Abstract
Objectives
To describe the technique of robot-assisted high-extended salvage retroperitoneal and pelvic lymphadenectomy (sRPLND+PLND) for ‘node-only’ recurrent prostate cancer.
Patients and Methods
In all, 10 patients underwent robot-assisted sRPLND+PLND (09/2015–03/2016) for ‘node-only’ recurrent prostate cancer, as identified by 11C-acetate positron emission tomography/computed tomography imaging. Our anatomical template extends from bilateral renal artery/vein cranially up to Cloquet’s node caudally, completely excising lymphatic-fatty tissue from aorto-caval and iliac vascular trees; RPLND precedes PLND. Meticulous node-mapping assessed nodes at four prospectively assigned anatomical zones.
Results
The median operative time was 4.8 h, estimated blood loss 100 mL and hospital stay 1 day. No patient had an intraoperative complication, open conversion or blood transfusion. Three patients had spontaneously resolving Clavien–Dindo grade II postoperative complications. The mean (range) number of nodes excised per patient was 83 (41–132) and mean (range) number of positive nodes per patient was 23 (0–109). Seven patients (70%) had positive nodes on final pathology. Node-positive rates per anatomical level I, II, III and IV were 28%, 32%, 33% and 33%, respectively. In patients with positive nodes, the median PSA level had decreased by 83% at the 2-month follow-up.
Conclusion
The initial series of robot-assisted sRPLND+PLND is presented, wherein we duplicate open surgery with superior nodal counts and decreased morbidity. Robot-assisted technical details for an anatomical LND template up to the renal vessels are presented. Longer follow-up is necessary to assess oncological outcomes.
Keywords: salvage RPLND, salvage PLND, robot-assisted surgery, #ProstateCancer, #PCSM
Introduction
Salvage lymphadenectomy (LND) has been proposed in patients with ‘node-only’ driven biochemical recurrence after definitive treatment of primary prostate cancer. In these carefully selected patients, if whole-body imaging indicates nodal-only involvement, without evidence of prostatic fossa, bony or systemic recurrence, salvage retroperitoneal and pelvic LND (sRPLND+PLND) may deliver biochemical response. This procedure has been typically performed using an open approach through a large midline incision, with its attendant morbidity. In the present study, we describe the detailed robot-assisted technique and present, to our knowledge, the initial series of 10 consecutive patients undergoing robot-assisted sRPLND+PLND for ‘node-only’ recurrent prostate cancer.
Patients and Methods
Data were prospectively collected in our Institutional Review Board-approved database. All patients presented with biochemical relapse after primary definitive treatment of prostate cancer at median of 4.3 years prior (Table 1). Clinical evaluation included biochemical testing, CT and/or MRI (chest/abdomen/pelvis) and sodium fluoride radionuclide bone scan, all of which were negative. All patients underwent dedicated 11C-acetate positron emission tomography (PET)/CT imaging, which identified node-only metastases (Fig. 1). Patients with local recurrence (prostate, prostatic fossa or seminal vesicles), or bony or visceral metastases were excluded. Risks, complications, alternatives, potential for open conversion, and the current status of data for sRPLND+PLND for node-metastatic prostate cancer were extensively discussed. Following informed consent, all patients wished to proceed.
Table 1.
Baseline demographics.
| Variable | Value |
|---|---|
|
| |
| Number of patients | 10 |
| Median (range) | |
| Age, years | 65 (55–76) |
| BMI, kg/m2 | 26.4 (22.8–30.5) |
| ASA Classification | 2 (2–3) |
| PSA at initial prostate cancer diagnosis, ng/mL | 6.95 (2.1–84.5) |
| PSA level at sRPLND, ng/mL* | |
| All patients (n = 10) | 2.78 (0.28–15.5) |
| Patients with positive nodes (n = 7) | 0.80 (0.28–15.5) |
| Patients with negative nodes (n = 3) | 1.80 (0.33–3.50) |
| n/N | |
| Primary Gleason score | |
| 7 (3 + 4/4 + 3) | 4/10 |
| 8 (4 + 4) | 1/10 |
| 9 (4 + 5/5 + 4) | 5/10 |
| TNM Stage | |
| T2cN0M0 | 6/10 |
| T3aN0M0 | 4/10 |
| Prior primary treatment | |
| Robotic RP | 7/10 |
| Open RP | 1/10 |
| Radiotherapy | 2/10 |
| Prior PLND (at initial RP), n/N | 8/10 |
| Median (range) | |
| Number of nodes | 7 (4–21) |
| Time from primary treatment, months | 52 (6–160) |
| n/N | |
| Prior adjuvant therapy | |
| Radiotherapy | 4/10 |
| Brachytherapy | 1/10 |
| Prior ADT | 5/10 |
| Positive PET/CT | 10/10 |
| Level I | 9/10 |
| Level II | 9/10 |
| Level III | 5/10 |
| Level IV | 0 |
BMI, body mass index; ASA, American Society of Anesthesiologists; ADT, androgen deprivation therapy;
Biochemical recurrence after primary treatment was defined as a PSA level of >0.2 ng/mL after RP, or Phoenix criteria or American Society for Therapeutic Radiology and Oncology (ASTRO) definition after RT.
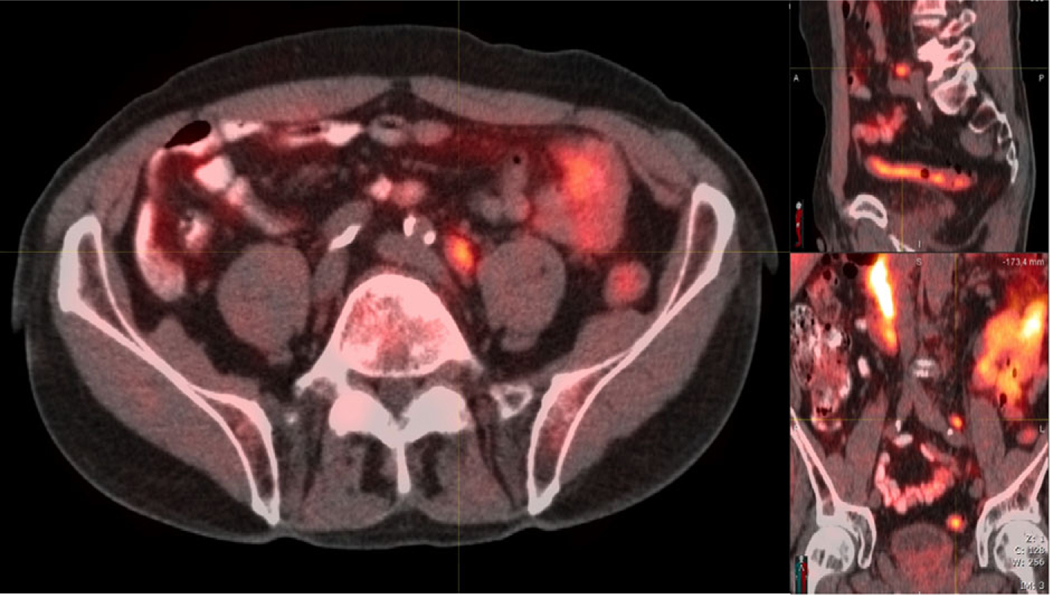
Fig. 1.
11C-acetate PET/CT imaging. PET/CT showing a 1.3-cm 11C-acetate avid left common iliac lymph node. 11C-acetate PET/CT imaging was performed on an integrated PET/CT scanner (Siemens Biograph 16; Malvern, PA, USA). Patients were positioned on the camera and then 740–1 480 MBq 11C-acetate (half-life 20.3 min) was administered as a bolus i.v. injection. A CT topogram was obtained from the vertex through the pelvis. Based on the topogram, the tube current for the CT scan was adjusted using a Care doseTM application to minimise exposure. The tube voltage was 130 kVp (peak kilovoltage). After the CT scan, emission images beginning at the pelvis and proceeding cranially were obtained (3–7 min after injection, mean 4.25 min). Images were reconstructed with iterative reconstruction (two iterations, eight subsets, matrix 168, Gaussian filter). The administration of 11C-acetate was well tolerated by all patients and there were no adverse events. All images were interpreted by nuclear medicine physicians with >20 years of PET imaging experience.
Robot-assisted Technique
Under general anaesthesia, bilateral 5-F open-ended ureteric catheters are inserted cystoscopically to facilitate intraoperative robotic identification of the ureters in patients with prior pelvic/retroperitoneal adhesions. Veress needle 15-mmHg CO2 pneumoperitoneum is established and six ports are placed (Fig. 2). With the patient in steep Trendelenburg position, the robot (da Vinci Si® or Xi Surgical System®, Intuitive Surgical, Sunnyvale, CA, USA) is docked. The posterior parietal peritoneum is incised from the caecum obliquely towards the duodeno-jejunal junction thereby gaining entry into the retroperitoneal space; the resultant twin edges of the posterior parietal peritoneum are retracted cephalad by judiciously located, percutaneously inserted retraction sutures. The third part of the duodenum is padded and retracted cephalad with the fourth arm. Our sRPLND+PLND template extends from the renal vein/artery cephalad to the Cloquet’s node caudally and both ureters laterally.
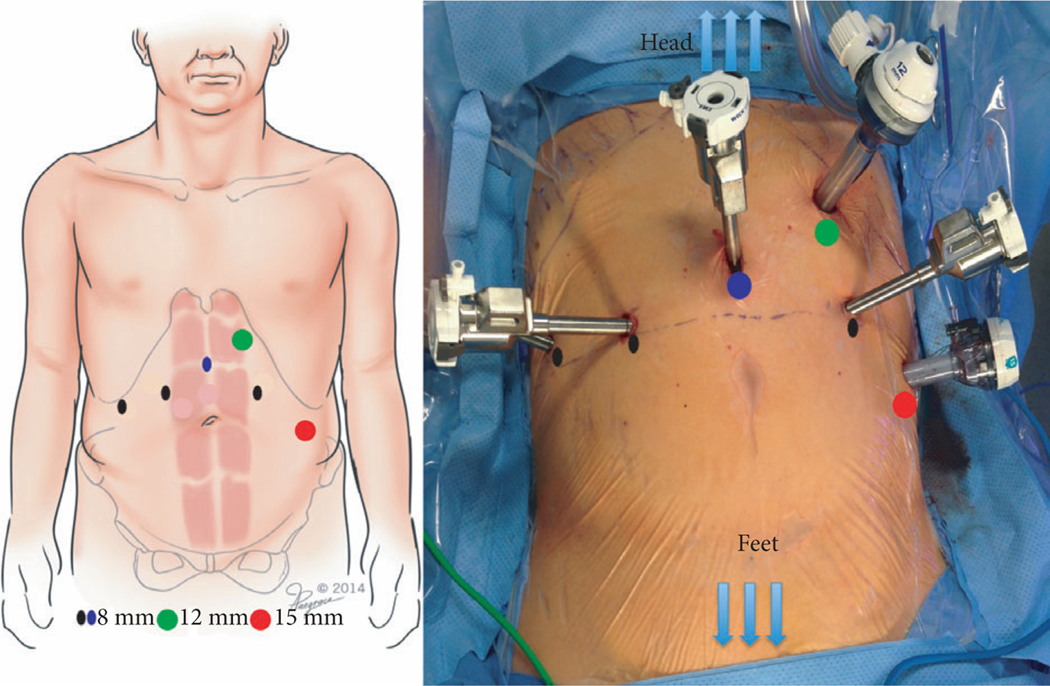
Fig. 2.
Port placement. A six-port transperitoneal approach is used: the camera port (blue circle) is placed 6–8 cm above the umbilicus, the robotic ports (black circles) are placed at the level of the 12th rib, and a 15-mm assistant port (red circle) is placed at the umbilicus level. A 12-mm AirSeal® port (green circle) is placed proximal and lateral to the camera port.
sRPLND is initiated using a ‘split-and-roll’ technique anterior to the inferior vena cava (IVC), immediately adjacent to the third part of the duodenum. sRPLND sequentially clears the right para-caval, pre-caval, inter-aorto-caval, pre-aortic, and left para-aortic regions at the level of the renal hilum. The lower edge of the right renal artery and the left renal vein and are skeletonised, establishing the cranial boundary of the dissection. The lumbar veins, and proximal lymphatic vessels are clipped and ligated. The lumbar arteries are mostly preserved.
The IVC and aorta are vessel-looped and elevated anteriorly to excise retro-caval and retro-aortic tissues, skeletonising the anterior spinous ligament. Subsequently, pre-aortic, inter-aorto-caval and left peri-aortic tissue is dissected caudally towards the inferior mesenteric artery (IMA), which is preserved; infra-IMA dissection is performed towards the aortic bifurcation. At this level of RPLND the lower edge of the renal hilum bilaterally, and the para-caval, pre-caval, retro-caval, inter-aorto-caval, pre-aortic, retro-aortic and para-aortic lymph nodes proximal and distal to IMA are completely dissected.
Extended salvage PLND starts from proximal to distal, beginning with the right side first. Bilateral ureteric mobilisation is performed. The aortic bifurcation, pre-sacral, para-rectal, common iliac, external and internal iliac, pre-sciatic fossa of Marseilles and obturator fossa, including the area posterior to the obturator nerve and the node of Cloquet are dissected (Fig. 3). Distal lymphatic vessels are clip-ligated bilaterally. Each anatomical nodal package is sent for individual pathological evaluation to allow node-mapping (Fig. 4).
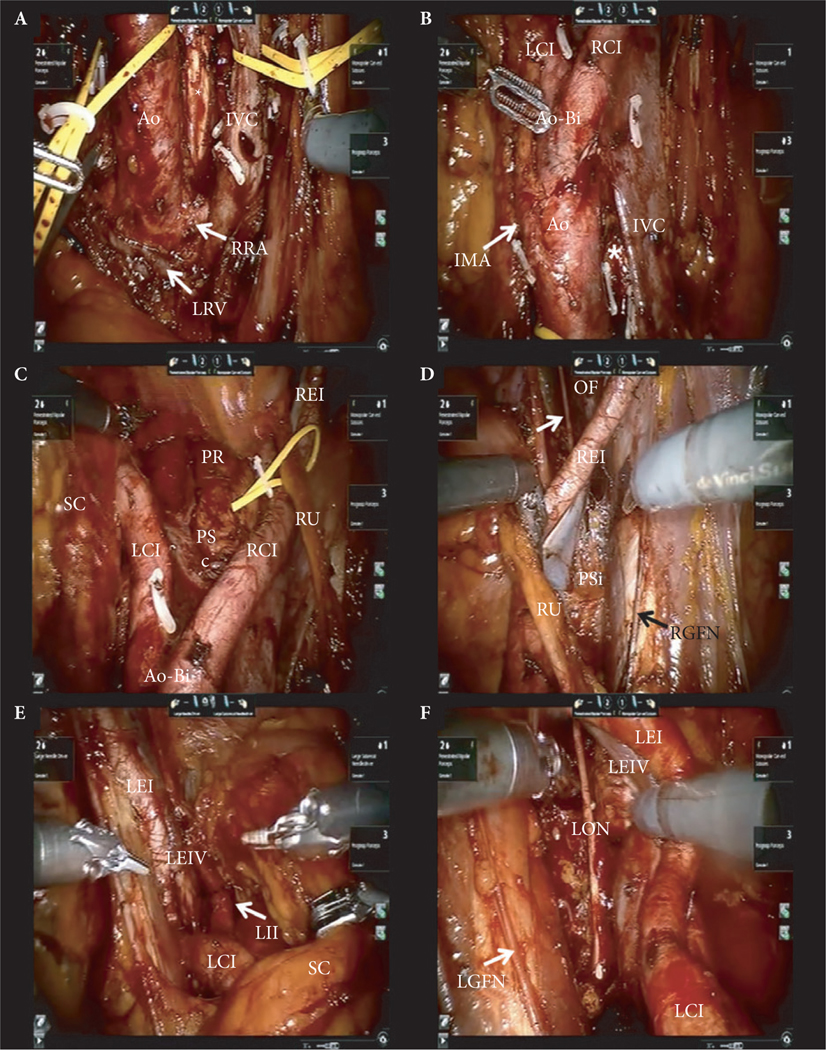
Fig. 3.
Intraoperative pictures of sRPLND/PLND. (A) Level IV – RPLND: The lower edge of the right renal artery (RRA) and left renal vein (LRV) are skeletonised, establishing the cranial boundary of the dissection. The IVC and aorta (Ao) are vessel-looped and elevated anteriorly to excise retro-caval and retro-aortic tissues, exposing the anterior spinous ligament (*) at the inter-aorto-caval area. (B) Level III – RPLND: The IMA is skeletonised; infra-IMA dissection is performed towards the Ao bifurcation (Ao-Bi) and the proximal parts of the right (RCI) and left (LCI) common iliac arteries are dissected. (C) Level II – RPLND/PLND: Bilateral extended PLND comprises pre-sacral (PSc), para-rectal (PR), and the RCI and LCI arteries. The right ureter (RU) is vessel-looped, the sigmoid colon (SC) is retracted laterally. REI, right external iliac artery. (D) Level I – right PLND: The REI and vein are skeletonised and retracted medially with the RU to expose the pre-sciatic (PSi) area. The obturator fossa (OF) is completely dissected exposing the obturator nerve (arrow). The right genito-femoral nerve (RGFN) is the lateral limit of dissection. (E) Level I – left PLND: With the SC and the left ureter retracted medially, the distal part of the LCI and the proximal part of the left external iliac artery (LEI) and vein (LEIV), and the left internal iliac artery (LII) are skeletonised. (F) Level I – left PLND: The left LEI and LEIV are skeletonised and retracted medially allowing dissection of the left pre-sciatic (‘fossa of Marseilles’) area and OF, exposing the left obturator nerve (LON).
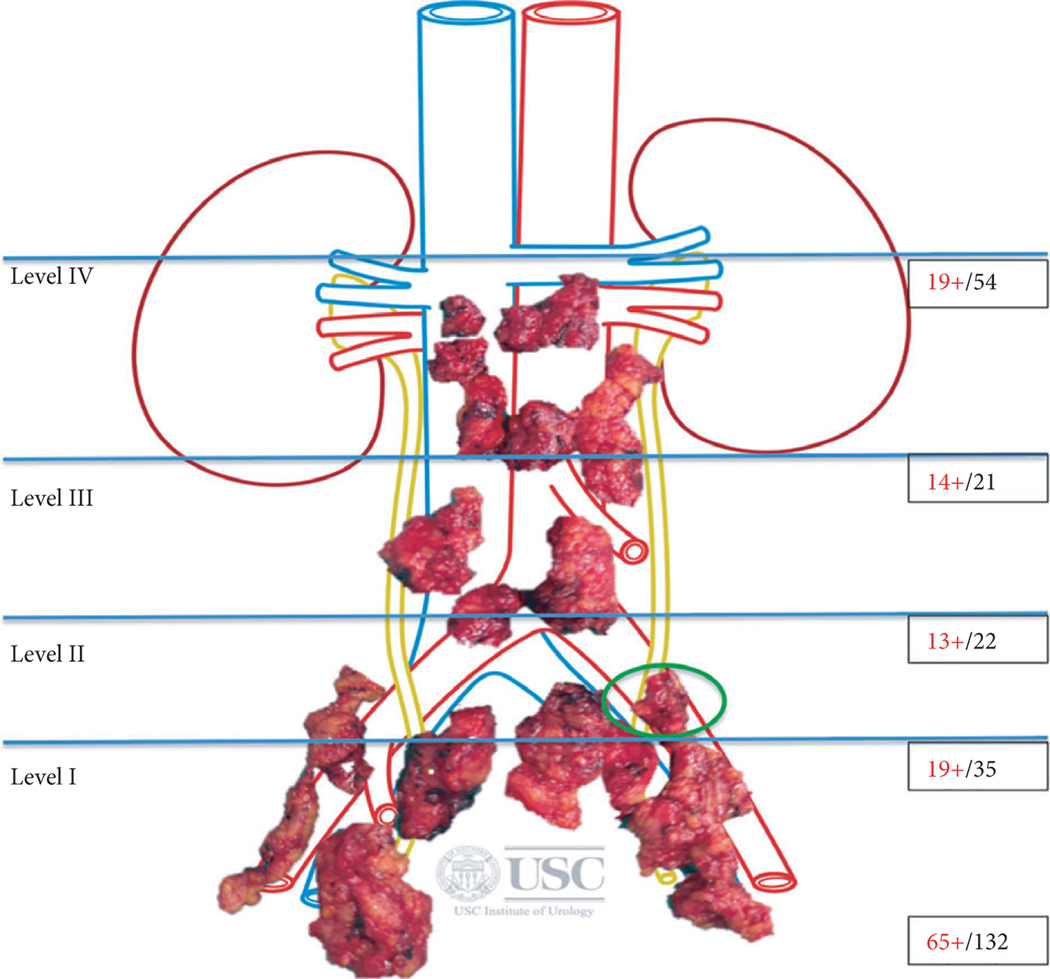
Fig. 4.
sRPLND/PLND template. We categorised the sRPLND/PLND template into four anatomical levels, as follows: Level I: Cephalad extent: internal iliac artery. Caudal limit: lymph node of Cloquet. Lateral limit: genito-femoral nerve. Posterior limit: Pre-rectal nodes and nodes posterior to the obturator nerve. Level I template includes nodes in the regions of the internal and external iliac vessels, obturator fossa, pre-sciatic fossa of Marseilles and pre-rectal nodes. Level II: Cephalad extent: aortic bifurcation. This region includes nodes in the regions of the common iliac vein and artery, upper pre-sciatic nodes, pre-sacral nodes and nodes in the region of the aortic bifurcation. Level III: Cephalad extent: inferior mesenteric artery (IMA). This region includes the distal para-caval, pre-caval, retro-caval, inter-aorto-caval, pre-aortic, retro-aortic and para-aortic nodes up to and including the IMA. Lateral limit: bilateral ureters. Level IV: Cephalad extent: left renal vein and the right renal artery along the undersurface of the third part of duodenum. This region includes nodes in the proximal para-caval, pre-caval, retro-caval, inter-aorto-caval, pre-aortic, retro-aortic and para-aortic tissue cephalad to the IMA. Lateral limit: bilateral ureters. This figure shows node-mapping from the patient shown in Fig. 1. The patient was a 76-year-old man with Gleason 8 (4 + 4) prostate cancer treated primarily with RT. His PSA level before sRPLND+PLND was 7.58 ng/mL. Of 132 nodes extracted, 65 were positive. At the 2-month postoperative follow-up his PSA level was 0.4 ng/mL. Green circle represents the positive node on preoperative PET-CT that was confirmed as node metastasis on final pathology.
Haemostasis is confirmed and haemostatic agents applied as needed. A 19-F Blake drain is inserted and robotic exit completed. Cefazolin and prophylactic heparin are routinely given. The Foley catheter and the ureteric stents are removed on postoperative day 1, before patient discharge.
Results
The median operative time was 4.8 h, estimated blood loss 100 mL and hospital stay 1 day. No patient had an intraoperative complication, open conversion or blood transfusion. Clavien–Dindo complications (Grade II) occurred in three of the 10 patients, all managed conservatively. No patient developed leg/pedal lymphoedema (Table 2).
Table 2.
Robotic sRPLND/PLND perioperative outcomes.
| Perioperative outcome | Value |
|---|---|
|
| |
| Median (range) | |
| Operative time, h | 4.8 (3.5—5.8) |
| Estimated blood loss, mL | 100 (50–250) |
| n/N | |
| Open conversion | 0/10 |
| Blood transfusion | 0/10 |
| Intra-operative complication | 0/10 |
| Jackson-Pratt drain placed | 5/10 |
| Median (range) | |
| Jackson-Pratt drain duration, days | 1.6 (0–6) |
| Hospital Stay, days | 1 (0–2) |
| n/N | |
| Complications | |
| None | 7/10 |
| Clavien-Dindo grade | |
| II | Flank/scrotal ecchymosis, 1/10 |
| II | Chylous ascites, 1/10 |
| II | Neuropraxia/Foot drop, 1/10 |
On histopathology, the mean number of total and positive nodes per patient were 83 and 23, respectively; overall, in our 10 patients, of the 829 total excised nodes, 31% were positive (Table 3). Seven of the 10 patients had positive nodes on final pathology; of the four patients with positive nodes cephalad to the IMA (level IV), two had histological evidence of extra-nodal extension. Pathology confirmed skip nodal lesions in two patients, one with isolated positive nodes only at level II and the second with positive nodes at levels I, II and IV, skipping level III.
Table 3.
Pathological outcomes and follow-up.
| Variable | Value |
|---|---|
|
| |
| Mean; median (range) | |
| Nodes excised/patient, n | 83; 83 (41–132) |
| Positive nodes/patient, n | 23; 8 (0–109) |
| n/N (%) | |
| Node-positivity rate per patient | |
| Overall | 7/10 (70) |
| Level I | 6/10 (60) |
| Level II | 7/10 (70) |
| Level III | 5/10 (50) |
| Level IV | 4/10 (40) |
| Node positivity rate per level | |
| Overall | 261/829 (32) |
| Level I | 62/221 (28) |
| Level II | 53/167 (32) |
| Level III | 73/223 (33) |
| Level IV | 73/218 (33) |
| Positive nodes per level per patient, mean; median (range) | |
| Level I | 4; 0 (0–18) |
| Level II | 7; 5 (0–21) |
| Level III | 6; 1 (0–39) |
| Level IV | 7; 0 (0–49) |
| Follow-up, days median, (range) | 154 (121–304) |
| Postoperative PSA level, ng/mL, median (range) | 0.80 (0.4–2) |
| % decrease in PSA level, median (range) | 83 (0–95) |
| Adjuvant ADT following sRPLND, n/N | 2/10 |
ADT, androgen deprivation therapy.
Overall, the median (range) PSA level before sRPLND+PLND was 2.78 (0.28–15.5) ng/mL. At 2 months postoperatively, the median (range) PSA level was 0.80 (0.4–2) ng/mL. This reflects an overall median PSA level decrease of 83%. PSA levels decreased by 84% in patients with histologically positive nodes and increased by 47% in patients with histologically negative nodes (P = 0.19). In no patient did the PSA level after sRPLND+PLND reach zero.
Discussion
The management of patients with recurrent prostate cancer is challenging. Despite primary treatment of prostate cancer with surgery or radiotherapy (RT), 20–40% of patients relapse within 5 years and 25–35% progress to metastatic disease [1]. In a select subset of patients with prostate cancer metastases, only the abdominal lymph nodes maybe involved as identified by functional imaging. In this select group, salvage LND has been proposed to surgically manage biochemical recurrence associated with ‘node-only’ disease. Some studies indicated decreased progression and improved 5-year recurrence-free survival in this select patient population; however, the optimal LND template remains a matter of debate. [2,3]. Extended, template-based sRPLND+PLND provides more accurate staging than salvage LND targeted only to the PET/CT positive nodes, due to the low accuracy of imaging studies for detecting low-volume nodal metastasis, especially in patients with low PSA levels [4].
Salvage LND has been performed in over 500 patients reported in 10 publications to date (Table 4). At 8 years, clinical recurrence-free survival and cancer-specific mortality-free survival were 38% and 81%, respectively [5]. Only two series have evaluated laparoscopic/robot-assisted surgery, with both performing only a limited PLND. Schilling et al. [6] performed laparoscopic PLND targeted to PET/CT suspicious nodes. The median number of total and positive nodes per patient was six and three, respectively; the mean hospital stay was 5.3 days. Claeys et al. [1] reported six robot-assisted and seven laparoscopic salvage PLNDs, wherein cephalad limit of the template was the common iliac artery at the level of ureteric crossing. The median number of total and positive nodes per patient was 11 and one, respectively. Surgical complications after salvage LND are usually more common than after primary LND, typically comprising lymphorrhoea, lymphocoele requiring drainage, and ileus [7,8]. A recent meta-analysis reported Clavien–Dindo complication rates of: Grade I, 0.8 –16.5%; Grade II, 11%; Grade IIIa, 8.6%; and Grade IIIb, 0.8% [9]. In the present study, our complication rates were comparable with other studies. Only one patient was re-admitted with bilateral flank and scrotal ecchymosis, managed conservatively and discharged after 2 days.
Table 4.
Salvage LND: literature review of series with ≥10 patients.
| Reference | N | Surgery, n (%) | Patients with positive nodes, n/N or n (%) | Mean (range) nodes, n | Mean (range) positive nodes, n | Positive node rate, % | PET/CT, n/N or % | Gleason score, n/N or n (%) | Primary treatment, n/N or n (%) | PSA level at LND, ng/mL | Cephalad limit | Clavien–Dindo complications, n/N or % | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Schilling et al., 2008 [6] | 10 | LAP | 7/10 | 7.1 (1–22) | 2.8 (0–8) | 39.4 | [11C]Choline | ≤7, 8/10 ≥8, 2/10 |
RP, 8/10 RT, 2/10 |
10.9 | NR | NA | 11 |
| Rinnab et al., 2008 [10] | 15 | Open | 8/15 | 13.9 (3–45) | NR | NR | [11C]Choline | ≤7, 11/15 ≥8, 4/15 |
RP, 15/15 | 2.0 | Common iliac (extended if needed) | NA | |
| Rigatti et al., 2011 [7] | 72 | Open | 60 (83.3) | 30.6 (4–87) | 9.08 (0–66) | 29.7 | [11C]Choline | ≤7, 45 (62.4) ≥8, 27 (37.6) |
RP, 72 (100) | 1.5 | Renal artery | Grade I, 20.8–25 Grade II, 1.4–19.4 Grade IIIa, 4.1–13.8 Grade IIIb, 1.4–2.7 |
39.4 |
| Jilg et al., 2012 [4] | 52 | Open | 47 (90.4) | 23.3 (1–57) | 9.74 (0–54) | 41.8 | [11C]Choline, 50 [18F]Choline, 50 |
≤7, 36 (69) ≥8, 16 (31) |
RP, 39 (83) RT, 8 (17) |
11.1 | Renal vein | Grade I, 1.9–7.7 Grade II, 5.8 Grade IIIa, 3.9 |
40.1 |
| Suardi et al., 2015 [5] | 59 | Open | 47 (79.7) | 29.5 (2–87) | 8.9 (0–66) | 30.2 | [11C]Choline | ≤7, 38 (64.4) ≥8, 21 (35.6) |
RP, 59 (100) | 2.0 | Inter-aorto-caval | Grade I, 20.3–30.5 Grade II, 1.7–20.3 Grade IIIa, 5.1–11.2 Grade IIIb, 1.7 |
81.1 |
| Tilki et al., 2015 [2] | 58 | Open | 45 (77.6) | 18.6 (1–88) | 6.3 | 33.9 | [11C]Choline | ≤7. 30 (52) ≥8, 28 (48) |
RP, 58 (100) | 9.8 | IMA | NR | 39 |
| Jilg et al., 2014 [11] | 72 | Open | 72 (100) | 29.5 (2–111) | 9.5 (0–81) | 32.2 | [11C]Choline, 37.5 [18F]Choline, 62.5 |
≤7, 34 (47) ≥8, 32 (48) |
RP, 63 (87.5) RT, 9 (12.5) |
10.6 | CavalInter-aorto-caval aortic | NR | 32 |
| Winter et al., 2015 [12] | 13 | Open | 11/13 | NR | 1 (0–3) | NR | [11C]Choline, 10/13 [18F]Choline, 3/13 |
≤7, 8/13 ≥8, 3/13 NA, 2/13 |
RP, 13/13 | 2.7 | NR | Grade IIIb, 1/13 | 31 |
| Karnes et al., 2015 [8] | 52 | Open | 52 (100) | 23.8 (16–30) | 5.3 (1–31) | 22.3 | [11C]Choline | ≤7, 30 (57.7) ≥8, 19 (36.5) NA, 3 (5.8) |
RP, 52 (100) | 4.0 | NR | Grade II, 1.9 Grade IIIa, 1.9 Grade IIIb, 1.9 |
20.9 |
| Claeys et al., 2014 [1] | 17 | Open, 4 (24) LAP, 7 (41) RA, 6 (35) |
15 (88.2) | 11.8 (1–21) | 2.3 (0–6) | 19.5 | [11C]Choline, 41 [18F]Choline, 35 No PET/CT, 24 |
≤7, 9 (53) ≥8, 8 (47) |
RP, 14 (82) RT, 8 (17) HIFU, 1 (6) |
2.0 | Common iliac | Grade I, 35 Grade II, 6 Grade IIIa, 6–12 |
22 |
| Present study 2016 | 10 | RA | 7/10 | 83 (41–132) | 26 (0–109) | 32 | [11C]Acetate | ≤7, 4/10 ≥8, 6/10 |
RP, 8/10 RT, 2/10 |
2.8 | Renal vein | Grade II, 3/10 | 2 |
LAP, laparoscopic; RA, robot-assisted; NR, not reported; HIFU, high-intensity focused ultrasound.
To our knowledge, this is the initial report of robot-assisted sRPLND+PLND for ‘node-only’ recurrent prostate cancer. Our mean (range) number of total and positive nodes per patient was 83 (41–132) and 23 (0–109), respectively. In our experience, 11C-acetate imaging accurately identified patients with nodal metastases, although it under-estimated the burden of nodal involvement. Although all our present 10 patients had positive 11C-acetate imaging, upon sRPLND only seven had positive nodes on final pathology. The three patients with negative nodes on final pathology underwent repeat (after sRPLND) 11C-acetate imaging, which identified a suspicious residual node in the left obturator fossa in two patients; intraoperatively, both patients had densely scarred obturator fossae due to prior PLND at initial prostatectomy, precluding thorough salvage obturator fossa dissection without transecting the obturator nerve. The third patient with negative nodes on final pathology had no evidence of residual disease on repeat 11C-acetate imaging.
In patients who have undergone primary RT for prostate cancer and subsequently failed with ‘node-only’ recurrence, interrogation of the in situ prostate with MRI and biopsy is warranted before considering sRPLND+PLND. Even when sRPLND reveals negative retroperitoneal nodes, these data can help confidently eliminate consideration of whole abdominal RT as adjuvant treatment for biochemical failure.
We present the detailed technique of robot-assisted sRPLND+PLD, which not only completely duplicates open surgery, but potentially extends it. Hence, in the reported open surgery series, the median total 14–29.5 and positive 1–9 nodal counts were significantly lower than our present study, at a mean (range) total number of nodes of 83 (41–132) and positive nodes 23 (0–109), attesting to the thoroughness of the robot-assisted dissection (Table 4). Despite such extensive surgery, no major intraoperative bowel or vascular injuries occurred, the mean hospital stay was 1 day and complications were low. However, we caution that this is a challenging procedure that should be reserved for tertiary centres with considerable robotic expertise. To address long-term oncological outcomes, and whether this concept can potentially alter the natural history of node-recurrent prostate cancer, a larger cohort with longer follow-up is necessary.
Conclusion
In the present study, we describe the detailed technique of robot-assisted high-extended sRPLND+PLND for ‘node-only’ recurrent prostate cancer and present the initial experience. Robot-assisted sRPLND+PLND duplicates open surgery, with superior nodal counts and decreased morbidity. 11C-acetate PET/CT imaging identifies patients appropriate for sRPLND+PLND, but may underestimate the extent of nodal involvement. As such, a standard anatomical LND template extending cranially to the renal vessels is recommended. Longer follow-up is necessary to assess durability of PSA response and oncological outcomes.
Supplementary Material
Video S1. Robotic salvage RPLND/PLND for ‘node-only’recurrent prostate cancer: Technique.
Abbreviations:
- (P)LND
(pelvic) lymph node dissection/lymphadenectomy
- IMA
inferior mesenteric artery
- IVC
inferior vena cava
- PET
positron emission tomography
- RP
radical prostatectomy
- RT
radiotherapy
- sRPNLD
salvage retroperitoneal PLND
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Conflicts of Interest
None declared.
References
- 1.Claeys T, Van Praet C, Lumen N et al. Salvage pelvic lymph node dissection in recurrent prostate cancer: surgical and early oncological outcome. Biomed Res Int 2015; 2015: 198543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilki D, Mandel P, Seeliger F et al. Salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy. J Urol 2015; 193: 484–90 [DOI] [PubMed] [Google Scholar]
- 3.Abdollah F, Briganti A, Montorsi F et al. Contemporary role of salvage lymphadenectomy in patients with recurrence following radical prostatectomy. Eur Urol 2015; 67: 839–49 [DOI] [PubMed] [Google Scholar]
- 4.Jilg CA, Rischke HC, Reske SN et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol 2012; 188: 2190–7 [DOI] [PubMed] [Google Scholar]
- 5.Suardi N, Gandaglia G, Gallina A et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol 2015; 67: 299–309 [DOI] [PubMed] [Google Scholar]
- 6.Schilling D, Schlemmer HP, Wagner PH et al. Histological verification of 11C-choline-positron emission/computed tomography-positive lymph nodes in patients with biochemical failure after treatment for localized prostate cancer. BJU Int 2008; 102: 446–51 [DOI] [PubMed] [Google Scholar]
- 7.Rigatti P, Suardi N, Briganti A et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol 2011; 60: 935–43 [DOI] [PubMed] [Google Scholar]
- 8.Karnes RJ, Murphy CR, Bergstralh EJ et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol 2015; 193: 111–6 [DOI] [PubMed] [Google Scholar]
- 9.Ploussard G, Almeras C, Briganti A et al. Management of node only recurrence after primary local treatment for prostate cancer: a systematic review of the literature. J Urol 2015; 194: 983–8 [DOI] [PubMed] [Google Scholar]
- 10.Rinnab L, Mottaghy FM, Simon J et al. [11C]Choline PET/CT for targeted salvage lymph node dissection in patients with biochemical recurrence after primary curative therapy for prostate cancer: preliminary results of a prospective study. Urol Int 2008; 81: 191–7 [DOI] [PubMed] [Google Scholar]
- 11.Jilg CA, Schultze-Seemann W, Drendel V et al. Detection of lymph node metastasis in patients with nodal prostate cancer relapse using (18)F/(11) C-choline positron emission tomography/computerized tomography. J Urol 2014; 192: 103–10 [DOI] [PubMed] [Google Scholar]
- 12.Winter A, Henke RP, Wawroschek F. Targeted salvage lymphadenectomy in patients treated with radical prostatectomy with biochemical recurrence: complete biochemical response without adjuvant therapy in patients with low volume lymph node recurrence over a long-term follow-up. BMC Urol 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Robotic salvage RPLND/PLND for ‘node-only’recurrent prostate cancer: Technique.