K, Jung Y, Cackowski FC, Decker AM, Li Y, Franceschi RT, Pienta KJ, Taichman RS. DNMT1 regulates epithelial-mesenchymal transition and cancer stem cells, which promotes prostate cancer metastasis. Neoplasia. 2016;18:553–566. doi: 10.1016/j.neo.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang H, Cao HJ, Ma N, Bao WD, Wang JJ, Chen TW, Zhang EB, Yuan YM, Ni QZ, Zhang FK, et al. Chromatin remodeling factor ARID2 suppresses hepatocellular carcinoma metastasis via DNMT1-Snail axis. Proc Natl Acad Sci USA. 2020;117:4770–4780. doi: 10.1073/pnas.1914937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang H, Liu P, Yang L, Xie X, Ye F, Wu M, Liu X, Chen B, Zhang L, Xie X. miR-185 suppresses tumor proliferation by directly targeting E2F6 and DNMT1 and indirectly upregulating BRCA1 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:3185–3197. doi: 10.1158/1535-7163.MCT-14-0243. [DOI] [PubMed] [Google Scholar]
- 57.Zhu A, Hopkins KM, Friedman RA, Bernstock JD, Broustas CG, Lieberman HB. DNMT1 and DNMT3B regulate tumorigenicity of human prostate cancer cells by controlling RAD9 expression through targeted methylation. Carcinogenesis. 2021;42:220–231. doi: 10.1093/carcin/bgaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao X, Sheng Y, Yang J, Wang C, Zhang R, Zhu Y, Zhang Z, Zhang K, Yan S, Sun H, et al. Osteopontin alters DNA methylation through up-regulating DNMT1 and sensitizes CD133+/CD44+ cancer stem cells to 5 azacytidine in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:179. doi: 10.1186/s13046-018-0832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai J, Zhang X, Hu K, Liu B, Wang H, Li A, Lin F, Zhang L, Sun X, Du Z, Song J. Silencing DNA methyltransferase 1 (DNMT1) inhibits proliferation, metastasis and invasion in ESCC by suppressing methylation of RASSF1A and DAPK. Oncotarget. 2016;7:44129–44141. doi: 10.18632/oncotarget.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie M, Nie FQ, Sun M, Xia R, Liu YW, Zhou P, De W, Liu XH. Decreased long noncoding RNA SPRY4-IT1 contributing to gastric cancer cell metastasis partly via affecting epithelial-mesenchymal transition. J Transl Med. 2015;13:250. doi: 10.1186/s12967-015-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y, Liu H, Shi X, Yao Y, Yang W, Song Y. The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma. Oncotarget. 2015;6:9160–9172. doi: 10.18632/oncotarget.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng F, Liu X, Lin C, Xu L, Liu J, Zhang P, Zhang X, Song J, Yan Y, Ren Z, Zhang Y. SMYD2 suppresses APC2 expression to activate the Wnt/β-catenin pathway and promotes epithelial-mesenchymal transition in colorectal cancer. Am J Cancer Res. 2020;10:997–1011. [PMC free article] [PubMed] [Google Scholar]
- 63.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 64.Chédin F. The DNMT3 family of mammalian de novo DNA methyltransferases. Prog Mol Biol Transl Sci. 2011;101:255–285. doi: 10.1016/B978-0-12-387685-0.00007-X. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZM, Lu R, Wang P, Yu Y, Chen D, Gao L, Liu S, Ji D, Rothbart SB, Wang Y, et al. Structural basis for DNMT3A-mediated de novo DNA methylation. Nature. 2018;554:387–391. doi: 10.1038/nature25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walton EL, Francastel C, Velasco G. Maintenance of DNA methylation: Dnmt3b joins the dance. Epigenetics. 2011;6:1373–1377. doi: 10.4161/epi.6.11.17978. [DOI] [PubMed] [Google Scholar]
- 67.Walton EL, Francastel C, Velasco G. Dnmt3b prefers germ line genes and centromeric regions: Lessons from the ICF syndrome and cancer and implications for diseases. Biology. 2014;3:578–605. doi: 10.3390/biology3030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Zhang W, Yan XJ, Lin XQ, Li W, Mi JQ, Li JM, Zhu J, Chen Z, Chen SJ. DNMT3A mutation leads to leukemic extramedullary infiltration mediated by TWIST1. J Hematol Oncol. 2016;9:106. doi: 10.1186/s13045-016-0337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui H, Hu Y, Guo D, Zhang A, Gu Y, Zhang S, Zhao C, Gong P, Shen X, Li Y, et al. DNA methyltransferase 3A isoform b contributes to repressing E-cadherin through cooperation of DNA methylation and H3K27/H3K9 methylation in EMT-related metastasis of gastric cancer. Oncogene. 2018;37:4358–4371. doi: 10.1038/s41388-018-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deivendran S, Marzook H, Santhoshkumar TR, Kumar R, Pillai MR. Metastasis-associated protein 1 is an upstream regulator of DNMT3a and stimulator of insulin-growth factor binding protein-3 in breast cancer. Sci Rep. 2017;7:44225. doi: 10.1038/srep44225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Niu H, Ma J, Yuan BY, Chen YH, Zhuang Y, Chen GW, Zeng ZC, Xiang ZL. The molecular mechanism of lncRNA34a-mediated regulation of bone metastasis in hepatocellular carcinoma. Mol Cancer. 2019;18:120. doi: 10.1186/s12943-019-1044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi W, Tang T, Li X, Deng S, Li R, Wang Y, Wang Y, Xia T, Zhang Y, Zen K, et al. Methylation-mediated silencing of miR-133a-3p promotes breast cancer cell migration and stemness via miR-133a-3p/MAML1/DNMT3A positive feedback loop. J Exp Clin Cancer Res. 2019;38:429. doi: 10.1186/s13046-019-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu K, Chen B, Li B, Li C, Zhang Y, Jiang N, Lang B. DNMT3B silencing suppresses migration and invasion by epigenetically promoting miR-34a in bladder cancer. Aging. 2020;12:23668–23683. doi: 10.18632/aging.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochimica et biophysica acta. Biochim Biophys Acta Mol Cell Res. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Takeshima H, Niwa T, Yamashita S, Takamura-Enya T, Iida N, Wakabayashi M, Nanjo S, Abe M, Sugiyama T, Kim YJ, Ushijima T. TET repression and increased DNMT activity synergistically induce aberrant DNA methylation. J Clin Invest. 2020;130:5370–5379. doi: 10.1172/JCI124070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ning B, Liu G, Liu Y, Su X, Anderson GJ, Zheng X, Chang Y, Guo M, Liu Y, Zhao Y, Nie G. 5-aza-2'-deoxycytidine activates iron uptake and heme biosynthesis by increasing c-Myc nuclear localization and binding to the E-boxes of transferrin receptor 1 (TfR1) and ferrochelatase (Fech) genes. J Biol Chemistry. 2011;286:37196–37206. doi: 10.1074/jbc.M111.258129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmelz K, Sattler N, Wagner M, Lübbert M, Dörken B, Tamm I. Induction of gene expression by 5-Aza-2'-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanisms. Leukemia. 2005;19:103–111. doi: 10.1038/sj.leu.2403552. [DOI] [PubMed] [Google Scholar]
- 78.Tong HY, Lin MF. Effect of 5-aza-2'-deoxycytidine on cell of high-risk patients with myelodysplastic syndrome in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:467–471. In Chinese. [PubMed] [Google Scholar]
- 79.Gagnon J, Shaker S, Primeau M, Hurtubise A, Momparler RL. Interaction of 5-aza-2'-deoxycytidine and depsipeptide on anti-neoplastic activity and activation of 14-3-3sigma, E-cadherin and tissue inhibitor of metalloproteinase 3 expression in human breast carcinoma cells. Anticancer Drugs. 2003;14:193–202. doi: 10.1097/00001813-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Jambhekar A, Dhall A, Shi Y. Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol. 2019;20:625–641. doi: 10.1038/s41580-019-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH, Yan C, Dong Z, Huang S, Zha Y, et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep. 2016;14:506–519. doi: 10.1016/j.celrep.2015.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hyun K, Jeon J, Park K, Kim J. Writing, erasing and reading histone lysine methylations. Exp Mol Med. 2017;49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 84.Skucha A, Ebner J, Grebien F. Roles of SETD2 in Leukemia-Transcription, DNA-Damage, and Beyond. Int J Mol Sci. 2019;20:1029. doi: 10.3390/ijms20051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 86.Montgomery ND, Yee D, Chen A, Kalantry S, Chamberlain SJ, Otte AP, Magnuson T. The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation. Curr Biol. 2005;15:942–947. doi: 10.1016/j.cub.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 87.Moore HM, Gonzalez ME, Toy KA, Cimino-Mathews A, Argani P, Kleer CG. EZH2 inhibition decreases p38 signaling and suppresses breast cancer motility and metastasis. Breast Cancer Res Treat. 2013;138:741–752. doi: 10.1007/s10549-013-2498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi X, Guo J, Guo J, Sun S, Yang P, Wang J, Li Y, Xie L, Cai J, Wang Z. EZH2-mediated epigenetic silencing of TIMP2 promotes ovarian cancer migration and invasion. Sci Rep. 2017;7:3568. doi: 10.1038/s41598-017-03362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahmoud F, Shields B, Makhoul I, Hutchins LF, Shalin SC, Tackett AJ. Role of EZH2 histone methyltrasferase in melanoma progression and metastasis. Cancer Biol Ther. 2016;17:579–591. doi: 10.1080/15384047.2016.1167291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lo Sardo F, Pulito C, Sacconi A, Korita E, Sudol M, Strano S, Blandino G. YAP/TAZ and EZH2 synergize to impair tumor suppressor activity of TGFBR2 in non-small cell lung cancer. Cancer Lett. 2021;500:51–63. doi: 10.1016/j.canlet.2020.11.037. [DOI] [PubMed] [Google Scholar]
- 91.Niu N, Lu P, Yang Y, He R, Zhang L, Shi J, Wu J, Yang M, Zhang ZG, Wang LW, et al. Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia-mesenchymal transition during pancreatic carcinogenesis. Gut. 2020;69:715–726. doi: 10.1136/gutjnl-2019-318362. [DOI] [PubMed] [Google Scholar]
- 92.Yuan H, Han Y, Wang X, Li N, Liu Q, Yin Y, Wang H, Pan L, Li L, Song K, et al. SETD2 restricts prostate cancer metastasis by integrating EZH2 and AMPK signaling pathways. Cancer Cell. 2020;38:350–365.e7. doi: 10.1016/j.ccell.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 93.Wu PC, Lu JW, Yang JY, Lin IH, Ou DL, Lin YH, Chou KH, Huang WF, Wang WP, Huang YL, et al. H3K9 histone methyl-transferase, KMT1E/SETDB1, cooperates with the SMAD2/3 pathway to suppress lung cancer metastasis. Cancer Res. 2014;74:7333–7343. doi: 10.1158/0008-5472.CAN-13-3572. [DOI] [PubMed] [Google Scholar]
- 94.Luan X, Wang Y. Long non-coding RNA XLOC_006390 promotes cervical cancer proliferation and metastasis through the regulation of SET domain containing 8. Oncol Rep. 2017;38:159–166. doi: 10.3892/or.2017.5663. [DOI] [PubMed] [Google Scholar]
- 95.Kang J, Shin SH, Yoon H, Huh J, Shin HW, Chun YS, Park JW. FIH Is an oxygen sensor in ovarian cancer for G9a/GLP-Driven epigenetic regulation of metastasis-related genes. Cancer Res. 2018;78:1184–1199. doi: 10.1158/0008-5472.CAN-17-2506. [DOI] [PubMed] [Google Scholar]
- 96.Qiang R, Cai N, Wang X, Wang L, Cui K, Wang X, Li X. MLL1 promotes cervical carcinoma cell tumorigenesis and metastasis through interaction with β-catenin. OncoTargets Ther. 2016;9:6631–6640. doi: 10.2147/OTT.S114370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li L, Zhang Z, Ma T, Huo R. PRMT1 regulates tumor growth and metastasis of human melanoma via targeting ALCAM. Mol Med Rep. 2016;14:521–528. doi: 10.3892/mmr.2016.5273. [DOI] [PubMed] [Google Scholar]
- 98.Chuang CY, Chang CP, Lee YJ, Lin WL, Chang WW, Wu JS, Cheng YW, Lee H, Li C. PRMT1 expression is elevated in head and neck cancer and inhibition of protein arginine methylation by adenosine dialdehyde or PRMT1 knockdown downregulates proliferation and migration of oral cancer cells. Oncol Rep. 2017;38:1115–1123. doi: 10.3892/or.2017.5737. [DOI] [PubMed] [Google Scholar]
- 99.Yin XK, Wang YL, Wang F, Feng WX, Bai SM, Zhao WW, Feng LL, Wei MB, Qin CL, Wang F, et al. PRMT1 enhances oncogenic arginine methylation of NONO in colorectal cancer. Oncogene. 2021;40:1375–1389. doi: 10.1038/s41388-020-01617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao R, Jiang H, Ma Y, Wang L, Wang L, Du J, Hou P, Gao Y, Zhao L, Wang G, et al. PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 2014;74:5656–5667. doi: 10.1158/0008-5472.CAN-14-0800. [DOI] [PubMed] [Google Scholar]
- 101.Bao X, Zhao S, Liu T, Liu Y, Liu Y, Yang X. Overexpression of PRMT5 promotes tumor cell growth and is associated with poor disease prognosis in epithelial ovarian cancer. J Histochem Cytochem. 2013;61:206–217. doi: 10.1369/0022155413475452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang J, Meng Q, Shi R, Xu Y. PRMT6 serves an oncogenic role in lung adenocarcinoma via regulating p18. Mol Med Rep. 2020;22:3161–3172. doi: 10.3892/mmr.2020.11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 104.Schneider J, Shilatifard A. Histone demethylation by hydroxylation: Chemistry in action. ACS Chem Biol. 2006;1:75–81. doi: 10.1021/cb600030b. [DOI] [PubMed] [Google Scholar]
- 105.Varier RA, Timmers HT. Histone lysine methylation and demethylation pathways in cancer. Biochim Biophys Acta. 2011;1815:75–89. doi: 10.1016/j.bbcan.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Hong Y, Li X, Zhu J. LSD1-mediated stabilization of SEPT6 protein activates the TGF-β1 pathway and regulates non-small-cell lung cancer metastasis. Cancer Gene Ther. 2022;29:189–201. doi: 10.1038/s41417-021-00297-6. [DOI] [PubMed] [Google Scholar]
- 107.Liu J, Feng J, Li L, Lin L, Ji J, Lin C, Liu L, Zhang N, Duan D, Li Z, et al. Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer. EMBO Rep. 2020;21:e48597. doi: 10.15252/embr.201948597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan HM, Lang WY, Yao LJ, Wang Y, Li XL. shRNA-interfering LSD1 inhibits proliferation and invasion of gastric cancer cells via VEGF-C/PI3K/AKT signaling pathway. World J Gastrointest Oncol. 2019;11:622–633. doi: 10.4251/wjgo.v11.i8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang Y, Liu Y, Yu L, Chen J, Hou J, Cui L, Ma D, Lu W. Histone demethylase KDM2A promotes tumor cell growth and migration in gastric cancer. Tumour Biol. 2015;36:271–278. doi: 10.1007/s13277-014-2630-5. [DOI] [PubMed] [Google Scholar]
- 110.Wanna-Udom S, Terashima M, Suphakhong K, Ishimura A, Takino T, Suzuki T. KDM2B is involved in the epigenetic regulation of TGF-β-induced epithelial-mesenchymal transition in lung and pancreatic cancer cell lines. J Biol Chem. 2021;296:100213. doi: 10.1074/jbc.RA120.015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu DH, Yang J, Gao LK, Min J, Tang JM, Hu M, Li Y, Li ST, Chen J, Hong L. Lysine demethylase 2A promotes the progression of ovarian cancer by regulating the PI3K pathway and reversing epithelial-mesenchymal transition. Oncol Rep. 2019;41:917–927. doi: 10.3892/or.2018.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tee AE, Ling D, Nelson C, Atmadibrata B, Dinger ME, Xu N, Mizukami T, Liu PY, Liu B, Cheung B, et al. The histone demethylase JMJD1A induces cell migration and invasion by up-regulating the expression of the long noncoding RNA MALAT1. Oncotarget. 2014;5:1793–1804. doi: 10.18632/oncotarget.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sechler M, Parrish JK, Birks DK, Jedlicka P. The histone demethylase KDM3A, and its downstream target MCAM, promote Ewing Sarcoma cell migration and metastasis. Oncogene. 2017;36:4150–4160. doi: 10.1038/onc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun S, Yang F, Zhu Y, Zhang S. KDM4A promotes the growth of non-small cell lung cancer by mediating the expression of Myc via DLX5 through the Wnt/β-catenin signaling pathway. Life Sci. 2020;262:118508. doi: 10.1016/j.lfs.2020.118508. [DOI] [PubMed] [Google Scholar]
- 115.Li S, Wu L, Wang Q, Li Y, Wang X. KDM4B promotes epithelial-mesenchymal transition through up-regulation of ZEB1 in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai) 2015;47:997–1004. doi: 10.1093/abbs/gmv107. [DOI] [PubMed] [Google Scholar]
- 116.Shen Y, Wei W, Zhou DX. Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci. 2015;20:614–621. doi: 10.1016/j.tplants.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Miao X, Liu Y, Li F, Liu Q, Sun J, Cai L. Dysregulation of histone acetyltransferases and deacetylases in cardiovascular diseases. Oxid Med Cell Longev. 2014;2014:641979. doi: 10.1155/2014/641979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gujral P, Mahajan V, Lissaman AC, Ponnampalam AP. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod Biol Endocrinol. 2020;18:84. doi: 10.1186/s12958-020-00637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W, Zhan Z, Zhang M, Sun B, Shi Q, Luo F, Zhang M, Zhang W, Hou Y, Xiao X, et al. KAT6A, a novel regulator of β-catenin, promotes tumorigenicity and chemoresistance in ovarian cancer by acetylating COP1. Theranostics. 2021;11:6278–6292. doi: 10.7150/thno.57455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Santos GC, Jr, da Silva AP, Feldman L, Ventura GM, Vassetzky Y, de Moura Gallo CV. Epigenetic modifications, chromatin distribution and TP53 transcription in a model of breast cancer progression. J Cell Biochem. 2015;116:533–541. doi: 10.1002/jcb.25003. [DOI] [PubMed] [Google Scholar]
- 121.Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003;4:944–947. doi: 10.1038/sj.embor.embor941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol. 2010;10:454–460. doi: 10.1016/j.coph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 123.Liu J, Gu J, Feng Z, Yang Y, Zhu N, Lu W, Qi F. Both HDAC5 and HDAC6 are required for the proliferation and metastasis of melanoma cells. J Transl Med. 2016;14:7. doi: 10.1186/s12967-015-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong L, Dong Q, Chen Y, Li Y, Zhang B, Zhou F, Lyu X, Chen GG, Lai P, Kung HF, He ML. Novel HDAC5-interacting motifs of Tbx3 are essential for the suppression of E-cadherin expression and for the promotion of metastasis in hepatocellular carcinoma. Signal Transduct Target Ther. 2018;3:22. doi: 10.1038/s41392-018-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–371. e1–e5. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 126.Cheng C, Yang J, Li SW, Huang G, Li C, Min WP, Sang Y. HDAC4 promotes nasopharyngeal carcinoma progression and serves as a therapeutic target. Cell Death Dis. 2021;12:137. doi: 10.1038/s41419-021-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang X, Li G, Su F, Cai Y, Shi L, Meng Y, Liu Z, Sun J, Wang M, Qian M, et al. HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Res. 2020;48:2912–2923. doi: 10.1093/nar/gkaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu XJ, Guo XZ, Li C, Chong Y, Song TN, Pang JF, Shao M. SIRT1-ZEB1-positive feedback promotes epithelial-mesenchymal transition process and metastasis of osteosarcoma. J Cell Biochem. 2019;120:3727–3735. doi: 10.1002/jcb.27653. [DOI] [PubMed] [Google Scholar]
- 129.Bai L, Lin G, Sun L, Liu Y, Huang X, Cao C, Guo Y, Xie C. Upregulation of SIRT6 predicts poor prognosis and promotes metastasis of non-small cell lung cancer via the ERK1/2/MMP9 pathway. Oncotarget. 2016;7:40377–40386. doi: 10.18632/oncotarget.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kugel S, Sebastián C, Fitamant J, Ross KN, Saha SK, Jain E, Gladden A, Arora KS, Kato Y, Rivera MN, et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165:1401–1415. doi: 10.1016/j.cell.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li R, Quan Y, Xia W. SIRT3 inhibits prostate cancer metastasis through regulation of FOXO3A by suppressing Wnt/β-catenin pathway. Exp Cell Res. 2018;364:143–151. doi: 10.1016/j.yexcr.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 132.Fu L, Dong Q, He J, Wang X, Xing J, Wang E, Qiu X, Li Q. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36:2724–2736. doi: 10.1038/onc.2016.425. [DOI] [PubMed] [Google Scholar]
- 133.Tang X, Shi L, Xie N, Liu Z, Qian M, Meng F, Xu Q, Zhou M, Cao X, Zhu WG, Liu B. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. 2017;8:318. doi: 10.1038/s41467-017-00396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun Y, Sun Y, Yue S, Wang Y, Lu F. Histone deacetylase inhibitors in cancer therapy. Curr Top Med Chem. 2018;18:2420–2428. doi: 10.2174/1568026619666181210152115. [DOI] [PubMed] [Google Scholar]
- 135.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors-development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 136.Greenberg VL, Williams JM, Cogswell JP, Mendenhall M, Zimmer SG. Histone deacetylase inhibitors promote apoptosis and differential cell cycle arrest in anaplastic thyroid cancer cells. Thyroid. 2001;11:315–325. doi: 10.1089/10507250152039046. [DOI] [PubMed] [Google Scholar]
- 137.Nishida K, Komiyama T, Miyazawa S, Shen ZN, Furumatsu T, Doi H, Yoshida A, Yamana J, Yamamura M, Ninomiya Y, et al. Histone deacetylase inhibitor suppression of autoanti-body-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50:3365–3376. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- 138.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens BV, Castronovo V. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 139.Dikic I. Proteasomal and autophagic degradation systems. Ann Rev Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 140.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiq-uitin-proteasome system. J Biosci. 2006;31:137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 141.Ikeda F, Dikic I. Atypical ubiquitin chains: New molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mevissen TET, Komander D. Mechanisms of deubiquitinase specificity and regulation. Ann Rev Biochem. 2017;86:159–192. doi: 10.1146/annurev-biochem-061516-044916. [DOI] [PubMed] [Google Scholar]
- 143.Snyder NA, Silva GM. Deubiquitinating enzymes (DUBs): Regulation, homeostasis, and oxidative stress response. J Biol Chem. 2021;297:101077. doi: 10.1016/j.jbc.2021.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Wijk SJ, Fulda S, Dikic I, Heilemann M. Visualizing ubiquitination in mammalian cells. EMBO Rep. 2019;20:e46520. doi: 10.15252/embr.201846520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu H, Ju L, Xiong Y, Yu M, Zhou F, Qian K, Wang G, Xiao Y, Wang X. E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability. Cell Death Dis. 2021;12:239. doi: 10.1038/s41419-021-03521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu H, Yang X, Xuan X, Wu D, Zhang J, Xu X, Zhao Y, Ma C, Li D. STAMBP promotes lung adenocarcinoma metastasis by regulating the EGFR/MAPK signaling pathway. Neoplasia. 2021;23:607–623. doi: 10.1016/j.neo.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao C, Wu G, Zhou Z, Zhang X, Wang Y, Song G, Ding E, Sun X, Zhong L, Li S, et al. RBBP6, a RING finger-domain E3 ubiquitin ligase, induces epithelial-mesenchymal transition and promotes metastasis of colorectal cancer. Cell Death Dis. 2019;10:833. doi: 10.1038/s41419-019-2070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xue S, Wu W, Wang Z, Lu G, Sun J, Jin X, Xie L, Wang X, Tan C, Wang Z, et al. USP5 Promotes metastasis in non-small cell lung cancer by inducing epithelial-mesenchymal transition via Wnt/β-catenin pathway. Front Pharmacol. 2020;11:668. doi: 10.3389/fphar.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yuan T, Chen Z, Yan F, Qian M, Luo H, Ye S, Cao J, Ying M, Dai X, Gai R, et al. Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein. Mol Oncol. 2020;14:197–210. doi: 10.1002/1878-0261.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther. 2017;10:681–689. doi: 10.2147/OTT.S124795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun H, Ou B, Zhao S, Liu X, Song L, Liu X, Wang R, Peng Z. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine. 2019;48:236–247. doi: 10.1016/j.ebiom.2019.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang C, Xie C, Wang X, Huang Y, Gao S, Lu J, Lu Y, Zhang S. Aberrant USP11 expression regulates NF90 to promote proliferation and metastasis in hepatocellular carcinoma. Am J Cancer Res. 2020;10:1416–1428. [PMC free article] [PubMed] [Google Scholar]
- 153.Xie P, Chen Y, Zhang H, Zhou G, Chao Q, Wang J, Liu Y, Fang J, Xie J, Zhen J, et al. The deubiquitinase OTUD3 stabilizes ACTN4 to drive growth and metastasis of hepatocellular carcinoma. Aging. 2021;13:19317–19338. doi: 10.18632/aging.203293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu R, Liu Y, Zhou H, Li L, Li Y, Ding F, Cao X, Liu Z. Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 2018;418:125–134. doi: 10.1016/j.canlet.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 155.Chen D, Wang Y, Lu R, Jiang X, Chen X, Meng N, Chen M, Xie S, Yan GR. E3 ligase ZFP91 inhibits Hepatocellular Carcinoma Metabolism Reprogramming by regulating PKM splicing. Theranostics. 2020;10:8558–8572. doi: 10.7150/thno.44873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu L, Dong L, Li H, Liu Z, Luo Z, Duan G, Dai X, Lin Z. Ubiquitination-mediated degradation of SIRT1 by SMURF2 suppresses CRC cell proliferation and tumorigenesis. Oncogene. 2020;39:4450–4464. doi: 10.1038/s41388-020-1298-0. [DOI] [PubMed] [Google Scholar]
- 157.Shen T, Cai LD, Liu YH, Li S, Gan WJ, Li XM, Wang JR, Guo PD, Zhou Q, Lu XX, et al. Ube2v1-mediated ubiquitination and degradation of Sirt1 promotes metastasis of colorectal cancer by epigenetically suppressing autophagy. J Hematol Oncol. 2018;11:95. doi: 10.1186/s13045-018-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo W, You X, Xu D, Zhang Y, Wang Z, Man K, Wang Z, Chen Y. PAQR3 enhances Twist1 degradation to suppress epithelial-mesenchymal transition and metastasis of gastric cancer cells. Carcinogenesis. 2016;37:397–407. doi: 10.1093/carcin/bgw013. [DOI] [PubMed] [Google Scholar]
- 159.Ye P, Chi X, Cha JH, Luo S, Yang G, Yan X, Yang WH. Potential of E3 ubiquitin ligases in cancer immunity: Opportunities and challenges. Cells. 2021;10:3309. doi: 10.3390/cells10123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, Huang X, Xu GT, Zhao S, Yang J, Liu S. Deubiquitinases in cancer. Oncotarget. 2015;6:12872–12889. doi: 10.18632/oncotarget.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, McDermott JL, Leach CA, Fulcinniti M, Kodrasov MP, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–358. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reiner T, Parrondo R, de Las Pozas A, Palenzuela D, Perez-Stable C. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: Possible role for inhibition of deubiquitinase activity. PLoS One. 2013;8:e56234. doi: 10.1371/journal.pone.0056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Ann Rev Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eichler J. Protein glycosylation. Curr Bio. 2019;29:R229–R231. doi: 10.1016/j.cub.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 166.Mammadova-Bach E, Jaeken J, Gudermann T, Braun A. Platelets and defective N-glycosylation. Int J Mol Sci. 2020;21:5630. doi: 10.3390/ijms21165630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Clerc F, Reiding KR, Jansen BC, Kammeijer GS, Bondt A, Wuhrer M. Human plasma protein N-glycosylation. Glycoconj J. 2016;33:309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shajahan A, Supekar NT, Gleinich AS, Azadi P. Deducing the N-and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology. 2020;30:981–988. doi: 10.1093/glycob/cwaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Magalhães A, Duarte HO, Reis CA. The role of O-glycosylation in human disease. Mol Aspects Med. 20210;79:100964. doi: 10.1016/j.mam.2021.100964. [DOI] [PubMed] [Google Scholar]
- 170.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 171.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Läubli H, Borsig L. Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis. Front Immunol. 2019;10:2120. doi: 10.3389/fimmu.2019.02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oliveira-Ferrer L, Legler K, Milde-Langosch K. Role of protein glycosylation in cancer metastasis. Semin Cancer Biol. 2017;44:141–152. doi: 10.1016/j.semcancer.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 174.Sengupta PK, Bouchie MP, Nita-Lazar M, Yang HY, Kukuruzinska MA. Coordinate regulation of N-glycosylation gene DPAGT1, canonical Wnt signaling and E-cadherin adhesion. J Cell Sci. 2013;126:484–496. doi: 10.1242/jcs.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao H, Liang Y, Xu Z, Wang L, Zhou F, Li Z, Jin J, Yang Y, Fang Z, Hu Y, et al. N-glycosylation affects the adhesive function of E-Cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J Cell Biochem. 2008;104:162–175. doi: 10.1002/jcb.21608. [DOI] [PubMed] [Google Scholar]
- 176.Pinho SS, Reis CA, Paredes J, Magalhães AM, Ferreira AC, Figueiredo J, Xiaogang W, Carneiro F, Gärtner F, Seruca R. The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin. Hum Mol Genet. 2009;18:2599–2608. doi: 10.1093/hmg/ddp194. [DOI] [PubMed] [Google Scholar]
- 177.Xu Y, Chang R, Xu F, Gao Y, Yang F, Wang C, Xiao J, Su Z, Bi Y, Wang L, Zha X. N-Glycosylation at Asn 402 Stabilizes N-cadherin and promotes cell-cell adhesion of glioma cells. J Cell Biochem. 2017;118:1423–1431. doi: 10.1002/jcb.25801. [DOI] [PubMed] [Google Scholar]
- 178.Binder MJ, McCoombe S, Williams ED, McCulloch DR, Ward AC. The extracellular matrix in cancer progression: Role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett. 2017;385:55–64. doi: 10.1016/j.canlet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 179.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park JJ, Lee M. Increasing the α 2,6 sialylation of glyco-proteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver. 2013;7:629–641. doi: 10.5009/gnl.2013.7.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suzuki O, Abe M, Hashimoto Y. Sialylation by β-galactoside α-2,6-sialyltransferase and N-glycans regulate cell adhesion and invasion in human anaplastic large cell lymphoma. Int J Oncol. 2015;46:973–980. doi: 10.3892/ijo.2015.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cui J, Huang W, Wu B, Jin J, Jing L, Shi WP, Liu ZY, Yuan L, Luo D, Li L, et al. N-glycosylation by N-acetylglucosaminyltra nsferase V enhances the interaction of CD147/basigin with integrin β1 and promotes HCC metastasis. J Pathol. 2018;245:41–52. doi: 10.1002/path.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li JH, Huang W, Lin P, Wu B, Fu ZG, Shen HM, Jing L, Liu ZY, Zhou Y, Meng Y, et al. N-linked glycosylation at Asn152 on CD147 affects protein folding and stability: Promoting tumour metastasis in hepatocellular carcinoma. Sci Rep. 2016;6:35210. doi: 10.1038/srep35210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang K, Li W, Zhang Q, Yan G, Guo K, Zhang S, Liu Y. GP73 N-glycosylation at Asn144 reduces hepatocellular carcinoma cell motility and invasiveness. Oncotarget. 2016;7:23530–23541. doi: 10.18632/oncotarget.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin TC, Chen ST, Huang MC, Huang J, Hsu CL, Juan HF, Lin HH, Chen CH. GALNT6 expression enhances aggressive phenotypes of ovarian cancer cells by regulating EGFR activity. Oncotarget. 2017;8:42588–42601. doi: 10.18632/oncotarget.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu C, Li Z, Xu L, Shi Y, Zhang X, Shi S, Hou K, Fan Y, Li C, Wang X, et al. GALNT6 promotes breast cancer metastasis by increasing mucin-type O-glycosylation of α2M. Aging. 2020;12:11794–11811. doi: 10.18632/aging.103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu WT, Yeh CC, Liu SY, Huang MC, Lai IR. The O-glycosylating enzyme GALNT2 suppresses the malignancy of gastric adenocarcinoma by reducing EGFR activities. Am J Cancer Res. 2018;8:1739–1751. [PMC free article] [PubMed] [Google Scholar]
- 188.Kariya Y, Kanno M, Matsumoto-Morita K, Konno M, Yamaguchi Y, Hashimoto Y. Osteopontin O-glycosylation contributes to its phosphorylation and cell-adhesion properties. Biochem J. 2014;463:93–102. doi: 10.1042/BJ20140060. [DOI] [PubMed] [Google Scholar]
- 189.Ponath P, Menezes D, Pan C, Chen B, Oyasu M, Strachan D, LeBlanc H, Sun H, Wang XT, Rangan VS, et al. A novel, fully human Anti-fucosyl-GM1 antibody demonstrates potent in vitro and in vivo antitumor activity in preclinical models of small cell lung cancer. Clin Cancer Res. 2018;24:5178–5189. doi: 10.1158/1078-0432.CCR-18-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Festuccia C, Mancini A, Gravina GL, Colapietro A, Vetuschi A, Pompili S, Ventura L, Delle Monache S, Iorio R, Del Fattore A, et al. Dual CXCR4 and E-selectin inhibitor, GMI-1359, shows Anti-bone metastatic effects and synergizes with docetaxel in prostate cancer cell intraosseous growth. Cells. 2019;9:32. doi: 10.3390/cells9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Taracha A, Kotarba G, Wilanowski T. Methods of analysis of protein phosphorylation. Postepy Biochem. 2017;63:137–142. In Polish. [PubMed] [Google Scholar]
- 192.Tokuda M, Hatase O. Regulation of neuronal plasticity in the central nervous system by phosphorylation and dephosphorylation. Mol Neurobiol. 1998;17:137–156. doi: 10.1007/BF02802028. [DOI] [PubMed] [Google Scholar]
- 193.Csolle MP, Ooms LM, Papa A, Mitchell CA. PTEN and other PtdIns(3,4,5)P3 lipid phosphatases in breast cancer. Int J Mol Sci. 2020;21:9189. doi: 10.3390/ijms21239189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zeng J, Li X, Liang L, Duan H, Xie S, Wang C. Phosphorylation of CAP1 regulates lung cancer proliferation, migration, and invasion. J Cancer Res Clin Oncol. 2022;148:137–153. doi: 10.1007/s00432-021-03819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang K, Wu R, Mei F, Zhou Y, He L, Liu Y, Zhao X, You J, Liu B, Meng Q, Pei F. Phosphorylated LASS2 inhibits prostate carcinogenesis via negative regulation of Wnt/β-catenin signaling. J Cell Biochem. 2021 Apr 14; doi: 10.1002/jcb.29926. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 196.Li J, Enomoto A, Weng L, Sun L, Takahashi M. Dephosphorylation of Girdin by PP2A inhibits breast cancer metastasis. Biochem Biophys Res Commun. 2019;513:28–34. doi: 10.1016/j.bbrc.2019.03.167. [DOI] [PubMed] [Google Scholar]
- 197.Hainaut P, Plymoth A. Targeting the hallmarks of cancer: Towards a rational approach to next-generation cancer therapy. Curr Opin Oncol. 2013;25:50–51. doi: 10.1097/CCO.0b013e32835b651e. [DOI] [PubMed] [Google Scholar]
- 198.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, et al. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5:196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Druker BJ. Imatinib mesylate in the treatment of chronic myeloid leukaemia. Expert Opin Pharmacother. 2003;4:963–971. doi: 10.1517/14656566.4.6.963. [DOI] [PubMed] [Google Scholar]
- 200.Ulivi P, Chiadini E, Dazzi C, Dubini A, Costantini M, Medri L, Puccetti M, Capelli L, Calistri D, Verlicchi A, et al. Nonsquamous, non-small-cell lung cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: Frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17:384–390. doi: 10.1016/j.cllc.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 201.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 202.Murray BW, Miller N. Durability of Kinase-directed therapies-A Network perspective on response and resistance. Mol Cancer Ther. 2015;14:1975–1984. doi: 10.1158/1535-7163.MCT-15-0088. [DOI] [PubMed] [Google Scholar]
- 203.Bagert JD, Mitchener MM, Patriotis AL, Dul BE, Wojcik F, Nacev BA, Feng L, Allis CD, Muir TW. Oncohistone mutations enhance chromatin remodeling and alter cell fates. Nat Chem Biol. 2021;17:403–411. doi: 10.1038/s41589-021-00738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 205.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Ann Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 206.Becker PB, Workman JL. Nucleosome remodeling and epigenetics. Cold Spring Harbor Perspect Biol. 2013;5:a017905. doi: 10.1101/cshperspect.a017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–5382. doi: 10.1002/j.1460-2075.1996.tb00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morrison AJ, Shen X. Chromatin remodelling beyond transcription: The INO80 and SWR1 complexes. Nat Rev Mol Cell Biol. 2009;10:373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gévry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev. 2007;21:1869–1881. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wong MM, Cox LK, Chrivia JC. The chromatin remodeling protein, SRCAP, is critical for deposition of the histone variant H2A.Z at promoters. J Biol Chem. 2007;282:26132–26139. doi: 10.1074/jbc.M703418200. [DOI] [PubMed] [Google Scholar]
- 211.Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 212.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/MCB.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Günther K, Rust M, Leers J, Boettger T, Scharfe M, Jarek M, Bartkuhn M, Renkawitz R. Differential roles for MBD2 and MBD3 at methylated CpG islands, active promoters and binding to exon sequences. Nucleic Acids Res. 2013;41:3010–3021. doi: 10.1093/nar/gkt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Poot RA, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, Gilbert N, Ferreira J, Varga-Weisz PD. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat Cell Biol. 2004;6:1236–1244. doi: 10.1038/ncb1196. [DOI] [PubMed] [Google Scholar]
- 215.Cavellán E, Asp P, Percipalle P, Farrants AK. The WSTF-SNF2h chromatin remodeling complex interacts with several nuclear proteins in transcription. J Biol Chem. 2006;281:16264–16271. doi: 10.1074/jbc.M600233200. [DOI] [PubMed] [Google Scholar]
- 216.Centore RC, Sandoval GJ, Soares LMM, Kadoch C, Chan HM. Mammalian SWI/SNF chromatin remodeling complexes: Emerging mechanisms and therapeutic strategies. Trends Genet. 2020;36:936–950. doi: 10.1016/j.tig.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 217.Yang Y, Liu L, Li M, Cheng X, Fang M, Zeng Q, Xu Y. The chromatin remodeling protein BRG1 links ELOVL3 transactivation to prostate cancer metastasis. Biochim Biophys Acta Gene Regul Mech. 2019;1862:834–845. doi: 10.1016/j.bbagrm.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 218.Liao XH, Zhang Y, Dong WJ, Shao ZM, Li DQ. Chromatin remodeling protein MORC2 promotes breast cancer invasion and metastasis through a PRD domain-mediated interaction with CTNND1. Oncotarget. 2017;8:97941–97954. doi: 10.18632/oncotarget.18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang J, Yan HB, Zhang Q, Liu WY, Jiang YH, Peng G, Wu FZ, Liu X, Yang PY, Liu F. Enhancement of E-cadherin expression and processing and driving of cancer cell metastasis by ARID1A deficiency. Oncogene. 2021;40:5468–5481. doi: 10.1038/s41388-021-01930-2. [DOI] [PubMed] [Google Scholar]
- 220.Hombach S, Kretz M. Non-coding RNAs: Classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 221.Zhang P, Wu W, Chen Q, Chen M. Non-coding RNAs and their integrated networks. J Integr Bioinform. 2019;16:20190027. doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ro-Choi TS. Nuclear snRNA and nuclear function (discovery of 5'cap structures in RNA) Crit Rev Eukaryot Gene Expr. 1999;9:107–158. doi: 10.1615/CritRevEukarGeneExpr.v9.i2.20. [DOI] [PubMed] [Google Scholar]
- 224.Deryusheva S, Talross GJS, Gall JG. SnoRNA guide activities: Real and ambiguous. RNA. 2021;27:1363–1373. doi: 10.1261/rna.078916.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ali T, Grote P. Beyond the RNA-dependent function of lncRNA genes. Elife. 2020;9:e60583. doi: 10.7554/eLife.60583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220:e202009045. doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yang Q, Li F, He AT, Yang BB. Circular RNAs: Expression, localization, and therapeutic potentials. Mol Ther. 2021;29:1683–1702. doi: 10.1016/j.ymthe.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 229.Chen B, Li Q, Zhou Y, Wang X, Zhang Q, Wang Y, Zhuang H, Jiang X, Xiong W. The long coding RNA AFAP1-AS1 promotes tumor cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR-133a. Cell Cycle. 2018;17:1949–1966. doi: 10.1080/15384101.2018.1496741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Petri BJ, Klinge CM. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev. 2020;39:837–886. doi: 10.1007/s10555-020-09905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: Therapeutic approaches based on metastasis-related miRNAs. J Mol Med. 2011;89:445–457. doi: 10.1007/s00109-010-0716-0. [DOI] [PubMed] [Google Scholar]
- 232.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am So Nephrol. 2010;21:438–447. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23:205–217. doi: 10.3727/096504016X14549667334007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 237.Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manage Res. 2018;10:6685–6693. doi: 10.2147/CMAR.S179189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, Zou C, Dong Y, Du J, Long X, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124. doi: 10.1186/s12943-017-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li Y, Egranov SD, Yang L, Lin C. Molecular mechanisms of long noncoding RNAs-mediated cancer metastasis. Genes Chromosomes Cancer. 2019;58:200–207. doi: 10.1002/gcc.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fang C, Wang L, Gong C, Wu W, Yao C, Zhu S. Long non-coding RNAs: How to regulate the metastasis of non-small-cell lung cancer. J Cell Mol Med. 2020;24:3282–3291. doi: 10.1111/jcmm.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Roe JS, Hwang CI, Somerville TDD, Milazzo JP, Lee EJ, Da Silva B, Maiorino L, Tiriac H, Young CM, Miyabayashi K, et al. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell. 2017;170:875–888.e20. doi: 10.1016/j.cell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information provided in this review is documented by relevant references. All references are open-ended articles.