Abstract
The high-molecular-weight sulfated or sulfonated polysaccharides or polymers cellulose sulfate, dextran sulfate, and polystyrene sulfonate were tested for microbicidal activity against bovine papillomavirus type 1 (BPV-1) and human papillomavirus type 11 (HPV-11) and type 40 (HPV-40). In vitro assays included the BPV-1-induced focus-forming assay and transient infection of human A431 cells with HPVs. The compounds were tested for microbicidal activity directly by preincubation with virus prior to addition to cell cultures and indirectly by addition of virus to compound-treated cells and to virus-coated cells to test inactivation of the virus after virus-cell binding. The data indicated that all three compounds showed direct microbicidal activity with 50% effective concentrations between 10 to 100 μg/ml. These concentrations were nontoxic to cell cultures for both assays. When a clone of C127 cells was tested for microbicidal activity, approximately 10-fold-less compound was required to achieve a 50% reduction in BPV-1-induced foci than for the uncloned parental C127 cells. Pretreatment of cells with compound prior to addition of virus also demonstrated strong microbicidal activity with dextran sulfate and polystyrene sulfonate, but cellulose sulfate required several orders of magnitude more compound for virus inactivation. Polystyrene sulfonate prevented subsequent infection of HPV-11 after virus-cell binding, and this inactivation was observed up to 4 h after addition of virus. These data indicate that the polysulfated and polysulfonated compounds may be useful nontoxic microbicidal compounds that are active against a variety of sexually transmitted disease agents including papillomaviruses.
Genital human papillomavirus (HPV) infections represent one of the most frequent sexually transmitted diseases (STDs). Although most infections spontaneously resolve within a year (12), most likely by host cell-mediated immunity, a proportion of persistent HPV infections can progress to invasive cervical cancer. Cervical cancer represents the second most frequent cause of cancer-related deaths in women, accounting for more than 200,000 deaths per year worldwide (21). Compounds with microbicidal activity against papillomaviruses, therefore, may reduce incident infections and decrease the rates of cervical cancer. To date, very few reagents with microbicidal activity against HPV infections have been described. These reagents include those that specifically target HPVs, such as monoclonal antibodies (MAbs) with virus-neutralizing activity (2, 3), and non-virus-specific agents, such as povidone-iodine (25), alkyl sulfates (13, 14), and monocaprin (14). Several reagents that have microbicidal activity against a broad range of STDs such as N-9 and C31G have proven to be ineffective against papillomaviruses (9, 13). Some of these latter agents also induce significant cellular cytotoxicity at high concentrations, although alkyl sulfates such as sodium dodecyl sulfate (SDS) were significantly less toxic than N-9 (13, 20).
The purpose of this study is to present data on papillomavirus microbicidal activity of a class of high-molecular-weight sulfated or sulfonated polysaccharides or polymers that do not show cellular cytotoxicity even at high concentrations and that have not been previously tested for microbicidal activity against either animal or human papillomaviruses. These reagents, which include cellulose sulfate (CS), dextran sulfate (DS), and polystyrene sulfonate (PSS), have been tested for microbicidal activity against a variety of STD agents, such as the enveloped viruses human immunodeficiency virus and herpesvirus 1 and 2, gonococci, chlamydia, and for sperm inactivation (1, 10, 11, 20, 29). PSS and CS are being developed as vaginal microbicides by the Program for the Topical Prevention of Conception and Disease (TOPCAD, Chicago, Ill.), in collaboration with the Contraceptive Research and Development (CONRAD) program (Washington, D.C.), and both are now in clinical trials (7). The mechanism by which these agents effect microbicidal activity against STDs could be either by binding directly to the infectious agent or by binding to the target cell, thus preventing successful infection of susceptible host cells or tissues. We found that these compounds strongly inhibited infection of mouse C127 cells by bovine papillomavirus type 1 (BPV-1) and blocked infection of human A431 epithelial cells by HPV type 11 (HPV-11) and HPV type 40 (HPV-40) as measured by an in vitro transient-infection assay (17, 23).
MATERIALS AND METHODS
Compounds tested for microbicidal activity.
Sodium cellulose sulfate (known as Ushercell J) (called cellulose sulfate or CS in this study) was synthesized under good manufacturing procedure (GMP) conditions by Dextran Products, Ltd. (Scarborough, Ontario, Canada) and has a peak molecular mass of 2,300 kDa and an average molecular mass of 1,900 kDa. DS was obtained from Dextran Products and has a peak molecular mass of 500 kDa. PSS was manufactured under GMP conditions by TOPCAD and has a peak molecular mass of 864 kDa and an average molecular mass of 751 kDa.
Microbicidal testing using the BPV-1 focus-forming assay.
Microbicidal activity of compounds was first tested using the well-characterized BPV-1 focus-forming assay (5), with modifications for microbicide testing (9, 13). Aliquots of BPV-1 containing approximately 100 to 200 focus-forming units were preincubated with dilutions of compounds for 10 min at 37°C prior to addition to cultures of mouse C127 cells. A 10-min preincubation period was chosen based on our previous studies with microbicide testing (13, 14). Cultures of C127 cells were set up in T25 tissue culture flasks (Corning Glass Works, Corning, N.Y.), containing 3 × 105 cells per flask. Virus-compound mixtures in a total volume of 50 μl were then added to flasks containing 1 ml of medium each, and an additional 3 ml of medium was added after 24 h of culture. The medium was changed every 3 or 4 days for a period of 2 weeks. Foci were enumerated by staining the monolayer with crystal violet and counting stained foci microscopically. Each concentration of compound was tested in duplicate, and the mean ± standard deviation (SD) of the number of foci was plotted against the preincubation virus-drug concentration for each compound.
Microbicidal activity of compounds was also tested by preincubation of cells with compounds followed by addition of virus to compound-coated C127 cells. In these experiments, dilutions of compounds were added to cultures of C127 cells, incubated for 1 h at 37°C, washed three times with medium to remove unbound compound, prior to addition of approximately 100 focus-forming units of BPV-1. The cultures were incubated for an additional hour and washed three times to remove unbound virus, and then the incubation was continued for 2 weeks with changes of the medium every 3 or 4 days and foci were counted as described above.
Microbicidal testing using transient infection with HPV-11 and HPV-40.
Compounds were tested for microbicidal activity using the in vitro HPV transient-infection assay originally described by Smith and colleagues (23) with some modifications (17). An enzyme-linked immunosorbent assay (ELISA)-based readout of optical density (OD) values using alkaline phosphatase cleavage of the substrate p-nitrophenyl phosphate was also used to measure HPV infection as described below.
In the standard reverse transcription-PCR (RT-PCR) assay (17, 23, 24) for detection of HPV-11 infection, aliquots of HPV-11 were preincubated with dilutions of compounds for 30 min at 37°C and then the mixtures were added to cultures of human A431 cells. Replicate cultures of A431 cells were set up by plating 5 × 105 cells per well into six-well culture plates. Virus-compound mixtures were added to individual A431 cultures, and the cultures were incubated for a further 4 days. Cells were harvested in 1 ml of Trizol (GIBCO/BRL), and then total RNA was prepared for RT and production of viral cDNA from spliced viral transcripts spanning a major splice site between E1 and E4 (17, 18, 23). Two rounds of PCR amplification using nested primers (see below) were conducted for detection of the spliced viral transcript, and the PCR products were detected as ethidium bromide-stained bands on agarose gels (17, 23). PCR products were cloned and sequenced to confirm the viral origin of the PCR product. The presence of the correct-size viral PCR product, as well as a failure to inactivate and/or block the virus by the test compound, was used to confirm successful infection by HPV-11 In contrast, the lack of a viral PCR product was interpreted to indicate virus inactivation and/or a failure of the virus to infect A431 cells. Amplified β-actin transcripts (23) were used as a control to establish the integrity of RNA isolation and RT-PCR procedures for uninfected cells and for cultures in which HPV-11 inactivation was achieved.
The RT-PCR assay to detect HPV-40 infection was designed similarly for the detection of HPV-11 infection as described above. Primers were prepared from the published sequence of HPV-40 (4) for amplification of a spliced E1∧E4 viral transcript for HPV-40. The RT reactions were primed using the downstream reverse primer 3570GGCGTGCGTGTTCTGTCT3554.The first PCR amplification used the following primers: for HPV-40 upstream, the outside primer was 817GGGCACATTACATATAGTGT836; for HPV-40 downstream, the outside primer was 3570GGCGTGCGTGTTCTGTCT3554. The second PCR amplification used inside (nested) primers: for HPV-40 upstream, the inside primer was 838CCCCAACTGTGCAGCTACA857; for HPV-40 downstream, the inside primer was 3520TGCCCACAGTAGTGGTGAT3502.
A modification of the RT-PCR assay that incorporates an ELISA-based readout (Boehringer-Mannheim) was also included to assess microbicidal activity. Replicate cell cultures of A431 cells were infected with an aliquot of infectious HPV virions as described above. After 4 days of culture, cells were harvested and RNA was extracted. RNA was subjected to RT using downstream antisense (reverse) primers for HPV-11 or HPV-40 and β-actin (as a control or housekeeping cellular transcript) to initiate cDNA synthesis. The cDNA was processed through two sets of 30 cycles of PCR amplification using nested primers: the second set of cycles used digoxigenin (DIG)-labeled dUTP to label the PCR products with DIG. DIG-labeled PCR products were denatured and then renatured together with a biotinylated oligonucleotide specific for the targeted PCR product (biotin-GCAGACTCTCCAGTACTATCGAGGAACAA for HPV-11, biotin-GGCGGACGATTCAGCACTGTACGAGAAGTA for HPV-40, and biotin-GGCCCGGACCTGACTGACTACCTCATGAAG for β-actin). Biotinylated products were detected by an ELISA with plates coated with streptavidin (to capture the biotinylated target PCR product) and then with anti-DIG antibody and substrate. Labeled PCR products were added directly to ELISA plates or titrated at 10-fold dilutions in duplicate for each cell culture for each virus dilution.
ELISA values above the background level were interpreted to indicate the presence of the viral (or β-actin) spliced cDNA fragment and thus, successful infection by HPV-11 or -40. Only semiquantitative data on viral infection were obtained because these assays (standard RT-PCR and ELISA-based RT-PCR) used nested PCR with two sets of amplifications following the RT steps.
Microbicidal activity of PSS after virus-cell binding.
Prevention of subsequent infection of postattachment HPV-11 or cell-surface-bound HPV-11 was tested using PSS in a manner similar to that described for the testing of postattachment neutralization by neutralizing MAbs (N-MAbs) (2). Aliquots of HPV-11 were added to cultures of A431 cells, and then PSS (100 μg/ml) was added at different time intervals from 10 min to 8 h after virus addition. All cultures were maintained at 37°C throughout the postattachment treatment periods. Four days later, the cultures were processed for the RT-PCR assay for detection of virus infection as described above.
Microbicidal activity.
The prevention of virus infection in the two in vitro assays (described above) by the tested compounds was interpreted as the compounds having microbicidal (virucidal) or virus-inactivating activity. This broad definition does not imply that the mechanism of action of the compounds on the virus and/or the mechanism of virus inactivation is known. The term microbicide (rather than virucide) for these compounds is used throughout this report because these compounds have shown activity against nonviral STD agents such as gonococci and chlamydia (1, 10, 11).
RESULTS
Microbicidal activity of CS, DS, and PSS on BPV-1.
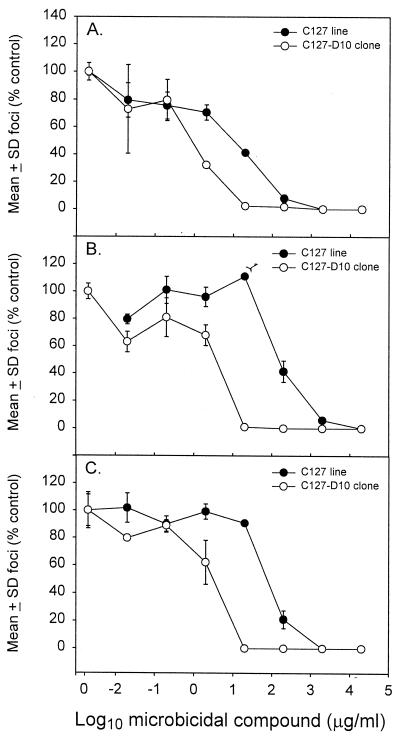
CS, DS, and PSS were tested for direct microbicidal activity against BPV-1 using the focus-forming assay described in Materials and Methods. Dilutions of compounds were incubated with aliquots of BPV-1 prior to addition to C127 cell cultures, and foci were counted after a 2-week culture period. The mean number of foci was plotted against drug concentration for each compound (Fig. 1). The results demonstrated that all three compounds showed microbicidal activity against BPV-1. The concentrations for CS, DS, and PSS that led to a 50% reduction in foci from cultures of C127 cells were between 10 and 100 μg/ml, with CS showing the strongest microbicidal activity. We also derived clones from the parental C127 cell line because of the consistent failure of BPV-1 to induce foci following several cell passages of the uncloned parental cell line. One clone, named C127-D10, which produced foci upon BPV-1 infection, was chosen for a repeat testing of the microbicide compounds. When this clone was tested for microbicidal activity, approximately 10-fold-less compound was required to achieve a 50% reduction in BPV-1-induced foci than for the uncloned parental C127 cells (Fig. 1). Concentrations of compounds required to reduce foci numbers by 50% in clone C127-D10 cells were between 1 and 10 μg/ml. Several other C127 clones failed to produce foci after BPV-1 infection (data not shown).
FIG. 1.
Microbicidal activities of CS (A), DS (B), and PSS (C) against BPV-1. The number of foci (mean ± SD; expressed as a percentage of foci in untreated control cultures) for each dilution of compound were plotted against the concentration of compound (in micrograms per milliliter) for parental C127 cells and clone C127-D10 cells. The number of foci in these experiments ranged from 100 to 200 per culture. A second experiment produced similar results.
Microbicidal activity of CS, DS, and PSS on HPV-11 and HPV-40.
CS, DS, and PSS were tested for microbicidal activity against HPV-11 and -40. The in vitro transient-infection assay described in Materials and Methods was performed. These tests were conducted to determine whether the microbicidal activity of these compounds against BPV-1 could extend to activity against HPVs. Dilutions of compounds were preincubated with aliquots of either HPV-11 or HPV-40 prior to addition to cultures of A431 cells. The microbicidal activity was assessed either as the detection of ethidium bromide-stained PCR products or as an ELISA-based readout as described above. Virus inactivation or lack of virus infection was evidenced by the failure to detect viral spliced RT-PCR products and/or the absence of ELISA values above the background level when the ELISA assay was used to detect labeled PCR products. An initial experiment was conducted to test the specificity of the ELISA-based RT-PCR assay by using HPV-11 and HPV-40 infection of A431 cells (Table 1). Highly specific detection of either HPV-11 or -40 was observed by the presence of high ELISA ODs for the HPV-11 probe from HPV-11-infected but not HPV-40-infected cultures and vice versa.
TABLE 1.
ELISA RT-PCR detection of transient infection of A431 cultures by HPV-11 and HPV-40
| Virus | Concn of PCR productsa | Mean (SD) of ELISA OD readings
|
|
|---|---|---|---|
| HPV-11 probe | HPV-40 probe | ||
| HPV-11 | 10 | 1.827 (0.174) | −0.013 (0.000) |
| 1 | 1.540 (0.034) | NTb | |
| 0.1 | 0.845 (0.039) | NT | |
| HPV-40 | 10 | 0.012 (0.002) | 2.000 (0.000) |
| 1 | NT | 1.842 (0.080) | |
| 0.1 | NT | 1.027 (0.028) | |
Volume (in microliters) of reaction products from the second set of PCR amplification products added to the ELISA wells.
NT, not tested.
All three compounds demonstrated strong microbicidal activity against both HPV-11 and HPV-40, and representative experiments are summarized in Table 2. The results showed that RT-PCR products from cells alone or from uninfected cultures treated with compounds consistently demonstrated low OD readings in the ELISA assay for the HPV products and high OD readings for the β-actin product. Upon HPV-11 and/or HPV-40 infection, cultures showed high levels of ELISA-detectable viral products, and addition of microbicides decreased the signal back to background (uninfected) levels. In tests for microbicidal activity against HPV-11 for CS, this occurred at 100 and 1,000 μg/ml; for DS, this occurred at 10 and 100 μg/ml; and for PSS, this occurred at 10, 100, and 1,000 μg/ml (100 μg/ml for experiment 2). In assays for HPV-40 infectivity, CS was microbicidal at 100 and 1,000 μg/ml, DS was microbicidal at 10 and 1,000 μg/ml, and PSS was microbicidal at 1,000 μg/ml. There was no cellular cytotoxicity for any of the doses of compounds as determined by microscopic examination of the cell cultures. In summary, the concentrations of CS, DS, and PSS required for complete inactivation of the HPV virions ranged from 10 to 100 μg/ml for both HPV-11 and HPV-40.
TABLE 2.
RT-PCR ELISA for detection of transient infection of human A431 cells with HPV-11 or HPV-40a
| Expt and treatment (μg/ml)b | Mean (SD) of OD reading for RT-PCR ELISA
|
|
|---|---|---|
| HPV probe | β-Actin probe | |
| Expt 1 | ||
| Cells alone | 0.043 (0.004) | 1.645 (0.052) |
| HPV-11 only | 1.417 (0.063) | 1.564 (0.051) |
| Without virus | ||
| CS (1,000) | 0.028 (0.004) | 1.427 (0.013) |
| DS (1,000) | 0.000 (0.000) | 1.470 (0.001) |
| PSS (1,000) | 0.038 (0.001) | 1.467 (0.158) |
| With virus | ||
| CS (1,000) | 0.038 (0.001) | 1.518 (0.020) |
| CS (100) | 0.049 (0.001) | 1.532 (0.025) |
| CS (10) | 1.485 (0.045) | 1.576 (0.021) |
| DS (100) | 0.035 (0.000) | 1.583 (0.022) |
| DS (10) | 0.023 (0.000) | 1.636 (0.105) |
| PSS (1,000) | 0.045 (0.001) | 1.565 (0.040) |
| PSS (100) | 0.036 (0.002) | 1.585 (0.022) |
| PSS (10) | 0.011 (0.004) | 1.629 (0.057) |
| Expt 2 | ||
| With virus | ||
| PSS (100) | 0.008 (0.006) | 1.002 (0.186) |
| PSS (10) | 1.045 (0.016) | 1.094 (0.074) |
| PSS (1) | 0.893 (0.100) | 0.145 (0.015) |
| PSS (0.1) | 0.714 (0.026) | 1.171 (0.042) |
| Expt 3 | ||
| With virus | ||
| CS (1,000) | 0.037 (0.004) | NDc |
| CS (100) | 0.128 (0.008) | ND |
| CS (10) | 0.397 (0.050) | ND |
| DS (1,000) | 0.006 (0.007) | ND |
| DS (100) | 0.375 (0.073) | ND |
| DS (10) | 0.042 (0.007) | ND |
| PSS (1,000) | 0.002 (0.003) | ND |
| PSS (100) | 0.732 (0.047) | ND |
| PSS (10) | 1.729 (0.384) | ND |
| HPV-40 only | 1.320 (0.074) | ND |
Human A431 cells were infected with HPV-11 (experiments 1 and 2) or HPV-40 (experiment 3), and HPV-11 probe (experiments 1 and 2) or HPV-40 probe (experiment 3) was used. Two additional experiments yielded similar results.
Concentrations shown are the viral pretreatment doses for CS, DS, and PSS.
ND, not determined.
Blockage of infection by CS, DS, and PSS.
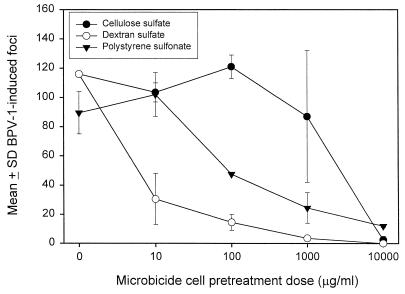
Preincubation of C127-D10 cells with compounds prior to addition of BPV-1 was tested to determine whether the microbicidal effects of CS, DS, and PSS extended to a blockage of virus interaction with cell surfaces. In these experiments, titrations of compounds were added to cell cultures, followed by washing away unbound reagent prior to addition of virus. After a 1-h incubation with virus, unbound virus was removed by washing and the cultures were monitored for foci after 2 weeks. The results (Fig. 2) indicated that these reagents showed some interference of virus with host cell surfaces, as evidenced by a dose-dependent reduction of BPV-1-induced foci. DS and PSS showed the strongest interference, with substantial reduction in foci at doses of 10 μg/ml. In contrast, CS showed only weak microbicidal effects when C127-D10 cells were pretreated with this compound.
FIG. 2.
Inactivation of postattachment BPV-1 following pretreatment of C127-D10 cells with CS, DS, or PSS. The number of foci (mean ± SD) from duplicate cultures of C127-D10 cells was plotted against the cell preincubation dose of compound. A second experiment produced similar results.
Postattachment inactivation of HPV-11 by PSS.
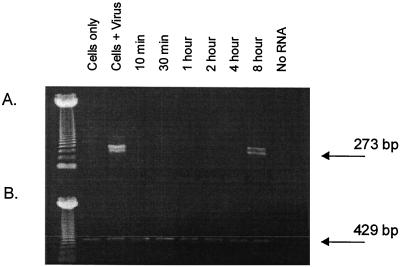
N-MAbs have been previously shown to effect a postattachment neutralization of BPV-1, cottontail rabbit papillomavirus (CRPV), and HPV-11, in which the postattachment neutralization could occur up to 8 h after addition of virus to susceptible cells or tissues (2). This postneutralization activity occurred in conditions in which the cell cultures were metabolically active (37°C). The mechanism by which this postattachment neutralization occurs is poorly understood. We tested PSS in a similar assay system to determine whether a compound other than an N-MAb could also effect a postattachment inactivation of a papillomavirus. HPV-11 virions were added to cultures of A431 cells, the cells were washed, and then a fixed concentration of PSS was added at different times after the addition of virus. The cultures were assayed for transient HPV-11 infection 4 days later using the RT-PCR assay described above, and the results are shown in Fig. 3. PSS inactivated HPV-11 when added as late as 4 h after virus addition. Two bands for detection of the spliced HPV-11 E1∧E4 transcript were occasionally detected because the PCR used nested primer sets, giving PCR products of 399 and 273 bp (17).
FIG. 3.
Postattachment inactivation of HPV-11 infection by PSS. Cultures of A431 cells were infected with HPV-11, and at set time points, 100 μg of PSS per ml was added. After 4 days of incubation, the cultures were assessed for E1∧E4 spliced viral mRNA using the RT-PCR assay described in Materials and Methods. Ethidium bromide-stained PCR products were detected on agarose gels. A 273-bp HPV-11 PCR product (A) and a 429-bp β-actin PCR product (B) can be seen. The leftmost lane contains molecular size markers. The other lanes contain the following: cells only; cells and HPV-11; or cells, HPV-11, and PSS (100 μg/ml) added at different time points after virus addition.
DISCUSSION
Reports describing compounds with effective microbicidal activity against HPV types have been limited to N-MAbs (2, 3), and SDS (13). A limited number of other compounds have shown microbicidal activity against BPV-1 and include povidone-iodine (25), monocaprin (14), SDS (13), and other members of the alkyl sulfate family (13). Together, these observations indicate that there is a dearth of identified compounds that are effective agents for the inactivation or blocking of infectious HPVs.
In this study, we have determined that several sulfated and/or sulfonated compounds have strong microbicidal activity against several papillomaviruses including BPV-1, HPV-11, and HPV-40. The BPV-1 focus-forming assay determined that three compounds, CS, DS, and PSS, had strong microbicidal activities with microbicidal 50% effective concentrations (EC50s) between 1 and 10 μg/ml. Transient-infection assays using human epithelial cells infected with HPV-11 and -40 showed microbicidal activity at concentrations between 10 and 100 μg/ml for these same compounds. These data indicated that the three compounds had microbicidal activity against papillomaviruses in general and validated the BPV-1 focus-forming assay as a relevant surrogate assay for testing non-virus-specific microbicidal agents for activity against infectious HPVs.
We conducted several experiments to begin to elucidate the mechanism by which these compounds inactivate infectious papillomaviruses. Pretreatment of infectious virions with compound led to strong virus inactivation. The microbicidal action therefore appeared to represent virus inactivation by direct association of the compounds with infectious virion. An alternative hypothesis is that the compounds prevented infection by attaching first to the cell surface and subsequently preventing infectious virus from binding. Both mechanisms may contribute to the prevention of papillomavirus infection in the in vitro assays. To determine whether the latter possible mechanism predominates, cells were pretreated with compound and washed multiple times, and then infectious virus was added. Under these conditions, all three compounds retained virus inactivation activity. CS pretreatment of cells required concentrations greater than 1,000 μg/ml for virus inactivation, and the effects obtained may simply have been accomplished by residual compound remaining in the culture dishes after the three washing steps. In contrast, strong virus inactivation by cells pretreated with DS and PSS was observed; this inactivation was equivalent to that obtained when BPV-1 was first incubated with compound. These data indicated that for the latter two compounds, significant virus inactivation activity occurred by blocking the attachment of (non-compound-coated) infectious virions to compound-coated cell surfaces. Blocking of the attachment of virus to cell surfaces cannot be the exclusive mechanism of action of these compounds however, because one of the compounds (PSS) was able to prevent infection of virus after the virus had attached to the cell surface.
We have conducted a number of focus-forming assays (in this and other studies) with cultures of C127 cells. However, the C127 parental cells often became unreliable after several passages such that the cultures appeared transformed without BPV-1 and/or no foci were discernible after a 2-week culture with BPV-1 (data not shown). We therefore subcloned the parental C127 cell cultures and derived several clones that were pretested for the maintenance of stable monolayers and for the ability to develop foci after BPV-1 infection. One clone, C127-D10, produced foci after BPV-1 infection, but several other clones did not (data not shown). When clone C127-D10 was tested for microbicidal activity, there was an approximate 10-fold decrease in the concentration of compound that was required to achieve a 50% reduction in BPV-1-induced foci from that for the uncloned parental C127 cells. The failure of several other C127 clones to produce foci upon BPV-1 infection would suggest that more BPV-1 virus is required to produce the same number of foci in the parental C127 cell cultures. In parental C127 cultures, therefore, the EC50 microbicidal dose would be expected to be greater (i.e., more compound is needed) because more virus is required to produce the same number of focus-forming units compared to that obtained in cultures of clone C127-D10. Other potential contributing mechanisms to explain the different EC50 doses include more-efficient infection of C127-D10 cells and/or more-efficient transformation of C127-D10 cells by infectious BPV-1.
When PSS was added to cultures of HPV-11-infected A431 cells, there was a similar postattachment inactivation to that observed with N-MAbs (2). These data confirmed previous observations that infectious papillomaviruses bind rapidly to cell surfaces but remain on the surface of the cell for several hours prior to internalization (2). The reason for the delay in internalization is unclear but may be related to a “dual” binding step in which virions first interact nonspecifically with cell surface glycosaminoglycans (8, 15) and then interact with a more specific receptor such α6 integrin (6). The importance of α6 integrin for papillomavirus binding, however, remains controversial (8, 22). Both nonspecific and specific coreceptor interactions between cell surfaces and viruses have been proposed in other virus infection systems (16, 19, 26–28). The microbicidal action of the three compounds against BPV-1 and HPVs (Fig. 1 and Table 2) therefore support previous observations that high-molecular-weight DS blocked binding of HPV-11 L1 virus-like particles to epithelial cells (15) and that HS blocked the infectivity of HPV-16 and -33 pseudovirions (8).
In summary, we describe three new compounds that show microbicidal activity against BPV-1, HPV-11, and HPV-40. The mechanism of inhibition of infection appears to include both direct interaction of the compounds with infectious virions and a blocking effect at the cell surface. The lack of significant cellular toxicity of CS, DS, and PSS make these compounds attractive potential microbicides for a variety of infectious pathogens including HPV.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service Program Project grant PO1 AI37829 from the National Institutes of Allergy and Infectious Diseases and by the Jake Gittlen Memorial Golf Tournament.
REFERENCES
- 1.Anderson R A, Feathergill K, Diao X, Cooper M, Kirkpatrick R, Spear P, Waller D P, Chany C, Doncel G F, Herold B, Zaneveld L J D. Evaluation of poly(styrene-4-sulfonate) as a preventative agent for conception and sexually transmitted diseases. J Androl. 2000;21:862–870. [PubMed] [Google Scholar]
- 2.Christensen N D, Cladel N M, Reed C A. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology. 1995;207:136–142. doi: 10.1006/viro.1995.1059. [DOI] [PubMed] [Google Scholar]
- 3.Christensen N D, Kreider J W, Cladel N M, Patrick S D, Welsh P A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990;64:5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Villiers E-M, Hirsch-Behnam A, Von Knebel-Doeberitz C, Neumann C, zur Hausen H. Two newly identified human papillomavirus types (HPV 40 and 57) isolated from mucosal lesions. Virology. 1989;171:248–253. doi: 10.1016/0042-6822(89)90532-1. [DOI] [PubMed] [Google Scholar]
- 5.Dvoretzky I, Shober R, Chattopadhyay S K, Lowy D R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980;103:369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- 6.Evander M, Frazer I H, Payne E, Qi Y M, Hengst K, McMillan N A J. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabelnick H L, Harper M J K. The promise of public/private sector collaboration in the development of microbicides. Int J Gynecol Obstet. 1999;67:S31–S38. doi: 10.1016/s0020-7292(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 8.Giroglou T, Florin L, Schafer F, Streeck R E, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermonat P L, Daniel R W, Shah K V. The spermicide nonoxynol-9 does not inactivate papillomavirus. Sex Transm Dis. 1992;19:203–205. doi: 10.1097/00007435-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Herold B C, Bourne N, Marcellino D, Kirkpatrick R, Strauss D M, Zaneveld L J D, Waller D P, Anderson R A, Chany C J, Barham B J, Stanberry L R, Cooper M D. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J Infect Dis. 2000;181:770–773. doi: 10.1086/315228. [DOI] [PubMed] [Google Scholar]
- 11.Herold B C, Siston A, Bremer J, Kirkpatrick R, Wilbanks G, Fugedi P, Peto C, Cooper M. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob Agents Chemother. 1997;41:2776–2780. doi: 10.1128/aac.41.12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho G Y F, Bierman R, Beardsley L, Chang C J, Burk R D. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 13.Howett M K, Neely E B, Christensen N D, Wigdahl B, Krebs F C, Malamud D, Patrick S D, Pickel M D, Welsh P A, Reed C A, Ward M G, Budgeon L R, Kreider J W. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howett M K, Wigdahl B, Malamud D, Christensen N D, Wyrick P B, Krebs F C, Catalone B J. Alkyl sulfates: a new family of broad spectrum microbicides. 2000. pp. 707–712. . Proceedings of the XIII International AIDS Conference 2000. Monduzzi Editoire, Durban, South Africa. [Google Scholar]
- 15.Joyce J G, Tung J S, Przysiecki C T, Cook J C, Lehman E D, Sands J A, Jansen K U, Keller P M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- 16.Liu C K, Wei G, Atwood W J. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2–6)-linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludmerer S W, McClements W L, Wang X M, Ling J C, Jansen K U, Christensen N D. HPV11 mutant virus-like particles elicit immune responses that neutralize virus and delineate a novel neutralizing domain. Virology. 2000;266:237–245. doi: 10.1006/viro.1999.0083. [DOI] [PubMed] [Google Scholar]
- 18.Nasseri M, Hirochika R, Broker T R, Chow L T. A human papilloma virus type 11 transcript encoding an El∧E4 protein. Virology. 1987;159:433–439. doi: 10.1016/0042-6822(87)90482-x. [DOI] [PubMed] [Google Scholar]
- 19.Pho M T, Ashok A, Atwood W J. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74:2288–2292. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Desormeaux A, Omar R F, Juhasz J, Bergeron M G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J Clin Microbiol. 2000;38:110–119. doi: 10.1128/jcm.38.1.110-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisani P, Parkin D M, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891–903. doi: 10.1002/ijc.2910550604. [DOI] [PubMed] [Google Scholar]
- 22.Sibbet G, Romero-Graillet C, Meneguzzi G, Campo M S. Alpha6 integrin is not the obligatory cell receptor for bovine papillomavirus type 4. J Gen Virol. 2000;81:327–334. doi: 10.1099/0022-1317-81-2-327. [DOI] [PubMed] [Google Scholar]
- 23.Smith L H, Foster C, Hitchcock M E, Isseroff R. In vitro HPV-11 infection of human foreskin. J Invest Dermatol. 1993;101:292–295. doi: 10.1111/1523-1747.ep12365409. [DOI] [PubMed] [Google Scholar]
- 24.Smith L H, Foster C, Hitchcock M E, Leiserowitz G S, Hall K, Isseroff R, Christensen N D, Kreider J W. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J Invest Dermatol. 1995;105:438–444. doi: 10.1111/1523-1747.ep12321173. [DOI] [PubMed] [Google Scholar]
- 25.Sokal D C, Hermonat P L. Inactivation of papillomavirus by low concentrations of povidone-iodine. Sex Transm Dis. 1995;22:22–24. doi: 10.1097/00007435-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Stehle T, Harrison S C. Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments. Structure. 1996;4:183–194. doi: 10.1016/s0969-2126(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 27.Summerford C, Bartlett J S, Samulski R J. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 28.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeitlin L, Whaley K J, Hegarty T A, Moench T R, Cone R A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–335. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]