Abstract
Background:
Focal ablative therapy may be a suboptimal option for anterior prostate cancers (APCs) reaching the prostate apex due to concerns for thermal injury to the external sphincter.
Objective:
To explore the technical feasibility of anterior partial prostatectomy (APP) for isolated APCs detected by magnetic resonance imaging (MRI), and to report short-term oncologic and functional outcomes.
Design, setting, and participants:
Following institutional review board approval, over an 8-yr period (2008–2015) 17 consenting patients were enrolled in a prospective single-arm single-center Innovation, Development, Exploration, Assessment, Long-term (IDEAL) phase 2a study. Inclusion criteria comprised preurethral, low- to intermediate-risk APC diagnosed by MRI, and targeted biopsies. Robotic template APP was performed; posterolateral aspect of the sub-montanal urethra, peripheral zone, and periprostatic tissues were preserved intact. Median follow-up was 30 mo (interquartile range [IQR]: 25–70).
Outcome measurements and statistical analysis:
We noted the incidence of perioperative complications and examined reports of pathology, prostate-specific antigen (PSA), imaging, biopsies, and questionnaires.
Results and limitations:
Preoperatively, median PSA was 9.8 ng/ml, Gleason score was 6–7 (3 + 4), and cancer volume was 3.7 cm3 (IQR: 1.7–4.6). The technique was feasible in all cases. Perioperative complications included anastomotic leak (12%; G2), urinary tract infection (6%; G2), and transient intestinal ileus in one case (6%; G2). At 3 mo, continence and potency rates were 100% and 83%, respectively. Median nadir PSA was 0.4 ng/ml (IQR: 0.3–0.7). All margins and posterolateral margins rates were 55% and 35%, respectively. APC recurrence-free survival at 2 yr was 0.86 (95% confidence interval [CI], 0.55–0.96). Four patients (24%) who recurred underwent an uncomplicated completion of robot-assisted prostatectomy. Regarding limitations, CIs are quite wide for reported outcomes.
Conclusions:
Robotic partial prostatectomy for isolated APC is feasible with good functional results. While promising, much more research is needed to verify our initial outcomes and appropriately position APP in the treatment paradigms for APC.
Patient summary:
We explored a novel approach for partial prostatic surgical ablation for prostate cancer located in the anterior part of the prostate as an alternative to other focal ablative techniques.
Keywords: Prostatectomy, Focal therapy, Prostate cancer, Magnetic resonance imaging, Image-guided intervention, Minimally invasive surgery, Robotic surgery
1. Introduction
Energy-based partial gland ablation is an emerging treatment for localized intermediate-risk prostate cancer (PCa) that aims to reduce the morbidity associated with radical whole-gland therapy while delivering cancer control [1,2]. These focal therapies are adapted to PCa location, such as the peripheral zone (PZ), transition zone (TZ), or anterior fibromuscular stroma (AFMS) [3].
Use of magnetic resonance imaging (MRI) and targeted biopsies has led to better detection and localization of PCa, such as diagnosing anterior prostate cancers (APCs) originating from the TZ. APCs account for 19% of new cancers [4–7]. We demonstrated that APCs originate from the anterior and medial TZ as well as anterior to the urethra in the midline [8,9]. Probably due to benign prostatic hyperplasia (BPH), some APCs spread anteriorly into the AFMS; then the anterior limit of the TZ acts as a barrier to APCs extending posteriorly [4].
An APC nodule can occasionally be located mainly within the AFMS and anterior to the TZ boundary without any cancer in the PZ. In these highly selected cases, which represent 3–5% of new cancers (Supplement 1), focal or partial treatment is appealing. However, delivering thermal energy to an apical APC may be undesirable [3], for fear of compromising the external sphincteric unit and/or the neurovascular bundles.
In this specific circumstance, we believe that en bloc surgical excision of the anterior prostate (ie, TZ, AFMS, anterior part of the PZ) would preserve intact the posterolateral aspect of the distal (submontanal) urethra, PZ, and periprostatic tissues. Doing so would effectively ablate the tumor with a safety margin of benign tissue posteriorly, deliver superior continence/potency outcomes versus radical prostatectomy (RP), and allow pathologic assessment of excised tissue. PSA nadir would still potentially be an accurate marker for oncologic control, and a complementary RP or ablative therapy could be performed in case of cancer recurrence, with oncologic and functional outcomes similar to RP.
The objective of our study was to evaluate the feasibility and oncologic and functional outcomes of robot-assisted anterior partial prostatectomy (APP) for isolated MRI-detected APC in a highly selected cohort.
2. Patients and methods
2.1. Study population
This study used a prospective single-arm single-center stage 2a Innovation, Development, Exploration, Assessment, Long-term (IDEAL) paradigm [10]. The robot-assisted APP technique innovation was carried out by a few surgeons, deemed to be probably safe after the first five cases, and was tested more broadly, although still experimental, in well-selected patients. The intervention needed to be refined. A regulatory process was required at this stage, and the study was approved by the institutional review board at the University of Lille, France, where all cases were treated. After detailed information was given to the patients, written signed consent was obtained. Assessing safety for the first five cases was based on bleeding (no transfusion), functional results at 6 mo (International Continence Society [ICS] score ≤4), and oncologic results (at least two of three cases with negative lateral/posterior margins). After five consecutive surgeries in an 18-mo period, the investigators decided to pursue the study. The decision to analyze the results after 17 patients was established empirically (median follow-up >24 mo was reached).
Inclusion criteria comprised a multiparametric MRI (mpMRI)–identified, predominantly anteriorly located tumor. It was proven at targeted biopsy (two cores per lesion) and determined to be at low or intermediate risk [11,12]. Cancer could be of any volume at MRI, provided its posterior limits were at least 17 mm (posterior biopsy core length) anterior to the rectal surface of the gland that defines the anterior location of cancers, and its lateral limits were within the TZ or AFMS. The whole prostate gland could be of any volume [13]. Exclusion criteria were if the posterior aspect of the APC at MRI was located <5 mm anterior to a coronal plane located at the level of the posterior TZ boundary (Fig. 1) or MRI-targeted biopsies Gleason score (GS) >7, an anterior bulging of the tumor beyond prostate boundaries or extension in the preprostatic fat or bladder neck (BN), a cancer length >3 mm at 12 systematic posterior biopsies in an adjacent sector to the anterior cancer, or an additional clinically significant PZ cancer (>3 mm of cancer on one core at 12 systematic posterior biopsies or at MRI-targeted biopsies to an secondary posteriorly located lesion at MRI). Patients who refused to consent were offered RP, radiation therapy, and/or active surveillance. Over an 8-yr period (January 2008 to December 2015), 28 patients fulfilled the entry criteria, of whom 17 (60%) gave informed consent and were enrolled in the study.
Fig. 1 –
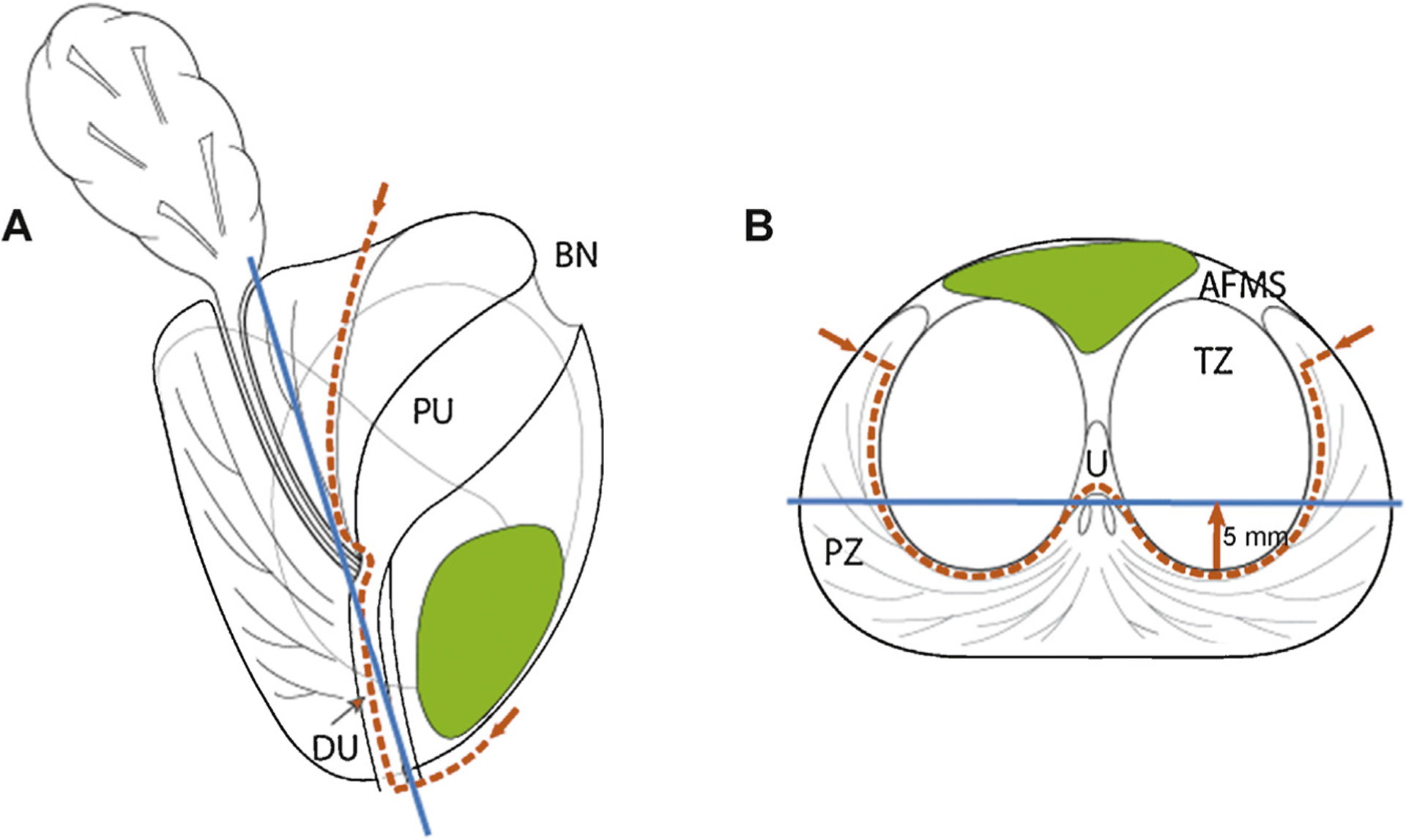
Schematic view of prostate (a) sagittal and (b) transverse aspects at midgland. Red dotted line shows dissection plane of anterior partial prostatectomy. Protocol comprises en bloc template excision of the anterior part of the prostate including anterior fibromuscular stroma, prostate adenoma (transition zone [TZ] and median lobe) with the proximal urethra, the anterior part of the distal (submontanal) urethra, the most anterior apical parts of the peripheral zone, and anterior bladder neck. Blue line represents a coronal plane 5 mm (arrow) anterior to the posterior aspect of the TZ. Average anterior cancer (in green) should be located at magnetic resonance imaging anterior to this coronal plane to ensure complete removal during partial surgery.
AFMS = anterior fibromuscular stroma; BN = bladder neck; DU = distal urethra; PU = proximal urethra; PZ = peripheral zone; TZ = transition zone; U = urethra.
All patients underwent prebiopsy mpMRI, followed by MR-targeted biopsies to any visible lesion, plus a 12-core systematic transrectal ultrasound–guided biopsy. Self-administered validated quality-of-life (QOL) questionnaires were used to assess preoperative urinary function (International Prostate Symptom Score [IPSS]), continence (ICS: 1–2), and potency (International Index of Erectile Function [IIEF]-5). Potency was defined as an IIEF-5 score ≥20 with or without drugs. Preoperative mpMRI protocol was performed within 3 mo of surgery and included axial (and sagittal, if necessary) gadolinium-enhanced sequences to assess the contour and the craniocaudal extent of the biopsy-proven cancerous area [8].
2.2. Surgical technique
Robotic surgery consisted of en bloc excision of the anterior part of the prostate composed of the AFMS, BN, prostate adenoma (TZ and median lobe) along with the proximal prostate urethra, PZ apical anterior horns, anterior aspect of the distal (submontanal) urethra, and anterior BN (Supplement 1, Fig. 1). Only three cases had lymph node dissection due to suspicious enlarged pelvic node enlargement at MRI (n = 2) and GS 4 + 3 (n = 1).
Perioperative data including peri- and postoperative treatment-related adverse events, and time to bladder catheter removal were recorded. Cancer location, volume, and margins were assessed according to the Stanford technique [14]. We differentiated positive posterior surgical margins at the posterior aspect of the excised specimen from anterior surgical margins that are an artifact occurring when preprostatic fat is removed during exposure in patients with APC. Patients with a positive posterior margin underwent mpMRI and biopsy of the PZ within 3 mo after surgery. We recommended that patients with a positive biopsy have a robot-assisted salvage nerve-sparing RP (Supplement 1).
All patients underwent PSA monitoring at 3 and 6 mo and then every 6 mo and had mpMRI at 6–12 mo. At 6 mo, protocol-based 12-core and/or targeted biopsies were performed in the first seven patients. Because biopsies were negative when MRI was not suspicious, only for-cause biopsies were performed in the remaining 10 patients. Self-administered questionnaires assessed urinary function (IPSS), continence (ICS: 1–2), and potency (IIEF-5) at months 6 and 12.
3. Results
3.1. Preoperative data
Clinical, pathologic, and biochemical preoperative data are shown in Table 1 and Supplementary Table 1. Clinical stage was T1c in all cases (normal digital rectal examination). APC location and volume are shown in Figure 2 and Supplementary Figure 1. In 11 of 17 cases (65%), tumor extended up to the apical part of the gland.
Table 1 –
Clinical, pathologic, and biochemical preoperative data of the 17 patients included for anterior partial prostatectomy
| Clinical | |
|---|---|
| Age, yr, mean (IQR) | 61 (54–66) |
| Preoperative PSA, ng/ml, median (IQR) | 9.8 (7.1–11.3) |
| Biopsies | |
| No. of cases with previous negative biopsy series, n (%) | 11 (65) |
| No. of cases with cancer at 12 systematic posterior biopsies, n (%) | 6 (35) |
| Maximum CCL at 12 systematic posterior biopsies, mm, median (IQR) | 1 (1–2) |
| Maximum CCL at targeted biopsies, mm, median (IQR) | 8 (7–9) |
| Gleason score, n | |
| 6 (3 + 3) | 8 |
| 7 (3 + 4) | 8 |
| 7 (4 + 3) | 1 |
| MRI | |
| Prostate volume, cm3, median (IQR) | 45 (37–59) |
| Cancer volume, cm3, median (IQR) | 4.15 (1.7–4.6) * |
| Tumor location, n | |
| Midline AFMS | 11 |
| Midline TZ/AFMS | 2 |
| Lateral TZ and AFMS | 3 |
| No visible lesion | 1* |
AFMS = anterior fibromuscular stroma; CCL = cancer core length; IQR = interquartile range; MRI = magnetic resonance imaging; PSA = prostate-specific antigen; RP = radical prostatectomy; TZ = transition zone.
MRI was not suspicious for case 4, and cancer volume could not be calculated.
Fig. 2 –
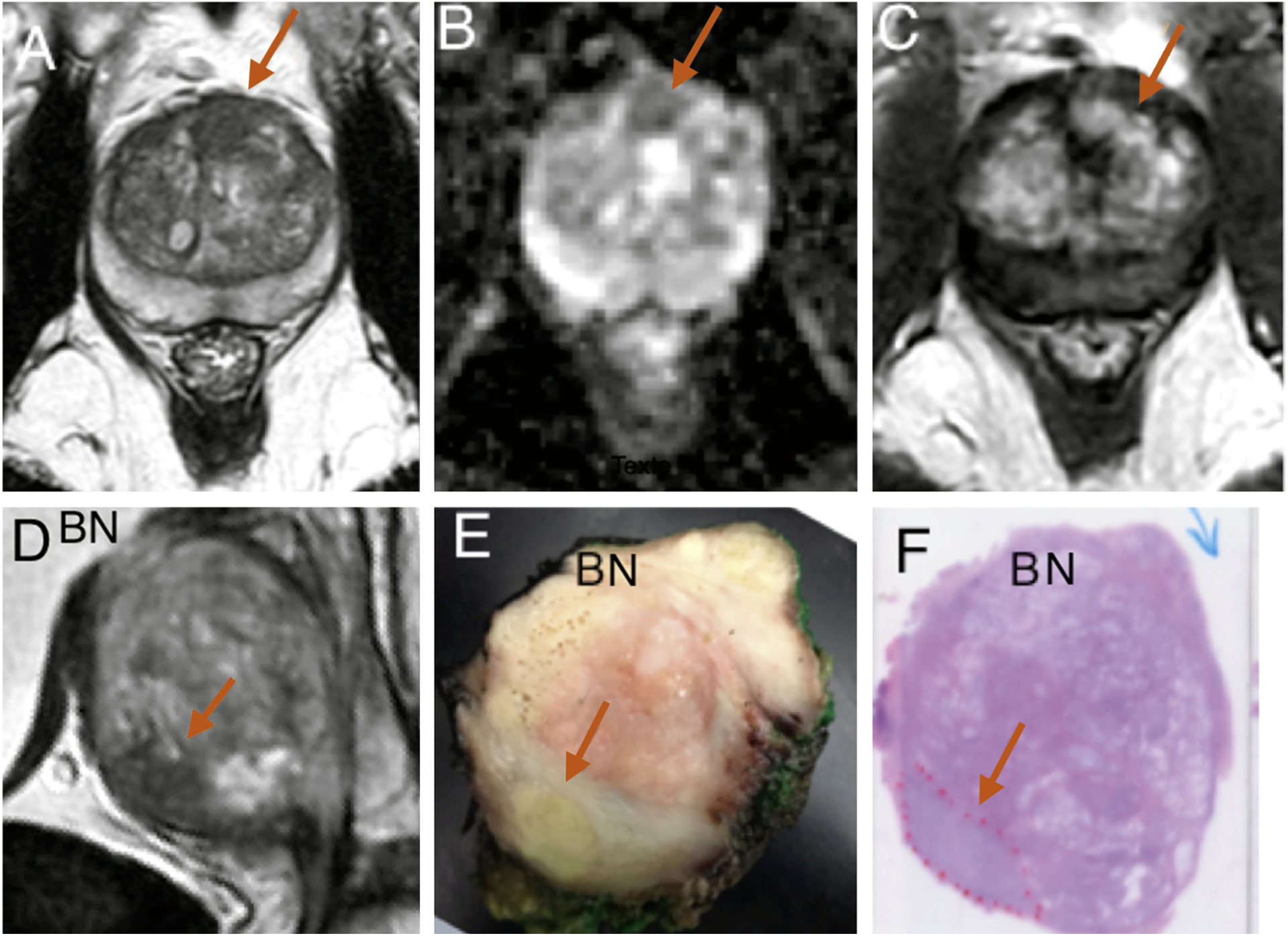
Case 17 (prostate-specific antigen [PSA] 7.13 ng/ml; prostate volume 73 cm3]. Isolated anterior 2.4-cm3 lesion suspicious at magnetic resonance imaging (MRI) in the anterior fibromuscular stroma on the midline and anterior left transition zone lobe (arrows). (a) MRI transverse T2. (b) MRI transverse apparent diffusion coefficient map. (c) MRI transverse dynamic contrast-enhanced sequences. Targeted biopsies were positive for 6-mm Gleason score (GS) 6 (3 + 3) cancer. (d) MRI T2 parasagittal view showed anterior cancer area at the anterior and inferior aspect (arrow). (e) Fixed midsagittal section showed yellow area suspected of malignancy at the anterior and inferior aspect (arrow). (f) Hematoxylin and eosin whole-mount sagittal histologic section confirmed cancer (red dotted line) area of 4 cm3, GS 7 (4 + 3), pT2, R0, and postoperative PSA of 0.4 ng/ml at 3 mo.
BN = bladder neck.
3.2. Perioperative results
Technique was feasible in all cases without open conversion or intraoperative complications. After the first five consecutive surgeries in an 18-mo period, there were no transfusions, ICS score was <4 in all cases, and four of five men had negative lateral/posterior margins. For the 17 patients, perioperative data showed a fluid loss (blood and urine) of 300 ml (interquartile range [IQR]: 200–400), and perioperative complications were grades 1–2 (Table 2).
Table 2 –
Perioperative and postoperative clinical data of the 17 patients included for anterior partial prostatectomy
| Perioperative data | |
|---|---|
| Operative time, min, median (IQR) | 150 (148–188) |
| Fluid loss (blood and urine), ml, median (IQR) | 300 (200–400)* |
| Bladder catheter removal, d, median (IQR) | 7 (6–7) |
| Postoperative complications | |
| Urinary infection, Clavien-Dindo grade 2 | Treated with antibiotics in two cases (12%) |
| Transient anastomotic leak, Clavien-Dindo grade 2 | Present in one case (case 2; 6%), resolved spontaneously with 10-d catheter drainage |
| Transient intestinal ileus, Clavien-Dindo grade 2 | Present in one case (case 1; 6%), resolved with transanal exsufflation tube |
IQR = interquartile range.
After the first five consecutive surgeries in an 18-mo period, there were no transfusions.
3.3. Postoperative clinical outcomes
Table 2 shows the clinical, biochemical, and pathologic outcomes. Median nadir PSA value was 0.4 ng/ml (0.3–0.7), representing a reduction of 8.7 ng/ml (94%; 95% confidence interval (CI), 7.3–10.2) compared with baseline PSA. Median time to achieve PSA nadir was 3 mo. Figure 3 shows PSA variations from the nadir value. PSA outcomes were not suspicious for cancer during follow-up in 13 of 17 patients (76%). Of these, three patients had a PSA rise with a velocity <0.10 ng/ml per year (cases 4, 5, and 7) and doubling time of 6.1 yr (case 5) (Fig. 3a); mpMRI showed residual BPH at the prostate base as the most likely cause of this PSA rise over time (Fig. 4) with protocol-based biopsies and MRI revealing no evidence of cancer. Of eight patients with stage pT3a after APP, 3 (37%) had a cancer recurrence. Among the nine pT2 cases, one (11%) was part of the four patients who recurred. The APC recurrence rate was 17%, 30%, and 0% for GS 6, 7 (3 + 4), and 7 (4 + 3) at RP, respectively.
Fig. 3 –
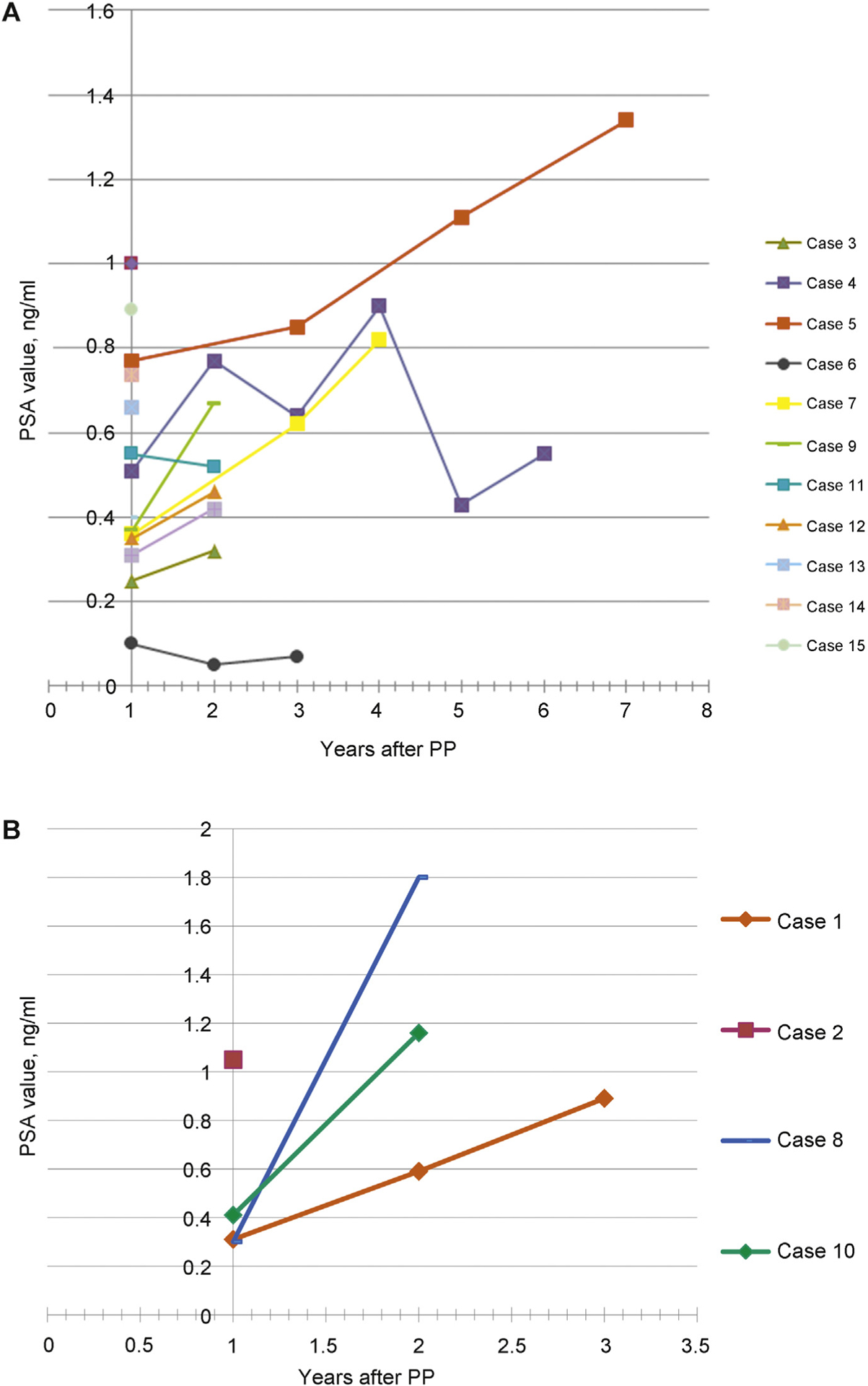
(a) Prostate-specific antigen (PSA) variations during follow-up starting from PSA nadir observed between months 3 and 9 postoperatively for 13 of 17 patients with no cancer recurrence. Cases 5, 7, and 9 showed slow PSA elevation and had residual benign prostatic hyperplasia at magnetic resonance imaging and no cancer at biopsies. (b) PSA variations during follow-up starting from PSA nadir were observed between months 3 and 9 postoperatively for 4 of 17 patients who had cancer recurrence diagnosed at months 2, 24, 25, and 30. Three of four patients had complementary radical prostatectomy with undetectable PSA postoperatively.
PP = partial prostatectomy; PSA = prostate-specific antigen.
Fig. 4 –
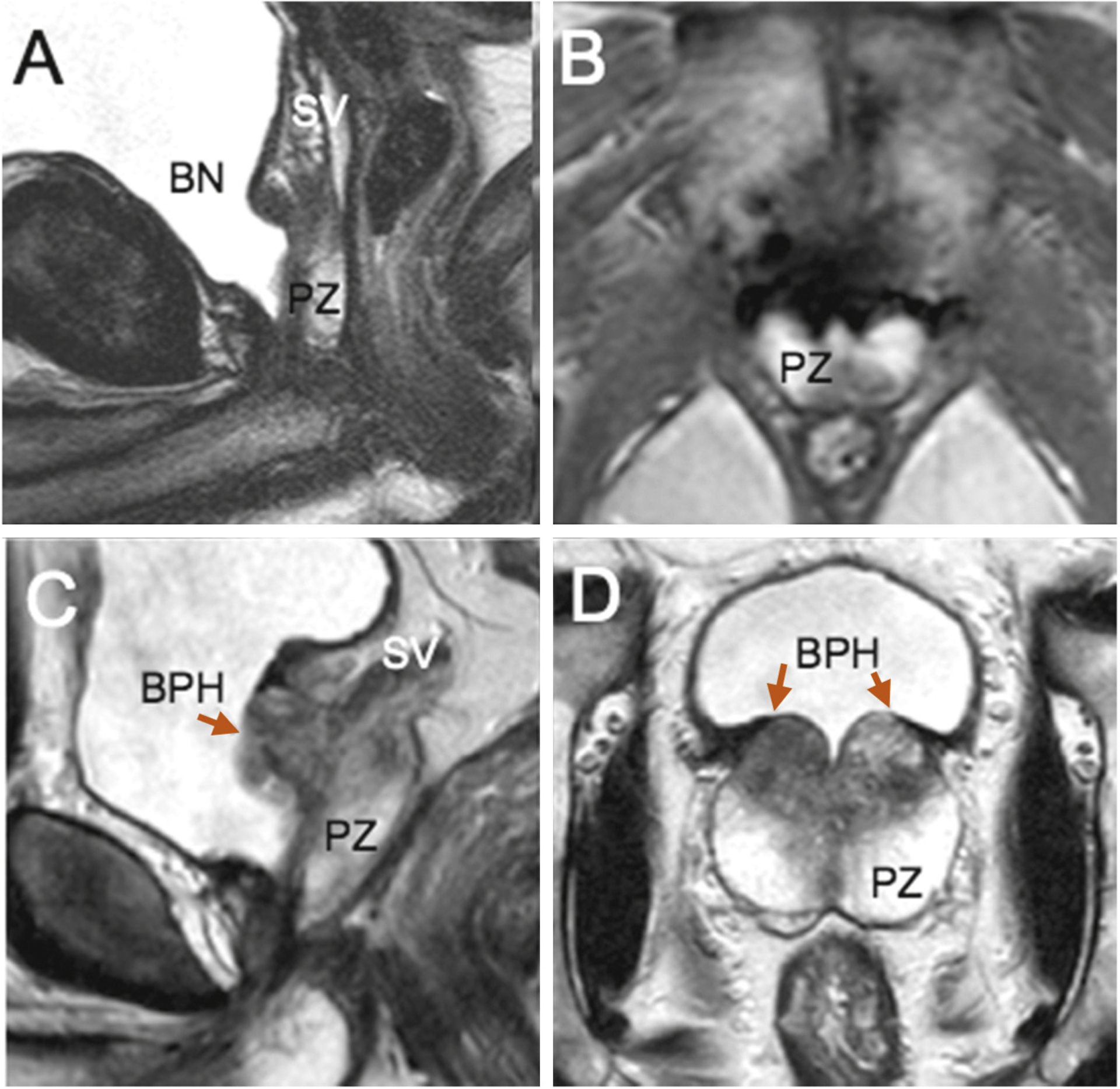
Cases 4 and 5 showing postoperative T2 magnetic resonance imaging (MRI) sequences at 6 mo, (a) sagittal and (b) transverse, and at 4 yr, (c) sagittal and (d) transverse; preserved peripheral zone (arrows) and seminal vesicles. Case 14 (a, b) had stable prostate-specific antigen (PSA) of 0.74 ng/ml at 6 mo and 0.70 ng/ml at 2 yr. Case 5 had rising PSA of 0.71 ng/ml at 6 mo and 1.34 ng/ml at 2 yr. MRI showed a symmetric area of benign prostatic hyperplasia recurrence/persistence at the prostate base on each side of the bladder neck that may explain this PSA rise with time. Protocol-based systematic biopsies were negative, and MRI was nonsuspicious for cancer.
BN = bladder neck; BPH = benign prostatic hyperplasia; PZ = peripheral zone; SV = seminal vesicle.
A rising PSA suspicious for cancer occurred in 4 of 17 men (24%; cases 1, 2, 8, and 10) (Fig. 3b) at 0.3, 2.5, 2, and 2 yr, respectively (Fig. 5). APC recurrence-free survival was 0.86 (range: 0.55–0.96) at 2 yr and 0.67 (range: 0.33–0.87) at 3 yr. MRI was suspicious and MRI-targeted biopsies were positive, all at the area corresponding to the location of the positive posterior margin (Table 3). Cancer volumes at MRI for the 13 patients with no recurrence and for the 4 patients who recurred and whole-gland volume are shown in Supplementary Figure 2.
Fig. 5 –
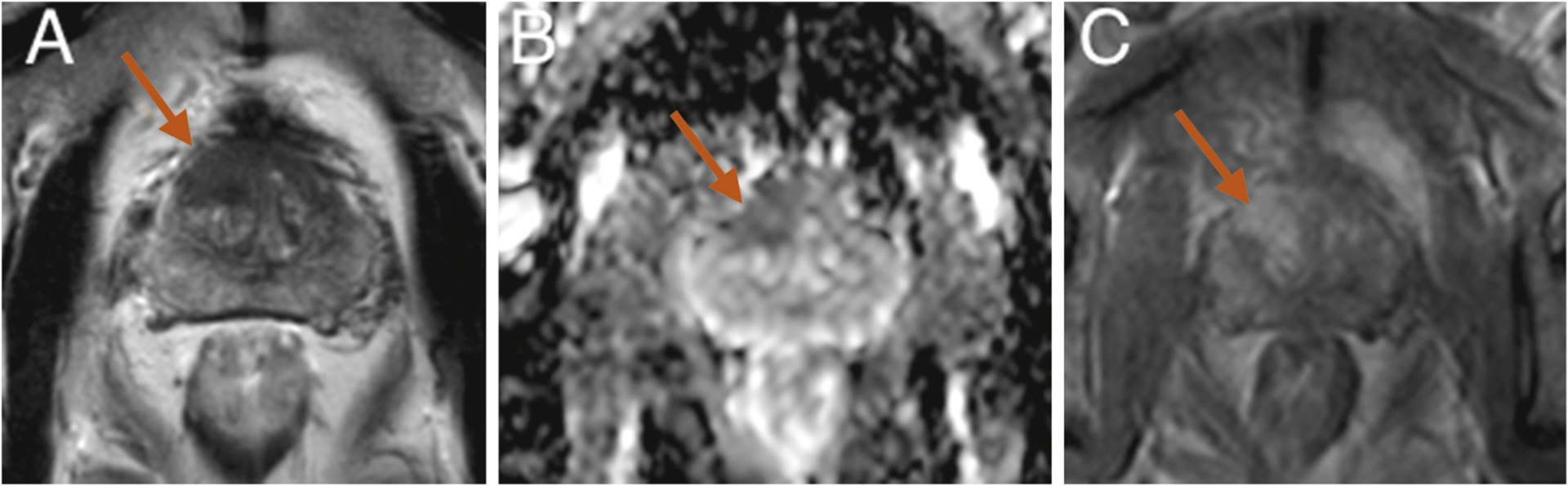
Case 10 (prostate-specific antigen [PSA] 7.24 ng/ml; prostate volume 27 cm3). Isolated anterior 2.6-cm3 lesion suspicious at magnetic resonance imaging in the anterior fibromuscular stroma on the midline and anterior right transition zone lobe (arrows): (a) T2; (b) apparent diffusion coefficient map; (c) dynamic contrast-enhanced sequences. Targeted biopsies were positive for 8-mm Gleason score (GS) 7 (4 + 3) cancer. At histology, cancer was at the anterior and inferior part of the specimen: 4.61-cm3 volume, GS 7 (3 + 4), pT3a, pN0, and R1. Positive margins were anterior 6 mm and lateral 6 mm. Postoperative PSA was 0.41 ng/ml at 3 mo and 1.16 ng/ml at 18 mo postoperatively. Biopsies at left anterior part of preserved PZ showed residual cancer on 3 mm GS 6 (3 + 3). Radical prostatectomy was performed at 2 yr with negative margins and detectable PSA.
Table 3 –
Biochemical and pathologic postoperative data and outcomes of the 17 patients included for anterior partial prostatectomy
| Follow-up, mo, median (Q1–Q3) | 30 (25–70) |
| Postoperative nadir PSA, ng/ml, median (Q1–Q3) | 0.4 (0.3–0.7) |
| Pathology at partial prostatectomy specimen, n (%) | |
| pT2 | 9 (53) |
| pT3a | 8 (47)* |
| pN0 (average number of removed nodes: 6) | 3 (100) |
| Margins | |
| Anterior: n (%); length, mm, median (Q1–Q3) | 5/17 (29); 4 (2–6) |
| Posterior/lateral: n (%); length, mm, median (Q1–Q3) | 6/17 (35); 8 (2–35) |
| All margins, n (%) | 9/17 (53)** |
| Cancer volume, cm3, median (Q1–Q3) | 5.3 (3.5–7.1) |
| GS | |
| 6 (3 + 3) | 6 |
| 7 (3 + 4) | 10 |
| 7 (4 + 3) | 1 |
| MRI and biopsies | |
| No. of cases with recurrence at postoperative protocol-based MRI at 1 yr | 0/16*** |
| No. of cases with recurrence at protocol-based 12-core posterior systematic biopsies at 1 yr | 1/7**** |
| No. of cases with recurrence at postoperative for-cause targeted biopsy to lateral/postmargin area and/or MRI lesion | 4/4 |
| Positive targeted biopsy core cancer length, mm | |
| Clinically insignificant cancer, GS 6 (3 + 3) | 2 |
| Clinically significant cancer, GS | |
| 7 (3 + 4) | 6 and 5 |
| 7 (4 + 3) | 2 |
| Pathology at secondary RP, pT2a/N0 or pN0, n | 4/4 |
| Positive margins at anterior aspect of specimen | 2 |
| Residual cancer largest dimension by GS, mm | |
| 6 (3 + 3) | 2 |
| 7 (3 + 4) | 8 and 20 |
| 7 (4 + 3) | 7 |
GS = Gleason score; MRI = magnetic resonance imaging; PSA = prostate-specific antigen; Q = quartile; RP = radical prostatectomy.
Median follow-up was 40 mo (range: 28–61) for these nine cases with pT2 stage and 60 mo (range: 30–72) for these eight cases with pT3 stage.
Median follow-up of R0 PCa was 26 mo (range: 15–38). No patients recurred among these eight cases.
Most recent case 17 recurred in only 3 mo postoperatively.
Only the first six cases had postoperative protocol-based 12-core posterior systematic biopsies.
Salvage robot-assisted RP resulted in undetectable PSA <0.1 ng/ml in three of four patients, durable over a mean follow-up of 6 yr. Case 10 had a detectable PSA at 3 mo after completion of RP. There were no intraoperative complications. Pathology results are shown in Table 3. In two cases, an additional separate clinically insignificant PZ cancer of 4 mm and 3 mm with GS 6 (3 + 3) was identified at pathology at a location not in contact with the anterior PZ margin site. No significant technical difficulties were encountered during the posterolateral aspect of the prostate dissection, posterior to the endopelvic fascia or in the prerectal space. One patient (case 1) had Clavien-Dindo grade 3b peritonitis due to spontaneous perforation of a sigmoid diverticulum on day 7 (6%), requiring colectomy and temporary colostomy. Two cases (7 and 15) had a focal posterolateral margin, at risk for incomplete tumor resection.
3.4. Functional outcomes
The pre- and postoperative questionnaire data are shown in Table 4, Figure 6, and Supplementary Figure 3. At 6–12 mo after surgery, potency remained uninterrupted in 10 of 12 patients (83%) who were potent preoperatively with an IIEF score ≥15, including one patient who had salvage RP. Of the five remaining patients with an IIEF score <15 preoperatively, two had improvement with use of phosphodiesterase type 5 inhibitor drugs with an IIEF score >15. Continence remained uninterrupted in all patients; however, three patients had urgency for 2–3 mo.
Table 4 –
Functionnal outcomes based on urinary and sexual function questionnaires
| Preoperative, median (IQR) | 6–12 mo, median (IQR) | Difference between preoperative and 6–12 mo, median (95% CI) | |
|---|---|---|---|
| ICS score | 0 (0–0) | 0 (0–4) | 4 (1–7) |
| IPSS score | 5 (0–11) | 2 (0–4.5) | 3 (−2 to 10) |
| IIEF-5 score | 19 (15–25) | 20 (11–24) | 4 (−4 to 13) |
| No. of cases | 16 | 14 |
CI = confidence interval; ICS = International Continence Society; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; IQR = interqartile range.
Fig. 6 –
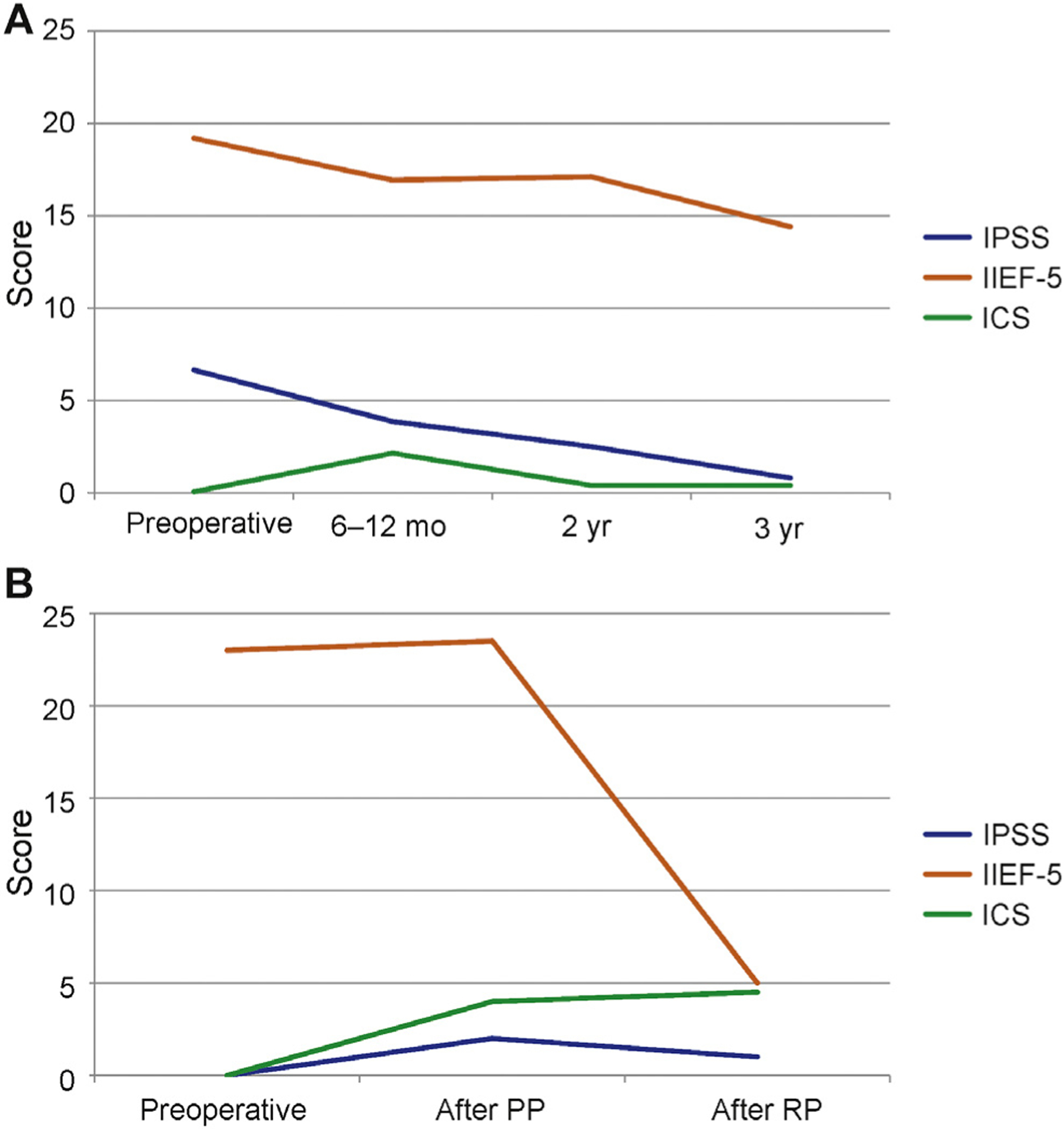
(a) Median International Prostate Symptom Score (IPSS), International Index of Erectile Function (IIEF)-5, and International Continence Society (ICS) scores from baseline to 3 yr. ICS score remained almost unchanged with no incontinence. There is gradual improvement of the median IPSS score and a gradual decrease of the median IIEF-5 scores. (b) Median IPSS, IIEF-5, and ICS scores from baseline to after anterior partial prostatectomy and to after complete radical prostatectomy for the 4 of 17 patients who had cancer recurrence diagnosed at months 2, 24, 25, and 30.
ICS = International Continence Society score; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; PP = partial prostatectomy; RP = radical prostatectomy.
4. Discussion
We explored a surgical, in lieu of a thermal, focal treatment option for highly selected consenting patients with anterior apical PCa. Surgical treatment planning was based on tumor location and intraprostatic anatomy derived from mpMRI and targeted biopsy data. We leveraged our prior experience with mpMRI and targeted biopsies to precisely identify APCs originating from the TZ.
Our group [4] and others [5,15,16] have published precise morphometric and anatomic descriptions that led us to explore this concept of APP in very carefully selected patients after detailed informed consent.
In each of our cases, the TZ-originated cancer invaded, or was entirely confined, to the AFMS. As such, simple prostatectomy (BPH enucleation) would have been insufficient because it would not have excised the AFMS. We learned that prostate volume (should be >42 cm3, which is the 25th percentile of whole-gland volume of the no-recurrence group) (Supplementary Fig. 2) is an important factor for the success of the procedure. The technical challenge of partial prostatectomy is not at the apex or at the anterolateral aspect of the gland where the dissection planes are similar to RP; the challenge is to ensure negative margins posterolaterally at the PZ site. One patient (case 10) with GS 7 (4 + 3) was included and was one of the four patients who recurred. This inclusion criteria of the GS score 7 (4 + 3) or higher should be considered oncologically incorrect. This case had a detectable PSA at 3 mo after RP completion. Therefore selection criteria in the four men who recurred were not so strict, and their results help refine the selection criteria of patients as candidates for the technique in future studies. Additional criteria for selection should include whole-gland volume >40–45 cm3. The technique may also include frozen section assessment of the PZ margin.
Focal ablative therapy is a suboptimal treatment option for PCa located in the anterior apex of the prostate because of potential thermal diffusion injury to the external striated sphincter, neurovascular bundles, and/or urethra, as well as interference from the pubic symphysis. To the best of our knowledge, the outcomes of focal high-intensity focal ultrasound, cryotherapy, or laser ablation focusing on this specific location are not available [17]. We are acutely aware that this concept of surgical focal excision is controversial. This concept is open to the valid critiques levied at focal ablation for PCa, such as uncertainty regarding the natural history of untreated cancer foci, uncertain post-treatment monitoring using PSA and MRI, lack of long-term oncologic outcomes data in a larger cohort, and lack of QOL data compared with traditional treatment strategies [18,19]. However, given that the cancer lesion is surgically excised, it can be subjected to accurate pathologic staging/grading, an aspect that is not feasible with ablative partial/focal therapy [20]. As for any focal treatment, the remainder of the gland should be devoid of any known foci of significant PCa, and it should be possible to perform a completion of radical procedure in case of recurrence or de novo cancer during follow-up. However, a strength of our study is the meticulous reporting of all relevant data regarding PSA, MRI, histopathology, follow-up continence and potency questionnaires, and our data with 30 mo of follow-up (range: 25–70).
This initial experience with robot-assisted APP provides the following insights. First, the technique is feasible and safe. Partial prostatectomy was not judged to be more difficult than RP by the participating surgeons, all of whom had expertise in robot-assisted surgery. Second, functional outcomes were satisfactory. No patient reported stress incontinence, in contrast to a 16% incontinence rate following robot-assisted RP [21]. Erectile function was maintained at 6 mo in 10 of 12 men (83%) who were potent preoperatively, which compares favorably with the 12-mo potency rates of 54–90% after RP [21]. Going forward, QOL questionnaires should be added to assess overall benefits or harms. Third, median PSA was 0.4 ng/ml (IQR: 0.3–0.7) at 3 mo. This value is close to the value of 0.6 ng/ml (range: 0–1.3) after a simple robot-assisted RP [22], but lower, probably because some of the PZ and most of the anterior and apical prostate were resected. There is currently no accepted definition for biochemical disease recurrence after focal therapy [23].
Fourth, our 29% anterior location rate of positive margins (PMs) should be compared with RP anterior location rates, in which the PZ was not preserved. However, our series is made of highly selected cases that do not reflect the whole spectrum of APC. Hence positive margin rates of 22% anteriorly and 37% at the BN locations were observed in a series of 55 RPs with TZ-originated cancer [24]. A 46% rate of PMs was observed in a series of 68 patients for which APC was diagnosed based on mpMRI and targeted biopsies and who had RP. The risk of PMs is higher for TZ- than for PZ-originated cancers, whereas there are no differences in biochemical recurrence (BCR)–free or overall survival [25]. Our 35% post/lateral rate of PMs (6 of 17 cases) led to recurrence in 4 of 6 cases. A frozen section margin assessment on the predominant side of the tumor might have led to a more extended excision of the PZ horn or to immediate RP. Fifth, completion RP was performed for four patients who recurred, was judged feasible with the possibility of the nerve-sparing technique, and excellent oncologic and outcomes were observed with a mean follow-up of 5 yr for three of four cases. Functional outcomes were similar to what is observed after RP as the primary treatment.
Limitations of the study include the small sample size associated with quite wide CIs for reported outcomes. It also includes the absence of an alternative biopsy scheme or approach such as template transperineal mapping for case selection.
5. Conclusions
Robotic partial prostatectomy for isolated anterior cancer is feasible, with good functional results and 86% BCR-free survival at 24 mo of follow-up. Partial prostatectomy is an option for highly selected men with anterior cancers as an alternative to other focal ablative therapy. CIs are quite wide for reported outcomes, suggesting that while promising, much more research is needed to verify our initial outcomes, adding QOL questionnaires to assess overall benefits or harms of this technique before being recommended or not advised as a reasonable alternative to standard therapies.
Supplementary Material
Acknowledgments:
We thank Laura Lecuyer for data collection and table construction and Gauthier Marcq for statistical help.
Financial disclosures:
Arnaud Villers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.08.057.
References
- [1].Marien A, Gill I, Ukimura O, Betrouni N, Villers A. Target ablation–image-guided therapy in prostate cancer. Urol Oncol 2014;32: 912–23. [DOI] [PubMed] [Google Scholar]
- [2].Orczyk C, Emberton M, Ahmed HU. What tumours should we treat with focal therapy based on risk category, grade, size and location? Curr Opin Urol 2015;25:212–9. [DOI] [PubMed] [Google Scholar]
- [3].Sivaraman A, Barret E. Focal therapy for prostate cancer: an “à la carte” approach. Eur Urol 2016;69:973–5. [DOI] [PubMed] [Google Scholar]
- [4].Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology 2011;78:1356–62. [DOI] [PubMed] [Google Scholar]
- [5].Lawrentschuk N, Haider MA, Daljeet N, et al. ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU Int 2010;105:1231–6. [DOI] [PubMed] [Google Scholar]
- [6].Vargas HA, Akin O, Franiel T, et al. Normal central zone of the prostate and central zone involvement by prostate cancer: clinical and MR imaging implications. Radiology 2012;262:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoeks CM, Hambrock T, Yakar D, et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 2013;266:207–17. [DOI] [PubMed] [Google Scholar]
- [8].Bouye S, Potiron E, Puech P, Leroy X, Lemaitre L, Villers A. Transition zone and anterior stromal prostate cancers: zone of origin and intraprostatic patterns of spread at histopathology. Prostate 2009; 69:105–13. [DOI] [PubMed] [Google Scholar]
- [9].Nevoux P, Ouzzane A, Ahmed HU, et al. Quantitative tissue analyses of prostate cancer foci in an unselected cystoprostatectomy series. BJU Int 2012;110:517–23. [DOI] [PubMed] [Google Scholar]
- [10].Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet 2009;374:1089–96. [DOI] [PubMed] [Google Scholar]
- [11].de la Rosette J, Ahmed H, Barentsz J, et al. Focal therapy in prostate cancer–report from a consensus panel. J Endourol 2010;24:775–80. [DOI] [PubMed] [Google Scholar]
- [12].Ahmed HU, Akin O, Coleman JA, et al. Transatlantic Consensus Group on active surveillance and focal therapy for prostate cancer. BJU Int 2012;109:1636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477–94. [DOI] [PubMed] [Google Scholar]
- [14].Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2006;176:2432–7. [DOI] [PubMed] [Google Scholar]
- [15].Radtke JP, Boxler S, Kuru TH, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis 2015; 18:288–96. [DOI] [PubMed] [Google Scholar]
- [16].Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng FM, Taneja SS. Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR Am J Roentgenol 2015;204:W266–72. [DOI] [PubMed] [Google Scholar]
- [17].Marshall S, Taneja S. Focal therapy for prostate cancer: the current status. Prostate Int 2015;3:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lindner U, Lawrentschuk N, Weersink RA, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol 2010;57:1111–4. [DOI] [PubMed] [Google Scholar]
- [19].Ahmed HU, Emberton M. Benchmarks for success in focal therapy of prostate cancer: cure or control? World J Urol 2010;28:577–82. [DOI] [PubMed] [Google Scholar]
- [20].Eggener S, Salomon G, Scardino PT, De la Rosette J, Polascik TJ, Brewster S. Focal therapy for prostate cancer: possibilities and limitations. Eur Urol 2010;58:57–64. [DOI] [PubMed] [Google Scholar]
- [21].Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405–17. [DOI] [PubMed] [Google Scholar]
- [22].Pokorny M, Novara G, Geurts N, et al. Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Eur Urol 2015; 68:451–7. [DOI] [PubMed] [Google Scholar]
- [23].Donaldson IA, Alonzi R, Barratt D, et al. Focal therapy: patients, interventions, and outcomes–a report from a consensus meeting. Eur Urol 2015;67:771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O’Neil LM, Walsh S, Cohen RJ, Lee S. Prostate carcinoma with positive margins at radical prostatectomy: role of tumour zonal origin in biochemical recurrence. BJU Int 2015;116(Suppl 3):42–8. [DOI] [PubMed] [Google Scholar]
- [25].Mygatt J, Sesterhenn I, Rosner I, et al. Anterior tumors of the prostate: clinicopathological features and outcomes. Prostate Cancer Prostatic Dis 2014;17:75–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


