Abstract
We report the first example of selective Pd(II)-catalyzed tertiary C−H activation of cyclobutylmethyl ketones using a transient directing group. An electron-deficient 2-pyridone ligand was identified as the optimal external ligand to enable tertiary C−H activation. A variety of cyclobutylmethyl ketones bearing quaternary carbon centers was readily accessed without preinstalling internal directing groups in up to 81% yield and >95:5 regioisomeric ratios of tertiary C‒H arylation to β-methylene (β-methyl) or γ-C‒H arylation.
Keywords: C‒H activation, arylation, palladium, transient directing group, synthetic methods
Entry for the Table of Contents
A Pd(II)-catalyzed tertiary C−H activation of cyclobutylmethyl ketones using a transient directing group is reported. An electron-deficient 2-pyridone ligand was identified an external ligand to enable tertiary C−H activation. A variety of cyclobutylmethyl ketones bearing quaternary carbon centers was readily accessed in up to 81% yield and >95:5 regioisomeric ratios of tertiary C‒H arylation to β-methylene (β-methyl) or γ-C‒H arylation.
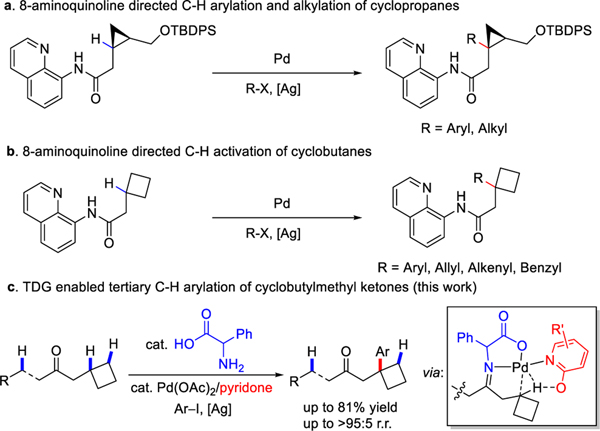
The activation of typically inert C–H bonds and their subsequent transformation into new functional groups via transition metal catalysis has emerged as a promising platform for enabling new disconnections in synthesis campaigns. While a wide range of Pd-catalyzed C‒H activation reactions has been discovered in the past decade,[1–2] the majority of these reports have targeted primary and secondary C‒H bonds, with only a handful of examples reporting tertiary C‒H activation.[3–4] Shuto and Hoshiya et al. reported a Pd-catalyzed tertiary C‒H arylation and alkylation of cyclopropanes using 8-aminoquinoline[5], a strongly coordinating auxiliary, as the preinstalled directing group (Scheme 1a).[3a, 3b] Rao and Sheng et al. also demonstrated a Pd-catalyzed cascade C‒H activation on the tertiary C‒H functionalization of cyclobutanes using the same bidentate 8-aminoquinoline directing group (Scheme 1b).[4] From the vantage point of practicality, C‒H activation reactions which do not require the installation and removal of bespoke, exogenous directing groups such as 8-aminoquinoline will invariably be much more attractive in terms of both atom and step economies. The constraints of such exogenous directing groups may be overcome in one of two ways: the development of ligand-accelerated C‒H activation reactions which may be directed by functional groups native to the target molecules,[6–7] or by the development of reactions which use reversibly attached transient directing groups.[8–11] Considering the widespread presence of cyclobutane motifs among biologically significant molecules,[12] we embarked on the development of a tertiary C−H bond arylations in cyclobutylmethyl ketones using a transient-directing-group (TDG- ) strategy (Scheme 1c).
Scheme 1.
Pd-catalyzed tertiary C‒H bond activation
Herein, we report the first example of Pd-catalyzed tertiary β- C‒H arylation of cyclobutylmethyl ketones using an α-amino acid as the transient directing group. The use of a pyridone ligand was found to be crucial in this reaction.
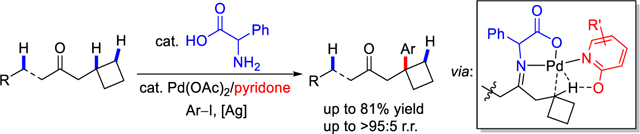
To test the feasibility of our approach, we first attempted the tertiary C‒H bond arylation of 1-cyclobutylpropan-2-one (1a) with methyl 4-iodobenzoate (2a) on the basis of our previous work in β-methylene C−H arylation.[8b, 8d] The results of the pyridone ligand evaluation are summarized in Table 1. In the absence of ligand, a 5% yield of the arylated product was observed. We found that electron-deficient pyridone ligands led to improved yields compared to the ligand-free conditions, which is in agreement with prior observations in Pd-catalyzed C‒H activation reactions.[7] It is also noteworthy that all the tested pyridone ligands gave the desired product with a high regioisomeric ratio (>95:5 r.r.) to γ-C‒H arylation.[7a] The use of 5-nitropyridone (L4) as the ligand gave the desired product in significantly increased yield (58%), and the yield could be further optimized to 71% by using 50 mol% L4 in the presence of 10 mol% Pd(OAc)2, 30 mol% DL-phenylglycine (TDG1), 0.7 equiv Ag3PO4, 2.5 equiv 1a. With 20 mol% DL-phenylglycine (TDG1), the yield dropped to 48%. When 30 mol% L4 or 50 mol% Ag3PO4 was used in this reaction, the desired products were obtained in 43% and 55% yield, respectively. While further screening of TDG derived from amino acids did not provide significant improvement, this finding guided us to design and synthesize other related amino acid amides as TDG for evaluation. Although preliminary exploration afforded inferior yields (see the Supporting Information for details of reaction optimization), the feasibility of developing other types of TDG for tertiary C–H bonds is valuable.
Table 1.
Ligand Evaluation for Tertiary C‒H Arylation of 1a[a]
|
Conditions: 1a (0.2 mmol, 2.0 equiv), methyl 4-iodobenzoate 2a (1.0 equiv), Pd(OAc)2 (10 mol %), TDG1 (30 mol %), ligand (50 mol %), Ag3PO4 (0.7 equiv.), HFIP (0.6 mL), 100 °C, under air, 24 h.
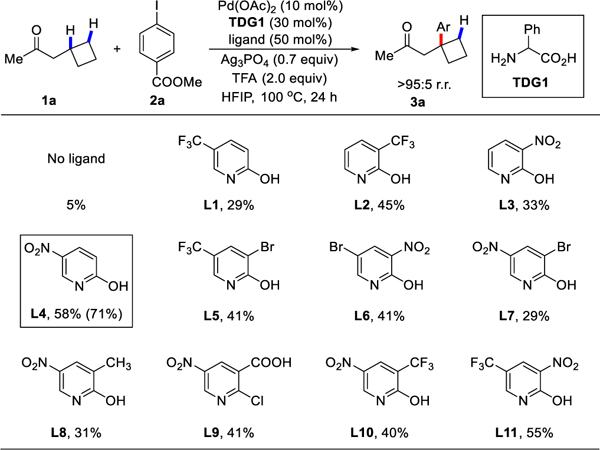
With the optimal conditions in hand, the substrate scope of the tertiary C‒H arylation reaction was examined with respect to the aryl iodides (Table 2). Most of the tested aryl iodides, bearing either electron-withdrawing groups (3a‒3h) or electron-donating groups (3i‒3j) on the phenyl ring, had little influence on the yield, providing the corresponding products in moderate to good yields and a high regioisomeric ratio (>95:5 r.r.). In this arylation reaction, fluoro- (3e, 3o), chloro- (3f, 3p), and bromo- (3g) substituted aryl iodides worked well, offering opportunities for the further derivatization of the reaction products. Functional groups such as ester (3a, 3m), nitro (3b, 3n), ketone (3k), and alcohol (3t) were also tolerated and showed comparable reactivity. Notably, formyl- and coordinative cyano- substituted aryl iodides still underwent the arylation to afford the desired products (3l, 3q), albeit in low yield.
Table 2.
Scope of Aryl Iodides[a]
|
Conditions: 1a (0.25 mmol, 2.5 equiv), 2 (0.1 mmol, 1.0 equiv), Pd(OAc)2 (10 mol %), TDG1 (30 mol %), L4 (50 mol %), Ag3PO4 (70 mol %), CsOAc (1.0 equiv.), HFIP (0.6 mL), 100 °C, under air, 24‒48 h.
3% di-arylation product on β-tertiary and γ-methylene C‒H bonds was observed
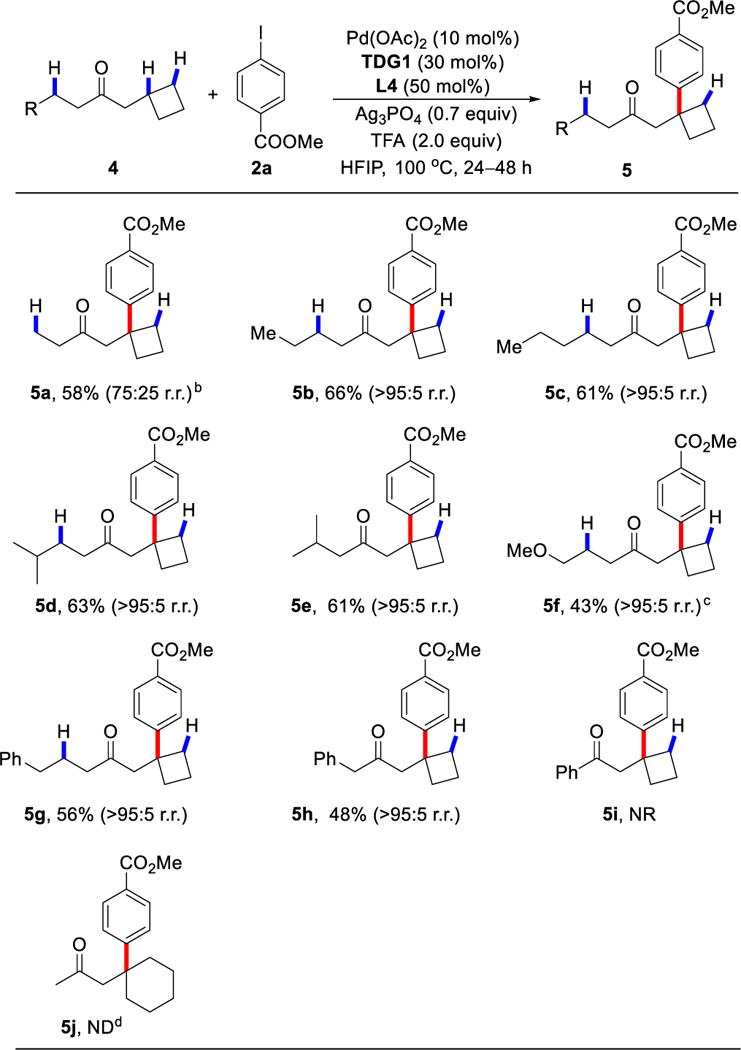
We next investigated the scope with respect to cyclobutylmethyl alkyl ketones, which are potentially more challenging substrates than 1a due to the possibility of β-C‒H bonds on either side of the ketone being activated (Table 3). Indeed, rerouting the selectivity from the β-methylene C‒H bond to the sterically hindered β-tertiary C‒H bond remains an unsolved challenge in Pd(II) catalysis. To our delight, this arylation enabled by pyridone ligand occurred highly selectively at the β-tertiary C‒H bond in the majority of cases. The arylation of β-methyl or β-methylene C–H bonds is largely disfavored, presumably due to the stereochemistry of the transient imines or the enhanced s character of the tertiary C‒H bond in cyclobutanes. For example, ketone substrates bearing alkyl (4b‒4e), methoxy (4f), phenylpropyl (4g) or benzyl (4h) functional groups selectively afforded the β-tertiary C‒H arylated product in 43 to 66% yield with up to > 95:5 r.r.. The ketone 4a, containing a readily accessible β-methyl C‒H bond, also afforded site selectivity for the tertiary C–H bond (75:25 r.r.). However, when we changed the alkyl group to a phenyl group (4i), no reaction was observed under the standard conditions. Likewise, no desired product was observed when 1-cyclohexylpropan-2-one (4j) was employed as the substrate.
Table 3.
Scope of Ketones[a]
|
Conditions: 4 (0.25 mmol, 2.5 equiv), 2a (0.1 mmol, 1.0 equiv), Pd(OAc)2 (10 mol %), TDG1 (30 mol %), L4 (50 mol %), Ag3PO4 (70 mol %), CsOAc (1.0 equiv.), HFIP (0.6 mL), 100 °C, under air, 24‒48 h.
The ratio of r.r. was determined by 1H NMR analysis of the crude reaction mixture.
2% di-arylation product on β-tertiary and γ-methylene C-H bonds was observed.
A trace amount of methylene C‒H activation product was observed
In conclusion, we have developed the first example of a tertiary C–H arylation of cyclobutylmethyl ketones using an α-amino acid as a transient directing group (TDG) to construct quaternary carbon centers. An electron-deficient 2-pyridone ligand was identified as an external ligand to enable tertiary C−H activation. This protocol will facilitate the synthesis of novel cyclobutane motifs containing quaternary carbon centers, which are potentially highly valuable in the creation of diverse cyclobutanecentered compound libraries in early drug discovery.
Supplementary Material
Acknowledgements
We gratefully acknowledge Scripps Research and the NIH (NIGMS, R01GM084019) for financial support. We thank Dr. Jason Chen, Ms. Brittany Sanchez, and Ms. Emily Sturgell (Automated Synthesis Facility, The Scripps Research Institute) for their assistance with HRMS analysis. J.-T. C. thanks the China Scholarship Council (CSC) fellowship program, Scientific and technological innovation project of China Academy of Chinese Medical Sciences (CI2021A04409), and the fundamental research funds for the central public welfare research institutes (No. ZZ13-YQ-061) for financial support. We thank Alastair N. Herron from Scripps Research for editorial help.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].For recent reviews on C‒H functionalization, see Lyons TW, Sanford MS, Chem. Rev. 2010, 110, 1147;Ackermann L, Chem. Rev. 2011, 111, 1315;Daugulis O, Roane J, Tran LD, Acc. Chem. Res. 2015, 48, 1053;He J, Wasa M, Chan KSL, Shao Q, Yu J-Q, Chem. Rev. 2017, 117, 8754;Lam NYS, Wu K, Yu J-Q, Angew. Chem. Int. Ed. 2021, 60, 15767; Angew. Chem. 2021, 133, 15901.
- [2].Selected reviews of enantioselective C‒H functionalization: Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q, Chem. Soc. Rev. 2009, 38, 3242;Newton CG, Wang S-G, Oliveira CC, Cramer N, Chem. Rev. 2017, 117, 8908;Saint-Denis TG, Zhu R-Y, Chen G, Wu Q-F, Yu J-Q, Science 2018, 359, 759;Shao Q, Wu K, Zhuang Z, Qian S, Yu J-Q, Acc. Chem. Res. 2020, 53, 833.
- [3].a) Hoshiya N, Takenaka K, Shuto S, Uenishi J, Org. Lett. 2016, 18, 48; [DOI] [PubMed] [Google Scholar]; b) Hoshiya N, Kobayashi T, Arisawa M, Shuto S, Org. Lett. 2013, 15, 6202; [DOI] [PubMed] [Google Scholar]; c) Ladd CL, Roman DS, Charette AB, Org. Lett. 2013, 15, 1350; [DOI] [PubMed] [Google Scholar]; d) Saget T, Perez D, Cramer N, Org. Lett. 2013, 15, 1354; [DOI] [PubMed] [Google Scholar]; e) Tsukano C, Okuno M, Takemoto Y, Chem. Lett. 2013, 42, 753. [Google Scholar]
- [4].Yang X, Shan G, Yang Z, Huang G, Dong G, Sheng C, Rao Y, Chem. Commun. 2017, 53, 1534. [DOI] [PubMed] [Google Scholar]
- [5].Zaitsev VG, Shabashov D, Daugulis O, J. Am. Chem. Soc. 2005, 127, 13154. [DOI] [PubMed] [Google Scholar]
- [6].For selected examples on 2-pyridone accelerated C(sp2)−H actvation reactions: Wang P, Farmer ME, Huo X, Jain P, Shen P-X, Ishoey M, Bradner JE, Wisniewski SR, Eastgate MD, Yu J-Q, J. Am. Chem. Soc. 2016, 138, 9269;Wang P, Verma P, Xia G, Shi J, Qiao JX, Tao S, Cheng PTW, Poss MA, Farmer ME, Yeung K-S, Yu J-Q, Nature 2017, 551, 489;Li G-C, Wang P, Farmer ME, Yu J-Q, Angew. Chem. Int. Ed. 2017, 56, 6874; Angew. Chem. 2017, 129, 6978;Farmer ME, Wang P, Shi H, Yu J-Q, ACS Catal. 2018, 8, 7362;Liu L-Y, Yeung K-S, Yu J-Q, Chem. Eur. J. 2019, 25, 2199.
- [7].For selected examples on 2-pyridone accelerated C(sp3)−H actvation reactions:Zhu R-Y, Li Z-Q, Park HS, Senanayake CH, Yu J-Q, J. Am. Chem. Soc. 2018, 140, 3564;Xia G, Weng J, Liu L-Y, Verma P, Li Z-Q, Yu J-Q, Nat. Chem. 2019, 11, 571;Park H, Yu J-Q, J. Am. Chem. Soc. 2020, 142, 16552;Xia G, Zhuang Z, Liu L-Y, Schreiber SL, Melillo B, Yu J-Q, Angew. Chem. Int. Ed. 2020, 59, 7783; Angew. Chem. 2020, 132, 7857.
- [8].For selected examples on TDG enabled C(sp3)−H activation on ketones: Zhang F-L, Hong K, Li T-J, Park H, Yu J-Q, Science 2016, 351, 252;Hong K, Park H, Yu J-Q, ACS Catal. 2017, 7, 6938;Park H, Verma P, Hong K, Yu J-Q, Nat. Chem. 2018, 10, 755;Xiao L-J, Hong K, Luo F, Hu L, Ewing WR, Yeung K-S, Yu J-Q, Angew. Chem. Int. Ed. 2020, 59, 9594; Angew. Chem. 2020, 132, 9681.
- [9].For selected examples on TDG enabled C(sp3)−H activation on aldehydes: Yang K, Li Q, Liu Y, Li G, Ge H, J. Am. Chem. Soc. 2016, 138, 12775;Ma F, Lei M, Hu L, Org. Lett. 2016, 18, 2708;St John-Campbell S, White AJP, Bull JA, Chem. Sci. 2017, 8, 4840;Gou B-B, Liu H-F, Chen J, Zhou L, Org. Lett. 2019, 21, 7084;Li B, Lawrence B, Li G, Ge H, Angew. Chem. Int. Ed. 2020, 59, 3078; Angew. Chem. 2020, 132, 3102;Wen F, Li Z, Adv. Synth. Catal. 2020, 362, 133.
- [10].For selected examples on TDG enabled C(sp3)−H activation on amines:Wu Y, Chen Y-Q, Liu T, Eastgate MD, Yu J-Q, J. Am. Chem. Soc. 2016, 138, 14554;Xu Y, Young MC, Wang C, Magness DM, Dong G, Angew. Chem. Int. Ed. 2016, 55, 9084; Angew. Chem. 2016, 128, 9230;Liu Y, Ge H, Nat. Chem. 2017, 9, 26;Yada A, Liao W, Sato Y, Murakami M, Angew. Chem. Int. Ed. 2017, 56, 1073; Angew. Chem. 2017, 129, 1093;Chen Y-Q, Wang Z, Wu Y, Wisniewski SR, Qiao JX, Ewing WR, Eastgate MD, Yu J-Q, J. Am. Chem. Soc. 2018, 140, 17884;Chen Y-Q, Singh S, Wu Y, Wang Z, Hao W, Verma P, Qiao JX, Sunoj RB, Yu J-Q, J. Am. Chem. Soc. 2020, 142, 9966;Chen Y-Q, Wu YWZ, Qiao JX, Yu J-Q, ACS Catal. 2020, 10, 5657;St John-Campbell S, Ou AK, Bull JA, Chem. Eur. J. 2018, 24, 17838;Lin H, Wang C, Bannister TD, Kamenecka TM, Chem. Eur. J. 2018, 24, 9535;Kapoor M, Liu D, Young MC, J. Am. Chem. Soc. 2018, 140, 6818.
- 11.For selected reviews on transient directing groups:Gandeepan P, Ackermann L. Chem 2018, 4, 199;Liao G, Zhang T, Lin Z-K, Shi B-F, Angew. Chem. Int. Ed. 2020, 59, 19773; Angew. Chem. 2020, 132, 19941.
- 12.a) “Naturally Occurring Cyclobutanes”: Hansen TV, Stenstrøm Y. in Organic Synthesis: Theory and Applications, Vol. 5 (Ed.: Hudlicky T), Elsevier Science, Oxford, 2001, p. 1; [Google Scholar]; b) Dembitsky VM, J. Nat. Med. 2008, 62, 1; [DOI] [PubMed] [Google Scholar]; c) Chi Y-M, Nakamura M, Zhao X-Y, Yoshizawa T, Yan W-M, Hashimoto F, Kinjo J, Nohara T, Chem. Pharm. Bull. 2005, 53, 1178; [DOI] [PubMed] [Google Scholar]; d) Kurosawa K, Takahashi K, Tsuda E, Antibiot J. 2001, 54, 541; [DOI] [PubMed] [Google Scholar]; e) Gutekunst WR, Baran PS, J. Am. Chem. Soc. 2011, 133, 19076; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Gutekunst WR, Gianatassio R, Baran PS, Angew. Chem. Int. Ed. 2012, 51, 7507; Angew. Chem. 2012, 124, 7625; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Gutekunst WR, Baran PS, J. Org. Chem 2014, 79, 2430; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Panish RA, Chintala SR, Fox JM, Angew. Chem. Int. Ed. 2016, 55, 4983; Angew. Chem. 2016, 128, 5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.