Abstract
Antimicrobial peptides are a source of novel agents that could be useful for treatment of the chronic lung infections that afflict cystic fibrosis (CF) patients. Efficacy depends on antimicrobial activity against the major pathogens of CF patients, Pseudomonas aeruginosa, Staphylococcus aureus, and Haemophilus influenzae, in the environment of the CF patient's airway. We describe the in vitro efficacies of derivatives of histatins, which are histidine-rich peptides produced by the salivary glands of humans and higher primates. P-113, a peptide containing 12 of the 24 amino acid residues of the parent molecule, histatin 5, retained full antibacterial activity and had a good spectrum of activity in vitro against the prominent pathogens of CF patients. However, P-113 was not active in the presence of purulent sputum from CF patients. In contrast, P-113d, the mirror-image peptide with the amino acid residues in the d configuration, was stable in sputum, was as active as P-113 against pathogens of CF patients in the absence of sputum and retained significant activity in the presence of sputum from CF patients. Recombinant human DNase, which effectively liquefies sputum, enhanced the activity of P-113d in undiluted sputum against both exogenous (added) bacteria and endogenous bacteria. Because of its properties, P-113d shows potential as an inhalant in chronic suppressive therapy for CF patients.
We are investigating the possibility that derivatives of histatins, which are naturally occurring peptides secreted by the salivary glands of humans (27), could be effective agents for treatment of the chronic lung infections that afflict cystic fibrosis (CF) patients. The derivative P-113 is composed of a sequence of 12 amino acid residues contained within the 24 amino acid residues of histatin 5 and is amidated at its C terminus (28). P-113 is as active as full-length histatins in terms of its anticandidal activity in vitro (37) and has also been shown to have in vivo activity in the prevention of gingivitis, but its use does not result in the adverse side effects of other dental products (24, 28). Histatins are known to have antibacterial properties (20), which, if retained in P-113, would make P-113 a potential candidate for treatment of the infections that afflict CF patients.
Chronic pulmonary disease subsequent to long-term bacterial infection accounts for more than 90% of the fatalities among the 30,000 CF patients in the United States (5, 6, 13, 34, 36). An ordered progression of microorganisms infects the lungs of CF patients. Infants are infected predominantly with Staphylococcus aureus and Haemophilus influenzae, whereas a chronic infection with Pseudomonas aeruginosa usually develops in older patients (6, 9, 29, 30, 39). The colonizing strain can acquire the mucoid phenotype (9, 21, 26, 30), caused by the overproduction of exopolysaccharide (9), which may contribute to the formation of a biofilm which contains P. aeruginosa cells that are considerably less susceptible to antimicrobial agents (1, 9, 30). The prognosis for patients who are infected with mucoid P. aeruginosa is markedly worse than that for those colonized with nonmucoid P. aeruginosa (1, 6, 9, 29, 30, 36).
Physicians rely heavily on antibiotic therapy to treat CF patients. Although intervention with antibiotic therapy may prevent or delay the onset of infection with P. aeruginosa (7, 41), once an infection with mucoid P. aeruginosa takes hold, it has thus far not been possible to eradicate the infection and the prognosis for the patient is markedly worse (1, 6, 9, 29, 30). Pulmonary exacerbations are partially relieved by dual therapy consisting of high doses of an aminoglycoside and a β-lactam antibiotic (5, 6, 34). Recently, chronic suppressive therapy, in which tobramycin is inhaled twice daily for 28 days, followed by 28 days without tobramycin treatment, has proved to be effective in retarding the loss of lung function in CF patients. Delivery by inhalation avoids the toxicity associated with intravenous tobramycin treatment (4, 35). However, heavy reliance on conventional antibiotics raises the possibility of selection of resistant mutants of P. aeruginosa (6, 8, 25, 34). An unconventional agent for treatment of the infections that afflict CF patients, such as an antimicrobial peptide, would not select for resistance to conventional antibiotics, which could be reserved for the treatment of pulmonary exacerbations. As an additional potential benefit, histatins and other antimicrobial peptides can neutralize lipopolysaccharide, a potent mediator of the host inflammatory response (10, 12, 40) responsible for much of the damage in chronically infected CF patients (5, 13, 15, 17, 39).
The potencies of antibiotics such as tobramycin are reduced in the presence of sputum from CF patients (14, 23, 39) due to binding to the DNA or mucin components of sputum (33) and to its ionic composition (19). Recombinant human DNase (rhDNase) reduces the viscoelasticity of sputum and is helpful in some CF patients because it may improve the access of antibiotics to bacteria (5, 34, 44). However, sputum, with its high content of proteases, mucus, and DNA, presents an obstacle to the efficacy of any therapeutic agent for CF patients. For these reasons, we tested P-113 and a related derivative, P-113 with the amino acids in the d configuration (P-113d), for their in vitro antibacterial activities and, in addition, for their activities and stabilities in the presence of sputum derived from CF patients.
MATERIALS AND METHODS
Bacterial strains.
A nonmucoid P. aeruginosa isolate (ATCC 27853) and a mucoid P. aeruginosa strain isolated from a CF patient (provided by the Clinical Microbiology Laboratory, The Hospital for Sick Children, Toronto, Ontario, Canada) were used as standard susceptible strains. Clinical isolates of P. aeruginosa, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans were obtained from Lisa Saiman (Columbia University), and additional strains of P. aeruginosa were obtained from Gerald Pier (Harvard University), as indicated in Table 1. Clinical isolates of S. aureus from CF patients were provided by Jane Burns (University of Washington), H. influenzae isolates were obtained from Arnold Smith (University of Missouri, Columbia), and isolates of Burkholderia cepacia from CF patients were obtained from David Speert (University of British Columbia, Vancouver, British Columbia, Canada).
TABLE 1.
Susceptibility testing of American Type Culture Collection strains and clinical isolates of P. aeruginosaa
| Strain | Age (yr) | Location | Susceptibilityb | Muciodc | Assayd | MIC
(μg/ml)
|
|
|---|---|---|---|---|---|---|---|
| P-113 | P-113d | ||||||
| ATCC 19142 | DA | 3.1 | |||||
| ATCC 19143 | DA | 3.1 | |||||
| ATCC 27853 | DA | 6.3 | 3.1 | ||||
| ATCC 43393 | DA | 6.3 | |||||
| ATCC 51674 | DA | 1.6 | |||||
| 332 | Yes | DA | 6.3 | 6.3 | |||
| 8050-2 | Yes | DA | 3.1 | 3.1 | |||
| 5681-1 | Yes | DA | 6.3 | 3.1 | |||
| 2192 | Yes | DA | 6.3 | 3.1 | |||
| 1-76 AM | 51 | Cleveland, Ohio | R | DA | 3.1 | 1.6 | |
| 1-77 AM | 27 | Dallas, Tex. | R | DA | 1.6 | 1.6 | |
| 1-78 AM | 20 | Birmingham, Al. | R | AB | 1.6 | ||
| 1-82 AM | 14 | St. Louis, Mo. | R | DA | 3.1 | ||
| 2-17 AM | 10 | Los Angeles, Calif. | RR | DA | 3.1 | 1.6 | |
| 2-24 AM | 15 | Atlanta, Ga. | R | AB | 1.6 | ||
| 2-25 AM | 21 | Durham, N.C. | R | DA | 1.6 | 1.6 | |
| 2-87 AM | 21 | Falls Church, Va. | R | DA | 1.6 | 1.6 | |
| 2-90 AM | 26 | Cincinnati, Ohio | R | DA | 1.6 | 3.1 | |
| 2-98 AL | 33 | Cleveland, Ohio | RR | Yes | DA | >100 | >50 |
| 3-16 AL | 23 | Providence, R.I. | R | AB | 1.6 | ||
| 3-20 AL | 23 | Fort Worth, Tex. | R | DA | 3.1 | 1.6 | |
| 3-36 AM | 26 | Tulsa, Okla. | R | DA | 3.1 | 1.6 | |
| 3-47 AM | 24 | Peoria, Ill. | R | AB | 1.6 | ||
| 3-66 AM | 23 | Oklahoma City, Okla. | R | DA | 3.1 | 3.1 | |
| 4-34 AL | 19 | Chattanooga, Tenn. | R | AB | 3.1 | ||
| 4-36 AL | 22 | Oklahoma City, Okla. | RR | DA | >50 | 25 | |
| 4-68 AL | 17 | Pittsburgh, Pa. | r1 | AB | 3.1 | ||
| 4-97 AL | 17 | San Francisco, Calif. | R | DA | 3.1 | 3.1 | |
| 5-39 AM | 21 | Phoenix, Ariz. | r3 | DA | 6.3 | ||
Strains obtained from the American Type Culture Collection (ATCC) are so designated. The other strains are clinical isolates from CF patients from Lisa Saiman at (Columbia University). Strains 332, 8050-2, 5681-1, and 2192, however, were from Gerald Pier (Harvard University). Geographic data and susceptibility data obtained by standard methods (38) are included.
R, resistant to all antibiotics tested (ceftazidime, amikacin, piperacillin, aztreonam, tobramycin, gentamicin, ticarcillin, and timentin); RR, resistant to high doses of tobramycin and gentamicin; r1, resistant to all antibiotics tested except ticarcillin and timentin; r3, resistant to all antibiotics tested except tobramycin and amikacin.
Yes indicates mucoid colonial morphology.
AB, Alamar Blue method; DA, direct assay, method (see Materials and Methods).
Antibacterial compounds.
P-113, a peptide with the sequence AKRHHGYKRKFH (amidated on the C terminus), P-113d (which has the same sequence as P-113 but with the amino acids in the d configuration), and histatin 5 were synthesized by Multiple Peptide Systems, San Diego, Calif. The purities (>95%) and authenticities of the sequences of all peptides were determined by analytical reverse-phase high-pressure liquid chromatography (HPLC) and maxtrix-assisted laser desorption ionization–time of flight mass spectroscopy (3). Tobramycin, imipenem, and ceftazidine were purchased from Sigma (St. Louis, Mo.).
Sputum samples.
The sputum used in microbiological studies was collected from hospitalized or outpatient CF patients (from The Hospital for Sick Children or St. Michael's Hospital, Toronto, Ontario, Canada) colonized with P. aeruginosa. For most studies, sputum from eight patients was pooled, mixed thoroughly, and stored in aliquots at −20°C until needed. When the effect of P-113 on endogenous P. aeruginosa was tested, however, sputum was used within 1 h of collection. The CF patient Sputum samples used to test the chemical stabilities of the peptides were obtained from Jane Burns (University of Washington, Seattle).
Determination of MICs.
MICs were determined by two methods: (i) the Alamar Blue uptake assay and (ii) the direct assay. By the Alamar Blue assay method, bacteria were inoculated onto a blood agar plate, the plate was incubated for 18 h at 35°C, and the bacteria were suspended in 10 mM potassium phosphate buffer (adjusted to pH 7.4) to a concentration of 1 × 105 to 8 × 105 CFU/ml. Fifty microliters of this suspension was transferred into each well of a 96-well microtiter plate, and the plate was incubated with 50 μl of various concentrations of P-113 or P-113d in 50 mM sodium acetate solution (pH 6) for 30 min at 37°C. Subsequently, 100 μl of cation-adjusted Mueller-Hinton (CAMH) broth (Difco) and Alamar Blue were then added, the bacteria were grown overnight, and the color change was monitored as instructed by the manufacturer (Alamar Biosciences, Inc., Sacramento, Calif.).
By the direct assay method, MICs were determined by direct measurement of the optical densities of the bacterial cultures after incubation of the bacteria in the presence of P-113 or P-113d in LM broth, which consisted of the following: cation-adjusted Mueller-Hinton broth (5%; Difco) supplemented with 2 mM Na2HPO4, 1.8 mM KH2PO4, 0.1 mM magnesium sulfate, and 1.0 mM sodium citrate. Glucose, an amino acid mixture lacking glutamine, and a vitamin mixture from the Life Technologies RPMI-1640 Select-Amine kit (Accumed International, Inc., Westlake, Ohio) were added to the medium according to the supplier's instructions. In addition, the medium contained 0.4 mg of ZnCl2, 2.0 mg of FeCl3 · 6H2O, 0.1 mg of CuSO4 · 5H2O, 0.1 mg of MnSO4 · H2O, and 0.1 mg of Na2B4O7 · 10H2O per liter. P. aeruginosa, S. aureus, B. cepacia, S. maltophilia, or A. xylosoxidans was inoculated onto a blood agar plate or brain heart infusion agar, incubated for 18 h at 35°C, and then stored at room temperature for use later in the day. The bacteria were suspended to a final concentration of 1 × 105 to 8 × 105 CFU/ml in LM broth containing twofold dilutions of P-113, P-113d, or conventional antibiotics. After 16 to 20 h at 35°C, the optical density was measured with a Thermomix plate reader (Molecular Devices, Sunnyvale, Calif.). After subtraction of the reading for a blank control containing growth medium but no bacteria, readings below 0.01 optical density units (which correlated with no visible growth) indicated growth inhibition. Independent MIC determinations with the same isolate and compound were usually identical or within a twofold range. If the results of two independent tests did not agree, a third test was conducted and the median value was reported.
Bacterial killing assays.
P. aeruginosa was grown on brain heart infusion agar or Pseudomonas isolation agar overnight at 37°C. The bacteria were suspended in assay buffer (8 mM sodium phosphate, 20 mM KCl, 80 mM NaCl [pH 7.4]) to a concentration of 2 × 107 to 5 × 108 CFU/ml. An aliquot of 0.3 ml was mixed with 1 μl containing P-113 or P-113d, and the mixture was incubated at 37°C for 1 h. Serial dilutions of the mixture were inoculated onto Pseudomonas isolation agar plates. P. aeruginosa incubated in assay buffer in the absence of P-113 or P-113d served as a control. After incubation for 18 to 24 h at 37°C, the colonies were counted to determine the percent survival of P. aeruginosa in the presence of P-113 or P-113d compared to the percent survival for the controls. For H. influenzae, the bacteria were grown on chocolate agar plates (tryptic soy agar supplemented with 50 ml of sheep blood per liter of hot agar, which lyses red blood cells, and yeast extract at 10 g/liter, as described previously [22], at 35°C in a Brewer jar with a GasPak carbon dioxide generator [Becton Dickinson, Cockeysville, Md.]). The bacteria were suspended in phosphate-buffered saline (10 mM sodium phosphate [pH 7.4], 130 mM NaCl), diluted to a concentration of 1 × 105 to 8 × 105 CFU/ml, and incubated with P-113 for 1 h at 37°C. The reaction mixture was serially diluted and inoculated onto chocolate agar plates, and the percent survival was determined as described above for P. aeruginosa.
The killing activity of P-113 or P-113d in the presence of sputum was determined by mixing 250 μl of undiluted sputum or sputum diluted 10-fold in the assay buffer together with P. aeruginosa at a final concentration of 2 × 107 to 5 × 108 CFU/ml. To this sputum-bacterium mixture was added 1 μl of P-113 or P-113d, and after additional mixing, the sample was incubated for 1 h at 37°C. The sample was then serially diluted and plated on Pseudomonas isolation agar. The survival of bacteria from incubations in the presence of sputum and P-113 or P-113d was calculated as the percentage of the number of CFU compared to that of control cells similarly exposed to sputum with no peptide.
In some experiments, sputum was preincubated with rhDNase (Pulmozyme; Genzyme Corp., San Francisco, Calif.) for 1 h at 37°C to decrease the viscosity of the sputum. Subsequently, the bacteria were added to the rhDNase-treated sputum, followed by addition of the peptide and incubation of the mixture for an additional 1 h at 37°C. Percent survival was calculated relative to survival for control samples that were not treated with rhDNase and that did not contain peptide, as described above.
To determine the antimicrobial activity of P-113d against endogenous P. aeruginosa, 250 μl of freshly obtained sputum was incubated with the desired concentration of P-113d in the presence or absence of rhDNase. The number of viable bacteria present in each reaction mixture and the percent survival were determined as described above.
Stability measurements in sputum.
Individual sputum samples from CF patients were diluted a total of 40-fold (by volume) in 10 mM potassium phosphate buffer (pH 7.4). One-half of the diluent buffer was added to a sputum sample, and the suspension was thoroughly mixed and then centrifuged at 1,000 rpm (IEC model 7000) for 10 min. The supernatant was saved, the pellet was washed with the remaining half of the diluent, and the procedure was repeated. The pooled supernatants were used in stability tests.
P-113 or P-113d (5 μl) was added to 45 μl of a diluted sputum sample to a final concentration of 100 μg/ml, and the mixture was incubated at 37°C. The reaction was stopped by the addition of 10 μl of 10% HCl. Peptide concentrations were were determined with a reversed-phase HPLC system containing a narrow-bore column (Delta-Pak C18, 300 Å, 2 mm by 15 cm; Waters) and a guard column (Widepore C18, 2 mm by 4 mm; Phenomenex). The mobile phase was a binary mixture of acetonitrile and water containing 0.1% trifluoroacetic acid. The composition of acetonitrile in the mobile phase was increased linearly from 6 to 27% for 28 min at a flow rate of 0.25 ml/min. P-113 and P-113d were monitored by measurement of the UV absorbance at 224 nm. Stability was calculated as the percentage of the initial concentration of peptide.
RESULTS
P-113 has potent killing activity against P. aeruginosa.
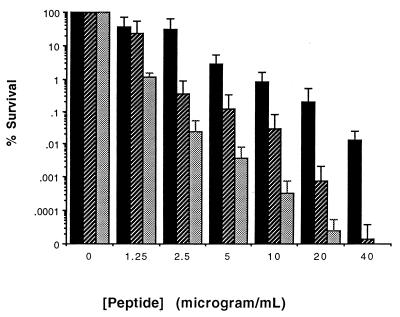
When P. aeruginosa strain ATCC 27853 was suspended in assay buffer and exposed to the P-113 peptide, a strong killing effect was observed (Fig. 1). The concentration sufficient to kill 90% of the bacterial cells (LD90) was 2.5 μg/ml (range, 1.25 to 5 μg/ml), as determined by measurement of the numbers of CFU.
FIG. 1.
Activities of P-113 and P-113d against P. aeruginosa. Bacteria were suspended in assay buffer, incubated with either P-113 or P-113d for 1 h at 37°C, and inoculated onto Pseudomonas isolation agar plates to determine the numbers of CFU. Survival with peptide is the percentage of the number of CFU relative to that for control cells suspended in buffer without peptide (2 × 107 to 5 × 108 CFU/ml). The error bars indicate standard deviations from the means for three experiments. ■, activity of P-113 against P. aeruginosa ATCC 27853; ▨, activity of P-113d against P. aeruginosa ATCC 27853; ░⃞, activity of P-113d against P. aeruginosa mucoid strain.
MIC testing.
In contrast to killing in buffer, P-113 exhibited little activity in MIC tests in medium such as CAMH broth, which is routinely used for susceptibility testing (data not shown). In order to efficiently determine the in vitro efficacy of P-113 against a panel of clinical isolates in growth medium rather than buffer, a broth dilution susceptibility test was developed.
When P. aeruginosa was exposed to P-113 in CAMH broth diluted 20-fold with deionized water, the MIC was found to be 3.1 μg/ml. This dramatic increase in susceptibility was attributed to dilution of one or more components in CAMH broth that were inhibitory to the antimicrobial activity of the peptide. To increase the density of growth in diluted medium while allowing the peptide to retain its antimicrobial activity, glucose, potassium phosphate, magnesium salt, amino acids, vitamins, and trace elements were added back to the diluted medium. LM broth, described in Materials and Methods, contains reduced concentrations of monovalent and divalent salts and contains an additional ingredient, citrate, which may act as a chelator of divalent cations. This broth supports growth to a higher cell density and with the same doubling time as cells grown in CAMH broth, while it retains the potent activity of P-113, with an MIC of 6.3 μg/ml (MIC range, 3.1 to 6.3 μg/ml) (Table 1). Thus, LM broth was judged to be suitable for testing of the MIC of P-113.
P. aeruginosa strain ATCC 27853 was also tested for its susceptibilities to the antibiotics ceftazidime, imipenem, and tobramycin determined with LM broth in comparison to those determined with standard susceptibility medium (CAMH broth). The MICs of both tobramycin and ceftazidime were the same in both media (0.4 and 1.6 μg/ml, respectively). The MIC of imipenem was 0.8 μg/ml in LM broth and 1.6 μg/ml in CAMH medium. These experiments suggested that the use of LM broth had an effect specifically on the susceptibility of P. aeruginosa to cationic peptides such as P-113.
Susceptibility testing of clinical isolates.
A series of P. aeruginosa clinical isolates from CF patients was tested with LM broth for most strains (by the direct assay, described in Materials and Methods). For a minority of strains that did not grow well in LM broth, we used the Alamar Blue method, described in Materials and Methods, to determine the MICs. Most strains were susceptible to P-113 (Table 1). The median MIC was 3.1 μg/ml, and the the MIC at which 90% of strains were inhibited was 6.3 μg/ml.
In addition to good activity against P. aeruginosa, P-113 was effective against the other pathogens most frequently encountered in CF patients, S. aureus and H. influenzae (Table 2). P-113 was not effective against B. cepacia, A. xylosoxidans, or S. maltophilia (Table 2).
TABLE 2.
Activities of P-113 and P-113d against other pathogens that infect CF patients
| Organism | Strain | MIC
(μg/ml)d
|
|
|---|---|---|---|
| P-113 | P-113d | ||
| S. aureus | 6538 | 6.3 | 3.1 |
| S. aureus | 99-68-0382 | 12.5 | 6.3 |
| S. aureus | 99-75-0311 | 12.5 | 6.3 |
| S. aureus | 99-75-0432 | 12.5 | 6.3 |
| S. aureus | 99-82-0575 | 12.5 | 6.3 |
| S. aureus | 99-88-0215 | 12.5 | 6.3 |
| S. aureus | 99-88-0261 | 12.5 | 6.3 |
| S. aureus | 102-0485 | 12.5 | 6.3 |
| S. aureus | 117-0350 | 12.5 | 6.3 |
| S. aureus | 110-0232 | 12.5 | 6.3 |
| S. aureus | 102-0484 | 12.5 | 6.3 |
| S. aureus | Smith | 12.5 | 6.3 |
| H. influenzaea | R2841 | 6.3 | 3.1 |
| H. influenzaea | R3001 | 3.1 | 3.1 |
| H. influenzaea | R3004 | 3.1 | 3.1 |
| H. influenzaea | 441513 | 12.5 | 12.5 |
| B. cepaciab,c | cep0533 | R | R |
| B. cepaciae | cep0455 | R | R |
| B. cepaciac,f | cep0509 | R | R |
| B. cepaciab | cep0055 | R | R |
| B. cepaciab,c | c5424 | R | R |
| B. cepaciag | c6061 | 50 | 25 |
| B. cepaciab | c4373 | R | R |
| B. cepaciab | c6424 | R | R |
| B. cepaciah | FC441 | R | R |
| B. cepaciab | cep0055s | R | R |
| S. maltophilia | 61AT | R | R |
| S. maltophilia | 72AT | R | R |
| S. maltophilia | 81BG | R | R |
| S. maltophilia | 85BG | R | R |
| S. maltophilia | 100BG | 25 | 6.3 |
| S. maltophilia | 47BH | 25 | 3.1 |
| S. maltophilia | 59BH | 12.5 | 12.5 |
| S. maltophilia | 17BI | R | 6.3 |
| S. maltophilia | 22BI | R | R |
| S. maltophilia | 44BI | R | 50 |
| S. maltophilia | 46BJ | R | 12.5 |
| S. maltophilia | 77BJ | R | R |
| A. xylosoxidans | 8AU | R | 100 |
| A. xylosoxidans | 32AU | R | R |
| A. xylosoxidans | 60AW | R | R |
| A. xylosoxidans | 61AW | R | R |
| A. xylosoxidans | 88AW | R | R |
| A. xylosoxidans | 29AX | R | R |
| A. xylosoxidans | 31AX | 6.3 | 6.3 |
| A. xylosoxidans | 34AX | R | R |
| A. xylosoxidans | 62AZ | R | R |
| A. xylosoxidans | 74BA | R | R |
| A. xylosoxidans | 39BB | R | R |
Tested by killing assay since the strains failed to grow in LM broth; the inhibitory concentrations represent LD90s.
Genomovar III.
Cable pilus strain. R = resistant (MIC > 100 μg/mL)
R, resistant (MIC, >100 μg/mL).
Genomovar II.
Genomovar I.
Genomovar IV.
Genomovar V.
Antimicrobial activity of P-113 in diluted sputum.
Because of the encouraging in vitro antimicrobial activity of P-113 against the prominent pathogens from CF patients, we tested its activity in sputum, an environment likely to be encountered in the airways of CF patients. Samples of purulent sputum were collected from eight CF patients and pooled. After storage for several months at −20°C, the sample was found to contain no viable P. aeruginosa cells. The sputum sample was then diluted 10-fold, and P. aeruginosa cells were added. The samples were then used to test for the effect of P-113 on bacterial viability. Incubation of the bacteria with diluted sputum and P-113 for 1 h at 37°C diminished the number of CFU by approximately 2 orders of magnitude (from 108 to 106 CFU per ml). Somewhat surprisingly, however, the viabilities of the added P. aeruginosa cells in the absence of P-113 were also diminished by 2 orders of magnitude after incubation in diluted sputum for 1 h. Thus, the addition of up to 100 μg of P-113 per ml to the mixture of bacteria and sputum resulted in little or no detectable reduction in the numbers of CFU beyond the killing effect of sputum itself (Fig. 2).
FIG. 2.
Activities of P-113 and P-113d in the presence of diluted sputum. An aliquot of the pooled sputum sample from eight CF patients was diluted 10-fold in assay buffer and mixed with cells of P. aeruginosa. Then, the peptide was added to the indicated concentration and the final mixture was incubated for 1 h at 37°C. The survival for each experiment was the percentage of the number of CFU compared to that for control cells similarly exposed to diluted sputum but no peptide, as described in Materials and Methods. Error bars indicate the standard deviations from the means for three experiments. ■, activity of P-113 against P. aeruginosa ATCC 27853; ▨, activity of P-113d against P. aeruginosa ATCC 27853; ░⃞, activity of P-113d against P. aeruginosa mucoid strain.
Stability of P-113 in sputum.
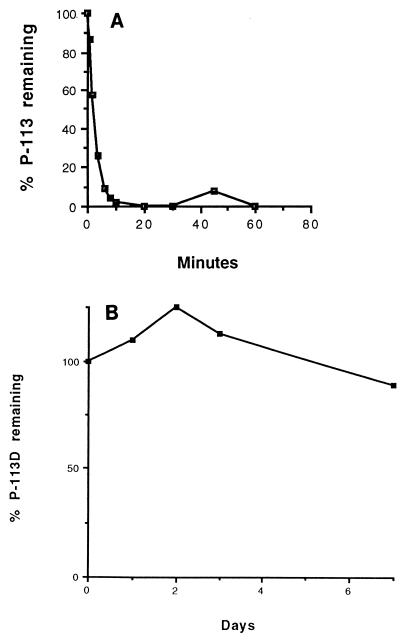
To determine whether the drastic reduction in the antimicrobial activity of P-113 in sputum could be attributed to instability, P-113 was incubated with a diluted supernatant of sputum derived from one CF patient, and the sample was then analyzed by HPLC. Figure 3A shows that P-113 was rapidly degraded, with a half-life of 2.8 min. Four other sputum samples derived from different CF patients showed half-lives of 17, 31.5, 40.5, and 58.5 min, respectively. Assuming that dilution has a direct linear effect on activity, we calculated the half-life of P-113 in undiluted sputum to range from 4.2 s to 1.5 min, depending on the sputum sample. These results suggest that the instability of P-113 in sputum probably prevents it from being efficacious as a therapeutic agent.
FIG. 3.
Stabilities of P-113 and P-113d in sputum. The proportion of peptide remaining after incubation with diluted sputum supernatant was determined by HPLC, as described in Materials and Methods. (A) P-113; (B) P-113d.
An isomer of P-113 retains favorable antimicrobial activity.
It has been found that the d configurations of some antimicrobial peptides retain activity (2). Peptide P-113d, which contains the same sequence of 12 amino acids as P-113 but in the d configuration, was synthesized with the expectation that it would be more resistant to protease degradation and therefore might be more stable in sputum. Figure 1 shows that P-113d had activity that was comparable to that of P-113 in killing P. aeruginosa. The LD90 was 1.25 to 2.5 μg/ml for nonmucoid P. aeruginosa strain ATCC 27853, whereas the LD90 of P-113 was 2.5 μg/ml (range, 1.25 to 5 μg/ml). The mucoid isolate was as susceptible to P-113d as strain ATCC 27853, with an LD90 of 1.25 μg/ml (Fig. 1); this activity was comparable to that of P-113 against the mucoid isolate (data not shown).
P-113d was also as active as P-113 against the battery of P. aeruginosa clinical isolates. Among the 17 strains tested, the MICs of the l and d configurations of the peptides were the same for 10 strains, and there was a twofold difference in the MICs for 7 strains (with P-113d having the lower MIC for all but one strain) (Table 1). The antimicrobial activity of P-113d against the two other species most commonly associated with infections in CF patients, S. aureus and H. influenzae, was also comparable to that of P-113 (Table 2).
P-113d was refractory to degradation by sputum with all five sputum samples tested. Even in the sputum sample with the highest degradation capacity (P-113 half-life, 2.8 min), P-113d was stable over a 7-day period (Fig. 3B).
When P-113d was tested for activity in 10% sputum, the LD90 was close to 10 μg/ml (range, 10 to 20 μg/ml) against both the nonmucoid and the mucoid strains of P. aeruginosa (Fig. 2). Thus, P-113d retained significant activity in sputum due to the stability of the d configuration in sputum. Because of this advantage, P-113d was therefore tested further for its activity in the presence of sputum.
Activity in undiluted sputum.
In view of the concentrations of inflammatory products found in the airways of CF patients in vivo, we believed that it would be clinically relevant to determine the effect of undiluted sputum on the activity of P-113d. In addition to proteases, mucin, and DNA, sputum contains high concentrations of ions, including divalent cations (19). The latter might be expected to inhibit the activity of P-113d markedly, on the basis of the antagonistic effects that divalent cations show in growth-inhibitory tests and in killing assays conducted with bacteria suspended in buffer (data not shown). Pooled, undiluted sputum was therefore mixed with exogenous P. aeruginosa strain ATCC 27853, and the mixture was incubated for 1 h at 37°C with or without P-113d. In the absence of P-113d, approximately 2 log units of killing was observed due to the in vitro antimicrobial properties of sputum. In the presence of P-113d, 3 log units of killing was observed (data not shown). Thus, the killing effect of P-113d could be observed as an increase in killing over and above the in vitro antimicrobial action of sputum. Because of the technical difficulties with the handling, mixing, and representative sampling of extremely viscous, gel-like sputum, another approach was required to test further the efficacy of P-113d in sputum.
Effect of DNase on antibacterial activity of P-113d.
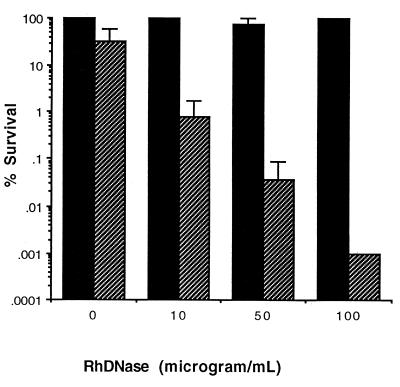
The therapeutic aerosol agent rhDNase digests DNA in tracheobronchial secretions from CF patients and often provides temporary relief of airway obstruction (5, 44). Sputum pretreated in vitro for 1 h at 37°C with 50 μg of rhDNase per ml caused a reduction of viscosity, as judged visually, and is referred to hereafter as liquefied sputum. rhDNase alone showed no antimicrobial activity against P. aeruginosa cells that had been suspended in killing assay buffer (data not shown). At concentrations up to 100 μg/ml, rhDNase also had no effect on the intrinsic antimicrobial activity of sputum (Fig. 4). However, when rhDNase was added to undiluted sputum in the presence of a fixed concentration (50 μg/ml) of P-113d, there was a dramatic enhancement in the antimicrobial activity of P-113d (Fig. 4).
FIG. 4.
Effect of rhDNase on P-113d activity in undiluted sputum. Sputum was liquefied by incubation with rhDNase at the indicated concentrations for 1 h at 37°C. P. aeruginosa strain ATCC 27853 cells were mixed, and the incubation proceeded for an additional hour in the absence or presence of 50 μg of P-113d per ml. For each experiment the survival was the percentage of the number of CFU compared to that for control cells exposed to untreated sputum for 1 h at 37°C in the absence of P-113d, as described in Materials and Methods. Error bars indicate the standard deviations from the means for three experiments. ■, pretreatment with rhDNase but no treatment with P-113d; ▨, pretreatment with rhDNase and treatment with P-113d.
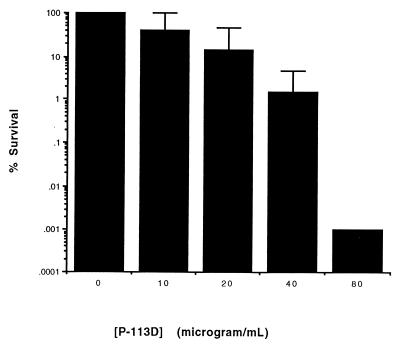
Figure 5 shows the concentration profile of P-113d against P. aeruginosa ATCC 27853 in liquefied sputum (pretreated with 50 μg of rhDNase per ml). In five independent experiments, the addition of P-113d to a mixture of P. aeruginosa cells and liquefied sputum resulted in a several-log reduction in the numbers of CFU per milliliter. The LD90 of P-113D was 20 μg/ml (range, 10 to 40 μg/ml). This compares to an LD90 of 1.25 to 2.5 μg/ml for P-113d against the same organism in the absence of sputum (Fig. 1).
FIG. 5.
Activity of P-113d in undiluted sputum pretreated with rhDNase. Sputum was liquefied by incubation with rhDNase at the indicated concentrations for 1 h at 37°C. P. aeruginosa strain ATCC 27853 cells were mixed, and the incubation proceeded for an additional hour in the presence of P-113d at the indicated concentrations. For each experiment the survival was the percentage of the number of CFU compared to that for control cells exposed to sputum in the absence of P-113d, as described in Materials and Methods. Error bars indicate the standard deviations from the means for three experiments.
Activities against endogenous microorganisms in sputum.
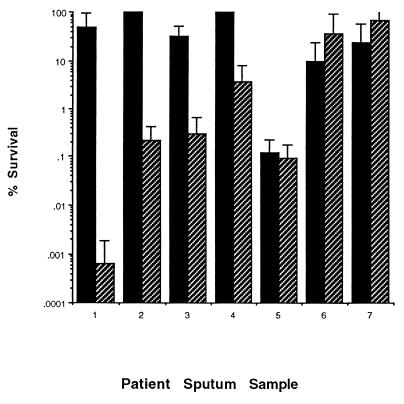
Because the genotype and physiological state of P. aeruginosa cells in the sputum of CF patients can differ from those of cells grown in culture (9, 21, 30), it was important to determine whether P-113d was also effective against endogenous bacteria found in sputum. For these experiments, freshly obtained sputum samples were required. Sputum was collected from seven different CF patients and was used within 1 h of collection. The samples were incubated for 2 h at 37°C. During the first hour, the samples were either untreated or were liquefied with 50 μg of rhDNase per ml. During the second hour, the samples were incubated with or without 100 μg of P-113d per ml. Control samples were incubated for 2 h without rhDNase treatment and without exposure to P-113d. Liquefied sputum samples without P-113d showed little or no killing compared to that for the control samples (data not shown), as was observed in Fig. 4. Sputum samples not liquefied during the first hour but then mixed with 100 μg of P-113d per ml during the second hour of incubation showed modest killing of P. aeruginosa (Fig. 6, samples 1, 3, 5, 6, and 7). Liquefied sputum incubated with 100 μg of P-113d per ml showed strong killing of endogenous bacteria from samples 1 to 5 (Fig. 6).
FIG. 6.
Activity of P-113d against endogenous P. aeruginosa in undiluted sputum. Fresh sputum was incubated for 1 h at 37°C with or without 50 μg of rhDNase per ml, as indicated, and for a second hour at 37°C with 100 μg of P-113d per ml. For each experiment the survival was the percentage of the number of CFU compared to that for sputum incubated for 2 h at 37°C in the absence of P-113d, as described in Materials and Methods. Error bars indicate the standard deviations from the means for three experiments. ■, no pretreatment with rhDNase; ▨, pretreatment with rhDNase.
In summary, P-113d significantly decreased the viabilities of the endogenous P. aeruginosa organisms present in most undiluted sputum samples from CF patients. In four of the seven samples tested, preincubation of the sputa with rhDNase enhanced the efficacy of P-113d, presumably by degrading the DNA and improving the access of the peptide to bacterial receptor sites.
DISCUSSION
The median survival age for CF patients has increased from 1 year to over 30 years in the last 50 years due, in part, to the aggressive use of antimicrobial agents to treat lung infections (5, 6, 34). The recent improvement in the status of CF patients who use inhaled tobramycin for chronic suppressive therapy for alternating 28-day periods on and off treatment (4, 35) suggests that the development of other therapeutic inhalants could further advance chronic suppressive therapy. Antimicrobial peptide P-113, a derivative of histatin 5, showed potent activity against the important pathogens of CF patients, P. aeruginosa, H. influenzae, and S. aureus (Fig. 1; Tables 1 and 2). Furthermore, the results of animal studies (28) and phase I and II human clinical trials to test the efficacy of P-113 in preventing gingivitis (24) indicate that the oral formulations have no adverse side effects. These studies suggested that the therapeutic index for P-113 in the treatment of lung infections in CF patients could be very favorable.
Despite its positive attributes, P-113 failed to retain its activity in sputum (Fig. 2) due to its instability (Fig. 3A). In order to salvage the possibility of using P-113 as an antimicrobial agent in CF patients, an attempt was made to stabilize the peptide with the protease inhibitors α-1 antitrypsin and serum leukoprotease inhibitor (SLPI), both of which are being evaluated in clinical trials as inhalants that might relieve CF patients from the effects of proteases (34). Although SLPI afforded some protection against proteolysis, the effect was judged to be too small to warrant further investigation at this time (data not shown). P-113d, however, proved to be stable in sputum (Fig. 3B) and retained significant activity in the presence of undiluted sputum (Fig. 5), including activity against endogenous microorganisms present in the sputum of CF patients (Fig. 6).
It was significant that P-113d retained good activity in the presence of sputum, in light of in vitro test results. Using standard conditions of susceptibility testing (i.e., CAMH broth), we detected very little antibacterial activity. However, potent activity was observed when we used LM broth, which contains low concentrations of salt and, presumably, low concentrations of free divalent cations, through the inclusion of citrate, a chelating agent. Higher concentrations of free divalent cations were shown to inhibit activity (data not shown). Significant P-113d activity, however, is retained in sputum (Fig. 5 and 6), despite the presence of Ca2+ and Mg2+ cations, each of which is expected to be present at concentrations in excess of 2 mM in sputum from CF patients (19). It is possible that divalent cations are also chelated by mucin, DNA, or other components found in sputum. This would explain why activity was observed in sputum and in MIC tests with LM broth but not under standard susceptibility testing conditions.
The use of rhDNase to liquefy sputum was an effective means of overcoming the technical issue of mixing of bacteria, peptide, and sputum. It is possible that rhDNase treatment not only liquefied sputum but also disrupted biofilms, which tend to be considerably more resistant to antibacterials (1), allowing greater access of the peptide to the bacteria.
Our data suggest that not all sputum samples containing P. aeruginosa will show an identical response to P-113d, nor will there be an equal enhancement of P-113d by rhDNase pretreatment (Fig. 6). This is perhaps not surprising, considering that interpatient variables (clinical severity of lung infection, therapy, time of day) were not standardized in this experiment and that there can be much variability in sputum sample composition and consistency even within the same patient.
It is interesting that P-113d retains potent activity, even though it contains only 12 amino acid residues, which is close to the minimum size required for antimicrobial cationic peptides to retain activity (31, 32). Histatins are classified as α-helical antimicrobial peptides (31, 32), and the sequence of P-113d, as well as structural studies, suggests that P-113 and P-113d could form an amphipathic α helix (37). A peptide must be 20 residues long to traverse the bacterial membrane as an α helix (43), precluding the simple model that P-113 or P-113d molecules form the sides of pores (10). However, experiments with Escherichia coli showed that P-113d rapidly made cells permeable to substrates that normally cannot traverse the outer or inner membrane, suggesting that P-113d is a surface-active agent (unpublished data).
The minimal size of P-113d has two possible advantages: it minimizes the costs of chemical synthesis and it minimizes the chance of elicitation of an immune response due to the lack of a defined structure in an aqueous environment (37).
There is no evidence of cross-resistance between antibiotics used in therapy for CF patients and antimicrobial peptides (10, 11). There is precedent for the use of cationic peptide-containing drugs from reports that the long-term use of inhaled colistin (a collection of polymyxins, each of which contains a large peptide component) diminishes the rate of deterioration of lung function, with little evidence of resistance-related issues from the clinical use of cationic peptide-containing drugs (16, 42).
P-113d has potential for use as inhalant therapy because it has good activity against the important pathogens found in CF patients (Tables 1 and 2) and because it retains activity in the presence of undiluted sputum (Fig. 5 and 6). The use of P-113d would avoid selection of resistance to classical antibiotics, which might then be reserved for use in combating exacerbations. Finally, the use of P-113d has another potential advantage, in that antimicrobial cationic peptides have the capacity to neutralize bacterial lipopolysaccharides (10, 11, 40), which are potent mediators of the host inflammatory response which is responsible for much of the lung damage in CF patients. Indeed, treatment of CF patients with anti-inflammatory agents such as ibuprofen appears to reduce the decline in lung function (5, 18, 34). Therefore, P-113d shows promise as an inhalant in chronic suppressive therapy for CF patients.
ACKNOWLEDGMENTS
We thank Lisa Saiman (Columbia University) for providing clinical isolates, valuable discussions, and providing susceptibility data for clinical isolates; Arnold Smith (University of Missouri), Gerald Pier (Harvard University), and David Speert (University of British Columbia) for providing clinical isolates; Jane Burns (University of Washington) for sending clinical isolates and sputum samples; and Elizabeth Tullis and Anna Tsang (St. Michael's Hospital, Toronto, Ontario, Canada) for providing sputum samples. Technical assistance was provided by Jason Raine (The Hospital for Sick Children, Toronto, Ontario, Canada). We also thank Marcia Osburne (Aventis Pharmaceuticals) for careful reading of the manuscript and valuable discussions.
This work was supported in part by a therapeutics development grant award (grant ROTHST98WO) from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Anwar H, Strap J L, Chen K, Costerton J W. Dynamic interactions of biofilms of mucoid Pseudomonas aeruginosawith tobramycin and piperacillin. Antimicrob Agents Chemother. 1992;36:1208–1214. doi: 10.1128/aac.36.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274:151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 3.Bieman K. Mass spectrometry of peptides and proteins. Annu Rev Biochem. 1992;61:977–1010. doi: 10.1146/annurev.bi.61.070192.004553. [DOI] [PubMed] [Google Scholar]
- 4.Burns J L, Emerson J, Stapp J R, Yim D L, Drzewinski J, Louden L, Ramsey B W, Clausen C R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 5.Davis P M, Drumm M, Konstan M W. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 6.Denton M, Wilcox M H. Antimicrobial treatment of pulmonary colonization and infection by Pseudomonas aeruginosain cystic fibrosis patients. J Antimicrob Chemother. 1997;40:468–474. doi: 10.1093/jac/40.4.468. [DOI] [PubMed] [Google Scholar]
- 7.Frederiksen B, Lanng S, Koch C, Hoiby N. Improved survival in the Danish center-treated cystic fibrosis patients: results of aggressive treatment. Pediatr Pulmonol. 1996;21:153–158. doi: 10.1002/(SICI)1099-0496(199603)21:3<153::AID-PPUL1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Giwercman B, Lambert P A, Rosdahl V T, Shand G H, Hoiby N. Rapid emergence of resistance in Pseudomonas aeruginosain cystic fibrosis patients due to in vivo selection of stable partially derepressed β-lactamase-producing strains. Antimicrob Agents Chemother. 1990;26:247–259. doi: 10.1093/jac/26.2.247. [DOI] [PubMed] [Google Scholar]
- 9.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock R E. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E, Chapple D S. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock R E, Scott M G. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilman B C. Genetic and immunological aspects of cystic fibrosis. Ann Allergy Asthma Immunol. 1997;79:379–394. doi: 10.1016/S1081-1206(10)63031-1. [DOI] [PubMed] [Google Scholar]
- 14.Hunt B E, Weber A, Berger A, Ramsey B, Smith A L. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39:34–39. doi: 10.1128/aac.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffar-Bandjee M C, Lazdunski A, Bally M, Carrere J, Chazalette J P, Galabert C. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aerginosa. J Clin Microbiol. 1995;33:924–929. doi: 10.1128/jcm.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen T, Pedersen S S, Garne S, Heilmann C, Hoiby N, Koch C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosalung infection. J Antimicrob Chemother. 1987;19:831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 17.Konstan M W, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Konstan M W, Byard P J, Hoppel C L, Davis P B. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 19.Levy J, Smith A L, Kenny M A, Ramsey B, Schoenknecht F D. Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: comparison with activity in serum. J Infect Dis. 1983;148:1069–1076. doi: 10.1093/infdis/148.6.1069. [DOI] [PubMed] [Google Scholar]
- 20.MacKay B J, Denepitiya L, Iacono V J, Krost S B, Pollock J J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984;44:695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahenthiralingam E, Campbell M E, Speert D P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosaisolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiden M F, Tanner A, Macuch P J. Rapid characterization of periodontal bacterial isolates by using fluorogenic substrate tests. J Clin Microbiol. 1996;34:376–384. doi: 10.1128/jcm.34.2.376-384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendelman P M, Smith A L, Levy J, Weber A, Ramsey B, Davis R L. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis. 1985;132:761–765. doi: 10.1164/arrd.1985.132.4.761. [DOI] [PubMed] [Google Scholar]
- 24.Mickels N, McManus C, Massaro J, Friden P M, Braman V, D'Agostino R, Oppenheim F, Warbington M, Dibart S, Van Dyke T. Clinical and microbial evaluation of a histatin containing mouthrinse in humans with experimental gingivitis. J Clin Periodont. 2001;28:404–410. doi: 10.1034/j.1600-051x.2001.028005404.x. [DOI] [PubMed] [Google Scholar]
- 25.Mouton J W, den Hollander J G, Horrevorts A M. Emergence of antibiotic resistance amongst Pseudomonas aeruginosaisolates from patients with cystic fibrosis. J Antimicrob Chemother. 1993;31:919–926. doi: 10.1093/jac/31.6.919. [DOI] [PubMed] [Google Scholar]
- 26.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosain cystic fibrosis lung infection. Science. 2000;288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheim F G, Xu T, McMillian F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 28.Paquette D W, Waters G S, Stefanidou V L, Lawrence H P, Friden P M, O'Connor S M, Sperati J D, Oppenheim F G, Hutchens L H, Williams R C. Inhibition of experimental gingivitis in beagle dogs with topical salivary histatins. J Clin Periodontol. 1997;24:216–222. doi: 10.1111/j.1600-051x.1997.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen S S, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosain cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pier G B. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosainfections. Proc Natl Acad Sci USA. 2000;97:8822–8828. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raj P A, Edgerton M, Levine M J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 32.Raj P A, Soni S D, Levine M J. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J Biol Chem. 1994;269:9610–9619. [PubMed] [Google Scholar]
- 33.Ramphal R, Lhermitte M, Filliat M, Roussel P. The binding of antipseudomonal antibiotics to macromolecules from cystic fibrosis sputum. J Antimicrob Chemother. 1988;22:483–490. doi: 10.1093/jac/22.4.483. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey B W. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey B W, Dorkin H L, Eisenberg J D, Gibson R L, Harwood I R, Kravitz R M, Schidlow D V, Wilmott R W, Astley S J, McBurnie M A, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstein B J, Zeitlin P L. Cystic fibrosis. Lancet. 1998;351:277–282. doi: 10.1016/S0140-6736(97)09174-5. [DOI] [PubMed] [Google Scholar]
- 37.Rothstein D M, Spacciapoli P, Tran L T, Xu T, Buxton D, Roberts F D, Dalla Serra M, Oppenheim F G, Friden P. Anticandidal activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother. 2001;45:1367–1373. doi: 10.1128/AAC.45.5.1367-1373.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saiman L, Mehar F, Niu W W, Neu H C, Shaw K J, Miller G, Prince A. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosaisolated from patients with cystic fibrosis, including candidates for transplantation. Clin Infect Dis. 1996;23:532–537. doi: 10.1093/clinids/23.3.532. [DOI] [PubMed] [Google Scholar]
- 39.Smith A. Pathogenesis of bacterial bronchitis in cystic fibrosis. Pediatr Infect Dis J. 1997;16:91–96. doi: 10.1097/00006454-199701000-00030. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama K. Anti-lipopolysaccharide activity of histatins, peptides from human saliva. Experientia. 1993;49:1095–1097. doi: 10.1007/BF01929920. [DOI] [PubMed] [Google Scholar]
- 41.Szaff M, Hoiby N, Flensborg E W. Frequent antibiotic therapy improves survival of cystic fibrosis patients with chronic Pseudomonas aeruginosainfection. Acta Paediatr Scand. 1983;72:651–657. doi: 10.1111/j.1651-2227.1983.tb09789.x. [DOI] [PubMed] [Google Scholar]
- 42.Valerius N H, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338:725–726. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 43.von Heijne G, Manoil C. Membrane proteins: from sequence to structure. Protein Eng. 1990;4:109–112. doi: 10.1093/protein/4.2.109. [DOI] [PubMed] [Google Scholar]
- 44.Welsh M J, Ramsey B W. Research on cystic fibrosis: a journey from the heart house. Am J Respir Crit Care Med. 1998;147:S148–S154. doi: 10.1164/ajrccm.157.4.nhlbi-13. [DOI] [PubMed] [Google Scholar]