Highlights
-
•
Inadequate sleep accelerates brain ageing (deviation above the chronological age).
-
•
Brain age delta is a highly sensitive marker linking poor sleep to brain ageing.
-
•
Multimodal analyses of brain ageing outperform unimodal approaches.
-
•
Bias correction (removal of age-related bias) improves estimates of brain age delta.
Keywords: Sleep, Ageing, Brain age, Gray matter, White matter, Magnetic resonance imaging
Abstract
Numerous studies indicate large heterogeneity in brain ageing, which can be attributed to modifiable lifestyle factors, including sleep. Inadequate sleep has been previously linked to gray (GM) and white (WM) matter changes. However, the reported findings are highly inconsistent. By contrast to previous research independently characterizing patterns of either GM or WM changes, we used here linked independent component analysis (FLICA) to examine covariation in GM, and WM in a group of older adults (n = 50). Next, we employed a novel technique to estimate the brain age delta (difference between chronological and brain age assessed using neuroimaging data) and study its associations with sleep quality and sleep fragmentation, hypothesizing that inadequate sleep accelerates brain ageing. FLICA revealed a number of multimodal components, associated with age, sleep quality, and sleep fragmentation. Subsequently, we show significant associations between brain age delta and inadequate sleep, suggesting 2 years deviation above the chronological age. Our findings indicate sensitivity of multimodal approaches and brain age delta in detecting link between inadequate sleep and accelerated brain ageing.
1. Introduction
The ageing brain undergoes widespread gray (GM) and white matter (WM) degeneration associated with functional brain changes and gradual cognitive decline. Numerous studies indicate large heterogeneity in the age-related brain changes in older adults (Cabeza et al., 2017; Eavani et al., 2018; Good et al., 2001; Raz & Rodrigue, 2006; Westlye et al., 2010). Understanding this heterogeneity is of high importance as it could provide key insights into why some older adults go through rapid cognitive deterioration progressing to dementia, while others experience only mild decline in cognitive functioning or no noticeable cognitive changes (Hayden et al., 2011). However, disentangling the heterogeneity in brain ageing has proved to be a challenge despite numerous research efforts. Some of the ongoing work focuses on phenotyping to capture a variation in brain ageing and predict trajectories of normal versus pathologic ageing (e.g., Eavani et al., 2018). Other studies explore compensatory and neuroadaptive mechanisms often linked to lifelong cumulative cognitive engagement (for review see Cabeza et al., 2018; Fabiani, 2012; Stern et al., 2020). Finally, a growing body of work links heterogeneity in brain ageing to modifiable lifestyle factors such as sleep, diet, and physical activity (Wassenaar et al., 2019).
Sleep disruptions constitute a potentially modifiable risk factor for dementia and reduced longevity. As we get older overall sleep quality deteriorates. Up to half of elderly population experiences various sleep problems and sleep disruptions, including difficulties in maintaining or initiating sleep, and fragmentation of sleep (Miner & Kryger, 2017; Varma et al., 2019). Short sleep duration and poor sleep quality in healthy older adults have been previously associated with grey matter atrophy and microstructural white matter changes, consequently leading to cognitive decline (Sexton et al., 2014; Sexton et al., 2017; Wassenaar et al., 2019). However, the reports linking sleep problems, brain changes, cognitive outcomes and overall increased risk of dementia are inconsistent (Sexton et al., 2020; Zitser et al., 2020). For example, a recent longitudinal ageing study, spanning over almost 3 decades failed to demonstrate a significant link between sleep duration, and either grey matter or white matter microstructure (Zitser et al., 2020). While some of the inconsistent findings (e.g., Fjell et al., 2021; Lo et al., 2014; Ramos et al., 2014; Sexton et al., 2014; Sexton et al., 2020; Sexton et al., 2017; Spira et al., 2016; Yaffe et al., 2016; Zitser et al., 2020) could be explained by differences in terms of how sleep problems have been defined and assessed or differences in studied population characteristics, it is also plausible that these could be attributed to methods employed to examine age-related brain changes.
Data analysis techniques rooted in structural magnetic resonance imaging (MRI), such as voxel-based morphometry (Ashburner & Friston, 2000) and cortical surface analysis (Dale et al., 1999; Fischl, 2012; Fischl & Dale, 2000) from T1-weighted scans, have been fundamental in identifying volumetric gray matter and cortical thickness changes, respectively, in relation to age-related cognitive decline (Giorgio et al., 2010; Good et al., 2001; Lemaitre et al., 2012). Additionally, diffusion MRI (dMRI)-based measures sensitive to microstructural properties of white matter have been successfully employed to characterize white matter alterations and changes in structural connectivity in relation to cognitive ageing (e.g., Barrick et al., 2010; Burzynska et al., 2010; Giorgio et al., 2010). Such approaches characterize patterns of either gray (e.g., voxel-based morphometry analysis, Ashburner & Friston, 2000) or white matter (e.g. tract-based spatial statistics analysis, Smith et al., 2006) changes independently, estimated from a single MRI modality. A potential shortcoming is that they fail to capture interlinked gray and white matter changes associated with cognitive decline as well as to model covariation in gray and white matter age-related deterioration. Furthermore, information provided by methods based on different modalities might be difficult to integrate into a single model of brain ageing and occasionally the resulting findings seem contradictory (e.g., McGinnis et al., 2011; Raz & Rodrigue, 2006). To overcome these drawbacks and limitations methods aimed at fusing information from multiple modalities have been developed (e.g., Groves et al., 2011; Liu et al., 2009; Xu et al., 2009). One of such methods, based on linked independent component analysis (Groves et al., 2011), enables decomposition of data from multiple modalities into spatial components to model variation in cross-modal imaging features across groups of participants. Linked independent component analysis has been previously applied to access patterns of structural brain changes during healthy and pathologic ageing (Douaud et al., 2014; Groves et al., 2011; Groves et al., 2012). To our best knowledge all the previous studies, examining the effects of sleep problems on brain ageing, separately examined patterns of either gray or white matter changes estimated from a single MRI modality.
Another approach to capturing the inter-individual differences in the rate of brain ageing is based on estimation of so called “brain age gap” or “brain age delta” i.e., difference between “chronological age” calculated from the date of birth and “brain age” computed based on neuroimaging data (Cole & Franke, 2017; Cole et al., 2017; Franke & Gaser, 2019; Liem et al., 2017; Smith et al., 2019). As this method enables to quantify the deviation from normative ageing, it has been used as a biomarker of brain ageing; to assess accelerated brain ageing associated with Alzheimer's disease as well as a predictor of progression from mild cognitive impairment to dementia (Boyle et al., 2021; Cole & Franke, 2017; Franke & Gaser, 2019; Gaser et al., 2013). Similarly, this method has a potential to provide understanding of long-term predictors of brain health, including socio-demographic and lifestyle factors. One recent study examined the effect of education and physical activity on the gap between “chronological age” and “brain age,” elegantly demonstrating that higher levels of education and physical activity have a positive impact on the ageing brain, supporting more “youthful” state (Steffener et al., 2016).
In this study, we investigated the associations of age-related brain changes with 2 measures indicative of sleep problems, sleep quality index (Pittsburgh Sleep Quality Index, Buysse et al., 1989) and actigraphy-derived measure of sleep fragmentation, hypothesizing that inadequate sleep (poor sleep quality and sleep fragmentation) accelerates brain ageing. First, using linked independent component analysis, we explored interconnected GM and WM microstructural changes due to brain aging and sleep problems (inadequate sleep) in a group of 50 neurotypical elderly participants using measures extracted from structural and diffusion MRI. Subsequently, to assess the effects of inadequate sleep on accelerated brain ageing, we employed a recently-introduced technique (Smith et al., 2019) to estimate brain age delta, the deviation from chronological brain age, in an unbiased manner (i.e., by applying linear and quadratic correction to remove age-related biases). While the most widely used approaches to study “brain age gap” (for review see Cole & Franke, 2017; Franke & Gaser, 2019) are based on predictors derived from a single MRI modality, we employed a multimodal approach, with a set of structural, and microstructural imaging-derived features (Smith et al., 2019). Altogether, we aimed to explore sensitivity of multimodal approaches and brain age delta in detecting the associations between the inadequate sleep (assessed based on overall sleep quality and sleep fragmentation) and brain ageing, which might not be evident using conventional unimodal analyses.
2. Methods and materials
2.1. Participants
Fifty older adults participated in the study (22 males; age range 65-84; mean ± SD age 73.5 ± 4.7). All participants were recruited either from the Neuropsychological panel of elderly volunteers, or the Birmingham 1000 Elders group, both established at the University of Birmingham. The 2 panels of elderly volunteers consist of adults aged 65 or over who have no pre-existing cognitive impairment. All participants had normal or corrected-to-normal vision, had no history of psychiatric or neurologic disease and were right-handed (self-report). All participants scored within normal range on all the subscales of the short version of the Depression, Anxiety and Stress Scale (DASS-21; Lovibond & Lovibond, 1995). Participants with contraindications to MRI and clinical diagnosis of sleep disorders were excluded.
The study was approved by the University of Birmingham Ethical Review Committee and all participants provided written informed consent and received monetary compensation for participation in agreement with approved ethics protocol. The demographic characteristics of participants are presented in Table 1.
Table 1.
Participant's characteristics
| Variable | Number (N = 50) | Mean (SD) | Range |
|---|---|---|---|
| Age (y) | — | 73.5 (4.7) | 65-84 |
| 65-69 | 11 | — | — |
| 70-74 | 17 | — | — |
| 75-79 | 16 | — | — |
| 80-85 | 6 | — | — |
| Gender (Male and/or Female) | 22/28 | — | — |
| PSQI (global score)a | — | 5.6 (3.3) | 0-15 |
| Subjective sleep qualityb | — | 0.9 (0.6) | 0-2 |
| Sleep latencyb | — | 0.9 (0.9) | 0-3 |
| Sleep durationb | — | 0.8 (0.7) | 0-2 |
| Sleep efficiencyb | — | 0.9 (0.9) | 0-3 |
| Sleep disruptionsb | — | 1.3 (0.5) | 0-2 |
| Sleep medicationb | — | 0.3 (0.6) | 0-3 |
| Daytime dysfunctionsb | — | 0.8 (0.6) | 0-3 |
| WASO (min) | — | 39.6 (19.2) | 15-107 |
PSQI, Pittsburgh Sleep Quality Index; WASO, wake after sleep onset.
Maximum score 21.
Maximum score 3).
2.2. Sleep assessment
Wrist actigraphy, sleep diaries and the Pittsburgh Sleep Quality Index (PSQI) questionnaire were used as objective and self-reported and/or subjective measures of habitual sleep. To objectively evaluate sleep patterns, participants were asked to wear wrist actigraphs (Actiwatch2, Philips Respironics Ltd) for a period of 2 weeks prior to the scheduled MRI scanning session. Actigraphs were set to 1-minute epochs (medium sensitivity setting), and collected data analyzed using Respironics Actiware 6 (Philips, Netherlands) software, which translates wrist movement data into sleep scores (sleep and/or wake cycles). The software output was validated using sleep diaries completed alongside actigraphy. For the purpose of the current study, we used Actiware outputs to calculate wake after sleep onset (WASO), a parameter measuring wakefulness time (in minutes) occurring after sleep onset, which indexes sleep fragmentation. In addition, participants were asked to self-evaluate their sleep quality by completing a widely used PSQI questionnaire (Buysse et al., 1989). PSQI is a validated scale consisting of 19 self-rated questions assessing sleep quality and sleep problems over a period of 1 month. Each question is rated on a 4-point Likert scale from 0 (not during the past month) to 3 (3 or more times a week). The scores are first combined into 7 components (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction), which are added to produce a global score of sleep quality, with a range of 0-21 points, with a higher score indexing worse sleep quality (Buysse et al., 1989; Carpenter & Andrykowski, 1998). PSQI has been shown to have a good reliability and validity in assessing sleep quality in the elderly population (Buysse et al., 1991; Gentili et al., 1995).
Throughout the manuscript we use term inadequate sleep, which is defined based on poor sleep quality indexed by the global PSQI score, and actigraphy-derived wakefulness time (WASO) indexing sleep fragmentation (not assessed by any of the PSQI questions). In order to ensure normal distribution, the WASO and PSQI global scores were log and square root transformed, respectively, prior to using them in the subsequent statistical analyses as measures of sleep fragmentation, and sleep quality.
2.3. MRI data acquisition
Structural T1-weighted and diffusion-weighted scans were acquired at the Birmingham University Imaging Centre (BUIC) using a Philips 3T Achieva scanner a 32-channel head coil. A T1-weighted MPRAGE with spatial resolution 1 × 1 × 1mm3 (176 sagittal slices, TR = 7.5 ms, TE = 3.5 ms, flip angle = 8°) was obtained for each participant, along with a multi-shell dMRI (single-shot EPI, 2 × 2 × 2mm3, TR = 9000 ms, TE = 81.5 ms, 5 x b = 0 s/mm2, 50 x b = 1000s/mm2, 50 x b = 2000s/mm2, plus 5 x b = 0 s/mm2 phase encoding-reversed to correct for susceptibility-induced artifacts; Andersson et al., 2003).
2.4. T1-weighted data pre-processing and voxel-based morphometry (Unimodal analysis)
The structural T1-weighted images were preprocessed using the UK Biobank T1-weighted pipeline (Alfaro-Almagro et al., 2018). The pipeline corrects for bias fields, performs skull-stripping and aligns data to the MNI152 standard template, before segmenting the T1 images into different tissue classes (e.g. GM/WM/CSF) as well as into to cortical and subcortical structures.
To examine whole brain age- and inadequate sleep (poor sleep quality and sleep fragmentation)-related differences in GM structure, the brain-extracted images were processed following the FSL voxel-based morphometry (VBM) pipeline (Douaud et al., 2007; Good et al., 2001). The resulting GM images were first non–linearly registered to the MNI152 standard space and they were concatenated and averaged to create a study-specific GM template. All of the 50 native images were then non–linearly registered to this study-specific template and they were modulated by the determinant of the Jacobian of the non–linear warp field to correct for local enlargement or contraction due to the transformation. The 50 modulated registered GM images were smoothed with an isotropic Gaussian kernel with a sigma of 2mm (∼5mm FWHM). We examined widespread GM volumetric changes in the brain by building a general linear model (GLM) using the demeaned age as a regressor. We also studied the effects of sleep problems on the GM changes by building 2 GLMs using the demeaned PSQI and WASO while regressing out age, respectively. We used FSLs randomize to perform non–parametric inference (Winkler et al., 2014). Threshold-free cluster enhancement (TFCE) was applied to avoid the selection of arbitrary initial cluster-forming threshold (Smith & Nichols, 2009) and 1000 permutations were performed for each contrast defined in the GLMs. The statistical maps were corrected for multiple comparisons using family-wise error rate (FWE).
2.5. Extraction of T1 features
We extracted 110 structural (T1) imaging derived phenotypes (T1 IDPs) using the UK Biobank T1 pipeline (Alfaro-Almagro et al., 2018). The T1 IDPs represented volumes of cortical and subcortical structures in each hemisphere in the standard MNI152 space which were based on the Harvard-Oxford structural atlases (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). The cortical IDPs were obtained using FAST (Zhang et al., 2001) to derive the GM volumes of the cortical regions of interest (ROIs). The subcortical IDPs were obtained using FMRIBs Integrated Registration and Segmentation Tool (FIRST; Patenaude et al., 2011) to produce the GM volumes of the subcortical ROIs including the limbic, basal ganglia and thalamic sub-regions, and extending to the brainstem. These T1-derived features, along with the dMRI-derived features (see Section 2.7), were used for brain age delta estimation (Section 2.9). The complete list of the 110 T1 IDPs that were used in the brain age delta models is presented in Supplementary Table 1.
2.6. Diffusion data pre-processing and tract-based spatial statistics (unimodal analysis)
The dMRI data were preprocessed using the UK biobank pipeline (Alfaro-Almagro et al., 2018). The procedure corrects for susceptibility induced distortion, eddy-current distortion and motion using the EDDY toolbox (Andersson & Sotiropoulos, 2016) and obtains transformations of the diffusion to structural and standard space. A diffusion tensor model (Basser et al., 1994) was fitted to low b-value (b = 0 and 1000s/mm2) shells of each voxel of the corrected diffusion data to obtain microstructural maps including fractional anisotropy (FA) and mean diffusivity (MD).
To examine whole brain age- and inadequate sleep-related differences in WM microstructure, Tract-Based Spatial Statistics (TBSS) were carried out to skeletonize and transform the FA volumes into a common space (Smith et al., 2006). The FA native images were non–linearly registered to the FMRIB58 FA standard space using FNIRT due to its good native-to-standard warping across different age groups (Andersson et al., 2019; Westlye et al., 2010). The mean FA volume from the 50 subjects was derived and thinned to create a study-specific mean FA skeleton which represents the centers of all common tracts. The mean FA skeleton was then thresholded and binarized at FA > 0.2 to minimize partial volume effects with the boundaries of GM and CSF tissues. The subject-wise FA volumes were warped onto this mean FA skeleton to produce skeletonized FA data and the same warping procedure was applied to the MD maps to yield skeletonized MD data from voxels with FA > 0.2. The resulting skeletonized FA and MD maps were then fed into voxelwise cross-subject statistics. We constructed GLMs to test for widespread effects of ageing on FA and MD while regressing out the effects of motion, as estimated by EDDY. We also examined the effects of inadequate sleep on FA and MD by building GLMs using the demeaned PSQI and WASO while regressing out motion and age. Threshold-free cluster enhancement (TFCE) was applied to avoid the selection of arbitrary initial cluster-forming threshold (Smith & Nichols, 2009) and 1000 permutations were performed for each contrast defined in the GLMs. The statistical maps were FWE-corrected for multiple comparisons.
2.7. Extraction of dMRI features
We performed automated probabilistic tractography using predefined protocols for identifying major WM tracts in the left and right hemispheres as described in FSLs XTRACT tool (Warrington et al., 2020; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/XTRACT). Prior to XTRACT, we fitted the crossing fiber model (FSLs BEDPOSTX; Behrens et al., 2007) to each subject's data to estimate up to 3 fiber orientations per voxel. XTRACT was then used to reconstruct a set of tracts including projection, association, commissural, and limbic fiber bundles for each subject. The tract probability density maps, normalized by the total number of valid streamlines, were thresholded at 0.1% and binarized to produce a tract mask for each tract in standard space. We then applied the tract mask of each subject with the TBSS derived FA skeleton to produce subject-wise skeletonized tract masks, depicting the core of each WM bundle. For each subject, the mean FA and MD within each skeletonized tract were obtained to produce 66 (33 FA and 33 MD) subject-wise microstructural IDPs. The predefined list of tracts that were reconstructed using XTRACT was: anterior commissure (AC), bilateral arcuate fasciculus (AF), bilateral acoustic radiation (AR), bilateral anterior thalamic radiation (ATR), bilateral cingulum (Cing), bilateral frontal aslant (FA), forceps major (FMA), forceps minor (FMI), fornix (FX), bilateral inferior fronto-occipital fasciculus (IFOF), bilateral inferior longitudinal fasciculus (ILF), middle cerebellar peduncle (MCP), bilateral middle longitudinal fasciculus (MdLF), bilateral optic radiation (OR), bilateral superior longitudinal fasciculus 1 (SLF1), bilateral superior longitudinal fasciculus 2 (SLF2), bilateral superior longitudinal fasciculus 3 (SLF3), bilateral superior thalamic radiation (STR), and bilateral uncinate fasciculus (UF).
2.8. Linked independent component analysis (multimodal analysis)
To jointly explore whole brain age- and inadequate sleep-related differences in GM structure and WM microstructure, we used the VBM and TBSS maps, extracted from T1 and diffusion MRI data respectively, as inputs to linked Independent Component Analysis (FLICA). FLICA is a data-driven approach which automatically decomposes multimodal data into independent components (ICs; Groves et al., 2011). Each IC characterizes a mode of inter-subject multimodal (e.g. brain GM structure and WM microstructure in our case) variability such that each subject loading, which is shared across the modalities, corresponds to statistically independent, and non–Gaussian multi-modal spatial maps (Groves et al., 2012). Importantly, an ICs subject loading may be dominated by a single modality as opposed to equal contribution of the modalities of interest. Given the sample size restriction, we ran a FLICA decomposition with 10 ICs on 3 inputs, derived from VBM (modulated GM density maps for each subject using a study-specific template in MNI152 space) and TBSS (skeletonized FA map and skeletonized MD map for each subject in MNI152 space) data to identify post-hoc ICs which may linearly and/or quadratically associate with age, PSQI, and WASO. We evaluated statistical significance as well as plausible relationships between each ICs loading and non–imaging measures using effect magnitude (R2) in addition to corrected p values (Douaud et al., 2014). The results were Bonferroni corrected for multiple comparisons across all 10 ICs.
2.9. Computation of brain age delta
Using multimodal features extracted from GM and WM, we estimated brain age delta () by employing multiple regression models as recently described in Smith et al (2019). This method allows for a linear and quadratic correction of , thus ensuring complete independence between and chronological age. For a vector Y (N x 1) representing chronological age for N participants, we created an imaging matrix X (N x M) which denotes the summary measures of the structural and microstructural features in the studied group of elderly participants (N = 50). We computed by using: (1) unimodal (i.e., 110 T1 or 66 dMRI) IDPs and (2) multimodal (i.e., 176 T1 + dMRI) IDPs, respectively (Fig. 1). The head size scaling factor was derived as an additional structural IDP in accordance with the UK Biobank T1 pipeline (Alfaro-Almagro et al., 2018) and introduced as a confound (Miller et al., 2016). Next, the imaging feature matrix X was dimensionality reduced by applying singular value decomposition (SVD). We then performed 5-fold cross validation (repeated 200 times and averaged results) to prevent the regression models from overfitting the imaging data and estimated , in a manner that makes it orthogonal to age and bias-free (Smith et al., 2019). Finally, to account for potential accelerated effects of ageing on imaging measures (advanced ageing), we estimated the quadratic correction of delta by adding a non–linear (quadratic) term in the multiple regression models. As a result, b was used to denote the biased (uncorrected) brain age delta (typically used in previous studies), was used to represent the linearly-corrected estimate of brain age delta, and finally, q was used to represent the quadratically-corrected estimate of brain age delta. All models are shown in Fig. 1.
Fig. 1.
Overview of the brain age prediction model. A recently published approach described by Smith et al. (2019) was used to estimate brain age delta in an unbiased manner. (A) The structural and diffusion MRI data were preprocessed using the UK Biobank pipeline. (B) The T1 imaging derived phenotypes (T1IDPs) were extracted from the parcellations of cortical and subcortical GM volumes based on the Harvard-Oxford structural atlases. (C) The diffusion imaging derived phenotypes (dMRI IDPs) were extracted using predefined protocols for identifying major WM tracts as described by the FSL's XTRACT tool. (D) The structural and microstructural IDPs were represented using an imaging matrix X (NxM) such that N = 50 participants and M = 176 (demeaned) imaging features. The head scaling factor was used as an additional structural IDP and introduced as a confound variable in the brain age prediction model. Next, a matrix Y was created to represent the (demeaned) chronological age for N = 50 participants. A matrix Y2 was also computed to account for quadratic ageing processes which was subsequently demeaned and orthogonalized with respect to Y. The initial brain age prediction model is YB = X1 + b such that 1 = X+Y and the uncorrected (biased) delta which is typically used in brain ageing studies is b = YB – Y. The corrected (unbiased) brain age prediction model is then computed as follows: b = Y22 + q such that 2 = Y2+b. The linearly-corrected estimate of delta is = b – Y2 and the quadratically-corrected estimate of delta is q = b – Y22. (E) The relationships between chronological age (Y) and brain age (uncorrected [YB]; linear [YL]; quadradic [YQ]) were assessed using Pearson's correlation coefficient. The relationships between brain age delta (uncorrected [b], linear [], quadratic [q]) and sleep measures (PSQI, WASO) were also evaluated using Pearson's correlation coefficient.
The R statistical package (http://r-pkgs.had.co.nz/intro.html) was used for statistical analyses of the brain age delta models with sleep patterns (i.e., PSQI, WASO) (e.g. obtaining R2). Similarly, we assessed the correlation strength of chronological age (Y) with the uncorrected brain age (YB), the predicted linear (YL) and quadratic (YQ) brain age estimates. To correct for multiple comparisons, a False Discovery Rate (FDR)-corrected threshold of p < 0.05 was applied.
3. Results
3.1. Unimodal VBM: GM volumetric alterations in the ageing brain
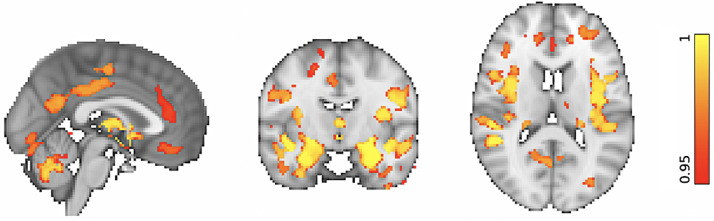
First, unimodal VBM and TBSS analyses were run to confirm that our data demonstrate anticipated trends in GM structure and WM microstructure with increasing age, as reported in previous studies. VBM analyses showed significant widespread GM volume reductions in our ageing cohort by recruiting the default mode network, primary motor cortex, primary somatosensory cortex, auditory cortex in addition to several language, and visual related areas (p < 0.05 corrected; Fig. 2). Significant morphologic reductions were also associated with increasing age in several prefrontal, limbic, and cerebellar structures (p < 0.05 corrected; Fig. 2). In agreement with the literature, similar GM alterations were observed in the basal ganglia which extended to the thalamus (p < 0.05 corrected; Fig. 2).
Fig. 2.
VBM: Whole-brain morphologic alterations in the ageing brain. Reduced GM volumes are significantly associated with increasing age. The colored voxels show widespread GM changes in the following regions: Default mode network: posterior cingulate cortex (PCC), precuneus (Pre), parahippocampal gyrus (PHG); Primary motor cortex: precentral gyrus (PreG); Primary somatosensory cortex: postcentral gyrus (PosG); Auditory cortex: Heschl's gyri (HG), superior temporal gyrus (STG), planum polare (PP); Visual areas: lingual gyrus (LG), fusiform gyrus (FG), inferior temporal gyrus (ITG); Language areas: angular gyrus (AG), inferior frontal gyrus (IFG), insular cortex (INC), middle temporal gyrus (MTG); Prefrontal regions: superior frontal gyrus (SFG), middle frontal gyrus (MFG), frontal polare (FP); Limbic regions: hippocampus (HIP), amygdala (AMYG), orbitofrontal cortex (OFC); Basal ganglia: dorsal striatum (caudate (CAU), putamen (PUT), ventral striatum (nucleus accumbens [NAcc]), pallidum (Pa). The spatial map is FWE-corrected for multiple comparisons set at p < 0.05.
3.2. Unimodal VBM: Sleep-related differences in GM volumes in the ageing brain
When we examined the link between sleep quality as indexed by PSQI and whole-brain morphometry, there was no evidence of significant associations with the GM volumes, after correcting for multiple comparisons. Similarly, no associations could be found when we tested the effects of sleep fragmentation as measured by WASO on the GM volumes. Taken together, the unimodal VBM analyses did not reveal sleep problems-related alterations in whole-brain morphometry in the ageing brain.
3.3. Unimodal TBSS: Age-related microstructural brain changes in WM
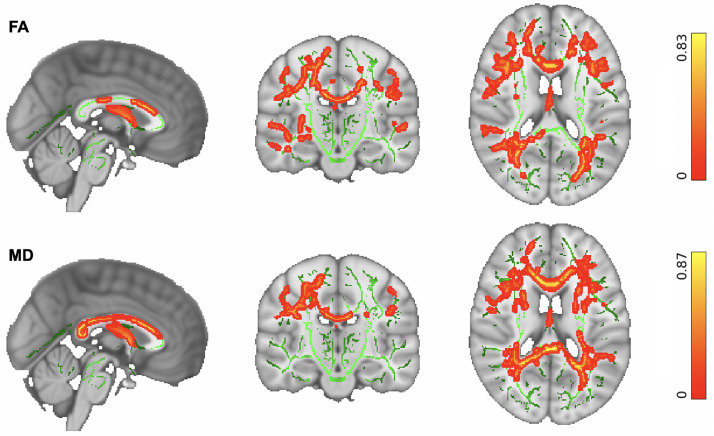
We then explored whether our cohort data support previous findings for widespread microstructural changes due to ageing. TBSS analyses demonstrated widespread age-related changes in the WM microstructure. Increasing age was significantly associated with reduced FA and increased MD (p < 0.05 corrected) within the association, projection, limbic, and commissural WM fiber bundles in the studied group of neurotypical older adults as illustrated in Fig. 3.
Fig. 3.
TBSS: Whole-brain skeletonized microstructural differences in older adults. (Top) Reduced FA is significantly associated with increasing age. (Bottom) Increased MD is significantly associated with increasing age in older adults. Changes were significant in a number of WM fiber bundles, including: Association fiber bundles: Inferior Longitudinal Fasciculus (ILF), Inferior Fronto-Occipital Fasciculus (IFOF), Superior Longitudinal Fasciculus (SLF), Uncinate Fasciculus (UF). Projection fiber bundles: Acoustic Radiation (AR), Anterior Thalamic Radiation (ATR), Posterior Thalamic Radiation (PTR), Corticospinal Tract (CST), Optic Radiation (OR). Limbic fiber bundles: Cingulum Gyrus part of Cingulum (Cing), Hippocampal part of Cingulum (CBH), Fornix (FX). Commissural fiber bundles: Forceps Major (FMA), Forceps Minor (FMI). The spatial maps are FWE-corrected for multiple comparisons set at p < 0.05.
3.4. Unimodal TBSS: Sleep-related differences in WM microstructure in the ageing brain
When we performed the unimodal TBSS analyses to examine sleep quality-related differences in FA or MD, there was no association between WM microstructure changes in the ageing brain with PSQI, after applying multiple comparison corrections. Similarly, no association was found when we investigated the effects of sleep fragmentation on FA, and MD. Overall, the unimodal TBSS analyses did not reveal sleep problems-related changes in WM microstructure in older adults.
3.5. Multimodal FLICA: Covariation in GM and WM changes associated with age
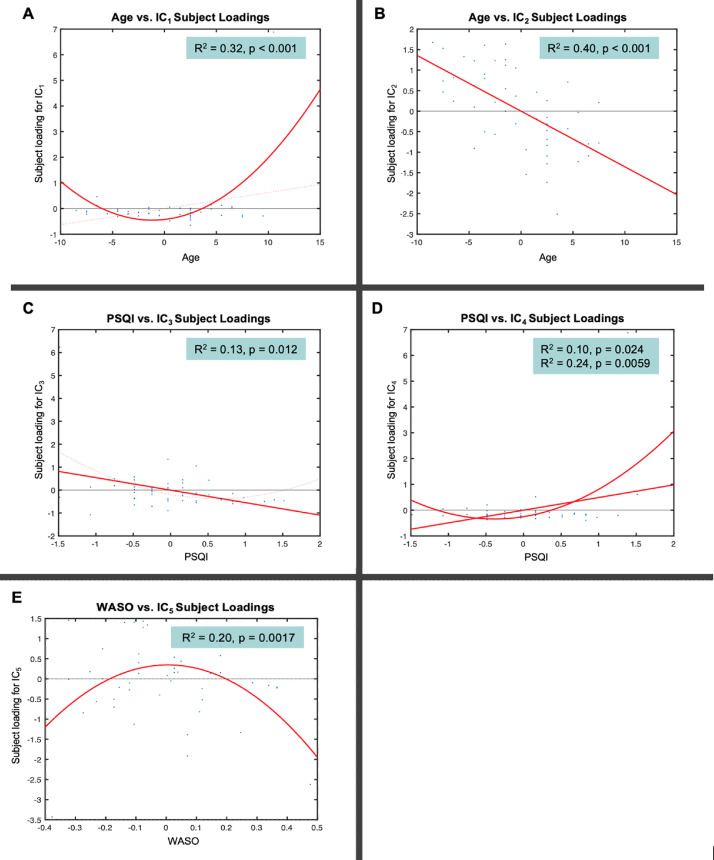
Similarly to the unimodal TBSS and VBM analyses, multimodal FLICA analysis revealed associations between changes in GM structure and WM microstructure, and age. Two components (i.e., IC1 and IC2) were significantly associated with increasing age. IC1 exhibited a U-shape profile with increasing age which was related to regional GM reduction and WM changes, i.e., FA decrease and MD increase (Fig. 4A). The IC1 subject loadings displayed a nonlinear pattern which decreased from 65 to 73 years and then increased from 73 to 84 years. While age explained 32% of the variance within IC1 (R2 = 0.32, p < 0.001 corrected), the U-shape relationship between the IC1 subject loading and age was dominated by MD (49%) and FA (34%) followed by GM volume (15%). The second age-related component, IC2, showed a linear decrease of global GM and WM microstructure with increasing age (Fig. 4B). Age explained 40% of the variance within IC2 (R2 = 0.40, p < 0.001 corrected). The linear relationship between the IC2 loading and age was mainly driven by the GM volume (42%) followed by FA (35%) and MD (22%).
Fig. 4.
FLICA: Strongest independent components (ICs) that capture different modes of covariations in VBM and WM microstructure in addition to their relationships with age (A-B), PSQI (C-D) and WASO (E). Note that the x-axis shows the demeaned demographic (i.e., age) and behavioral (i.e., PSQI, WASO) values. The effect magnitude is represented by R2. The significant relationships between the IC subject loadings and demographic and behavioral measures are Bonferroni-corrected for multiple comparisons set at p < 0.05.
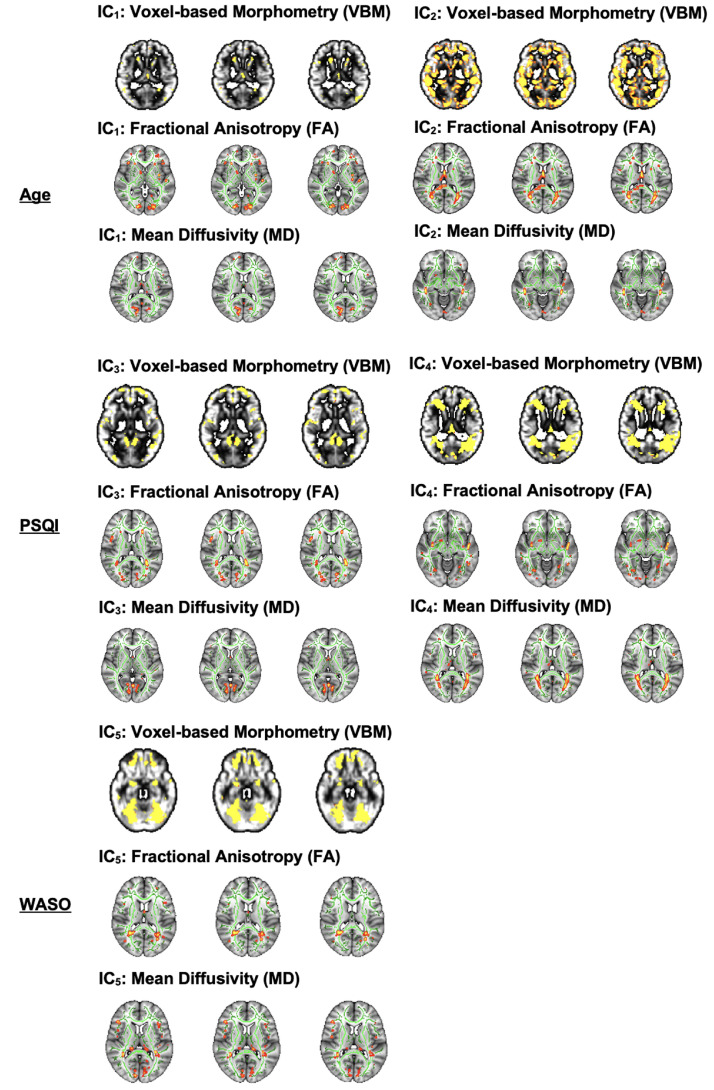
Although the IC1 subject loadings supported that GM volumetric features contributed less to age-related changes in this component compared to WM microstructural features, both cortical (AG, SFG, STG, MFG, FP, lateral occipital cortex, parietal operculum cortex), and subcortical (caudate, thalamus) regions were involved in localized GM alterations (Fig. 5). FA and MD maps revealed high degree of overlap in small clusters that correspond to the association (ILF, SLF), projection (ATR) and commissural (FMA, FMI) fiber bundles. Of note, the FA map showed ageing effects in other association (IFO and UF) fiber bundles whereas the MD map showed some unique changes in the limbic (CBH, FX) fiber bundles. On the other hand, the spatial map of IC2 showed widespread GM volumetric alterations with increasing age which were evident in the primary motor and somatosensory cortices, auditory cortex, limbic system, in addition to several prefrontal, language and visual related areas (Fig. 5). Similarly, FA and MD shared considerable overlap in the tracts that form part of the limbic (CBH) and association (ILF, IFO, SLF) fibers. However, FA showed distinct age-related alterations in other limbic (FX), projection (FMA), and commissural (ATR, PTR) fiber bundles.
Fig. 5.
FLICA: Spatial maps of the independent components (ICs) that reveal GM volumetric and WM microstructural changes measured by FA and MD in Age (Top Panel), PSQI (Middle Panel) and WASO (Bottom Panel). The spatial maps are Bonferroni-corrected for multiple comparisons set at p < 0.05.
3.6. Multimodal FLICA: Covariation in GM and WM changes associated with sleep problems
Contrary to the unimodal TBSS and VBM analyses, multimodal FLICA analysis revealed associations between changes in GM structure and WM microstructure and sleep quality measures. FLICA revealed 2 multimodal components (i.e., IC3, and IC4) which captured interlinked GM and WM changes that were associated with the global sleep quality score, i.e., PSQI. IC3 showed a dominant mode of linear decrease in GM volumes and WM microstructure with poorer sleep quality (Fig. 4C). PSQI explained 13% of the variance within IC3 (R2 = 0.13, p = 0.012 corrected). The linear relationship between the IC3 subject loadings and PSQI was mainly driven by GM volume (40%) followed by FA (31%) and MD (26%). The second PSQI-related component, IC4, shared 2 modes of linear and nonlinear covariation in regional GM and WM microstructure which were related to decreasing sleep quality (Fig. 4D). The IC4 subject loadings showed a U-shape profile such that weaker loadings were associated with higher sleep quality (PSQI 5) whereas stronger loadings were related to a deterioration in sleep quality (PSQI > 5). While PSQI explained 10% of the linear variation within IC4 (R2 = 0.10, p = 0.024 corrected), it explained 24% of the U-shape variation within the same component (R2 = 0.24, p = 0.0059 corrected). Specifically, the linear and U-shape relationships between the IC4 subject loadings and PSQI were greatly dominated by morphologic reductions (72%) followed by FA (15%) and MD (12%) alterations.
The spatial distribution of IC3 showed GM networks that have been previously associated with several cognitive domains such as language, sensorimotor, visual, emotion, attention, and default mode functions (Fig. 5). FA and MD maps demonstrated WM alterations with poor sleep quality in tracts that overlapped in the association (ILF, IFO) and projection (ATR) bundles. We also observed that FA captured distinct sleep-related effects in the FMA, AR, SLF and UF fibre bundles whereas MD showed some changes that were specific to the FX. Our data-driven approach also showed that the GM spatial map of IC4 overlapped with that of IC3 to a certain extent (Fig. 5). Further, FA and MD revealed sleep-related alterations which overlapped in the association (ILF, IFO, SLF) and commissural (FMA) fiber bundles. Nonetheless, FA showed specific sleep-related changes in the UF and CBH fiber bundles whereas MD revealed non–overlapping alterations in the ATR.
Lastly, we observed a multimodal component (i.e., IC5) which revealed a significant nonlinear relationship with sleep fragmentation, i.e., WASO. IC5 exhibited a markedly inverted U-shape profile that reflects widespread GM and localized WM microstructural alterations with WASO (Fig. 4E). The IC5 subject loadings displayed a nearly symmetrical inverted U-shape profile as a result of stronger loadings being associated with less sleep disturbance (WASO 36 minutes) and weaker loadings relating to more frequent sleep disturbance (WASO > 36 minutes). WASO explained 20% of the variance within IC5 (R2 = 0.20, p = 0.0017 corrected). The inverted U-shape relationship between the IC5 loading and WASO was predominantly driven by the GM volume (54%) followed by FA (27%) and MD (16%).
Spatially, the GM changes in IC5 were evident in the frontal, temporal, and occipital areas including portions of the cerebellum (Fig. 5). FA and MD maps showed significant WM alterations that overlapped in small clusters that form part of the association (ILF, IFO, SLF), projection (ATR), and commissural (FMA) fiber bundles. In addition, there were evidence of distinct sleep-related effects in the FA map that correspond to the limbic (CBH, FX) fiber bundles. Similarly, the MD map showed the presence of non–overlapping sleep-related effects in the PTR.
3.7. Brain age predictions
Using multimodal imaging features representing regional GM volumes and WM tract microstructure, we calculated the uncorrected (b), corrected linear (), and quadratic (q) estimates of brain age delta (Table 2). The magnitude of and q showed that in the studied cohort, the estimated brain age (using the multimodal neuroimaging features) was on average approximately 2 years older than the chronological age. Both and q ( = 1.9; = 2.0) were notably smaller than b ( = 3.3), i.e., the biased/uncorrected estimate that is typically used in brain age prediction studies. Given that brain age delta corresponds to the residuals in the brain prediction model, the smaller and q (which are orthogonal to age) improve the associations between the respective chronological age and predicted brain age (YL and YQ respectively) compared to the predicted brain age YB using b (Table 2), as expected, and shown before (Smith et al., 2019).
Table 2.
Brain age delta
| IDP | -value | -value | -value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | 3.2 | 2.0 | 2.1 | 0.58 | <0.001 | 0.84 | <0.001 | 0.82 | <0.001 |
| dMRI | 4.5 | 1.3 | 1.6 | 0.41 | 0.0028 | 0.95 | <0.001 | 0.93 | <0.001 |
| T1 + dMRI | 3.3 | 1.9 | 2.0 | 0.54 | <0.001 | 0.86 | <0.001 | 0.83 | <0.001 |
Magnitude of the uncorrected , linearly-corrected () and quadratically-corrected estimates of delta obtained from unimodal (T1 or dMRI IDPs) and multimodal (T1+dMRI) IDPs, and the associations of chronological age (Y) with the uncorrected brain age prediction (YB), linearly-corrected brain age prediction (YL) and quadratically-corrected (YQ) brain age prediction.
Brain age estimates using linear corrections showed significant associations with inadequate sleep indices. Notably, there was no significant association between uncorrected b with either PSQI or WASO.
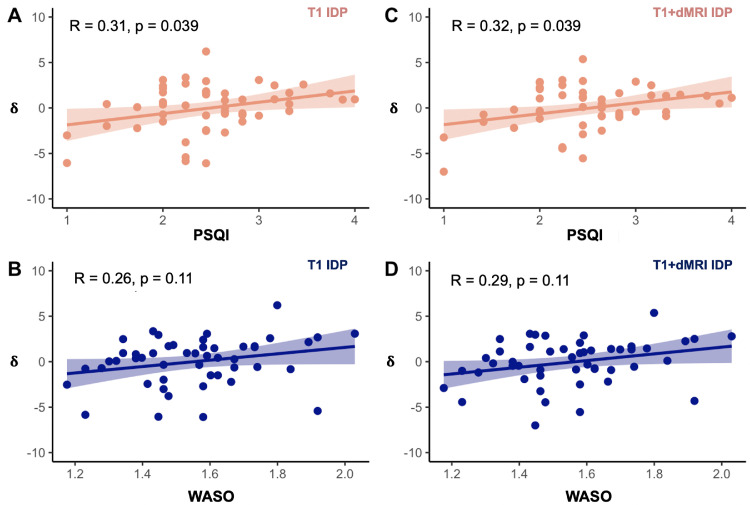
When using unimodal (either T1 or dMRI) IDPs to estimate brain age delta, there was a significant relationship between the linearly-corrected and PSQI (R = 0.31, p = 0.039 FDR) but only when the T1 IDPs were applied in the brain age prediction model (Fig. 6A; there was no significant relationship between unimodal and WASO, Fig. 6B). There was no significant relationship between the quadratically-corrected δq and PSQI when the unimodal IDPs were used.
Fig. 6.
Relationships between brain age delta and sleep measures using the unimodal (T1) and multimodal (T1+dMRI) IDPs. (A) was significantly associated with PSQI using the unimodal T1 IDPs. (B) was not significantly associated with WASO after applying FDR correction when the unimodal T1 IDPs were used. (C) was significantly related to PSQI with the multimodal (T1+dMRI) IDPs. (D) was not significantly associated with WASO when the multimodal (T1+dMRI) IDPs were used. All relationships between brain age delta and sleep measures are FDR-corrected for multiple comparisons set at p < 0.05. Note that the raw PSQI and WASO scores are square root and log-transformed, respectively (see Section 2.2).
When using multimodal (T1+dMRI) IDPs to estimate brain age delta, the linearly-corrected estimate was significantly associated with PSQI (R = 0.32, p = 0.039 FDR, Fig. 6C; multimodal δ was not significantly associated with WASO, Fig. 6D). There was no significant relationship between the quadratically-corrected q and PSQI when the multimodal IDPs were used.
Taken together, the results indicate that poor sleep quality is associated with accelerated brain ageing, i.e., brain age which is ∼2 years older than its chronological age.
4. Discussion
The ageing process, which results in structural brain deterioration and affects cognitive performance and daily functioning, is inevitable. However, not all older adults experience sharp cognitive decline. Some individuals undergo only gradual drop in cognitive functioning or even retain high levels of mental capacity throughout lifespan (Hayden et al., 2011). Substantial inter-individual differences in the pace of age-related brain changes underlying cognitive decline have been reported and attributed to modifiable lifestyle factors (for review see Cabeza et al., 2017; Eavani et al., 2018; Raz & Rodrigue, 2006). The existing evidence suggest a potential link between sleep problems and brain ageing (e.g., Sexton et al., 2014; Sexton et al., 2017; Wassenaar et al., 2019). Consequently, disentangling the heterogeneity of brain ageing, and the effects of sleep on differential trajectories of normal versus pathologic brain ageing is of high interest.
The current study explored volumetric and microstructural brain changes in healthy ageing and used estimates of brain age delta (difference between chronological and apparent brain age assessed using neuroimaging data) to investigate the associations of age-related brain changes with sleep quality and sleep fragmentation. Linked independent component analysis revealed a significant interlinked linear decrease of the global GM and WM microstructure with increasing age and sleep problems (both poor sleep quality and sleep fragmentation) as well as a degree of inter-individual variability in the observed age-related and inadequate sleep-related brain deterioration. These joint associations between brain structural and microstructural features with inadequate sleep indices were not evident using unimodal analyses (VBM and TBSS).
Furthermore, brain age delta, estimated with linear age-bias correction (Smith et al., 2019) from GM structural and WM microstructural neuroimaging features, revealed significant association between poor sleep quality, and the accelerated brain aging. These associations were not evident when using the uncorrected (biased) estimates often used in brain-age prediction studies (for discussion see Le et al., 2018; Liang et al., 2019). Specifically, our findings demonstrated a 2-year deviation above the chronological age (i.e., accelerated ageing) linked to sleep problems. We discuss our findings in context of prior research investigating the effects of sleep on age-related brain deterioration as well as research focusing on the use “brain age gap” as a biomarker of brain ageing.
The reported heterogeneity of age-related brain changes raises a possibility of maintaining more youthful brain later in life. While, both genetic and environmental influences likely account for variation in ageing, recent research strongly suggests that modifiable lifestyle factors, such as sleep, diet and physical activity, could hold the key to slowing down brain, and cognitive ageing (for review see Wassenaar et al., 2019). As we get older waking up refreshed after a good night sleep becomes a challenge due to difficulties in maintaining or initiating sleep, fragmentation of sleep, increased daytime napping and changes in wake-sleep cycle (Miner & Kryger, 2017; Varma et al., 2019). The loss of good night sleep has a detrimental effect on overall health, reduces longevity, and worsens cognitive performance (André et al., 2019; Gangwisch et al., 2008; Mattis & Sehgal, 2016). While older adults might have increased vulnerability to sleep problems and they often sleep less, the sleep recommendations for that age group are similar to these given to general adult population (7-9 hours per day; Hirshkowitz et al., 2015). Difficulties falling asleep, short sleep duration, excessive daytime sleepiness and napping can be regarded as modifiable behavioural sleep problems exacerbated by inadequate sleep hygiene, a set of behavioural practices, everyday habits, and environmental factors required to achieve good night sleep (Lin et al., 2007). And sleep hygiene interventions have been shown to be effective in alleviating sleep problems in older adults, particularly increasing the efficiency of sleep, and decreasing sleep fragmentation (e.g., Martin et al., 2017). Taken together, a growing body of evidence strongly suggests that reduced sleep time and poor sleep quality should no longer be viewed as intrinsically related to normal ageing but as potentially modifiable factors putting older adults at risk of cognitive decline and dementia (e.g., Ju et al., 2014; Keage et al., 2012; Lim et al., 2013; Wassenaar et al., 2019). While there is a substantial number of studies linking short sleep duration and/or poor sleep quality to grey matter atrophy and microstructural white matter changes, the evidence is inconsistent (Fjell et al., 2021; Lo et al., 2014; Ramos et al., 2014; Sexton et al., 2014; Sexton et al., 2020; Sexton et al., 2017; Spira et al., 2016; Yaffe et al., 2016; Zitser et al., 2020). Some of the reported discrepancies could be attributed to how sleep patterns are assessed, how brain changes are characterized and/or differences in studied population characteristics and/or sample size.
A recent large longitudinal study (613 participants), spanning over 28 years found no significant link between self-reported sleep duration and either grey matter or white matter microstructure (Zitser et al., 2020). However, it should be noted that Zitser and colleagues only collected longitudinal measures of sleep duration, while the brain changes were assessed at a single time-point. There are 3 potential explanations of these null results. Firstly, their study used unimodal analyses; we also could not find associations when performing unimodal analyses and only when probing the joint variance across modalities effects could be revealed. Analyses based on a single-item self-report of sleep duration in combination of single modality derived measures of GM or WM changes might not be sensitive enough to detect any brain deterioration beyond the effect of age itself. Secondly, it is plausible that not sleep duration per se but sleep quality or combination of these 2 affects brain changes in older adults without any diagnosis of sleep disorders. Thirdly, reliability of self-report of sleep duration as the discrepancies between objective and frequently overestimated self-reported measures of sleep duration, and their associations with health outcomes, are well documented (Lauderdale et al., 2008; McSorley et al., 2019; Silva et al., 2007).
The current study used a combination of objective (actigraphy based assessment of sleep fragmentation; WASO) and self-reported (assessed by commonly used PSQI questionnaire) measures of inadequate sleep to explore associations between sleep problems and age-related brain changes, hypothesizing that inadequate sleep accelerates brain ageing. We showed that self-reported but objectively measured sleep disruptions were associated with accelerated brain ageing. Specifically, global PSQI score indexing several common sleep dysfunctions (e.g., short sleep duration, sleep latency, poor sleep efficiency, sleep disturbances and daytime dysfunctions), was positively correlated with estimated brain age delta. Thus, indicating that overall “poorer” sleep quality is indeed linked to a larger “brain age gap” i.e., deviation from chronological age. Our findings suggest that subjective measures of sleep disruptions and/or composite measures of self-reported sleep problems are more sensitive to detect the associations between sleep and brain ageing as compared to single item self-reported measures. Alternatively, it is plausible that overall poor sleep quality rather than sleep duration itself (see Zitser et al., 2020) has a substantially greater effect on brain deterioration in neurotypical older adults. As the observed heterogeneity of brain ageing can be attributed to both the large variability in the pace of ageing and to a wide range of onset ages for ageing process, the detection of any brain deterioration beyond the effect of age itself, e.g., the impact of sleep, might be challenging. This perhaps explains the discrepancies between our findings and some of the prior studies examining sleep-related differences in brain ageing, which all independently examined patterns of either gray or white matter changes estimated from a single MRI modality. Furthermore, to increase sensitivity, we used here measures derived from both structural and diffusion MRI to examine interlinked GM and WM changes as well as estimated deviation from chronological age (brain age delta; Smith et al., 2019) to investigate the link between age-related brain changes and inadequate sleep in a group of neurotypical elderly participants. We explored that further using exploratory FLICA method (Douaud et al., 2014; Groves et al., 2011).
The FLICA approach applied here demonstrated an interlinked global GM and WM decrease with increasing age and a variability in the observed age-related brain deterioration in the studied group of elderly participants. Importantly, FLICA revealed interlinked GM, and WM changes driven by poor sleep quality as assessed by PSQI. This is in line with both single modality and multimodal estimates of brain age delta, which proved to be a sensitive biomarker of accelerated brain ageing linked to poor sleep quality. FLICA also identified a multimodal component associated with sleep fragmentation (WASO). The U-shape versus linear profile of the unique multimodal components revealed by FLICA are result of differential contribution of GM volume, FA, and MD to each individual component (Douaud et al., 2014; Groves et al., 2011). It should be noted here that in contrast to FLICA, commonly used unimodal approaches such as VBM or TBSS, separately using measures of either GM or WM changes, failed to detect any links between inadequate sleep indices, and brain deterioration in the examined group of elderly participants. Thus, these results strongly indicate that multimodal analysis increases sensitivity when assessing association between sleep problems, and brain ageing.
“Brain age gap” estimates are increasingly being used as a biomarker of brain's health signaling increased risk of brain deterioration and predictors of progression from mild cognitive impairment to dementia (Cole & Franke, 2017; Gaser et al., 2013). This method has been also applied to predict cognitive functioning in non–demented older adults and to identify lifestyle factors associated with maintaining a more youthful brain in old age (Boyle et al., 2021; Steffener et al., 2016). While many, especially earlier, “brain age gap” studies used estimates based on a single MRI modality (for review see Cole & Franke, 2017; Franke & Gasser, 2019), more recent approaches employed multimodal datasets (Cole, 2020; Smith et al., 2020; Smith et al., 2019). Here, we used a recently developed multimodal technique to calculate unbiased estimates of brain age delta (see Smith et al., 2019), in order to explore the effects of sleep on the ageing brain, stipulating that inadequate sleep would accelerate brain ageing. As proposed by Smith and colleagues, this approach enables, to remove typical biases affecting brain-age estimates (and lead to overestimation in younger and underestimation in older individuals; for discussion see Le et al., 2018; Liang et al., 2019; see also Beheshti et al., 2019) for similar approach) and increases sensitivity of associations with lifestyle and/or socio-demographic variables. Indeed, we found that the corrected (i.e., removal of age-related bias) brain age delta estimates outperformed the uncorrected (biased) estimates in their predictive power and revealed associations with poor sleep quality (PSQI), not evident when the uncorrected delta estimates were used. In the studied group of neurotypical older adults we found 2 years deviation above the chronological age, which was correlated with poor sleep quality. A 2-year deviation from chronological age might seem relatively small. However, to put our findings in a broader perspective, it should be noted that a recent large epidemiologic study identified a 4-year brain age gap to be associated with dementia and predictive of low cognitive functioning (Kaufmann et al., 2019).
Our study jointly model's covariation in GM and WM structural and/or microstructural features to examine links between inadequate sleep (assessed by a combination of objective and self-reported measures) and accelerated brain ageing. While one potential limitation of our study is the sample size (n = 50), it should be noted that our brain delta estimations and all other analyses are linear regression models with a few model parameters. One caveat to our findings is that multiple lifestyle factors, socio-economic factors (education, physical activity for instance) and genetic influences, not just inadequate sleep, have been implicated and shown to a varied degree influence accelerated brain ageing (e.g., Smith et al., 2020; see also Steffener et al., 2016 vs Nyberg et al., 2021 for contradictory evidence). Having more participants and data (e.g. using the UK Biobank) will allow in the future to disentangle such unique and additive effects. Still, our study is informative in demonstrating for the first time the value of incorporating multi-modal neuroimaging information and joint modeling for increasing sensitivity in capturing such associations. And doing so in a focused, sleep-phenotyped (considerably more detailed than done in larger-scale hypothesis-free initiatives, such as the UK Biobank), aging participants cohort. Finally, a recent study (Butler et al., 2021) based on a series of simulations and analysis using T1-weighted scans from the Philadelphia Neurodevelopmental Cohort (age 8-22) concluded that current “brain age gap” methods are not free from estimation errors as either might be prone to age-related biases or by mitigating such biases might inflate results. Thus, despite methodological progress such as correction for dependence of estimates on age itself and use of multimodal data (employed here brain age delta, Smith et al., 2019), further methodological advances in estimating deviations from developmental and normal ageing trajectories, and evaluation of applicability of such methods to different age groups and clinical populations are needed.
In conclusion, taking into account a recent evidence that a few years deviation from normative brain ageing is one of the hallmarks of dementia (Kaufmann et al., 2019), we suggest that sleep problems in healthy older adults should be considered a modifiable risk factor for dementia. Our findings also point to the aptitude of behavioral intervention to combat the effects of inadequate sleep on the ageing brain. However, it should be noted that any conclusions drawn from our findings are limited by cross-sectional design and thus further longitudinal studies, preferably based on multimodal approaches are needed.
Verification
The manuscript presents original findings and the work described in the paper has not been published previously and is not under consideration for publication elsewhere. The paper has been made available as pre-print on the bioRxiv.
Disclosure statement
The authors declare no potential conflict of interest.
Acknowledgements
We warmly thank the volunteers from the School of Psychology panel and the Birmingham 1000 Elders group for participation in this study. This work was supported by the Birmingham-Nottingham Strategic Collaboration Fund (BNSCF336 to SNS and MC) and by a Wellcome Trust Institutional Strategic Support Fund critical data award (204846/Z/16/Z to MC). SS was also supported by the Wellcome Trust (217266/Z/19/Z) and by an ERC Consolidator Grant (101000969). MC was also supported by a BRIDGE (Birmingham-Illinois Partnership for Discovery, Engagement and Education) Fellowship.
Data availability statement
Participant data will be made available to researchers upon request.
CRediT authorship contribution statement
Jivesh Ramduny: Methodology, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. Matteo Bastiani: Methodology, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. Robin Huedepohl: Methodology, Formal analysis. Stamatios N. Sotiropoulos: Conceptualization, Funding acquisition, Methodology, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing, Supervision, Project administration. Magdalena Chechlacz: Conceptualization, Funding acquisition, Methodology, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing, Supervision, Project administration.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2022.02.005.
Contributor Information
Stamatios N. Sotiropoulos, Email: stamatios.sotiropoulos@nottingham.ac.uk.
Magdalena Chechlacz, Email: m.chechlacz.1@bham.ac.uk.
Appendix. Supplementary materials
References
- Alfaro-Almagro F., Jenkinson M., Bangerter N.K., Andersson J.L.R., Griffanti L., Douaud G., Sotiropoulos S.N., Jbabdi S., Hernandez-Fernandez M., Vallee E., Vidaurre D., Webster M., McCarthy P., Rorden C., Daducci A., Alexander D.C., Zhang H., Dragonu I., Matthews P.M., Miller K.L., Smith S.M. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/s1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. High resolution nonlinear registration with simultaneous modelling of intensities. bioRxiv. 2019;646802 doi: 10.1101/646802. [DOI] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André C., Tomadesso C., de Flores R., Branger P., Rehel S., Mézenge F., Landeau B., Sayette V.d.l., Eustache F., Chételat G., Rauchs G. Brain and cognitive correlates of sleep fragmentation in elderly subjects with and without cognitive deficits. Alzheimers Dement. 2019;11:142–150. doi: 10.1016/j.dadm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barrick T.R., Charlton R.A., Clark C.A., Markus H.S. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51(2):565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., Lebihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beheshti I., Nugent S., Potvin O., Duchesne S. Bias-adjustment in neuroimaging-based brain age frameworks: a robust scheme. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102063. 102063-102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R., Jollans L., Rueda-Delgado L.M., Rizzo R., Yener G.G., McMorrow J.P., Knight S.P., Carey D., Robertson I.H., Emek-Savaş D.D., Stern Y., Kenny R.A., Whelan R. Brain-predicted age difference score is related to specific cognitive functions: a multi-site replication analysis. Brain Imaging Behav. 2021;15(1):327–345. doi: 10.1007/s11682-020-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska A.Z., Preuschhof C., Bäckman L., Nyberg L., Li S.C., Lindenberger U., Heekeren H.R. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Butler E.R., Chen A., Ramadan R., Le T.T., Ruparel K., Moore T.M., Satterthwaite T.D., Zhang F., Shou H., Gur R.C., Nichols T.E., Shinohara R.T. Pitfalls in brain age analyses. Human Brain Mapp. 2021;42(13):4092–4101. doi: 10.1002/hbm.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Hoch C.C., Yeager A.L., Kupfer D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh sleep quality index (PSQI) Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- Cabeza R., Albert M., Belleville S., Craik F.I.M., Duarte A., Grady C.L., Lindenberger U., Nyberg L., Park D.C., Reuter-Lorenz P.A., Rugg M.D., Steffener J., Rajah M.N. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L., Park D.C. Second edition. Oxford University Press; New York, NY: 2017. Cognitive neuroscience of aging: linking cognitive and cerebral aging. [Google Scholar]
- Carpenter J.S., Andrykowski M.A. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Cole J.H. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.H., Franke K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci. 2017;40(12):681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Cole J.H., Poudel R.P.K., Tsagkrasoulis D., Caan M.W.A., Steves C., Spector T.D., Montana G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Douaud G., Groves A.R., Tamnes C.K., Westlye L.T., Duff E.P., Engvig A., Walhovd K.B., James A., Gass A., Monsch A.U., Matthews P.M., Fjell A.M., Smith S.M., Johansen-Berg H. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci. 2014;111(49):17648–17653. doi: 10.1073/pnas.1410378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Eavani H., Habes M., Satterthwaite T.D., An Y., Hsieh M.K., Honnorat N., Erus G., Doshi J., Ferrucci L., Beason-Held L.L., Resnick S.M., Davatzikos C. Heterogeneity of structural and functional imaging patterns of advanced brain aging revealed via machine learning methods. Neurobiol Aging. 2018;71:41–50. doi: 10.1016/j.neurobiolaging.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M. It was the best of times, it was the worst of times: a psychophysiologist's view of cognitive aging. Psychophysiology. 2012;49(3):283–304. doi: 10.1111/j.1469-8986.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Sørensen Ø., Amlien I.K., Bartrés-Faz D., Brandmaier A.M., Buchmann N., Demuth I., Drevon C.A., Düzel S., Ebmeier K.P., Ghisletta P., Idland A.-V., Kietzmann T.C., Kievit R.A., Kühn S., Lindenberger U., Magnussen F., Macià D., Mowinckel A.M., Nyberg L., Sexton C.E., Solé-Padullés C., Pudas S., Roe J.M., Sederevicius D., Suri S., Vidal-Piñeiro D., Wagner G., Watne L.O., Westerhausen R., Zsoldos E., Walhovd K.B. Poor Self-reported sleep is related to regional cortical thinning in aging but not memory decline—results from the lifebrain consortium. Cerebral Cortex. 2021;31(4):1953–1969. doi: 10.1093/cercor/bhaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K., Gaser C. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol. 2019;10:789. doi: 10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch J.E., Heymsfield S.B., Boden-Albala B., Buijs R.M., Kreier F., Opler M.G., Pickering T.G., Rundle A.G., Zammit G.K., Malaspina D. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- Gaser C., Franke K., Klöppel S., Koutsouleris N., Sauer H., Alzheimer's Disease Neuroimaging, I BrainAGE in mild cognitive impaired patients: predicting the conversion to alzheimer's disease. PLOS ONE. 2013;8(6):e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili A., Weiner D.K., Kuchibhatla M., Edinger J.D. Test-retest reliability of the Pittsburgh sleep quality index in nursing home residents. J Am Geriatr Soc. 1995;43(11):1317–1318. doi: 10.1111/j.1532-5415.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Santelli L., Tomassini V., Bosnell R., Smith S., De Stefano N., Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Groves A.R., Beckmann C.F., Smith S.M., Woolrich M.W. Linked independent component analysis for multimodal data fusion. Neuroimage. 2011;54(3):2198–2217. doi: 10.1016/j.neuroimage.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Groves A.R., Smith S.M., Fjell A.M., Tamnes C.K., Walhovd K.B., Douaud G., Woolrich M.W., Westlye L.T. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage. 2012;63(1):365–380. doi: 10.1016/j.neuroimage.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Hayden K.M., Reed B.R., Manly J.J., Tommet D., Pietrzak R.H., Chelune G.J., Yang F.M., Revell A.J., Bennett D.A., Jones R.N. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40(6):684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O'Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Adams Hillard P.J. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Ju Y.-E.S., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I., Alnæs D., Barch D.M., Baur-Streubel R., Bertolino A., Bettella F., Beyer M.K., Bøen E., Borgwardt S., Brandt C.L., Buitelaar J., Celius E.G., Cervenka S., Conzelmann A., Córdova-Palomera A., Dale A.M., de Quervain D.J.F., Di Carlo P., Djurovic S., Dørum E.S., Eisenacher S., Elvsåshagen T., Espeseth T., Fatouros-Bergman H., Flyckt L., Franke B., Frei O., Haatveit B., Håberg A.K., Harbo H.F., Hartman C.A., Heslenfeld D., Hoekstra P.J., Høgestøl E.A., Jernigan T.L., Jonassen R., Jönsson E.G., Farde L., Flyckt L., Engberg G., Erhardt S., Fatouros-Bergman H., Cervenka S., Schwieler L., Piehl F., Agartz I., Collste K., Victorsson P., Malmqvist A., Hedberg M., Orhan F., Kirsch P., Kłoszewska I., Kolskår K.K., Landrø N.I., Le Hellard S., Lesch K.-P., Lovestone S., Lundervold A., Lundervold A.J., Maglanoc L.A., Malt U.F., Mecocci P., Melle I., Meyer-Lindenberg A., Moberget T., Norbom L.B., Nordvik J.E., Nyberg L., Oosterlaan J., Papalino M., Papassotiropoulos A., Pauli P., Pergola G., Persson K., Richard G., Rokicki J., Sanders A.-M., Selbæk G., Shadrin A.A., Smeland O.B., Soininen H., Sowa P., Steen V.M., Tsolaki M., Ulrichsen K.M., Vellas B., Wang L., Westman E., Ziegler G.C., Zink M., Andreassen O.A., Westlye L.T., Karolinska Schizophrenia P. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature Neuroscience. 2019;22(10):1617–1623. doi: 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage H.A., Banks S., Yang K.L., Morgan K., Brayne C., Matthews F.E. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Lauderdale D.S., Knutson K.L., Yan L.L., Liu K., Rathouz P.J. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.T., Kuplicki R.T., McKinney B.A., Yeh H.-W., Thompson W.K., Paulus M.P., Aupperle R.L., Bodurka J., Cha Y.-H., Feinstein J.S., Khalsa S.S., Savitz J., Simmons W.K., Victor T.A. A nonlinear simulation framework supports adjusting for age when analyzing BrainAGE [Methods] Front Aging Neurosci. 2018;(317):10. doi: 10.3389/fnagi.2018.00317. T. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H., Goldman A.L., Sambataro F., Verchinski B.A., Meyer-Lindenberg A., Weinberger D.R., Mattay V.S. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617.e611–617.e619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Zhang F., Niu X. Investigating systematic bias in brain age estimation with application to post-traumatic stress disorders. Hum Brain Mapp. 2019;40(11):3143–3152. doi: 10.1002/hbm.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F., Varoquaux G., Kynast J., Beyer F., Kharabian Masouleh S., Huntenburg J.M., Lampe L., Rahim M., Abraham A., Craddock R.C., Riedel-Heller S., Luck T., Loeffler M., Schroeter M.L., Witte A.V., Villringer A., Margulies D.S. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–188. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Lim A.S., Kowgier M., Yu L., Buchman A.S., Bennett D.A. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.C., Cheng C., Yang C.-M., Hsui S.C. Psychometric properties of the sleep hygiene practice scale. Sleep. 2007;30:A262. [Google Scholar]
- Liu J., Pearlson G., Windemuth A., Ruano G., Perrone-Bizzozero N.I., Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2009;30(1):241–255. doi: 10.1002/hbm.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J.C., Loh K.K., Zheng H., Sim S.K., Chee M.W. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond S.H., Lovibond P.F. second ed. Psychology Foundation of Australia; Sydney: 1995. Manual for the Depression Anxiety Stress Scales. [Google Scholar]
- Martin J.L., Song Y., Hughes J., Jouldjian S., Dzierzewski J.M., Fung C.H., Rodriguez Tapia J.C., Mitchell M.N., Alessi C.A. A four-session sleep intervention program improves sleep for older adult day health care participants: results of a randomized controlled trial. Sleep. 2017;(8):40. doi: 10.1093/sleep/zsx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J., Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab. 2016;27(4):192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis S.M., Brickhouse M., Pascual B., Dickerson B.C. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24(3-4):279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley V.E., Bin Y.S., Lauderdale D.S. Associations of sleep characteristics with cognitive function and decline among older adults. Am J Epidemiol. 2019;188(6):1066–1075. doi: 10.1093/aje/kwz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.L., Alfaro-Almagro F., Bangerter N.K., Thomas D.L., Yacoub E., Xu J., Bartsch A.J., Jbabdi S., Sotiropoulos S.N., Andersson J.L.R., Griffanti L., Douaud G., Okell T.W., Weale P., Dragonu I., Garratt S., Hudson S., Collins R., Jenkinson M., Matthews P.M., Smith S.M. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nature Neuroscience. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner B., Kryger M.H. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Magnussen F., Lundquist A., Baaré W., Bartrés-Faz D., Bertram L., Boraxbekk C.J., Brandmaier A.M., Drevon C.A., Ebmeier K., Ghisletta P., Henson R.N., Junqué C., Kievit R., Kleemeyer M., Knights E., Kühn S., Lindenberger U., Penninx B.W.J.H., Pudas S., Sørensen Ø., Vaqué-Alcázar L., Walhovd K.B., Fjell A.M. Educational attainment does not influence brain aging. Proc Natl Acad Sci. 2021;118(18) doi: 10.1073/pnas.2101644118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A.R., Dong C., Rundek T., Elkind M.S., Boden-Albala B., Sacco R.L., Wright C.B. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res. 2014;23(5):524–530. doi: 10.1111/jsr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Rodrigue K.M. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Storsve A.B., Walhovd K.B., Johansen-Berg H., Fjell A.M. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. doi: 10.1212/wnl.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Sykara K., Karageorgiou E., Zitser J., Rosa T., Yaffe K., Leng Y. Connections between insomnia and cognitive aging. Neuroscience Bulletin. 2020;36(1):77–84. doi: 10.1007/s12264-019-00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Zsoldos E., Filippini N., Griffanti L., Winkler A., Mahmood A., Allan C.L., Topiwala A., Kyle S.D., Spiegelhalder K., Singh-Manoux A., Kivimaki M., Mackay C.E., Johansen-Berg H., Ebmeier K.P. Associations between self-reported sleep quality and white matter in community-dwelling older adults: a prospective cohort study. Hum Brain Mapp. 2017;38(11):5465–5473. doi: 10.1002/hbm.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva G.E., Goodwin J.L., Sherrill D.L., Arnold J.L., Bootzin R.R., Smith T., Walsleben J.A., Baldwin C.M., Quan S.F. Relationship between reported and measured sleep times: The Sleep Heart Health Study (SHHS) J Clin Sleep Med. 2007;3(6):622–630. doi: 10.5664/jcsm.26974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Elliott L.T., Alfaro-Almagro F., McCarthy P., Nichols T.E., Douaud G., Miller K.L. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. eLife. 2020;9:e52677. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vidaurre D., Alfaro-Almagro F., Nichols T.E., Miller K.L. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–539. doi: 10.1016/j.neuroimage.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.P., Gonzalez C.E., Venkatraman V.K., Wu M.N., Pacheco J., Simonsick E.M., Ferrucci L., Resnick S.M. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep. 2016;39(5):1121–1128. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Habeck C., O'Shea D., Razlighi Q., Bherer L., Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging. 2016;40:138–144. doi: 10.1016/j.neurobiolaging.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Arenaza-Urquijo E.M., Bartrés-Faz D., Belleville S., Cantilon M., Chetelat G., Ewers M., Franzmeier N., Kempermann G., Kremen W.S., Okonkwo O., Scarmeas N., Soldan A., Udeh-Momoh C., Valenzuela M., Vemuri P., Vuoksimaa E. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma P., Jackson M.L., Meaklim H. Dreaming of the good old days: sleep in older adults. J Pharm Pract Res. 2019;49(3):209–211. doi: 10.1002/jppr.1578. [DOI] [Google Scholar]
- Warrington S., Bryant K.L., Khrapitchev A.A., Sallet J., Charquero-Ballester M., Douaud G., Jbabdi S., Mars R.B., Sotiropoulos S.N. XTRACT - Standardised protocols for automated tractography in the human and macaque brain. Neuroimage. 2020;217 doi: 10.1016/j.neuroimage.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T.M., Yaffe K., van der Werf Y.D., Sexton C.E. Associations between modifiable risk factors and white matter of the aging brain: insights from diffusion tensor imaging studies. Neurobiol Aging. 2019;80:56–70. doi: 10.1016/j.neurobiolaging.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A., Grydeland H., Tamnes C.K., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Xu L., Pearlson G., Calhoun V.D. Joint source based morphometry identifies linked gray and white matter group differences. Neuroimage. 2009;44(3):777–789. doi: 10.1016/j.neuroimage.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Nasrallah I., Hoang T.D., Lauderdale D.S., Knutson K.L., Carnethon M.R., Launer L.J., Lewis C.E., Sidney S. Sleep duration and white matter quality in middle-aged adults. Sleep. 2016;39(9):1743–1747. doi: 10.5665/sleep.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S.M. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zitser J., Anatürk M., Zsoldos E., Mahmood A., Filippini N., Suri S., Leng Y., Yaffe K., Singh-Manoux A., Kivimaki M., Ebmeier K., Sexton C. Sleep duration over 28 years, cognition, gray matter volume, and white matter microstructure: a prospective cohort study. Sleep. 2020;(5):43. doi: 10.1093/sleep/zsz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participant data will be made available to researchers upon request.