Abstract
Background:
Salvage lymph node dissection (SLND) represents a possible treatment option for prostate cancer patients affected by nodal recurrence after local treatment. However, SLND may be associated with intra- and postoperative complications, and the oncological benefit may be limited to specific groups of patients.
Objective:
To identify the optimal candidates for SLND based on preoperative characteristics.
Design, setting, and participants:
The study included 654 patients who experienced prostate-specific antigen (PSA) rise and nodal recurrence after radical prostatectomy (RP) and underwent SLND at nine tertiary referral centers. Lymph node recurrence was documented by positron emission tomography/computed tomography (PET/CT) scan using either 11C-choline or 68Ga-labeled prostate-specific membrane antigen ligand.
Intervention:
SLND.
Outcome measurements and statistical analysis:
The study outcome was early clinical recurrence (eCR) developed within 1 yr after SLND. Multivariable Cox regression analysis was used to develop a predictive model. Multivariable-derived coefficients were used to develop a novel risk calculator. Decision-curve analysis was used to evaluate the net benefit of the predictive model.
Results and limitations:
Median follow-up was 30 (interquartile range, 16–50) mo among patients without clinical recurrence (CR), and 334 patients developed CR after SLND. In particular, eCR at 1 yr after SLND was observed in 150 patients, with a Kaplan-Meier probability of eCR equal to 25%. The development of eCR was significantly associated with an increased risk of cancer-specific mortality at 3 yr, being 20% versus 1.4% in patients with and without eCR, respectively (p < 0.0001). At multivariable analysis, Gleason grade group 5 (hazard ratio [HR]: 2.04; p < 0.0001), time from RP to PSA rising (HR: 0.99; p = 0.025), hormonal therapy administration at PSA rising after RP (HR: 1.47; p = 0.0005), retroperitoneal uptake at PET/CT scan (HR: 1.24; p = 0.038), three or more positive spots at PET/CT scan (HR: 1.26; p = 0.019), and PSA level at SLND (HR: 1.05; p < 0.0001) were significant predictors of CR after SLND. The coefficients of the predictive model were used to develop a risk calculator for eCR at 1 yr after SLND. The discrimination of the model (Harrel’s C index) was 0.75. At decision-curve analysis, the net benefit of the model was higher than the “treat-all” option at all the threshold probabilities.
Conclusions:
We reported the largest available series of patients treated with SLND. Roughly 25% of men developed eCR after surgery. We developed the first risk stratification tool to identify the optimal candidate to SLND based on routinely available preoperative characteristics. This tool can be useful to avoid use of SLND in men more likely to progress despite any imaging-guided approach.
Patient summary:
The risk of early recurrence after salvage lymph node dissection (SLND) was approximately 25%. In this study, we developed a novel tool to predict the risk of early failure after SLND. This tool will be useful to identify patients who would benefit the most from SLND from other patients who should be spared from surgery.
Keywords: Prostatic neoplasms, Neoplasm recurrence, Positron emission tomography, computed tomography, Lymph node excision, Salvage therapy
1. Introduction
Approximately 25% of patients with biochemical recurrence (BCR) after radical prostatectomy (RP) experience clinical progression at long-term follow-up [1,2]. However, the outcome of these patients is not invariably poor, varying significantly according to the site and the extent of recurrence [3].
In this context, the increasing use of positron emission tomography/computed tomography (PET/CT) has led to a shift towards early detection of low-volume metastatic prostate cancer (PCa) (eg, isolated nodal recurrences) that, in turn, has contributed to a shift in the treatment paradigm of these patients [4,5]. Recently, the European Association of Urology guidelines have indeed introduced salvage lymph node dissection (SLND) as a possible treatment option for men with nodal recurrence after local treatment [6]. The rationale of this approach is based on the evidence that men with nodal recurrences have better prognosis compared to their counterparts with bone or visceral metastasis [7–10]. However, considering the high risk of clinical relapse after SLND [11–16], one of the main endpoints would be represented by the delay of systemic treatment administration, as recently shown in a phase 2 trial [17]. Despite the possible benefit on hormonal therapy (HT)–free survival, it is currently unknown whether SLND is associated with better cancer control compared to standard treatments. This is mainly due to the lack of large, prospective, randomized trials focusing on hard clinical endpoints. While waiting for robust evidence, it would be important to identify patients who may have good outcomes after SLND [18] and to spare the surgical intervention and its possible related side effects in those patients who invariably progress despite the use of SLND [19]. As such, the identification of the optimal candidate for SLND is of utmost importance to maximize cancer control and to avoid overtreatment. We thus aimed at identifying the optimal candidate for SLND based on a large, multi-institutional series of patients treated for node-only recurrent PCa detected at PET/CT scan after RP.
2. Materials and methods
2.1. Patient population
Institutional Review Board approval was obtained for data sharing through the different centers. We identified 840 patients who experienced prostate-specific antigen (PSA) rise and nodal recurrence after RP and underwent SLND at nine tertiary referral centers by expert surgeons between 2002 and 2016. Lymph node recurrence was documented by PET/CT scan using either 11C-choline or 68Ga-labeled prostate-specific membrane antigen (68Ga-PSMA) ligand. All patients were preoperatively staged with abdominal CT scan and bone scan using technetium Tc 99m methylene diphosphonate (Tc 99m MDP) to exclude any other sites of recurrence.
Patients with missing information for Gleason grade group (n = 29), site of positive uptake at PET/CT scan (n = 21), number of positive spots at PET/CT scan (n = 6), HT administration before SLND (n = 48), PSA level at SLND (n = 1), PSA level after SLND (n = 15), and follow-up data (n = 30) were excluded. Furthermore, 36 patients received targeted SLND to the positive nodes at PET/CT scan only. These patients were excluded because of the high risk of missed lymph node metastases [13,20]. These selection criteria yielded 654 evaluable individuals with complete clinical, pathological, and follow-up data.
2.2. Surgical technique
The surgical technique was previously described [20] and was consistent through the different centers. In brief, pelvic SLND consisted of the excision of external iliac, obturator, internal iliac, common iliac, and presacral nodes. The distal limit of the dissection was represented by the femoral canal, whereas the proximal limit consisted of the aortic bifurcation. All fibro-fatty tissue within the obturator fossa was removed to completely skeletonize the obturator nerve. The medial and the lateral limits of the dissection consisted of the peri-vesical fat and the pelvic sidewall, respectively. At the discretion of the surgeon, intraoperative frozen section analysis of the common iliac nodes was performed. When positive, lymphadenectomy was extended to the retroperitoneum, based on the high probability of retroperitoneal lymph node metastasis [21].
Retroperitoneal SLND consisted of the excision of all nodal tissue located between the renal artery (cranially) and the aortic bifurcation (caudally). Lateral limits consisted of the right ureter (on the right) and the left ureter (on the left). All nodal specimens were mapped according to their anatomic location and sent for pathological assessment in multiple packages.
2.3. Follow-up
Follow-up consisted of PSA testing 1 mo after surgery; 3, 6, 9, and 12 mo after SLND; and biannually thereafter. Postoperative 11C-choline PET/CT scan and eventual bone scan using Tc 99m MDP were performed in case of PSA rising after SLND. The administration of additional treatments after SLND was left at the discretion of the surgeon.
2.4. Variable definition
Data consisted of variables related to RP; SLND, pretreatment; and SLND, post-treatment.
RP: age at RP (years), preoperative PSA level (nanograms per milliliter), primary tumor (pT) stage (pT2 vs pT3a vs ≥pT3b), Gleason grade group (≤3 vs 4 vs 5), surgical margin status (negative vs positive), lymph node (pN) stage (pN0 vs pN1 vs pNx), number of lymph nodes removed, number of positive nodes, first PSA level at 1 mo after RP (nanograms per milliliter; PSA persistence was defined as a serum concentration ≥0.1 ng/ml at 1 mo after RP [22]), adjuvant HT (no vs yes; type and duration of HT), and adjuvant RT (no vs yes; dose and field of RT).
SLND, pretreatment: time from RP to PSA rising (months), HT administration at the time of PET/CT scan (no vs yes; type and duration of HT), PET/CT tracer (11C-choline vs 68Ga-PSMA), site of positive uptake at PET/CT scan (pelvic vs retroperitoneal vs both), number of positive spots at PET/CT (1 vs 2 vs 3 vs ≥4), age at SLND (years), and PSA level at SLND (nanograms per milliliter).
SLND, post-treatment: number of lymph nodes removed, number of positive nodes, PSA level at 1 mo after SLND (nanograms per milliliter), and PSA difference (PSA pre-SLND and PSA post-SLND).
2.5. Outcome definition
The primary outcome of the study was early clinical recurrence (eCR) after SLND that was defined as positive imaging in presence of a rising PSA within 12 mo after surgery. Such a definition of eCR was derived by the control group of a randomized phase 2 trial testing the role of metastasis-directed therapy (including SLND) in oligometastatic recurrent PCa [17]. Furthermore, in the recently published Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients (ICECaP) study, metastasis-free survival was a strong surrogate for overall survival for localized pCa [23]. Follow-up time consisted of the time between SLND and clinical recurrence or last follow-up. Secondary outcomes consisted of BCR after SLND (defined as PSA level ≥0.2 ng/ml and rising), and HT-free survival.
2.6. Statistical analyses
Statistical analyses consisted of three main steps. First, Kaplan-Meier analysis was used to assess clinical recurrence (CR), BCR, and HT-free survival after SLND. Second, multivariable Cox regression analyses were used to predict CR after SLND. Predictors consisted of Gleason grade group (≤4 vs 5), time from RP to PSA rising (months), HT administration at the time PET/CT scan (no vs yes), site of positive imaging (pelvis vs retroperitoneum ± pelvis), number of positive spots at PET/CT scan (one or two vs three or more), and PSA level at SLND (nanograms per milliliter). The discrimination of the model was tested using Harrel’s C index. The ten-fold cross-validation was used to correct Harrell’s C index for overfit. The predictive model coefficients were used to calculate the risk of eCR at 1 yr after SLND for each patient and to develop the corresponding risk calculator. Third, decision-curve analysis was used to evaluate the net benefit of the predictive model.
All statistical analyses were performed using Stata version 12.0 (StataCorp LP, College Station, TX, USA).
3. Results
Descriptive characteristics of patients are illustrated in Tables 1–3. At RP, 466 (71%) patients were pN0, 104 (16%) were pN1, and 84 (13%) were pNx (Table 1). At PET/CT scan (Table 2), 534 (82%) patients had pelvic uptake. Of these, 442 (82.7%) received pelvic SLND only and 92 (17.3%) received both pelvic and retroperitoneal SLND. By contrast, 62 patients (9%) had retroperitoneal uptake and 58 patients (9%) had both pelvic and retroperitoneal uptake. All patients with retroperitoneal involvement at PET/CT scan received both pelvic and retroperitoneal SLND. Median (interquartile range [IQR]) number of lymph nodes removed at SLND was 26 (15–38). The number of positive nodes at SLND was zero, one, two, and three or more in 62 (9%), 150 (23%), 92 (14%), and 350 (54%) patients, respectively (Table 3).
Table 1 –
RP characteristics of 654 patients treated with salvage lymph node dissection for nodal recurrence of prostate cancer
| Variable | Overall population (n = 654; 100%) |
|---|---|
| Age at RP, yr | 59 (55–65) |
| Pre-RP PSA level, ng/ml | 8.4 (5.9–13.0) |
| Pathologic T stage, n (%) | |
| pT2 | 210 (32) |
| pT3a | 214 (33) |
| pT3b | 214 (33) |
| Unknown | 16 (2) |
| Surgical margins, n (%) | |
| Negative | 396 (61) |
| Positive | 232 (35) |
| Unknown | 26 (4) |
| Gleason grade group, n (%) | |
| ≤3 | 22 (3) |
| 4 | 352 (54) |
| 5 | 280 (43) |
| Pathologic N stage, n (%) | |
| pN0 | 466 (71) |
| pN1 | 104 (16) |
| pNx | 84 (13) |
| Lymph nodes removed at RP | 8 (5–14) |
| Undetectable PSA after RP, n (%) | |
| No | 254 (39) |
| Yes | 284 (43) |
| Unknown | 116 (18) |
| Post-RP radiation therapy, n (%) | |
| No | 258 (39) |
| Yes | 396 (61) |
| Type of radiation therapy, n (%) | |
| No | 258 (39) |
| Adjuvant | 56 (9) |
| Salvage for PSA rising | 296 (45) |
| Salvage for PSA persistence | 44 (7) |
PSA = prostate-specific antigen; RP = radical prostatectomy.
All values are medians (interquartile range) or frequencies (proportions).
Table 3 –
Post-SLND characteristics of 654 patients treated with salvage lymph node dissection for nodal recurrence of prostate cancer
| Variable | Overall population (n = 654; 100%) |
|---|---|
| Lymph nodes removed at SLND | 26 (15–38) |
| Positive lymph nodes at SLND, n (%) | |
| 0 | 62 (9) |
| 1 | 150 (23) |
| 2 | 92 (14) |
| ≥3 | 350 (54) |
| PSA after SLND, ng/ml | 0.3 (0.0–1.0) |
| PSA difference (after–pre), ng/ml | −1.4 (−2.8 to−0.3) |
| PSA response after SLND | |
| (<0.2 ng/ml), n (%) | |
| No | 368 (56) |
| Yes | 286 (44) |
| Undetectable PSA after SLND | |
| (<0.1 ng/ml), n (%) | |
| No | 456 (70) |
| Yes | 198 (30) |
PSA = prostate-specific antigen; SLND = salvage lymph node dissection.
All values are medians (interquartile range) or frequencies (proportions).
Table 2 –
Pre-SLND characteristics of 654 patients treated with salvage lymph node dissection for nodal recurrence of prostate cancer
| Variable | Overall population (n = 654; 100%) |
|---|---|
| Time from RP to PSA rising, mo | 19 (4–44) |
| HT administration at PSA rising, n (%) | |
| No | 538 (82) |
| Yes | 116 (18) |
| Type of PET/CT tracer, n (%) | |
| 11C-Choline | 460 (70) |
| 68Ga-PSMA | 194 (30) |
| Positive sites at PET/CT scan, n (%) | |
| Pelvic | 534 (82) |
| Retroperitoneal | 62 (9) |
| Both | 58 (9) |
| Positive spots at PET/CT scan, n (%) | |
| 1 | 332 (51) |
| 2 | 150 (23) |
| 3 | 112 (17) |
| ≥4 | 60 (9) |
| Age at SLND, yr | 66 (60–70) |
| PSA at SLND, ng/ml | 2.1 (1.0–4.0) |
HT = hormonal therapy; PET/CT = positron emission tomography/computed tomography; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; RP = radical prostatectomy; SLND = salvage lymph node dissection.
All values are medians (interquartile range) or frequencies (proportions).
Surgical route consisted of open, laparoscopic, and robotic technique in 572 (87%), 6 (1%), and 76 (12%) patients, respectively. Intra- and postoperative complications of SLND were illustrated in Supplementary Table 1. Type of intraoperative complications were reported, and Clavien-Dindo classification was considered for postoperative complications.
Median follow-up among patients who did not develop CR was 30 (IQR, 16–50) mo; 334 patients developed CRafter SLND. CR-free survival, BCR-free survival, and HT-free survival are illustrated in Figures 1, 2, and 3, respectively. In particular, eCR at 1 yr after SLND was observed in 150 patients, with a Kaplan-Meier probability of eCR equal to 25% (95% confidence interval [CI]: 20–30). The development of eCR was significantly associated with increased risk of 3-yr cancer-specific mortality (Fig. 4), being 20% versus 1.4% in men with and without eCR, respectively (−p < 0.0001).
Fig. 1 –
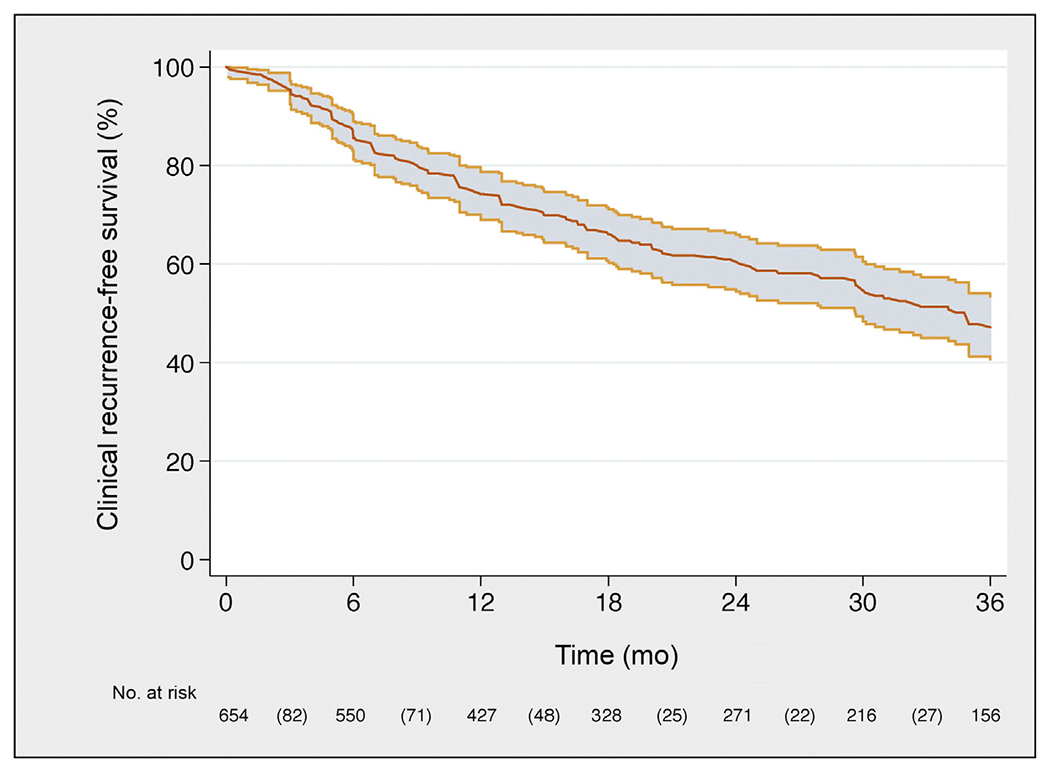
Kaplan-Meier plot depicting clinical recurrence-free survival in the overall population.
Fig. 2 –
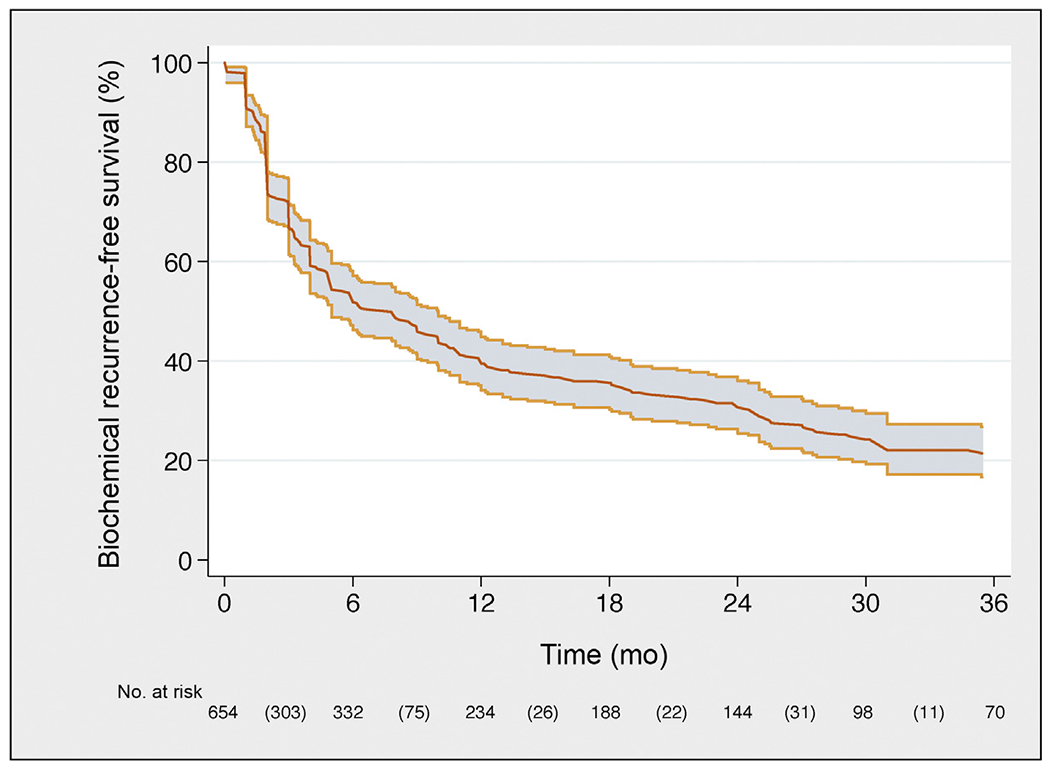
Kaplan-Meier plot depicting biochemical recurrence-free survival in the overall population.
Fig. 3 –
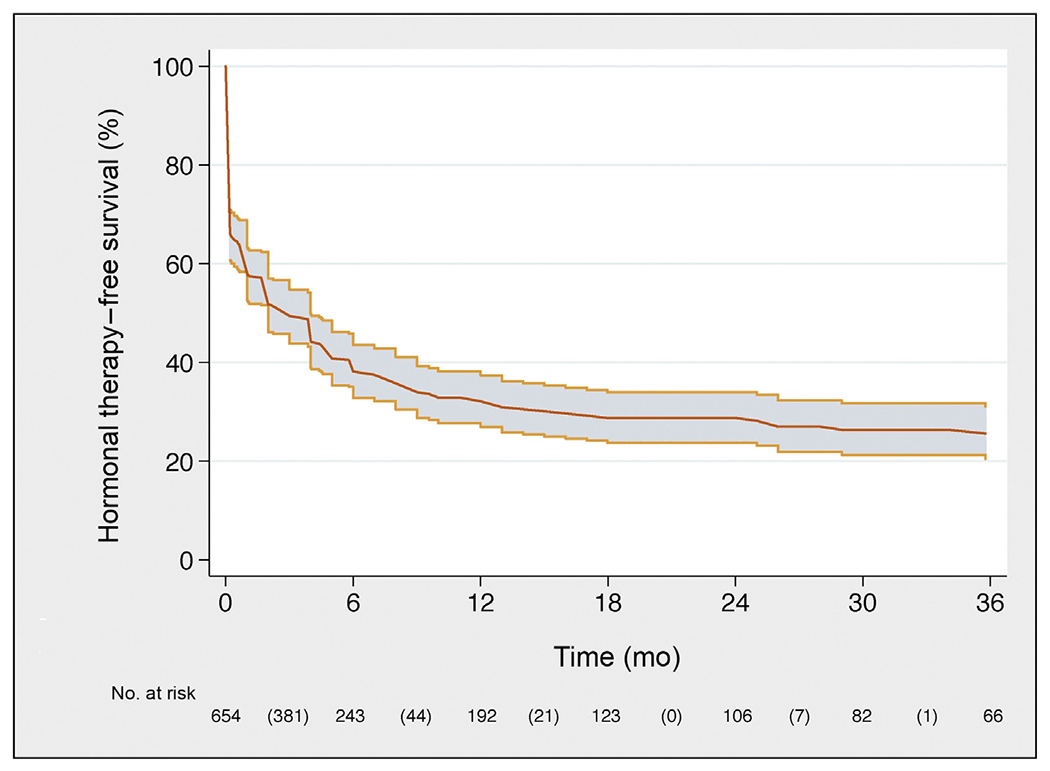
Kaplan-Meier plot depicting hormonal therapy-free survival in the overall population.
Fig. 4 –
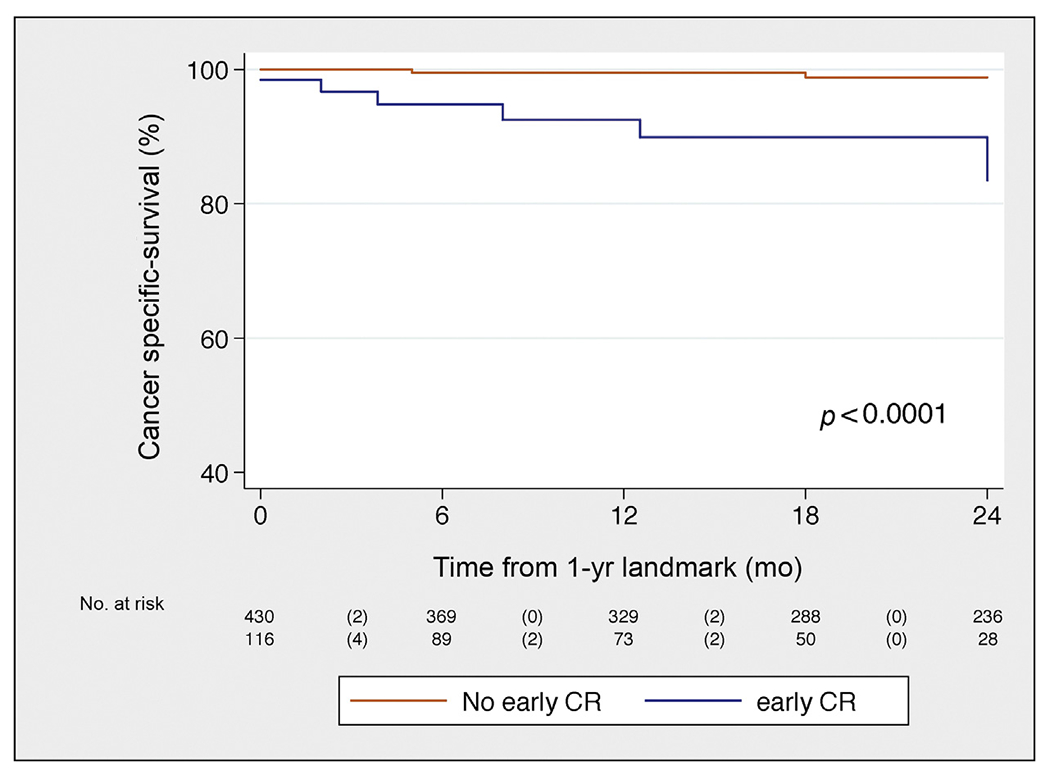
Kaplan-Meier plot depicting cancer-specific mortality, stratified in two groups: patients who developed early clinical recurrence versus patients who did not.
CR = clinical recurrence.
At multivariable Cox regression analysis, Gleason grade group 5 (hazard ratio [HR]: 2.04; 95% CI: 1.66–2.50; p < 0.0001), time from RP to PSA rising (HR: 0.99; 95% CI: 0.98–0.99; p = 0.025), HT administration at PSA rising after RP (HR: 1.47; 95% CI: 1.19–1.82; p = 0.0005), retroperitoneal uptake at PET/CT scan (HR: 1.24; 95% CI: 1.01–1.52; p = 0.038), three or more positive spots at PET/CT scan (HR: 1.26; 95% CI: 1.05–1.61; p = 0.019), and PSA level at SLND (HR: 1.05; 95% CI: 1.04–1.07; p < 0.0001) were significant predictors of CR after SLND (Table 4).
Table 4 –
Multivariable Cox regression analysis predicting clinical recurrence after SLND in 654 patients treated for nodal recurrence of prostate cancer
| Predictor | HR | 95% CI | p value |
|---|---|---|---|
| Gleason grade group | |||
| ≤4 | 1.00 | Ref. | – |
| 5 | 2.04 | 1.66–2.50 | <0.0001 |
| Time from RP to PSA rising, per 6 mo | 0.98 | 0.96–0.99 | 0.025 |
| HT administration at the time of PET/CT scan | |||
| No | 1.00 | Ref. | – |
| Yes | 1.47 | 1.19–1.82 | 0.0005 |
| Retroperitoneum involvement at PET/CT scan | |||
| No | 1.00 | Ref. | – |
| Yes | 1.24 | 1.01–1.52 | 0.038 |
| Positive spots at PET/CT scan | |||
| ≤2 | 1.00 | Ref. | – |
| ≥3 | 1.26 | 1.05–1.61 | 0.019 |
| PSA at SLND, ng/ml | 1.05 | 1.04–1.07 | <0.0001 |
CI = confidence interval; HR = hazard ratio; HT = hormonal therapy; PET/CT = positron emission tomography/computed tomography; PSA = prostate-specific antigen; Ref. = reference; RP = radical prostatectomy; SLND = salvage lymph node dissection.
The coefficients of the predictive model were used to develop a risk calculator (Supplementary data) for eCR at 1 yr after SLND. The discrimination of the model (Harrel’s C index) was 0.75. Decision-curve analysis illustrated the net benefit of the model, which was higher than the “treat-all” option at all the threshold probabilities (Fig. 5).
Fig. 5 –
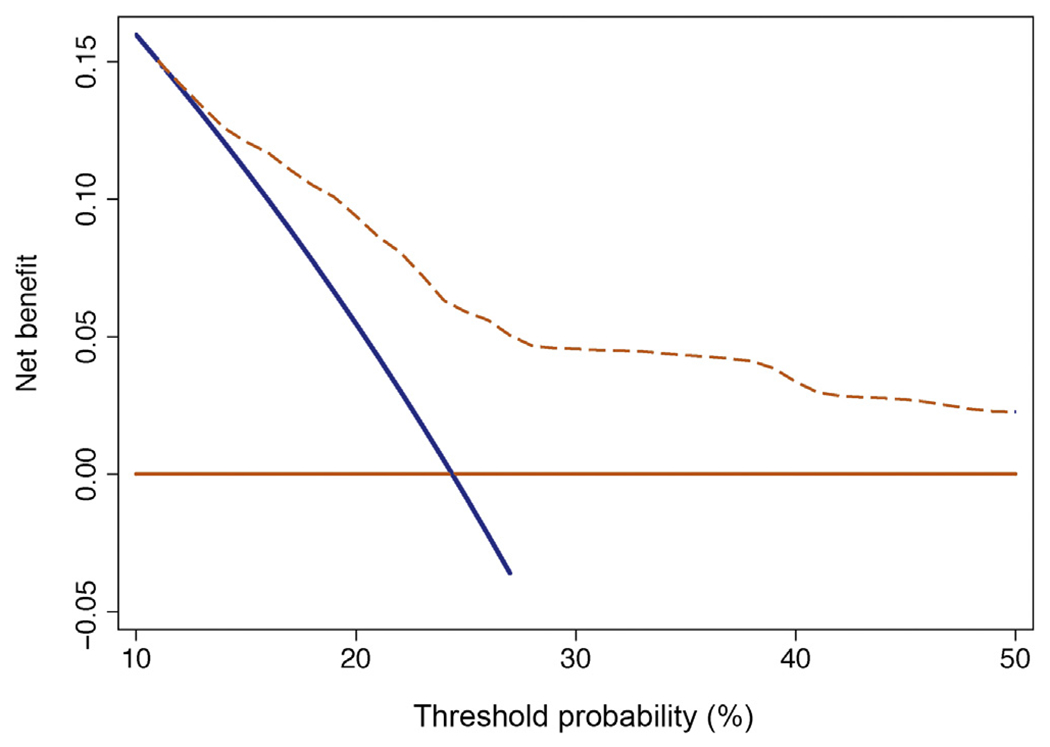
Decision-curve analysis assessing the net benefit of the model.
4. Discussion
In this study, we aimed at identifying the optimal candidate for SLND among men with node-only recurrent PCa after RP based on the largest available series. Although this surgical approach has now been included in the currently available guidelines as a possible treatment option for these men, it is currently unknown which patient could benefit the most from this treatment. Such void is due to two main reasons: (i) the lack of large phase 3 trials testing the role of SLND compared to standard management, and (ii) unavailability of any risk prediction model able to assess the outcomes of men treated with SLND. Men with node recurrent PCa have indeed heterogeneous outcomes according to clinical and pathological characteristics. Although some of these patients are affected by a systemic disease upfront leading to early recurrence after SLND, others have a true oligo-recurrent disease. This has, in turn, significant therapeutic implications. Indeed, while in the first patient group any metastasis-directed therapy might be of limited value, in the second group such an approach might be beneficial, allowing at least to postpone the use of systemic treatments. In this study, we hypothesized that patients with lymph node recurrence from PCa can be accurately stratified according to their risk of eCR after SLND, thus allowing for accurate selection of the optimal candidate for SLND. Our results confirmed our initial hypothesis, as we were able to develop the first risk prediction model for eCR after SLND based on the largest multi-institutional series. Several facets of our findings deserve attention.
First, we identified clinical and pathological predictors of eCR after SLND that formed the basis of a novel and accurate (area under the curve, 75%) risk prediction model (Supplementary data). This risk calculator can be used to assess individual risk of early failure after SLND, having the predictive model a higher clinical net-benefit compared to the “treat-all” option at decision curve analysis. The endpoint of our study was eCR based on the assumption that men with eCR are those likely to have systemic, disseminated disease already at the time of SLND. The definition of eCR was derived from the control group of a recent prospective randomized phase 2 trial focused on men with oligo-recurrent PCa [17]: in the observational arm, patients had a median time to progression of 13 mo. Although men with bone and visceral metastases also were included in that trial, we postulated that any metastasis-directed therapy such as SLND should at least prolong the onset of clinical progression of oligo-recurrent disease. This is important, given the protracted natural history of men with node-recurrent-only disease. Our model can be used to evaluate the individual patient risk of eCR and, in turn, to optimize patient selection for SLND. For example, a patient with two positive spots detected at PET/CT in the pelvis, a PSA of 2.2 ng/ml at 36 mo after RP with Gleason 4 + 3 without any previous hormonal manipulation showed a risk of eCR of 15%. Conversely, a man with three positive spots detected at PET/CT in the para-aortic nodes, a PSA of 4.8 ng/ml at 24 mo after RP with a Gleason 4 + 5 with previous hormonal manipulation showed a risk of eCR of 59%. Although risk of eCR for men with isolated nodal recurrence managed conservatively is unknown in our study due to the lack of a control group, we argue that SLND would be hardly justifiable in men with high risk of early CR for the presence of concomitant systemic disease likely undetected by imaging. Therefore, the impact of any imaging-guided therapy may be highly diluted in this patient group. Conversely, men with lower risk of eCR are those in whom SLND may be associated with higher impact on patient outcomes. However, whether favorable outcomes were due to the impact of SLND or simply related to disease biology is currently unknown and cannot be answered by our study, given the lack of a control group not receiving SLND.
Second, our results are in line with those obtained by metastasis-directed therapy in the above-mentioned phase 2 randomized trial of oligo-recurrent PCa [17]. In our study, median time to progression after SLND was 18 mo, comparable to the time reported by Ost et al in the group of men randomized to metastasis-directed therapy. Therefore, despite the lack of a control group, our results indicate the possible role of SLND in delaying further clinical progression in men with node-only PCa recurrence. Indeed, at 3-yr follow-up, approximately one in four patients was free from both BCR and HT. Interestingly, in the study by Ost et al, use of metastasis-directed therapy had comparable effect on HT-free survival in men with nodal versus non-nodal metastases, opening new perspectives in future large phase 3 trials testing the role of metastasis-directed therapy in the entire oligo-recurrent setting.
Third, our results seem to confirm previously identified prognostic factors for SLND reported in more limited series, such as site of nodal involvement at imaging, PSA value at SLND, and the number of positive spots at PET/CT [11–16,20]. However, in addition to previous studies, we were able to combine these predictors into the first risk prediction model using eCR as endpoint and developed in the largest available series of SLND. Our results reiterate the importance of a multivariable risk assessment of these patients, an assessment that should consider several clinical and pathological variables to optimize risk assessment and patient selection. Finally, our results are hypothesis generating and can be used to select the optimal candidate for SLND to be included future prospective randomized trials.
Despite several strengths, our study is not devoid of limitations. First, no control group of patients receiving standard of care was included. Therefore, based on these results, it is not possible to compare oncological outcomes with patients treated with standard management. This procedure is still experimental, and patients should be informed accordingly. Second, the lack of standardized post-RP management may represent a limitation of this study. But it reflects the real-life clinical scenarios, where patients with nodal recurrence have extremely heterogeneous urological and oncological histories. Therefore, the simple exclusion of patients receiving (or not receiving) androgen deprivation therapy and/or RT may represent an additional selection bias. For this reason, we decided to include all patients treated with SLND and to use multivariable analysis to adjust for these specific factors. Third, although all men were surgically treated at high-volume centers, given the retrospective nature of our study diagnostic biases could have been introduced from possible differences in surgical templates among surgeons. Fourth, not all patients received the same imaging protocols, since either 11C-choline or 68Ga-PSMA was used as a tracer for PET/CT scan that could be associated with different diagnostic performances. Finally, central pathological review of the specimens was not conducted in this multi-institutional study.
5. Conclusions
We reported the largest available series of patients treated with SLND. Roughly 25% of men developed eCR after surgery. We developed the first risk stratification tool to identify the optimal candidate to SLND based on routinely available preoperative characteristics. This tool can be useful to avoid use of SLND in men more likely to progress despite any imaging-guided approach.
Supplementary Material
Footnotes
Financial disclosures: Alberto Briganti certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2018.09.009.
References
- [1].Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama 1999;281:1591–7. [DOI] [PubMed] [Google Scholar]
- [2].Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol 2011;59:893–9. [DOI] [PubMed] [Google Scholar]
- [3].Glass TR, Tangen CM, Crawford ED, Thompson I. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol 2003;169:164–9. [DOI] [PubMed] [Google Scholar]
- [4].Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol 2013;189:1308–13. [DOI] [PubMed] [Google Scholar]
- [5].Ceci F, Herrmann K, Castellucci P, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging 2014;41:2222–31. [DOI] [PubMed] [Google Scholar]
- [6].Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2016;71:630–42. [DOI] [PubMed] [Google Scholar]
- [7].Fossati N, Trinh Q-D, Sammon J, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol 2014;1–4. [DOI] [PubMed] [Google Scholar]
- [8].Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 2014;65:3–6. [DOI] [PubMed] [Google Scholar]
- [9].Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol 2015;68:325–34. [DOI] [PubMed] [Google Scholar]
- [10].Ost P, Decaestecker K, Lambert B, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate 2014;74:297–305. [DOI] [PubMed] [Google Scholar]
- [11].Jilg CA, Rischke HC, Reske SN, et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol 2012;188:2190–7. [DOI] [PubMed] [Google Scholar]
- [12].Karnes RJ, Murphy CR, Bergstralh EJ, et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol 2014;193:111–6. [DOI] [PubMed] [Google Scholar]
- [13].Suardi N, Gandaglia G, Gallina A, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol 2015;67:299–309. [DOI] [PubMed] [Google Scholar]
- [14].Tilki D, Mandel P, Seeliger F, et al. Salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy. J Urol 2015;193:484–90. [DOI] [PubMed] [Google Scholar]
- [15].Osmonov DK, Aksenov AV, Trick D, et al. Cancer-specific and overall survival in patients with recurrent prostate cancer who underwent salvage extended pelvic lymph node dissection. BMC Urol 2016;16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Porres D, Pfister D, Thissen A, et al. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis 2017;20:85–92. [DOI] [PubMed] [Google Scholar]
- [17].Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2017. 10.1200/JCO.2017.75.4853, JCO2017754853. [DOI] [PubMed] [Google Scholar]
- [18].Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015;67:852–63. [DOI] [PubMed] [Google Scholar]
- [19].Jilg CA, Leifert A, Schnell D, et al. Toxicity and quality of life after choline-PET/CT directed salvage lymph node dissection and adjuvant radiotherapy in nodal recurrent prostate cancer. Radiat Oncol 2014;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rigatti P, Suardi N, Briganti A, et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol 2011;60:935–43. [DOI] [PubMed] [Google Scholar]
- [21].Briganti A, Suardi N, Capogrosso P, et al. Lymphatic spread of nodal metastases in high-risk prostate cancer: the ascending pathway from the pelvis to the retroperitoneum. Prostate 2011;72:186–92. [DOI] [PubMed] [Google Scholar]
- [22].Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2016;71:618–29. [DOI] [PubMed] [Google Scholar]
- [23].Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017;35:3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


