Abstract
BACKGROUND
Multiparametric magnetic resonance imaging (MRI), with or without targeted biopsy, is an alternative to standard transrectal ultrasonography–guided biopsy for prostate-cancer detection in men with a raised prostate-specific antigen level who have not undergone biopsy. However, comparative evidence is limited.
METHODS
In a multicenter, randomized, noninferiority trial, we assigned men with a clinical suspicion of prostate cancer who had not undergone biopsy previously to undergo MRI, with or without targeted biopsy, or standard transrectal ultrasonography–guided biopsy. Men in the MRI-targeted biopsy group underwent a targeted biopsy (without standard biopsy cores) if the MRI was suggestive of prostate cancer; men whose MRI results were not suggestive of prostate cancer were not offered biopsy. Standard biopsy was a 10-to-12–core, transrectal ultrasonography–guided biopsy. The primary outcome was the proportion of men who received a diagnosis of clinically significant cancer. Secondary outcomes included the proportion of men who received a diagnosis of clinically insignificant cancer.
RESULTS
A total of 500 men underwent randomization. In the MRI-targeted biopsy group, 71 of 252 men (28%) had MRI results that were not suggestive of prostate cancer, so they did not undergo biopsy. Clinically significant cancer was detected in 95 men (38%) in the MRI-targeted biopsy group, as compared with 64 of 248 (26%) in the standard-biopsy group (adjusted difference, 12 percentage points; 95% confidence interval [CI], 4 to 20; P = 0.005). MRI, with or without targeted biopsy, was noninferior to standard biopsy, and the 95% confidence interval indicated the superiority of this strategy over standard biopsy. Fewer men in the MRI-targeted biopsy group than in the standard-biopsy group received a diagnosis of clinically insignificant cancer (adjusted difference, −13 percentage points; 95% CI, −19 to −7; P<0.001).
CONCLUSIONS
The use of risk assessment with MRI before biopsy and MRI-targeted biopsy was superior to standard transrectal ultrasonography–guided biopsy in men at clinical risk for prostate cancer who had not undergone biopsy previously. (Funded by the National Institute for Health Research and the European Association of Urology Research Foundation; PRECISION ClinicalTrials.gov number, NCT02380027.)
Men with a clinical suspicion of prostate cancer on the basis of an elevated prostate-specific antigen (PSA) level or an abnormal digital rectal examination are typically offered a standard transrectal ultrasonography–guided biopsy of the prostate during which 10 to 12 cores are obtained. This approach is associated with the underdetection of higher-grade (clinically significant) prostate cancers and the overdetection of low-grade (clinically insignificant) cancers.1 Despite randomized trials showing that men with clinically insignificant cancer do not benefit from treatment,2,3 its identification still results in the overtreatment of some men. Some men will receive radical treatment that has side effects,4,5 and others will undergo active surveillance with repeated assessment over time that has costs for patients and health care systems.6,7
An alternative diagnostic pathway in men with a clinical suspicion of prostate cancer involves multiparametric magnetic resonance imaging (MRI). With better standardization of the conduct and reporting of multiparametric MRI, the ability to detect clinically significant cancer and to rule it out has improved over the past decade.1,8,9 Multiparametric MRI could be used as a triage test to avoid a biopsy if the results were negative,1 whereas positive results could be used for targeting abnormal areas in the prostate during biopsy.10,11
In single-center studies, the approach of obtaining MRI-targeted biopsy cores alone, without performing standard biopsies, has shown similar or higher rates of detection of clinically significant cancer12–15 and lower rates of detection of clinically insignificant cancer15 than standard biopsy. We compared MRI-targeted biopsy with standard transrectal ultrasonography–guided biopsy in a pragmatic, multicenter, randomized trial. The PRECISION (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) trial aimed to evaluate prospectively whether multiparametric MRI, with targeted biopsy in the presence of an abnormal lesion, was noninferior to standard transrectal ultrasonography–guided biopsy in the detection of clinically significant prostate cancer in men with a clinical suspicion of prostate cancer who had not undergone biopsy of the prostate previously.
METHODS
TRIAL DESIGN
We conducted this multicenter, randomized, noninferiority trial at 25 centers in 11 countries (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Men who provided written informed consent were randomly assigned in a 1:1 ratio to either the MRI-targeted biopsy group or the standard-biopsy group (Fig. S1 in the Supplementary Appendix). The assignment sequence used computer-generated, randomly permuted blocks of unequal size, stratified according to center. Group assignments were revealed by the Web-based system once a participant had been assessed as eligible and had provided written informed consent.
The full trial protocol, available at NEJM.org, has been published previously16 and was approved by the ethics review board at each participating institution. The trial was monitored by an independent trial steering committee and data and safety monitoring committee. The trial was designed by the Standards of Reporting for MRI-Targeted Biopsy Studies (START) working group,10 and final decisions were made by the first author and the last two authors. Data were gathered by the trial team members who are listed in Section S1 in the Supplementary Appendix. One author analyzed the data, and the analysis was independently verified by another author. The authors assume responsibility for the accuracy and completeness of the data and analyses and for the adherence of the trial to the protocol. The first draft of the manuscript was written by the first author.
No commercial entity was involved in the trial. The trial was funded by the National Institute for Health Research and the European Association of Urology Research Foundation, with trial governance from University College London. The funders had no role in the protocol development, data analysis or interpretation, or manuscript preparation.
PARTICIPANTS
Participants were recruited in outpatient clinics and were eligible for enrollment if they had not undergone biopsy of the prostate previously and had been referred with a clinical suspicion of prostate cancer on the basis of an elevated PSA level, an abnormal digital rectal examination, or both (Table S2 in the Supplementary Appendix). Participants were required to have a PSA level of 20 ng per milliliter or less, to have results on digital rectal examination that did not suggest extracapsular disease, and to be suitable candidates for biopsy of the prostate and for MRI.
MRI AND MRI-TARGETED BIOPSY
Multiparametric MRI was performed with the use of a 1.5-T or 3.0-T scanner with a pelvic phased-array coil, with or without an endorectal coil (Table S3 in the Supplementary Appendix). T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences were acquired according to minimum standards that have been set by consensus guidelines.8 Areas on the multiparametric MRI that were suggestive of prostate cancer were categorized by a local radiologist according to the Prostate Imaging–Reporting and Data System, version 2 (PI-RADS v2),9 on a scale from 1 to 5, with higher numbers indicating a greater likelihood of clinically significant cancer. Table S4 in the Supplementary Appendix provides details regarding the experience of the clinicians who took part in the trial.
Men who had a positive result on the multiparametric MRI — that is, in whom an area with a score of 3 (equivocal regarding the likelihood of prostate cancer), 4 (likely to be prostate cancer), or 5 (highly likely to be prostate cancer) was identified — underwent MRI-targeted biopsy with the use of real-time ultrasonographic guidance. A maximum of three areas that were suggestive of prostate cancer were permitted to be chosen for targeted biopsy, with a maximum of 4 biopsy cores obtained per area, resulting in a maximum of 12 biopsy cores obtained per participant. MRI-targeted biopsy registration (i.e., matching of the image of the target on MRI with the real-time image of the prostate during biopsy) could be performed by means of visual registration or software-assisted registration (also known as MRI–ultrasonographic fusion)10 and could be carried out through the transrectal or transperineal route, according to local expertise (Table S5 in the Supplementary Appendix). In the absence of abnormal areas on the multiparametric MRI (i.e., a negative result, with a score of 1 or 2), the participant was not offered a protocol biopsy.
STANDARD TRANSRECTAL ULTRASONOGRAPHY–GUIDED BIOPSY
Biopsy was carried out by experienced operators who used a standard transrectal technique. A total of 10 to 12 biopsy cores were obtained from the peripheral zone of the prostate at the base, mid gland, and apex.17
PARTICIPANT-REPORTED OUTCOME MEASURES
Participant-reported questionnaires were used to collect data about intervention-specific side effects immediately and at 30 days after biopsy and after MRI.16,18 Health-related quality of life was assessed with the use of the EuroQol–5 Dimension Self-Report Questionnaire at baseline, 24 hours after the intervention, and 30 days after the intervention.19–21
OUTCOMES
The primary outcome was the proportion of men with clinically significant cancer, defined as the presence of a single biopsy core indicating disease of Gleason score 3+4 (Gleason sum of 7) or greater (the Gleason score is composed of a primary [most predominant] grade plus a secondary [highest nonpredominant] grade; the range for a primary or secondary grade is from 3 to 5, with the Gleason sum ranging from 6 to 10, and with higher scores indicating a more aggressive form of prostate cancer). Secondary outcomes included the proportion of men with clinically insignificant cancer (Gleason score 3+3), the proportion of men in the MRI-targeted biopsy group who did not undergo biopsy, and the proportion of men with adverse events after the intervention. All the secondary outcomes are listed in Table S6 in the Supplementary Appendix. Outcomes were reported according to the START guidelines,10 which are the consensus criteria for reporting studies of MRI-targeted prostate biopsies.
FOLLOW-UP
Participants were followed until the visit at which their treatment decisions were made or until their 30-day postintervention questionnaires were completed, whichever was later. Participants who underwent further diagnostic tests as a result of the outcome of the treatment-decision visit were additionally followed until after the results of the further investigation were made available and recorded. These participants included men who had negative test results in either the standard-biopsy group or the MRI-targeted biopsy group and underwent additional testing. Participants who had negative test results in either group at the end of the trial period returned to standard-care monitoring at each center, which typically involved surveillance of the PSA level. Participants who underwent radical prostatectomy on the basis of their treatment decision were also followed until the pathological testing results of their radical prostatectomy were available. Participants provided written informed consent for long-term follow-up as part of future studies involving additional contact from the trial center and linkage to national databases.
QUALITY CONTROL
Uroradiologists and pathologists at the coordinating center, who were unaware of the results of the original reports, reviewed 25% of the multiparametric MRIs and 15% of the original pathological specimens. These MRIs and specimens had been chosen at random from participants at every site.
STATISTICAL ANALYSIS
Using a noninferiority margin of 5 percentage points that was agreed on at an expert consensus group meeting10 and a one-sided alpha level of 2.5%, we calculated that the randomization of 422 men would provide the trial with 90% power to show the noninferiority of MRI, with or without targeted biopsy, to standard biopsy, assuming a detection rate of clinically significant cancer of 40% in the group that underwent MRI, with or without targeted biopsy, and 30% in the standard-biopsy group. This sample size was increased to 470 to allow for a 10% rate of withdrawal and loss to follow-up. Detailed justification of the sample size is provided in the protocol.16
The statistical analysis plan was prespecified and approved by the data and safety monitoring committee before the analysis of any data. For the primary outcome, if the lower boundary of the two-sided 95% confidence interval for the difference in the rates of detection of clinically significant cancer in the MRI-targeted biopsy group relative to the standard-biopsy group was greater than −5 percentage points, then MRI, with or without targeted biopsy, would be deemed to be noninferior. Furthermore, if the lower boundary was greater than zero, superiority would be claimed. The difference was estimated with the use of a generalized linear mixed model (with the use of an identity link function with a binomial distribution) that included trial center as a random effect.
All the participants who underwent randomization were included in the primary intention-to-treat analysis. Analyses were repeated in the modified intention-to-treat population and the per-protocol population as sensitivity analyses (Table S7A, S7B, and S7C in the Supplementary Appendix). The modified intention-to-treat analysis excluded participants who did not complete a diagnostic test strategy; this analysis was carried out to prevent unequal withdrawal in the two groups from contributing to a difference between the groups. The per-protocol analysis included only men who underwent the randomly assigned testing procedure as specified in the protocol; this analysis was carried out because this was a noninferiority trial, so a per-protocol analysis would reduce the chance of biasing the result toward the null. If, after participants had undergone the trial test procedures, further tests provided different information about the presence of cancer, no adjustment was made to the analyses of the primary and secondary outcomes. A post hoc Bonferroni correction was used to adjust for three secondary outcomes (proportion of men with clinically insignificant cancer, maximum cancer core length, and health-related quality of life), with a two-sided P value of less than 0.017 considered to indicate statistical significance. The methods of analysis of the other outcomes are described in Section S2 in the Supplementary Appendix.
RESULTS
TRIAL POPULATION
From February 2016 through August 2017, a total of 500 participants underwent randomization at 23 of the 25 sites, with 252 participants being assigned to the MRI-targeted biopsy group and 248 to the standard-biopsy group (Fig. 1, and Table S1 in the Supplementary Appendix). The characteristics of the participants at baseline were similar in the two groups (Table 1).
Figure 1. Enrollment, Randomization, and Follow-up of the Participants.
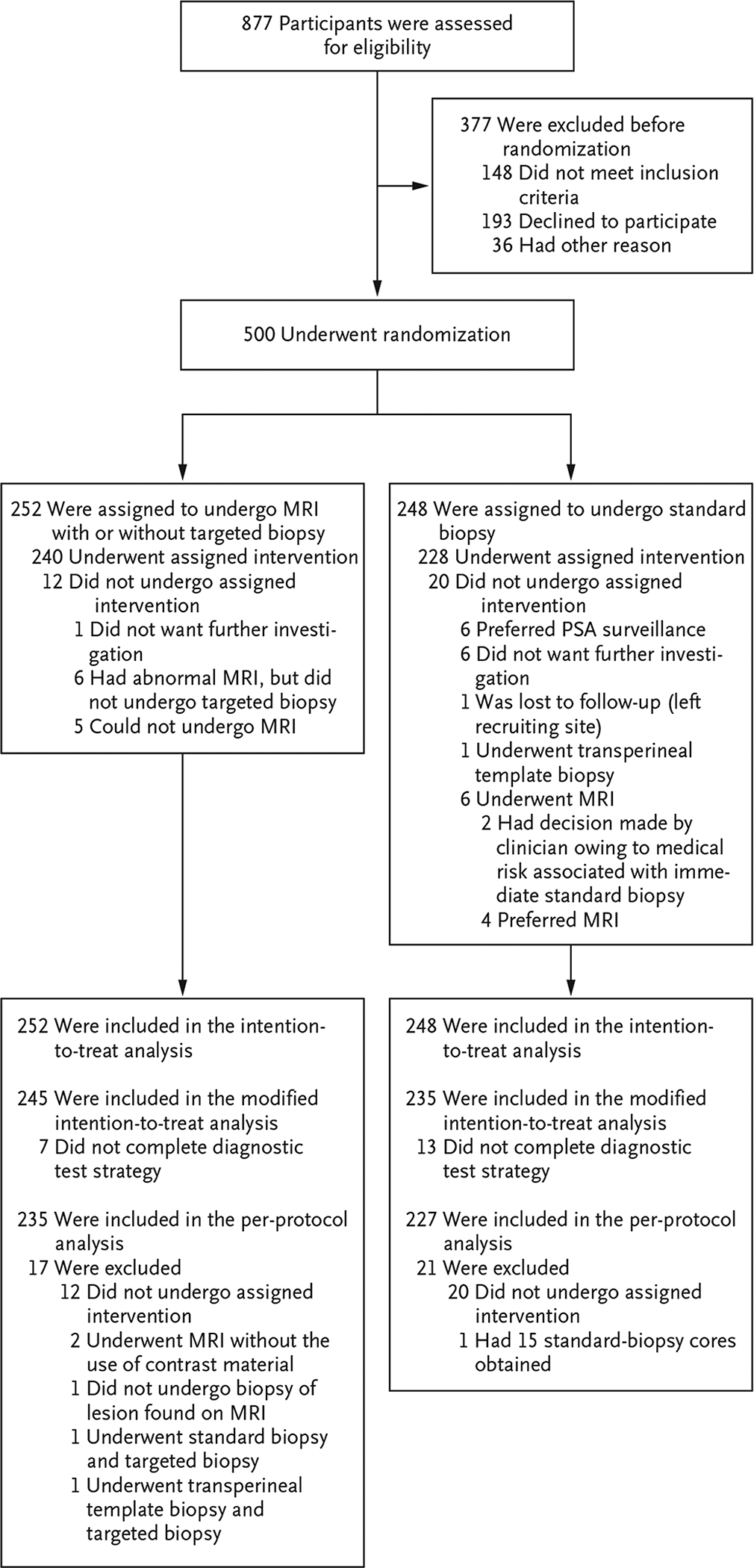
Men who were randomly assigned to the magnetic resonance imaging (MRI)–targeted biopsy group underwent MRI. If the MRI revealed results that were suggestive of prostate cancer, the participant underwent a targeted biopsy; men whose MRI results were not suggestive of prostate cancer were not offered biopsy. Men who were assigned to the standard-biopsy group underwent standard transrectal ultrasonography–guided biopsy. PSA denotes prostate-specific antigen.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | MRI-Targeted Biopsy Group (N = 252) |
Standard-Biopsy Group (N = 248) |
|---|---|---|
| Age — yr | 64.4±7.5 | 64.5±8.0 |
| PSA level — ng/ml | ||
| Median | 6.75 | 6.50 |
| Interquartile range | 5.16–9.35 | 5.14–8.65 |
| Family history of prostate cancer — no. (%) | 48 (19) | 40 (16) |
| Abnormal digital rectal examination — no. (%) | 36 (14) | 38 (15) |
Plus–minus values are means ±SD. Men who were randomly assigned to the MRI-targeted biopsy group underwent MRI. If the MRI revealed results that were suggestive of prostate cancer, the participant underwent a targeted biopsy; men whose MRI results were not suggestive of prostate cancer were not offered biopsy. Men who were assigned to the standard-biopsy group underwent standard transrectal ultrasonography–guided biopsy. The characteristics of the participants at baseline were similar in the two groups. PSA denotes prostate-specific antigen.
A total of 71 of 252 participants (28%) in the MRI-targeted biopsy group had a result on multiparametric MRI that was not suggestive of prostate cancer (PI-RADS v2 score, ≤2), and so they did not undergo biopsy. Among the participants with a positive result on multiparametric MRI, 51 of 175 (29%) had a PI-RADS v2 score of 3, 70 (40%) had a score of 4, and 54 (31%) had a score of 5 (Table S8 in the Supplementary Appendix). The remaining 6 men did not complete the MRI assessment (Fig. 1). Among the participants who underwent biopsy, a median of 4 biopsy cores were obtained in the MRI-targeted biopsy group, as compared with a median of 12 cores in the standard-biopsy group (Table S9 in the Supplementary Appendix).
OUTCOMES
Clinically significant cancer was detected in 95 men (38%) in the MRI-targeted biopsy group, as compared with 64 (26%) in the standard-biopsy group (adjusted difference, 12 percentage points; 95% confidence interval [CI], 4 to 20; P = 0.005) (Table 2). The lower boundary of the 95% confidence interval for the difference was greater than −5 percentage points, so MRI, with or without targeted biopsy, was deemed to be noninferior to standard transrectal ultrasonography–guided biopsy in the detection of clinically significant cancer. Furthermore, the 95% confidence interval showed the superiority of MRI, with or without targeted biopsy, over transrectal ultrasonography–guided biopsy. The results were consistent in the modified intention-to-treat and per-protocol populations (Fig. 2).
Table 2.
Comparison of Cancer Detection between Groups.*
| Outcome | MRI-Targeted Biopsy Group (N = 252) |
Standard-Biopsy Group (N = 248) |
Difference† | P Value |
|---|---|---|---|---|
| Biopsy outcome — no. (%) | — | — | ||
| No biopsy because of negative result on MRI | 71 (28) | 0 | ||
| Benign tissue | 52 (21) | 98 (40) | ||
| Atypical small acinar proliferation | 0 | 5 (2) | ||
| High-grade prostatic intraepithelial neoplasia | 4 (2) | 10 (4) | ||
| Gleason score | ||||
| 3+3 | 23 (9) | 55 (22) | ||
| 3+4 | 52 (21) | 35 (14) | ||
| 3+5 | 2 (1) | 1 (<1) | ||
| 4+3 | 18 (7) | 19 (8) | ||
| 4+4 | 13 (5) | 6 (2) | ||
| 4+5 | 7 (3) | 2 (1) | ||
| 5+5 | 3 (1) | 1 (<1) | ||
| No biopsy‡ | 4 (2) | 3 (1) | ||
| Withdrawal from trial§ | 3 (1) | 13 (5) | ||
| Clinically significant cancer¶ | ||||
| Intention-to-treat analysis — no. (%) | 95 (38) | 64 (26) | 12 (4 to 20) | 0.005 |
| Modified intention-to-treat analysis — no./total no. (%) | 95/245 (39) | 64/235 (27) | 12 (3 to 20) | 0.007 |
| Per-protocol analysis — no./total no. (%) | 92/235 (39) | 62/227 (27) | 12 (3 to 20) | 0.007 |
| Clinically insignificant cancer — no. (%) | 23 (9) | 55 (22) | −13 (−19 to −7) | <0.001 |
| Maximum cancer core length — mm | 7.8±4.1 | 6.5±4.5 | 1.0 (0.0 to 2.1) | 0.053 |
| Core positive for cancer — no./total no. of cores (%) | 422/967 (44) | 515/2788 (18) | — | — |
| Men who did not undergo biopsy — no. (%)‖ | 78 (31) | 16 (6) | — | — |
Clinically significant cancer was defined as the presence of a single biopsy core indicating disease of Gleason score 3+4 (Gleason sum of 7) or greater, and clinically insignificant cancer as a biopsy sample with a Gleason score of 3+3 (Gleason sum of 6). The Gleason score is composed of a primary (most predominant) grade plus a secondary (highest nonpredominant) grade; the range for a primary or secondary grade is from 3 to 5, with the Gleason sum ranging from 6 to 10, and with higher scores indicating a more aggressive form of prostate cancer.
Differences between rates are shown in percentage points, and the difference in maximum cancer core length is shown in millimeters. Differences in the percentages of men with clinically significant cancer detected and men with clinically insignificant cancer were calculated with a generalized linear mixed model (with the use of an identity link function with a binomial distribution) that included trial center as a random effect. The between-center variance estimates for the intention-to-treat analysis of the proportion of men with clinically significant cancer was 0.002 and for the proportion of men with clinically insignificant cancer was 0; the 95% prediction intervals for the detection rates of clinically significant and clinically insignificant cancer, incorporating between-center variation, were 14 to 39% and 17 to 28%, respectively, for standard biopsy, and 26 to 51% and 4 to 11%, respectively, for MRI-targeted biopsy. The difference in the maximum cancer core length was calculated with the use of a linear mixed model with trial center as a random effect. The between-center estimate of variance was 2.14; the 95% prediction interval for the maximum cancer core length, incorporating between-center variation, was 3.3 to 9.8 mm for standard biopsy and 4.4 to 10.8 mm for MRI-targeted biopsy.
In four participants in the MRI-targeted biopsy group, MRI identified at least one area with a score on the Prostate Imaging–Reporting and Data System, version 2, of 3 or greater (on a scale from 1 to 5, with higher numbers indicating a greater likelihood of clinically significant cancers), but targeted biopsy was not performed. In the standard-biopsy group, three participants declined transrectal ultrasonography–guided biopsy and underwent an MRI. The MRI revealed no areas that were suggestive of prostate cancer, and the participants did not undergo biopsy.
These participants did not complete any diagnostic test.
The intention-to-treat analysis included all the participants who underwent randomization, the modified intention-to-treat analysis excluded participants who did not complete a diagnostic test strategy, and the per-protocol analysis included only participants who underwent the randomly assigned testing procedure as specified in the protocol.
Data include men who did not undergo biopsy because they withdrew before undergoing any diagnostic test or because they did not complete the diagnostic strategy.
Figure 2. Intention-to-Treat, Modified Intention-to-Treat, and Per-Protocol Analyses of the Primary Outcome for the Detection of Clinically Significant Prostate Cancer.
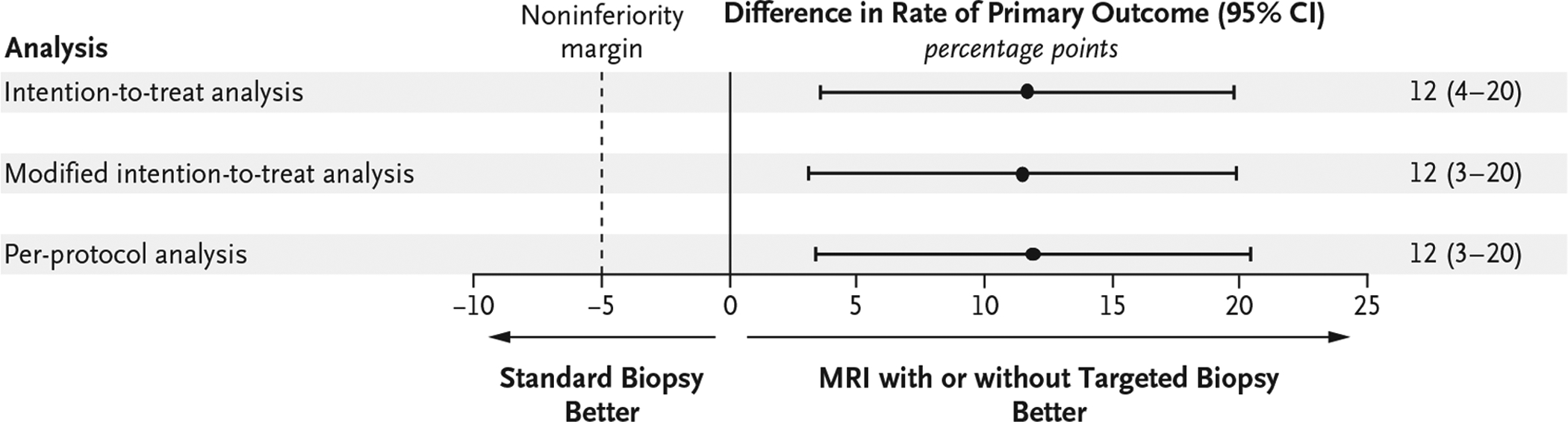
Shown are the absolute differences between the MRI-targeted biopsy group and the standard-biopsy group in the rates of detection of clinically significant cancer. The intention-to-treat analysis included all the participants who underwent randomization, the modified intention-to-treat analysis excluded participants who did not complete a diagnostic test strategy, and the per-protocol analysis included only participants who underwent the randomly assigned testing procedure as specified in the protocol. If the lower boundary of the two-sided 95% confidence interval for the difference (MRI-targeted biopsy group minus standard-biopsy group) was greater than −5 percentage points (dashed line), then MRI, with or without targeted biopsy, would be deemed to be noninferior. If the lower boundary was greater than zero (solid line), superiority would be claimed.
Fewer participants received a diagnosis of clinically insignificant cancer in the MRI-targeted biopsy group than in the standard-biopsy group (23 men [9%] vs. 55 [22%]; adjusted difference, −13 percentage points; 95% CI, −19 to −7; P<0.001). In men with cancer, the mean maximum cancer core length was 7.8 mm in the MRI-targeted biopsy group and 6.5 mm in the standard-biopsy group (adjusted mean difference, 1.0 mm; 95% CI, 0.0 to 2.1; P = 0.053). Interpretations of the results for these secondary outcomes were unchanged by post hoc Bonferroni correction (see Section S3 in the Supplementary Appendix).
A greater percentage of cores were positive for cancer in the MRI-targeted biopsy group (422 of 967 cores [44%]) than in the standard-biopsy group (515 of 2788 [18%]). Among men with a positive result on MRI, the percentage of men with clinically significant cancer was highest among participants with a PI-RADS v2 score of 5 (83%), followed by those with a score of 4 (60%) and those with a score of 3 (12%). Conversely, the percentage of men without cancer was highest among participants with a PI-RADS v2 score of 3 (67%), followed by those with a score of 4 (31%) and those with a score of 5 (6%) (Fig. 3).
Figure 3. Percentages of Men with Clinically Significant, Clinically Insignificant, and No Cancer, Identified According to PI-RADS v2 Score.
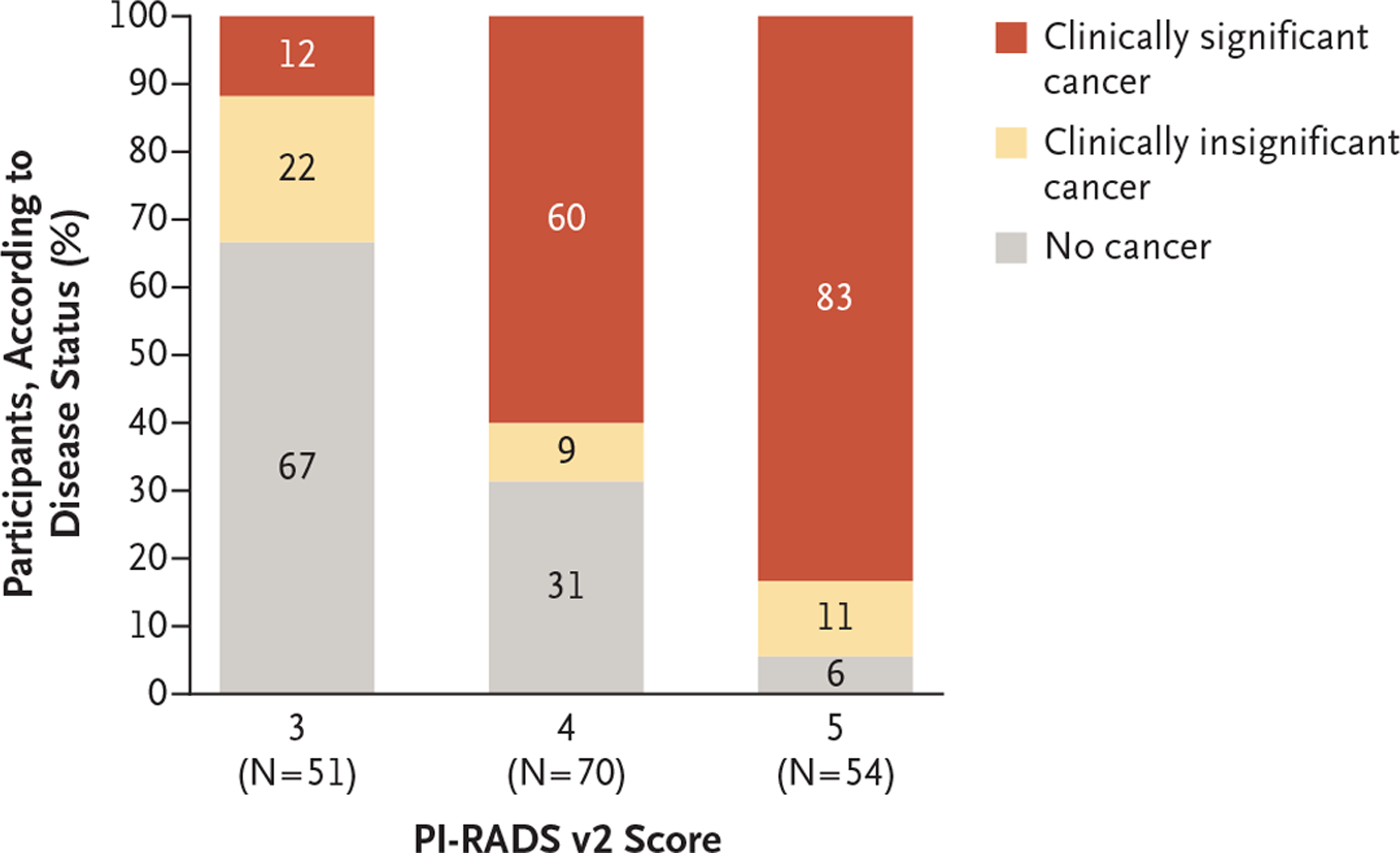
For men randomly assigned to the MRI-targeted biopsy group, the areas of the prostate were scored with the use of the Prostate Imaging–Reporting and Data System, version 2 (PI-RADS v2). Scores range from 1 to 5, with higher numbers indicating a greater likelihood of clinically significant cancer; a score of 3 indicates equivocal results, 4 results that are likely to be prostate cancer, and 5 results that are highly likely to be prostate cancer. Men who had a score of 3 or higher underwent MRI-targeted biopsy. Clinically significant cancer was defined as the presence of a single biopsy core indicating disease of Gleason score 3+4 (Gleason sum of 7) or greater, and clinically insignificant cancer as a biopsy sample with a Gleason score of 3+3 (Gleason sum of 6). The Gleason score is composed of a primary (most predominant) grade plus a secondary (highest nonpredominant) grade; the range for a primary or secondary grade is from 3 to 5, with the Gleason sum ranging from 6 to 10, and with higher scores indicating a more aggressive form of prostate cancer. Percentages may not total 100 because of rounding.
QUALITY OF LIFE AND SAFETY
Health-related quality of life at 24 hours and at 30 days after the intervention did not differ significantly between the MRI-targeted biopsy group and the standard-biopsy group. The intervention was associated with similar results regarding immediate postintervention discomfort and pain in the two groups. The participant-reported complications at 30 days were less frequent in the MRI-targeted biopsy group than in the standard-biopsy group, including events of blood in the urine (30% vs. 63%), blood in the semen (32% vs. 60%), pain at the site of the procedure (13% vs. 23%), rectal bleeding (14% vs. 22%), and erectile dysfunction (11% vs. 16%). These findings reflected the lower percentage of men undergoing biopsy and fewer biopsy cores obtained in the MRI-targeted biopsy group than in the standard-biopsy group. A total of 2% of the men in the MRI-targeted biopsy group and 2% in the standard-biopsy group had serious adverse events. Details regarding health-related quality-of-life scores, participant-reported complications, and adverse events are provided in Tables S10 through S12 in the Supplementary Appendix.
FURTHER DIAGNOSTIC TESTING
After the discussion of the test results with each participant, more men in the standard-biopsy group (39 men [16%]) than in the MRI-targeted biopsy group (7 [3%]) underwent further diagnostic tests. Of the 39 further diagnostic tests that were performed in the standard-biopsy group, 38 (in 15% of the participants in the group) were diagnostic multiparametric MRIs that were carried out in men with negative results on the transrectal ultrasonography–guided biopsy. In the MRI-targeted biopsy group, only 3 participants (1%) with negative results on MRI subsequently underwent standard transrectal ultrasonography–guided biopsy. More men who underwent MRI-targeted biopsy (104 men [41%]) than men who underwent standard transrectal ultrasonography–guided biopsy (74 [30%]) adopted a strategy of monitoring of the PSA level, although the percentage of men undergoing active surveillance or radical treatment was similar in the two groups.
Among the participants who underwent further biopsy, clinically significant cancer was detected in none of the 4 men in the MRI-targeted biopsy group and in 3 of 9 men (33%) in the standard-biopsy group. Of the 71 men with negative results on MRI and no biopsy, 3 (4%) were discharged, 62 (87%) were referred for monitoring of the PSA level, 3 (4%) underwent further prostate biopsy (all had negative results), 1 (1%) underwent an additional multiparametric MRI, and 2 (3%) had missing information. The percentage of men whose Gleason score was upgraded (i.e., found to be higher) after radical prostatectomy was similar in the MRI-targeted biopsy group (5 of 30 men [17%]) and the standard-biopsy group (4 of 27 [15%]). Details are provided in Tables S13 through S15 in the Supplementary Appendix.
QUALITY CONTROL
Results of the quality-control review of multiparametric MRI showed that the percentage of cases that were scored with agreement for concordant biopsy decision by the central radiology team and the site radiologist was 78% (50 of 64 cases). The percentage of cases that were scored with agreement on the Gleason score by the central pathologists and the site pathologist was 88% (53 of 60 cases). Details are provided in Table S16A, S16B, and S16C in the Supplementary Appendix.
DISCUSSION
The ideal test for prostate cancer would be minimally invasive, have few side effects, identify a high proportion of men who would benefit from treatment, and minimize the identification of men with clinically insignificant cancer in order to prevent overtreatment. In men with a clinical suspicion of prostate cancer who had not undergone biopsy of the prostate previously, the PRECISION trial showed that MRI, with or without targeted biopsy, appeared to achieve these goals better than the traditional standard of care, transrectal ultrasonography–guided biopsy. MRI, with or without targeted biopsy, led to fewer men undergoing biopsy, more clinically significant cancers being identified, less overdetection of clinically insignificant cancer, and fewer biopsy cores being obtained than did standard transrectal ultrasonography–guided biopsy. Slightly more than one quarter of the men avoided a biopsy altogether, and the 30-day participant-reported side-effect profile appeared to be more favorable in the MRI-targeted biopsy group than in the standard-biopsy group. The MRI-targeted biopsy approach was also well adhered to by the participants and clinicians, with only 7 of 252 men (3%) not completing the diagnostic test strategy (Fig. 1).
The results of single-center studies have been mixed. Some studies have not shown the superiority of an MRI-based pathway over transrectal ultrasonography–guided biopsy, although these comparisons were likely to have been underpoered.12,22 Other single-center studies have shown advantages of an MRI-based diagnostic pathway over transrectal ultrasonography–guided biopsy,13,14,23 and a meta-analysis of published studies had findings concordant with those of our trial.15 These single-center studies have limitations in their lack of generalizability, and the majority of the studies were small and nonrandomized.
The PRECISION trial was an international trial, and key strengths included its size and pragmatism.24 We did not limit the performance of MRI-targeted biopsy to highly experienced operators, and most of the participating investigators had modest experience with MRI-targeted biopsy, particularly as compared with standard transrectal ultrasonography–guided biopsy. We also allowed nonacademic centers outside the original expert group to take part. In addition, either 1.5-T or 3.0-T MRI machines were permitted, and the use of an endorectal coil was permitted but not required. Also, various techniques of MRI-targeted biopsy, with visual registration or software-assisted registration with either transrectal or transperineal access routes, were permitted. This approach is supported by a meta-analysis of studies that showed a lack of superiority of any one registration approach.25 We believed that the results would be more generalizable if we permitted centers to use their local expertise and resources than if we required that they use a particular operating system or access route that may not have been available to all centers outside of the trial. We observed differences among centers in the detection of clinically significant cancers. However, on average, MRI with or without targeted biopsy was conclusively superior to standard transrectal ultrasonography–guided biopsy.
Our trial has limitations. First, despite the use of standardized reporting of MRI results,9 the central quality-control review of multiparametric MRIs (Table S16A in the Supplementary Appendix) showed moderate agreement (78%) between the site and the central radiologist reading, a finding that highlights that there is still room for improvement in attaining consistency in the reporting of the results of multiparametric MRI. Regardless, the degree of agreement with central interpretation was similar to the interrater agreement that has been seen in other studies involving expert readers of multiparametric MRIs.1,26 This finding highlights the need for further research regarding improvements to the standardization, reproducibility, and reporting of multiparametric MRIs.
Second, a small proportion of the pathological test results were upgraded or downgraded on central pathological review. However, the differences were not substantial between groups, and the agreement that was seen on central review was consistent with that seen in the literature.27
Third, there are concerns about the men with negative results on multiparametric MRI who do not undergo biopsy. It has been shown that these men have a low risk of clinically significant cancer,1 but nonetheless, follow-up with monitoring of the PSA level is routine, reasonable, and safe. Participants provided consent for long-term follow-up in national registries. Moreover, this trial showed that, among men with negative results on initial tests, a far greater proportion of the participants in the standard-biopsy group underwent further diagnostic tests than did those in the MRI-targeted biopsy group, a finding that confirms that a negative result on multiparametric MRI was more reassuring to the participants and clinicians than a negative result on standard transrectal ultrasonography–guided biopsy.
Fourth, it is possible that clinically significant cancers may have been missed by the omission of standard biopsy cores in men in the MRI-targeted biopsy group. Previous well-designed studies have highlighted that the percentage of cases of clinically significant cancer that are missed by MRI-targeted biopsy but detected by standard transrectal ultrasonography–guided biopsy is low, between 0% and 10%.12,13,23,28 Despite more than one quarter of the men avoiding a biopsy, this trial showed that when clinicians limited themselves to the use of MRI-targeted biopsies only, the rates of detection of clinically significant cancer were higher than those seen with the standard of care. Furthermore, because systematic biopsy was avoided, clinically insignificant cancer was detected in fewer men, which may have a substantial benefit in reducing the overtreatment of men with prostate cancer. If both systematic biopsy and MRI-targeted biopsy were carried out in the same man at the same time, the performance of one test could be influenced by the other, which would make it difficult to evaluate the unbiased performance of each test individually.
We acknowledge that the acquisition and reporting of MRI of the prostate are specialist skills with a learning curve and that the radiologists involved in this trial were reporting a high volume of MRIs per year (median, 300 MRIs per year). We suggest that those who report MRIs of the prostate report a high volume of scans under the supervision of a radiologist who is experienced in MRI of the prostate. We acknowledge that a change in the standard of care for prostate-cancer diagnosis would entail changes in health care systems to accommodate appropriate MRI capacity and to meet the training needs of radiologists and urologists. From a health economics perspective, the cost savings with MRI, with or without targeted biopsy, over standard transrectal ultrasonography–guided biopsy may emerge from the earlier detection of clinically significant cancers, fewer cases of insignificant cancer diagnosed, and fewer repeat biopsies. Reports from other studies and in different contexts suggest that this pathway may be cost-effective in the long term.29–31
In conclusion, in men with a clinical suspicion of prostate cancer, we found that a diagnostic pathway including risk assessment with MRI before biopsy and MRI-targeted biopsy in the presence of a lesion suggestive of cancer was superior to the diagnostic pathway of standard transrectal ultrasonography–guided biopsy.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research (NIHR), the U.K. Department of Health, or the European Association of Urology Research Foundation (EAURF).
Supported by the NIHR through a doctoral fellowship award (DRF-2014-07-146, to Dr. Kasivisvanathan) and by a grant (2015001) from the EAURF. This article presents independent research funded by the NIHR and EAURF. Dr. Deeks is a United Kingdom NIHR Senior Investigator Emeritus and has received support from the NIHR Birmingham Biomedical Research Centre. Dr. Takwoingi is supported by the NIHR through a postdoctoral fellowship award (PDF-2017-10-059). Dr. Emberton is a United Kingdom NIHR Senior Investigator and receives research support from UCL Hospitals-UCL NIHR Biomedical Research Centre.
We thank the participants who volunteered to take part in this trial and the trial teams that cared for them, the investigators for their contribution, the UCL Surgical and Interventional Trials Unit for coordination of the trial, and the trial steering committee and data and safety monitoring committee for oversight of the trial.
Appendix
The authors’ full names and academic degrees are as follows: Veeru Kasivisvanathan, M.R.C.S., Antti S. Rannikko, Ph.D., Marcelo Borghi, M.D., Valeria Panebianco, M.D., Lance A. Mynderse, M.D., Markku H. Vaarala, Ph.D., Alberto Briganti, Ph.D., Lars Budäus, M.D., Giles Hellawell, F.R.C.S.(Urol.), Richard G. Hindley, F.R.C.S.(Urol.), Monique J. Roobol, Ph.D., Scott Eggener, M.D., Maneesh Ghei, F.R.C.S.(Urol.), Arnauld Villers, M.D., Franck Bladou, M.D., Geert M. Villeirs, Ph.D., Jaspal Virdi, F.R.C.S.(Urol.), Silvan Boxler, M.D., Grégoire Robert, Ph.D., Paras B. Singh, F.R.C.S.(Urol.), Wulphert Venderink, M.D., Boris A. Hadaschik, M.D., Alain Ruffion, Ph.D., Jim C. Hu, M.D., Daniel Margolis, M.D., Sébastien Crouzet, Ph.D., Laurence Klotz, M.D., Samir S. Taneja, M.D., Peter Pinto, M.D., Inderbir Gill, M.D., Clare Allen, F.R.C.R., Francesco Giganti, M.D., Alex Freeman, F.R.C.Path., Stephen Morris, Ph.D., Shonit Punwani, F.R.C.R., Norman R. Williams, Ph.D., Chris Brew-Graves, M.Sc., Jonathan Deeks, Ph.D., Yemisi Takwoingi, Ph.D., Mark Emberton, F.R.C.S.(Urol.), and Caroline M. Moore, F.R.C.S.(Urol.).
The authors’ affiliations are as follows: University College London (UCL) and UCL Hospitals NHS Foundation Trust (V.K., C.A., F.G., A.F., S.M., S.P., M.E., C.M.M.), London North West Healthcare NHS Trust (G.H.), Whittington Health NHS Trust (M.G.), Royal Free London NHS Foundation Trust (P.B.S.), and UCL Surgical and Interventional Trials Unit (N.R.W., C.B.-G.), London, Hampshire Hospitals NHS Foundation Trust, Basingstoke (R.G.H.), Princess Alexandra Hospital NHS Trust, Harlow (J.V.), and the Institute of Applied Health Research and the NIHR Birmingham Biomedical Research Centre, University of Birmingham, Birmingham (J.D., Y.T.) — all in the United Kingdom; Helsinki University and Helsinki University Hospital, Helsinki (A.S.R.), and Medical Research Center Oulu, University of Oulu and Oulu University Hospital, Oulu (M.H.V.) — all in Finland; Centro de Urología, Buenos Aires (M.B.); Sapienza University, Rome (V.P.), and IRCCS Ospedale San Raffaele and Vita-Salute San Raffaele University, Milan (A.B.) — all in Italy; Mayo Clinic, Rochester, MN (L.A.M.); Martini Klinik, Hamburg (L.B.), University Hospital Essen, Essen (B.A.H.), and University Hospital Heidelberg, Heidelberg (B.A.H.) — all in Germany; Erasmus University Medical Center, Rotterdam (M.J.R.), and Radboud University Medical Center, Nijmegen (W.V.) — both in the Netherlands; University of Chicago, Chicago (S.E.); Université de Lille and Centre Hospitalier Universitaire Lille, Lille (A.V.), Université de Bordeaux and Bordeaux Pellegrin University Hospital, Bordeaux (G.R.), and Hospices Civils de Lyon, Centre Hospitalier Lyon-Sud (A.R.), and Hospices Civils de Lyon of the Hôpital Edouard Herriot (S.C.), Lyon — all in France; Jewish General Hospital, Montreal (F.B.), and Sunnybrook Health Sciences Centre, Toronto (L.K.) — both in Canada; Ghent University Hospital, Ghent, Belgium (G.M.V.); University Hospital Bern, Bern, Switzerland (S.B.); Weill Cornell Medicine, New York–Presbyterian Hospital (J.C.H., D.M.), and New York University Langone Medical Center (S.S.T.), New York; National Institutes of Health, Bethesda, MD (P.P.); and the University of Southern California Institute of Urology, Keck School of Medicine, Los Angeles (I.G.).
Footnotes
A complete list of members of the PRECISION Study Group is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24. [DOI] [PubMed] [Google Scholar]
- 3.Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377: 132–42. [DOI] [PubMed] [Google Scholar]
- 4.Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary nationwide patterns of active surveillance use for prostate cancer. JAMA Intern Med 2015; 175: 1569–71. [DOI] [PubMed] [Google Scholar]
- 5.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 2017; 317: 1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldefrawy A, Katkoori D, Abramowitz M, Soloway MS, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol 2013; 31: 576–80. [DOI] [PubMed] [Google Scholar]
- 7.Egger SJ, Calopedos RJ, O’Connell DL, Chambers SK, Woo HH, Smith DP. Long-term psychological and quality-of-life effects of active surveillance and watchful waiting after diagnosis of low-risk localised prostate cancer. Eur Urol 2017. August 26 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 8.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011; 59: 477–94. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging — Reporting and Data System: 2015, Version 2. Eur Urol 2016; 69: 16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of Reporting for MRI-Targeted Biopsy Studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013; 64: 544–52. [DOI] [PubMed] [Google Scholar]
- 11.Rhudd A, McDonald J, Emberton M, Kasivisvanathan V. The role of the multiparametric MRI in the diagnosis of prostate cancer in biopsy-naïve men. Curr Opin Urol 2017; 27: 488–94. [DOI] [PubMed] [Google Scholar]
- 12.Baco E, Rud E, Eri LM, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol 2016; 69: 149–56. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol 2017; 72: 282–8. [DOI] [PubMed] [Google Scholar]
- 15.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015; 68: 438–50. [DOI] [PubMed] [Google Scholar]
- 16.Kasivisvanathan V, Jichi F, Klotz L, et al. A multicentre randomised controlled trial assessing whether MRI-targeted biopsy is non-inferior to standard transrectal ultrasound guided biopsy for the diagnosis of clinically significant prostate cancer in men without prior biopsy: a study protocol. BMJ Open 2017; 7(10): e017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011; 108(8 Pt 2): E171–E178. [DOI] [PubMed] [Google Scholar]
- 18.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012; 344: d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Devlin NJ, Shah KK, Mulhern B, van Hout B. New methods for modelling EQ-5D-5L value sets: an application to English data. Health Econ 2018; 27: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013; 22:1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonttila PP, Lantto J, Pääkkö E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol 2016; 69: 419–25. [DOI] [PubMed] [Google Scholar]
- 23.Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015; 33(1): 17.e1–17.e7. [DOI] [PubMed] [Google Scholar]
- 24.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
- 25.Wegelin O, van Melick HHE, Hooft L, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration — is there a preferred technique? Eur Urol 2017; 71: 517–31. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology 2016; 280: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brimo F, Schultz L, Epstein JI. The value of mandatory second opinion pathology review of prostate needle biopsy interpretation before radical prostatectomy. J Urol 2010; 184: 126–30. [DOI] [PubMed] [Google Scholar]
- 28.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS Trial. Eur Urol 201466: 343–51. [DOI] [PubMed] [Google Scholar]
- 29.Faria R, Soares MO, Spackman E, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol 2018; 73: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014; 66: 430–6. [DOI] [PubMed] [Google Scholar]
- 31.Cerantola Y, Dragomir A, Tanguay S, Bladou F, Aprikian A, Kassouf W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol 2016; 34(3): 119.e1–e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


