Abstract
Background:
Long-term outcomes of patients treated with salvage lymph node dissection (sLND) for nodal recurrence of prostate cancer (PCa) remain unknown.
Objective:
To investigate long-term oncological outcomes after sLND in a large multi-institutional series.
Design, setting, and participants:
The study included 189 patients who experienced prostate-specific antigen (PSA) rise and nodal-only recurrence after radical prostatectomy (RP) and underwent sLND at 11 tertiary referral centers between 2002 and 2011. Lymph node recurrence was documented by positron emission tomography/computed tomography (PET/CT) scan using either 11C-choline or 68Ga prostate-specific membrane antigen ligand.
Outcome measurements and statistical analysis:
The primary outcome of the study was cancer-specific mortality (CSM). The secondary outcomes were overall mortality, clinical recurrence (CR), biochemical recurrence (BCR), and androgen deprivation therapy (ADT)-free survival after sLND. The probability of freedom from each outcome was calculated using Kaplan-Meier analyses. Cox regression analysis was used to predict the risk of prostate CSM after accounting for several parameters, including the use of additional treatments after sLND.
Results and limitations:
At long term, 110 and 163 patients experienced CR and BCR, respectively, with CR-free and BCR-free survival at 10 yr of 31% and 11%, respectively. After sLND, a total of 145 patients received ADT, with a median time to ADT of 41 mo. At a median (interquartile range) follow-up for survivors of 87 (51, 104) mo, 48 patients died. Of them, 45 died from PCa. The probabilities of freedom from cancer-specific and all-cause death at 10 yr were 66% and 64%, respectively. Similar results were obtained in sensitivity analyses in patients with pelvic-only positive PET/CT scan, as well as after excluding men on ADT at PET/CT scan and patients with PSA level at sLND higher than the 75th percentile. At multivariable analyses, patients who had PSA response after sLND (hazard ratio [HR]: 0.45; p = 0.001), and those receiving ADT within 6 mo from sLND (HR: 0.51; p = 0.010) had lower risk of death from PCa.
Conclusions:
A third of men treated with sLND for PET-detected nodal recurrence of PCa died at long term, with PCa being the main cause of death. Salvage LND alone was associated with durable long-term outcomes in a minority of men who significantly benefited from additional treatments after surgery. Taken together, all these data argue against the use of metastasis-directed therapy alone for patients with node-only recurrent PCa. These men should instead be considered at high risk of systemic dissemination already at the time of sLND.
Patient summary:
We assessed long-term outcomes of patients treated with salvage lymph node dissection (sLND) for node-recurrent prostate cancer (PCa). In contrast with prior evidence, we found that the majority of these men recurred after sLND and eventually died from PCa. A significant survival benefit associated with the administration of androgen deprivation therapy after sLND suggests that sLND should be considered part of a multimodal approach rather than an exclusive treatment strategy.
Keywords: Prostate cancer, Neoplasm recurrence, Positron emission tomography scan, Metastasis-directed therapy, Salvage lymph node dissection, Androgen deprivation therapy
1. Introduction
Introduction of new imaging modalities such as choline or prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has led to early identification of recurrent prostate cancer (PCa) after primary treatment [1–4]. However, patients with PET-detected recurrence represent a heterogeneous population of men sharing different outcomes according to the site and number of suspicious spots. It has indeed been shown that men with nodal metastases have better prognosis than those with bone or visceral recurrences [5]. Despite this evidence, controversies exist about the optimal management of these patients considering the lack of solid prospective randomized evidence supporting any imaging-guided approach in men with PET-detected recurrence on hard clinical outcomes. In this context, it has been proposed that metastasis-directed therapy (MDT) might be beneficial for patients with limited metastatic burden, reducing the risk of further progression and delaying the need for systemic treatments [6–11]. A number of phase 2 clinical trials investigated mainly stereotactic body radiation therapy (SBRT) as an MDT option [12–14]. However, large phase 3 randomized trials to support MDT as a possible treatment strategy for these men are still awaited.
Salvage lymph node dissection (sLND) has been shown to be a possible MDT option for patients with nodal recurrence after primary treatment for PCa. Even in the absence of solid prospective data, encouraging perioperative and short-term outcomes have been reported in retrospective series [15–18]. However, long-term outcomes of patients treated with sLND are virtually unknown. This is key given the relatively long natural history of PCa in these patients. A recent systematic review on 27 studies and 1370 patients showed that only 41% of studies reported a follow-up of >2 yr after surgery [16]. Moreover, a common limitation of papers on this topic is small sample size. For these reasons, there is a clear need for investigations with adequate power and follow-up to assess long-term outcomes, providing additional evidence on sLND and, more generally, on MDT for oligometastatic disease. We here report long-term oncological outcomes of a large multi-institutional cohort of patients treated with sLND for nodal recurrence of PCa.
2. Patients and methods
2.1. Patient population
We identified 211 patients who experienced prostate-specific antigen (PSA) rise and nodal-only recurrence after radical prostatectomy (RP) and underwent sLND at 11 tertiary referral centers between 2002 and 2011. Institutional Review Board approval was obtained for data sharing through different centers. Lymph node recurrence was documented by PET/CT scan using either 11C-choline or 68Ga-labeled PSMA (68Ga-PSMA) ligand. To exclude other sites of recurrence, preoperative imaging included abdominal CT scan, whole-body magnetic resonance imaging, or bone scan using technetium-99 m methylene diphosphonate (Tc99m MDP) according to the standard of care at each treating institution. As a result, all patients included in this series did not have preoperative imaging suspicious for local recurrence (ie, recurrence in the prostatic fossa) and/or M1b-M1c disease. We excluded patients with missing data for the number of nodes removed at sLND (n = 3), PSA level after sLND (n = 10), and androgen deprivation therapy (ADT) administration after sLND (n = 9). These selection criteria yielded 189 evaluable individuals with complete clinical, pathological, and follow-up data.
2.2. Surgical technique
The surgical technique was previously described [15,19], and it was consistent across different centers. Briefly, pelvic sLND consisted of the excision of external iliac, obturator, internal iliac, common iliac, and presacral nodes. The distal limit of the dissection was represented by the femoral canal, whereas the proximal limit consisted of the aortic bifurcation. All fibro-fatty tissue within the obturator fossa was removed, to completely skeletonize the obturator nerve. The medial and lateral limits of the dissection consisted of the perivesical fat and pelvic sidewall, respectively. At the discretion of the surgeon, intraoperative frozen section analysis of the common iliac nodes was performed. When positive, lymphadenectomy was extended to the retroperitoneum, based on the high probability of retroperitoneal lymph node metastases [20].
Retroperitoneal sLND consisted of the excision of all nodal tissue located between the renal artery (cranially) and the aortic bifurcation (caudally). Medial and lateral limits consisted of the midline of the inferior caval vein and the right ureter (on the right), as well as the midline of the aorta and the left ureter (on the left). All nodal specimens were mapped according to their anatomic location and sent for pathological assessment in multiple packages.
Patients received pelvic sLND when PET/CT showed positive spots in the pelvic area. Retroperitoneal sLND was performed in case of positive spots at PET/CT scan or positive intraoperative frozen section analysis of the common iliac nodes [20,21].
2.3. Definition of variables
Available data consisted of variables related to the following:
RP: age at RP (years), PSA level at RP (ng/mL), pT stage (pT2 vs. pT3a vs. ≥ pT3b), pathological International Society of Urological Pathology (ISUP) group (≤3 vs. 4 vs. 5), pN stage (pN0 vs. pN1 vs. pNx), surgical margin status (negative vs. positive), number of nodes removed, number of positive nodes (0 vs. 1 vs. 2 vs. ≥3), post-prostatectomy radiation therapy (RT; no vs. yes), and post-prostatectomy ADT (no vs. yes).
Salvage LND, pre-treatment: time from RP to PSA rising (months), type of PET/CT tracer (11C-choline vs. 68Ga-PSMA), ongoing ADT at the time of PET/CT scan (no vs. yes), site of positive spots at PET/CT scan (pelvic vs. retroperitoneal vs. both), number of positive spots at PET/CT (0 vs. 1 vs. 2 vs. ≥3), age at sLND (years), and PSA level at sLND (ng/mL).
Salvage LND, post-treatment: number of lymph nodes removed at sLND, number of positive nodes at sLND (0 vs. 1 vs. 2 vs. ≥3), PSA level after sLND (ng/mL; measured at 1 mo after surgery), PSA response after sLND (no vs. yes; defined as PSA level <0.2 ng/mL), post-sLND complications according to Clavien-Dindo classification [22] (≤1 vs. ≥2), and additional therapies after sLND.
2.4. Follow-up
Follow-up consisted of PSA testing 1 mo after surgery, and subsequently, further measurements were performed at time intervals of 3–6 mo. Postoperative PET/CT scan and eventual bone scan using Tc99m MDP were performed in case of PSA rising after sLND. Administration of additional treatments after sLND was left at the discretion of each surgeon.
2.5. Outcome definition
The primary outcome of the study was cancer-specific mortality. The secondary outcomes were overall mortality, clinical recurrence (CR; defined as positive imaging in presence of a rising PSA level), biochemical recurrence (BCR; defined as PSA level ≥0.2 ng/mL and rising), and ADT-free survival after sLND. Vital status and cause of death were identified from death certificates and physician correspondence.
2.6. Statistical analyses
The probability of freedom from each outcome of interest and corresponding 95% confidence interval (CI) were calculated using Kaplan-Meier analyses. For non-mortality outcomes, patients were censored on the date of last evidence of freedom from BCR, CR, and ADT. Moreover, we investigated predictors of cancer-specific mortality using a multivariable Cox proportional hazards model. Predictors were selected among potential factors associated with oncological outcomes after sLND and consisted of ISUP grade group at RP (≤4 vs. 5) [15], PSA level at sLND (ng/mL), number of nodes removed at sLND, number of positive nodes after sLND (≤2 vs. ≥3) [23], PSA response after sLND (no vs. yes), ADT administration within 6 mo from sLND (no vs. yes), and RT administration within 6 mo from sLND (no vs. yes). This temporal window was chosen to reproduce a multimodal treatment strategy delivered right after surgery, according to a previously defined cutoff [24]. Patients were considered at risk of cancer-specific mortality once the 6-mo landmark was reached. As a consequence, this analysis did not include patients with follow-up <6 mo (n = 5). Sensitivity analyses were performed in patients with positive PET/CT scan only in the pelvis, after excluding patients on ADT at PET/CT scan and those whose PSA level at sLND was higher than the 75th percentile. Since data from different institutions are correlated, we incorporated institution clustering in our analysis using the cluster option in Stata statistical software.
All statistical analyses were performed using Stata version 14.0 (StataCorp LP, College Station, TX, USA).
3. Results
Descriptive characteristics of the patients are reported in Tables 1–3. At RP, 35 (19%) patients had ISUP grade group 5 tumor, whereas 33 (17%) had nodal involvement (Table 1).
Table 1 –
Descriptive characteristics of patients related to radical prostatectomy (RP).
| Variable | Overall population (n = 189; 100%) |
|---|---|
| Age at RP (yr) | 61 (55, 66) |
| PSA level at RP (ng/mL) | 10.1 (7.0, 16.0) |
| pT stage, n (%) | |
| pT2 | 64 (34) |
| pT3a | 66 (35) |
| pT3b/pT4 | 56 (30) |
| Unknown | 3 (1) |
| Pathological ISUP group, n (%) | |
| ≤3 | 119 (62) |
| 4 | 35 (19) |
| 5 | 35 (19) |
| LND performed during RP, n (%) | |
| No | 17 (9) |
| Yes | 163 (86) |
| Unknown | 9 (5) |
| pN stage, n (%) | |
| pN0 | 130 (69) |
| pN1 | 33 (17) |
| pNx | 17 (9) |
| Unknown | 9 (5) |
| Surgical margins, n (%) | |
| Negative | 115 (61) |
| Positive | 63 (33) |
| Unknown | 11 (6) |
| Number of nodes removeda | 8 (4, 13) |
| Number of positive nodesa, n (%) | |
| 0 | 130 (69) |
| 1 | 12 (6) |
| 2 | 11 (6) |
| ≥3 | 10 (5) |
| Unknown | 26 (14) |
| Postprostatectomy RT, n (%) | |
| No | 68 (36) |
| Yes | 116 (61) |
| Unknown | 5 (3) |
| Postprostatectomy ADT, n (%) | |
| No | 74 (39) |
| Yes | 114 (60) |
| Unknown | 1 (1) |
ADT = androgen deprivation therapy; ISUP = International Society of Urological Pathology; LND = lymph node dissection; PSA = prostate-specific antigen; RP = radical prostatectomy; RT = radiation therapy.
All numbers are medians (interquartile range) and frequencies (proportions).
Data were reported for patients who received pelvic lymph node dissection at the time of radical prostatectomy.
Table 3 –
Descriptive postoperative characteristics of patients related to salvage lymph node dissection (sLND).
| Variable | Overall population (n = 189; 100%) |
|---|---|
| Surgical approach, n (%) | |
| Open | 183 (97) |
| Laparoscopic | 6 (3) |
| Lymph nodes removed at sLND | 19 (12, 35) |
| Positive nodes at sLND, n (%) | |
| 0 | 40 (21) |
| 1 | 33 (17) |
| 2 | 18 (10) |
| ≥3 | 98 (52) |
| PSA level after sLND (ng/mL) | 0.6 (0.0, 2.0) |
| PSA response (<0.2 ng/mL) after sLND, n (%) | |
| No | 120 (63) |
| Yes | 69 (37) |
| Post-sLND complications, n (%) | |
| Clavien-Dindo ≤1 | 137 (72) |
| Clavien-Dindo ≥2 | 25 (13) |
| Unknown | 27 (14) |
| Additional therapies after sLND | |
| None | 14 |
| ADT alone | 81 |
| RT alone | 18 |
| ADT + RT | 29 |
| ADT + CT | 6 |
| ADT + RT + CT | 11 |
| Other combinations | 30 |
| ADT administration within 6 mo from sLND, n (%) | |
| No | 74 (39) |
| Yes | 115 (61) |
| RT administration within 6 mo from sLND, n (%) | |
| No | 166 (88) |
| Yes | 23 (12) |
ADT = androgen deprivation therapy; CT = chemotherapy; PSA = prostate-specific antigen; RT = radiation therapy.
All numbers are medians (interquartile range) and frequencies (proportions).
Data on the site of positive spots at PET/CT were available for the majority of patients (84%; Table 2). Among patients with only pelvic uptake (n = 113), 49 (43%) men received pelvic sLND only and 64 (57%) received both pelvic and retroperitoneal sLND. Conversely, retroperitoneal uptake was present in 45 patients. All patients with retroperitoneal uptake at PET/CT scan received both pelvic and retroperitoneal sLND. Among 31 patients with missing data on the site of positive PET/CT scan, 27 (87%) patients received both pelvic and retroperitoneal sLND, whereas four (13%) men received only pelvic sLND.
Table 2 –
Descriptive preoperative characteristics of patients related to salvage lymph node dissection (sLND).
| Variable | Overall population (n = 189; 100%) |
|---|---|
| Time from RP to PSA rising (mo) | 22 (7, 38) |
| Type of PET/CT tracer, n (%) | |
| 11C-choline | 154 (81) |
| 68Ga-PSMA | 6 (3) |
| Unknown | 29 (16) |
| Ongoing ADT at the time of PET/CT, n (%) | |
| No | 98 (52) |
| Yes | 34 (18) |
| Unknown | 57 (30) |
| Site of positive spots at PET/CT, n (%) | |
| Pelvic | 113 (60) |
| Retroperitoneal | 18 (10) |
| Both | 27 (14) |
| Unknown | 31 (16) |
| Number of positive spots at PET/CT, n (%) | |
| 0 | 9 (5) |
| 1 | 91 (48) |
| 2 | 26 (14) |
| ≥3 | 38 (20) |
| Unknown | 25 (13) |
| Age at sLND (yr) | 65 (60, 70) |
| PSA level at sLND (ng/mL) | 2.5 (1.1, 5.4) |
ADT = androgen deprivation therapy; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; PET/CT = positron emission tomography/computed tomography; RP = radical prostatectomy.
All numbers are medians (interquartile range) and frequencies (proportions).
The median number of lymph nodes removed at sLND was 19 (interquartile range [IQR]: 12, 35), and almost half of the patients (n = 98; 52%) had three or more positive nodes at final pathology (Table 3). Overall, 69 (37%) patients had PSA response after sLND. Intra- and post-operative complications are described in the Supplementary material.
There were 163 cases of BCR. The 10and 12-yr probabilities of freedom from BCR were 11% (95% CI: 7–17) and 9% (95% CI: 5–15), respectively (Fig. 1C). Median time to BCR after sLND was 22 mo (95% CI: 11–28).
Fig. 1 –
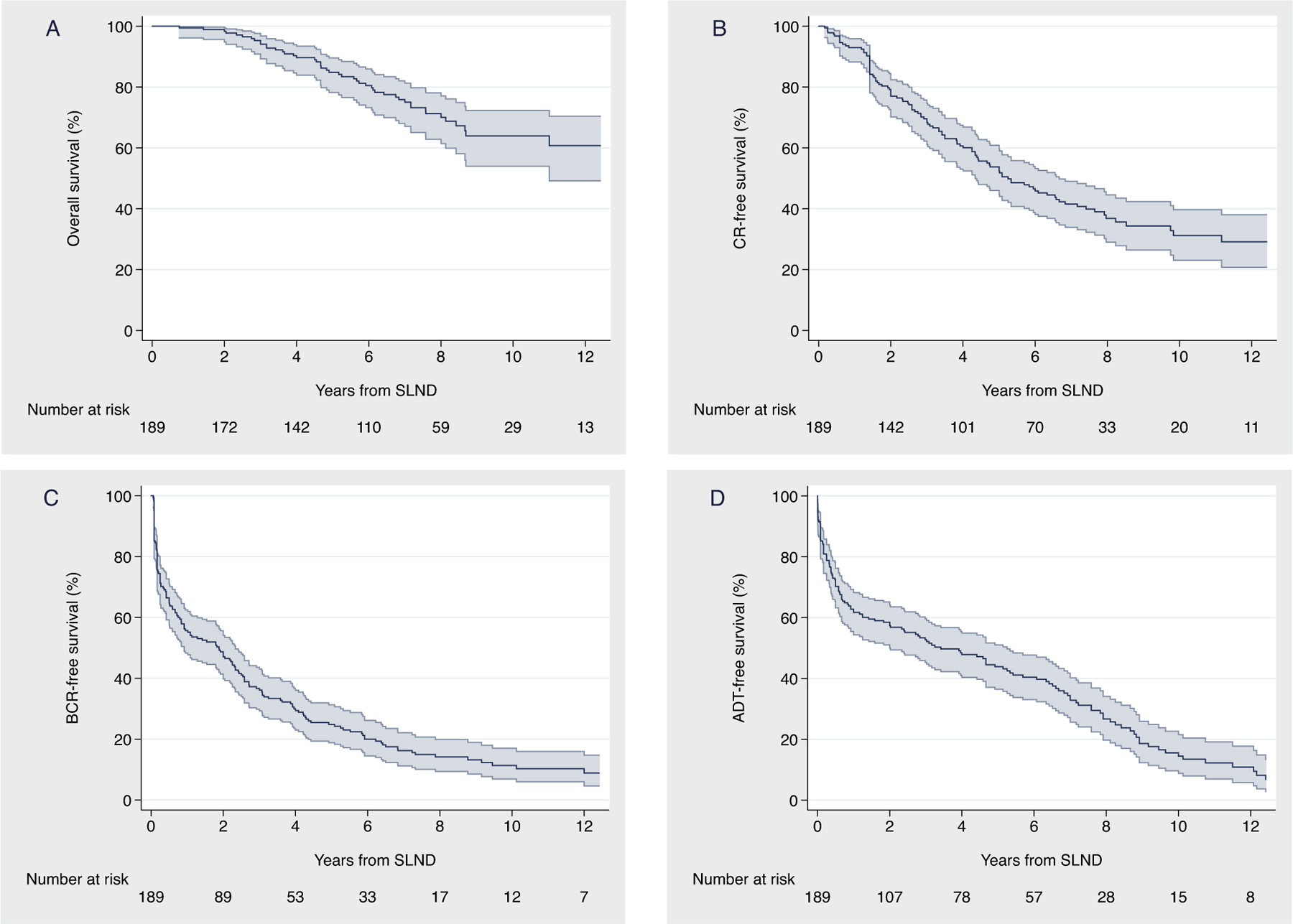
Kaplan-Meier analysis depicting (A) overall survival, (B) clinical recurrence, (C) biochemical recurrence, and (D) ADT-free survival after salvage lymph node dissection. ADT = androgen deprivation therapy; BCR = biochemical recurrence; CR = clinical recurrence; SLND = salvage lymph node dissection.
A total of 110 patients developed CR. Among 84 (76%) patients with available data on the site of CR, the locations were skeletal (n = 30, 36%), retroperitoneal (n = 24, 29%), pelvic (n = 22, 26%), visceral (n = 6, 7%), and prostatic fossa (n = 2, 2%). The median time to CR was 63 mo (95% CI: 52–80). The 10and 12-yr probabilities of freedom from metastases were 31% (95% CI: 23–40) and 29% (95% CI: 21–38), respectively (Fig. 1B). Most patients recurred within 5 yr after sLND. However, if a patient did not recur within the first 5 yr, the probability of remaining free from CR at 10 yr was 58% (95% CI: 44–70; Supplementary material).
After sLND, a total of 145, 70, 31, 18, and five patients received hormonal therapy, radiotherapy, chemotherapy, additional surgery, and other therapy, respectively. Table 3 describes the most frequent combinations of post-sLND therapies; a full report is shown in the Supplementary material. Median time to ADT was 41 mo (95% CI: 24–63). The 1-, 3-, and 5-yr probabilities of freedom from hormonal therapy were 62% (95% CI: 54–68), 53% (95% CI: 45–60), and 44% (95% CI: 36–51), respectively (Fig. 1D).
Overall, 48 patients died during follow-up, 45 of them from PCa. The median follow-up for survivors was 87 (IQR: 51, 104) mo. The predicted 8-, 10-, and 12-yr cancer-specific survival was 72% (95% CI: 63–79), 66% (95% CI: 55–74), and 62% (95% CI: 50–72), respectively (Fig. 2). The predicted 8-, 10-, and 12-year overall survival was 70% (95% CI: 61–77), 64% (95% CI: 54–72), and 61% (95% CI: 49–70), respectively (Fig. 1A). These results were confirmed at sensitivity analyses in patients with pelvic-only positive PET/CT scan, and after excluding men on ADT at PET/CT scan and those with PSA level at sLND higher than the 75th percentile (Supplementary material).
Fig. 2 –
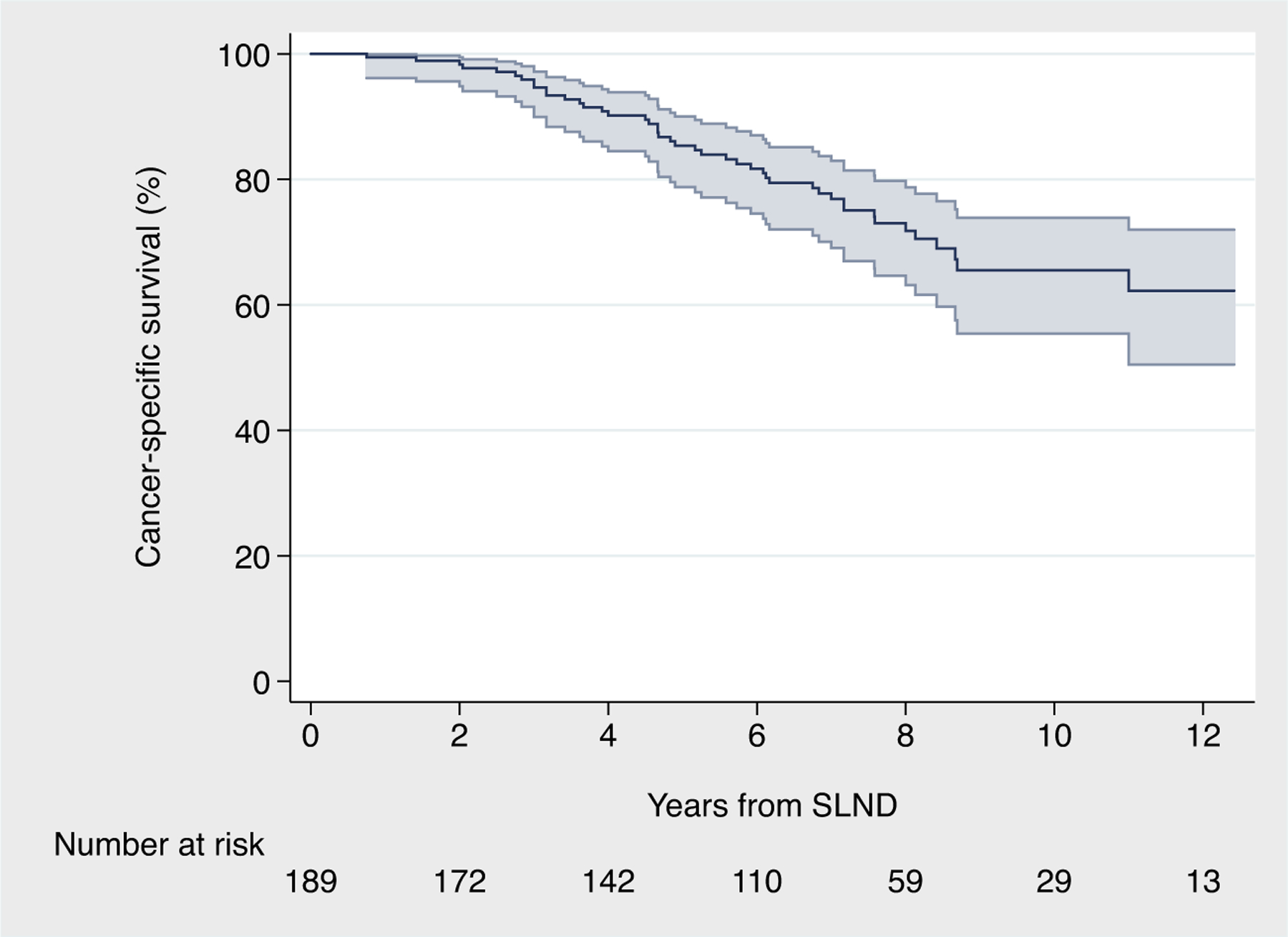
Kaplan-Meier curves depicting cancer-specific survival after salvage lymph node dissection for node-recurrent prostate cancer. SLND = salvage lymph node dissection.
At multivariable Cox regression analyses, patients who had PSA response after sLND (hazard ratio [HR]: 0.45; 95% CI: 0.28, 0.73; p = 0.001) and those receiving ADT within 6 mo from sLND (HR: 0.51; 95% CI: 0.31, 0.85; p = 0.010) had lower risk of death from PCa (Table 4).
Table 4 –
Multivariable Cox regression to predict cancer-specific mortality for after salvage lymph node dissection.
| Variable | Hazards ratio | 95% CI | p value |
|---|---|---|---|
| ISUP grade at RP | |||
| ≤4 | Ref. | - | |
| ≥5 | 1.34 | 0.89, 2.03 | 0.2 |
| PSA at sLND | 1.01 | 1.00, 1.02 | 0.2 |
| Number of nodes removed at sLND | 1.00 | 0.97, 1.02 | 0.7 |
| Number of positive nodes at sLND | |||
| ≤2 | Ref. | - | |
| 3+ | 2.13 | 0.87, 5.20 | 0.10 |
| PSA response | |||
| No | Ref. | - | |
| Yes | 0.45 | 0.28, 0.73 | 0.001 |
| ADT administration within 6 mo from sLND | |||
| No | Ref. | - | |
| Yes | 0.51 | 0.31, 0.85 | 0.010 |
| RT administration within 6 mo from sLND | |||
| No | Ref. | - | |
| Yes | 0.55 | 0.19, 1.58 | 0.3 |
ADT = androgen deprivation therapy; CI = confidence interval; ISUP = International Society of Urological Pathology; RP = radical prostatectomy; PSA = prostate-specific antigen; Ref. = reference; RT = radiation therapy; sLND = salvage lymph node dissection.
4. Discussion
In this study, we described the long-term outcomes of men with node-recurrent PCa treated with sLND. Notably, in contrast with previous findings reporting more favorable outcomes, we found high rates of cancer-specific mortality at long term. Indeed, one out of three patients died of PCa 10 yr after surgery.
Our findings provide relevant insights into the natural history of PCa following sLND for node-recurrent disease and thus have relevant implications for clinical practice. Initial single-center series on sLND found favorable outcomes for men with lymph node–only recurrent disease, supporting the hypothesis that patients with limited metastatic burden might be amenable to treatment strategies with curative intent [19,25]. Although this is not in contrast with our results, we found that the long-term prognosis of patients treated with sLND seems to be much worse than previously thought. This may be a consequence of the long-term follow-up in our series, which included a median follow-up of >7 yr for survivors. Under this assumption, it is thus possible that the encouraging results found by other investigators [6,26–28] might derive from an immature follow-up. For instance, Steuber et al [6] described favorable outcomes for 263 men with nodal metastasis treated with either sLND or SBRT, with 12 of them dying from PCa during the study period. However, a closer look at their survival analyses shows that only 65 men were at risk 10 yr after treatment, suggesting that the majority of patients did not contribute to long-term survival rates. That said, the large population included in our study and the adequate length of follow-up make us confident that our results provide solid evidence on long-term outcomes of sLND.
Our data entail a second critical point for the management of patients with node-only recurrent PCa. The significant association between improved survival and administration of ADT after sLND suggests that the majority of these patients might not be cured by surgery alone. Accordingly, although MDT has the potential to improve outcomes of these patients, it remains an investigational area and its use has still to be considered part of a multimodal approach rather than an exclusive treatment [29]. In this context, confirmatory evidence is awaited from ongoing prospective trials. The NCT02974075 trial is assessing perioperative and oncological outcomes of 70 patients treated with sLND in a phase I/II study. The STORM trial (NCT03569241) is a phase II trial randomizing patients with a maximum of three pelvic nodal recurrences between MDT (including sLND) with short-term ADT and MDT with short-term ADT in combination with whole pelvic radiotherapy. The primary endpoint of the study is metastasis-free survival. These prospective studies should increase the power to uncover a survival benefit associated with MDT in the setting of oligometastatic PCa.
Our study also has implications for clinical research, and it represents a benchmark for future investigations on this topic. The natural history of PCa after sLND is indeed incompletely understood. While prior studies identified predictors of recurrence [15,30], future research should examine the distribution of such recurrences, and validate biomarkers (in addition to clinical parameters) able to identify patients who will and will not benefit from MDT at long term. This also reiterates the importance of risk assessment and patient selection. Although predictive models are available in the literature [15], future investigations should focus on optimization of preoperative patient selection, with the final aim of sparing overtreatment in patients unlikely to benefit from surgery. The increasing availability of genetic tests offers the opportunity to reach this goal [31,32]. In this regard, although most of the available tools were developed for decision making in the primary treatment setting [31], our data suggest that patient selection among men with PSA rise after surgery followed by positive imaging still needs to be implemented, and thus, future validations of genetic assays in this patient population are awaited. This may open new perspectives in the oligorecurrent setting, with the potential of identifying patients who might benefit from sLND alone as a result of better risk stratification. Moreover, there is a clear need for studies assessing patterns of metastatic diffusion, as the optimal template of sLND is still unclear. In this regard, the ProsTone Trial (NCT04271579) is a prospective randomized study investigating the oncological outcome (namely, biochemical response after sLND) of universus bilateral pelvic sLND. There is also growing interest toward imaging-guided surgery such as 68Ga-PSMA radio-guided surgery, which showed promising preliminary results [33]. This implementation, together with the use of a robotic approach [21], has the potential to mitigate the morbidity of sLND and improve the detection of suspicious lymph nodes at imaging at the same time. Finally, while a number of trials testing the oncological efficacy of SBRT in the oligometastatic setting (NCT02759783, NCT02417662) are ongoing, prospective, high-level evidence on sLND is urgently needed.
Our study has several limitations. First, no control group including men managed with either observation or systemic treatment was available. Moreover, the use of additional treatments after sLND was left at the discretion of physicians. As such, it is hard to extrapolate the relative contribution of sLND and subsequent treatments to oncological outcomes given possible patient selection biases. However, the role of a multimodal approach was confirmed at multivariable analyses accounting for all possible significant confounders. A central re-evaluation of PET/CT scans was not performed. Still, all patients received PET/CT scan at high-volume centers, and the variability in imaging evaluation may be limited. We also have to acknowledge that, given the limited availability of 68Ga-PSMA tracer during the period of study, the majority of patients in our series received 11C-choline PET/CT scan, and as such, there might have been an underestimation of systemic disease at the time of sLND. Similarly, despite the presence of negative pre-sLND imaging for local (prostate bed) recurrence, it is possible that a portion of patients had undetected local disease recurrence. We also noted that, although preoperative characteristics were similar to those described in a recent systematic review [16], approximately half of our population had more than three positive lymph nodes at final pathology after sLND, a finding that might explain the worse-than-expected outcomes of our study. Still, the rate of pathologically confirmed positive nodes was not higher than that of previously published series [16]. Moreover, our multivariable model predicting oncological outcomes included the number of positive nodes at final pathology after sLND, and finally, our results remained consistent in a number of sensitivity analyses after the exclusion of patients with unfavorable characteristics. For these reasons, we are positive that our findings were not a consequence of unbalances in casemix. That said, we cannot exclude residual confounding from known and unknown variables. For instance, the multi-institutional data collection did not account for central specimen review or surgical experience of each treating surgeon. Since there is evidence that these factors might influence results and outcomes after surgery [34–36], it is plausible that the inclusion of such features might affect our results. Despite these limitations, our study represents the largest and only sLND series with mature follow-up after sLND.
5. Conclusions
Based on the largest available sLND series with long term follow-up, we remarkably found that approximately two out of three patients experienced CR and a third of them died from PCa at 10-yr follow-up. These results are much worse than what reported in all previous series with shorter follow-up and fewer patients included. Moreover, our results support the role of a multimodal approach after sLND, including the use of ADT in order to maximize patient outcomes. Taken together, these data suggest that MDT might be curative in a very small proportion of these patients who should instead be considered at high risk of systemic dissemination already at the time of sLND. Future trials testing the combination of different local and systemic approaches in men with node-only recurrent disease are eagerly awaited.
Supplementary Material
Footnotes
Financial disclosures: Alberto Briganti certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.06.043.
References
- [1].Battaglia A, De Meerleer G, Tosco L, et al. Novel insights into the management of oligometastatic prostate cancer: a comprehensive review. Eur Urol Oncol 2019;2:174–88. [DOI] [PubMed] [Google Scholar]
- [2].De Visschere PJL, Standaert C, Fütterer JJ, et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol 2019;2:47–76. [DOI] [PubMed] [Google Scholar]
- [3].Rauscher I, Düwel C, Haller B, et al. Efficacy, predictive factors, and prediction nomograms for 68ga-labeled prostate-specific membrane antigen–ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol 2018;73:656–61. 10.1016/j.eururo.2018.01.006. [DOI] [PubMed] [Google Scholar]
- [4].Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the Management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol 2018;74:179–90. [DOI] [PubMed] [Google Scholar]
- [5].Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol 2015;68:325–34. [DOI] [PubMed] [Google Scholar]
- [6].Steuber T, Jilg C, Tennstedt P, et al. Standard of care versus metastases-directed therapy for PET-detected nodal oligorecurrent prostate cancer following multimodality treatment: a multi-institutional case-control study. Eur Urol Focus 2019;5:1007–13. [DOI] [PubMed] [Google Scholar]
- [7].Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol 2018;1:531–7. [DOI] [PubMed] [Google Scholar]
- [8].Aluwini SS, Mehra N, Lolkema MP, et al. Oligometastatic prostate cancer: results of a Dutch multidisciplinary consensus meeting. Eur Urol Oncol 2020;3:231–8. [DOI] [PubMed] [Google Scholar]
- [9].Montorsi F, Gandaglia G, Fossati N, et al. Robot-assisted salvage lymph node dissection for clinically recurrent prostate cancer. Eur Urol 2017;72:432–8. [DOI] [PubMed] [Google Scholar]
- [10].De Bleser E, Jereczek-Fossa BA, Pasquier D, et al. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: a multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol 2019;76:732–9. [DOI] [PubMed] [Google Scholar]
- [11].Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015;67:852–63. [DOI] [PubMed] [Google Scholar]
- [12].Ost P, Reynders D, Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2018;36:446–53. [DOI] [PubMed] [Google Scholar]
- [13].Siva S, Bressel M, Murphy DG, et al. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol 2018;74:455–62. [DOI] [PubMed] [Google Scholar]
- [14].Phillips R, Lim SJ, Shi WY, et al. Primary outcomes of a phase II randomized trial of Observation Versus Stereotactic Ablative RadiatIon for OLigometastatic Prostate CancEr (ORIOLE). Radiat Oncol Biol 2019;105:681. [Google Scholar]
- [15].Fossati N, Suardi N, Gandaglia G, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol 2019;75:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ploussard G, Gandaglia G, Borgmann H, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: a systematic review. Eur Urol 2019;76:493–504. [DOI] [PubMed] [Google Scholar]
- [17].Mottet N, Bellmunt J, Briers E, et al. EAU – ESTRO – ESUR – SIOG guidelines on prostate cancer. https://Uroweb.org/Guideline/Prostate-Cancer/.
- [18].Horn T, Krönke M, Rauscher I, et al. Single lesion on prostate-specific membrane antigen-ligand positron emission tomography and low prostate-specific antigen are prognostic factors for a favorable biochemical response to prostate-specific membrane antigen-targeted radioguided surgery in recurrent prostate cancer. Eur Urol 2019;76:517–23. [DOI] [PubMed] [Google Scholar]
- [19].Rigatti P, Suardi N, Briganti A, et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol 2011;60:935–43. [DOI] [PubMed] [Google Scholar]
- [20].Briganti A, Suardi N, Capogrosso P, et al. Lymphatic spread of nodal metastases in high-risk prostate cancer: the ascending pathway from the pelvis to the retroperitoneum. Prostate 2012;72:186–92. [DOI] [PubMed] [Google Scholar]
- [21].Devos G, Muilwijk T, Raskin Y, et al. Comparison of peri-operative and early oncological outcomes of robot-assisted vs. open salvage lymph node dissection in recurrent prostate cancer. Front Oncol 2019;9:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications. Ann Surg 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [23].Briganti A, Karnes JR, Da Pozzo LF, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. a new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol 2009;55:261–70. [DOI] [PubMed] [Google Scholar]
- [24].Touijer KA, Karnes RJ, Passoni N, et al. Survival outcomes of men with lymph node-positive prostate cancer after radical prostatectomy: a comparative analysis of different postoperative management strategies. Eur Urol 2018;73:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suardi N, Briganti A, Gandaglia G, Fossati N, Montorsi F. Salvage lymph node dissection for node-only recurrence of prostate cancer: ready for prime time? Eur Urol 2017;71:693–4. [DOI] [PubMed] [Google Scholar]
- [26].Osmonov DK, Aksenov AV, Trick D, et al. Cancer-specific and overall survival in patients with recurrent prostate cancer who underwent salvage extended pelvic lymph node dissection. BMC Urol 2016;16 (September):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Porres D, Pfister D, Thissen A, et al. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis 2016;20:85–92. [DOI] [PubMed] [Google Scholar]
- [28].Hiester A, Nini A, Niegisch G, et al. Oncological outcome of patients treated with spot-specific salvage lymph node dissection (sLND) for positron-emission tomography (PET)-positive prostate cancer (PCa) relapse. World J Urol 2019;37(January):2081–90. [DOI] [PubMed] [Google Scholar]
- [29].Abdollah F, Briganti A, Montorsi F, et al. Contemporary role of salvage lymphadenectomy in patients with recurrence following radical prostatectomy. Eur Urol 2015;67:839–49. [DOI] [PubMed] [Google Scholar]
- [30].Suardi N, Gandaglia G, Gallina A, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol 2015;67:299–309. [DOI] [PubMed] [Google Scholar]
- [31].Cucchiara V, Cooperberg MR, Dall’Era M, et al. Genomic markers in prostate cancer decision making. Eur Urol 2018;73:572–82. [DOI] [PubMed] [Google Scholar]
- [32].Xu MJ, Kornberg Z, Gadzinski AJ, et al. Genomic risk predicts molecular imaging-detected metastatic nodal disease in prostate cancer. Eur Urol Oncol 2019;2:685–90. [DOI] [PubMed] [Google Scholar]
- [33].Knipper S, Tilki D, Mansholt J, et al. Metastases-yield and prostate-specific antigen kinetics following salvage lymph node dissection for prostate cancer: a comparison between conventional surgical approach and prostate-specific membrane antigen-radioguided surgery. Eur Urol Focus 2019;5:50–3. [DOI] [PubMed] [Google Scholar]
- [34].European Organisation for Research and Treatment of Cancer Radiotherapy and Genito-Urinary Cancer Groups, Van der Kwast TH, Collette L, et al. Impact of pathology review of stage and margin status of radical prostatectomy specimens (EORTC trial 22911). Virchows Arch 2006;449:428–34. [DOI] [PubMed] [Google Scholar]
- [35].Bravi CA, Tin A, Vertosick E, et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol 2019;202:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bravi CA, Vertosick E, Tin A, et al. Relative contribution of sampling and grading to the quality of prostate biopsy: results from a single high-volume institution. Eur Urol Oncol 2018. 10.1016/j.euo.2018.10.007, Nov 24;S2588–9311(18)30188–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


