Abstract
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors are effective agents in lowering cholesterol and triglycerides and are being used by human immunodeficiency virus-positive patients to treat the lipid elevation that may be associated with antiretroviral therapy. Many HMG-CoA reductase inhibitors and protease inhibitors are metabolized by the same cytochrome P450 enzyme 3A4 (CYP3A4). In addition, many protease inhibitors are potent inhibitors of CYP3A4. Therefore, coadministration of these two classes of drugs may cause significant drug interactions. This open-label, multiple-dose study was performed to determine the interactions between nelfinavir, a protease inhibitor, and two HMG-CoA reductase inhibitors, atorvastatin and simvastatin, in healthy volunteers. Thirty-two healthy subjects received either atorvastatin calcium (10 mg once a day) or simvastatin (20 mg once a day) for the first 14 days of the study. Nelfinavir (1,250 mg twice a day) was added on days 15 to 28. Pharmacokinetic assessment was performed on days 14 and 28. The study drugs were well tolerated. Nelfinavir increased the steady-state area under the plasma concentration-time curve during one dosing period (AUCτ) of atorvastatin 74% and the maximum concentration (Cmax) of atorvastatin 122% and increased the AUCτ of simvastatin 505% and the Cmax of simvastatin 517%. Neither atorvastatin nor simvastatin appeared to alter the pharmacokinetics of nelfinavir. It is recommended that coadministration of simvastatin with nelfinavir should be avoided, whereas atorvastatin should be used with nelfinavir with caution.
The combination of a human immunodeficiency virus (HIV) protease inhibitor or a nonnucleoside reverse transcriptase inhibitor with two nucleoside reverse transcriptase inhibitors, so-called highly active antiretroviral therapy, is the recommended treatment for HIV-positive patients (6, 9, 22). The adoption of highly active antiretroviral therapy has reduced the mortality and morbidity of HIV-positive patients dramatically (14, 19). However, with the long-term administration of these agents, metabolic changes such as hyperglycemia (2, 8), hyperlipidemia (M. J. Jimenez-Exposito, A. Paul, A Laville, and L. Masana, Abstr. 6th Eur. Conf. Clin. Aspect Treatment HIV Infect., 1997; package insert for Sustiva [efavirenz]), and lipodystrophy (13, 21) have been observed in HIV-positive patients. The etiology of these changes is poorly understood. However, medications are prescribed to treat these metabolic changes because of concerns about the long-term cardiovascular risks. Lipid-lowering agents, especially HMG-CoA reductase inhibitors (15), are being used to reduce cholesterol and triglycerides in HIV-positive patients.
Many HMG-CoA reductase inhibitors are metabolized by the liver cytochrome CYP3A4 enzyme. Coadministration of HMG-CoA reductase inhibitors with CYP3A4 inhibitors/substrates such as itraconazole (16, 17) and cyclosporine (1, 11) significantly increases the levels of HMG-CoA reductase inhibitors in plasma. Simvastatin and lovastatin appear to be especially sensitive to P450 3A4 inhibition. For example, itraconazole increases the mean peak concentration and area under the concentration-time curve (AUC) of simvastatin and lovastatin approximately fivefold but increased the AUC of atorvastatin only 60% (16, 17). The greatly elevated concentrations of simvastatin and lovastatin caused by itraconazole or other CYP3A4 inhibitors may increase the risk of rhabdomyolysis (18, 20; R. S. Lees and A. M. Lees, Letter, N. Engl. J. Med. 333:664–665, 1995).
All of the HIV protease inhibitors that are commercially available are metabolized by CYP3A4 and are inhibitors of CYP3A4. The dual protease inhibitor combination of saquinavir plus ritonavir has been shown to increase the concentration of simvastatin and atorvastatin (C. Fichtenbaum, J. Gerber, S. Rosenkranz et al., Abstr. 7th Conf. Retrovir. Opportunistic Infect., abstr LB 6, 2000). Nelfinavir, like the other protease inhibitors, is an inhibitor of CYP3A4, although it is not as strong a CYP3A4 inhibitor as ritonavir. Nelfinavir is a widely prescribed protease inhibitor. It is therefore important to study the interaction of nelfinavir and HMG-CoA reductase inhibitors. Two HMG-CoA reductase inhibitors were chosen for this study. Atorvastatin was studied because it is the most prescribed HMG-CoA reductase inhibitor, and simvastatin was studied because it is particularly susceptible to CYP3A4 inhibition.
MATERIALS AND METHODS
Study design.
This was an open-label, sequential, multiple-dose, single-center study to determine the pharmacokinetic interaction between nelfinavir and atorvastatin or nelfinavir and simvastatin in healthy volunteers. Thirty-two subjects were enrolled in the study. The study was performed at Phoenix International Life Sciences, Inc. (Neptune, N.J.).
Inclusion and exclusion criteria.
Healthy male or female subjects 18 to 55 years old were enrolled after the Investigation Review Board approved the study protocol and subjects signed the informed consent form. Subjects were excluded if they took medications that might affect CYP3A4 activities, took alcohol or illegal drugs or smoked during the study, or were positive for HIV, hepatitis B, or hepatitis C.
Treatment.
Subjects were divided into two groups. The two groups were roughly matched for gender, race, and age. The treatments were as follows.
Treatment group 1.
In period 1, subjects received atorvastatin calcium (Lipitor; 10 mg once a day [QD]) in the morning for the first 14 days of the study. In period 2, subjects received atorvastatin calcium (10 mg QD) plus nelfinavir (Viracept; 1,250 mg twice a day [BID]) for an additional 14 days (days 15 to 28). All atorvastatin and nelfinavir doses were taken with food.
Treatment group 2.
In period 1, subjects received simvastatin (Zocor; 20 mg QD) in the morning for the first 14 days of the study. In period 2, subjects received simvastatin (20 mg QD) plus nelfinavir (1,250 mg BID) for an additional 14 days (days 15 to 28). All simvastatin and nelfinavir doses were taken with food.
Pharmacokinetic sample collection. (i) Atorvastatin and simvastatin sample collections
Trough samples (7 ml) were collected prior to dosing on days 1, 5, 10, 20, and 25. On days 14 and 28, serial blood samples (7 ml) were collected predose and at 1, 2, 3, 4, 5, 6, 8, 10, 12, 18, and 24 h postdose.
(ii) Nelfinavir sample collections
Trough samples (7 ml) were collected prior to initiation of nelfinavir on day 14 and prior to dosing on days 20 and 25. Serial blood samples (7 ml) were collected predose and at 1, 2, 3, 4, 5, 6, 8, 10, and 12 h postdose.
Bioanalytical procedures. (i) Nelfinavir and AG1402.
Concentrations of nelfinavir and its active metabolite AG1402, previous named M8 (25), in plasma were measured by a validated reverse-phase high-performance liquid chromatography method with UV detection at PPD Development Inc. (Richmond, Va.). Plasma samples (0.25 ml) containing various concentrations of nelfinavir and AG1402 were mixed with an internal standard [6,7-dimethyl-2,3-di(2-pydridyl)-quinoxaline] solution (0.25 ml). Each sample was mixed with 0.5 ml of 0.1 N ammonium hydroxide (pH 10.5) and then extracted by 2.0 ml of ethyl acetate/acetonitrile (9:1 ratio) mixture. The mixture was centrifuged for 5 min at 1,000 × g, and the organic layer was transferred to a new tube and evaporated to dryness under a nitrogen stream at 50°C. The residue was reconstituted with 0.15 ml of mobile phase (25 mM sodium phosphate, monobasic pH 3.4, acetonitrile-methanol; 60:32.5:7.5 by volume) and subjected to high-performance liquid chromatography. Separation was achieved on a Waters symmetry C18 column at 30°C with a flow rate of 1.5 ml and detection of nelfinavir and AG1402 via UV detection at 220 nm. The typical elution times of nelfinavir, AG1402, and the internal standard were 14.5, 6, and 11 min, respectively. This method was validated within a concentration range of 0.05 to 10.0 μg/ml for nelfinavir and AG1402. The standard curves were linear with R2 values of >0.997. Nelfinavir and AG1402 were stable (<20% loss from the baseline) for at least 21 months when stored at −20°C. The precision (percent coefficient of variation) of the assay for nelfinavir and AG1402 was ≤7.2 and 6.6%, respectively. The accuracy (deviation from the nominal concentrations) of the assay for nelfinavir and AG1402 was −16.0 to −6.6% and −5.9 to −1.7%, respectively.
(ii) Atorvastatin and simvastatin.
Plasma samples were analyzed for atorvastatin equivalent concentrations (as the total of atorvastatin acid and its active metabolites) or simvastatin equivalent concentrations (as the total of simvastatin acid and its active metabolites) by a validated enzyme inhibition assay for HMG-CoA reductase inhibitors (7) at Phoenix International Life Sciences, Inc. (Saint-Laurent, Quebec, Canada). Atorvastatin or simvastatin was isolated from 0.25 ml of human plasma by protein precipitation with acetonitrile/acetone (95:5, vol/vol). The atorvastatin or simvastatin equivalent concentration was estimated by inhibition of the production of [14C]mevalone from [14C]HMG-CoA in the presence of HMG-CoA reductase from rat liver microsomes and cofactor. Since it was an enzyme inhibition assay, all of the atorvastatin or simvastatin and its active metabolites in plasma capable of inhibiting HMG-CoA reductase was quantified. Therefore, atorvastatin or simvastatin concentrations were expressed as atorvastatin or simvastatin equivalents. The standard curves were linear with R2 values of >0.997 for both atorvastatin and simvastatin. This method was validated within concentration ranges of 0.18 to 7.35 ng/ml for atorvastatin and 0.3 to 15.2 ng/ml for simvastatin. The precision levels (percent coefficient of variation) of the assay for atorvastatin and simvastatin were ≤11.3 and 25.4%, respectively. The accuracy levels (deviation from the nominal concentrations) of the assay for atorvastatin and simvastatin were within 0.1 to 3.7% and −6.4 to 3.4%, respectively.
Safety evaluations.
Adverse events and changes from the baseline laboratory parameters were monitored and evaluated. Metabolic laboratory parameters, including total cholesterol, low-density lipoproteins (LDL), triglycerides, glucose, insulin, and C-peptide, were determined after an overnight fast on screening, before first dosing, and on days 14 and 28.
Statistical analysis.
Standard noncompartmental methods were used to calculate pharmacokinetic parameters. The geometric mean and its associated 95% confidence interval (CI) of the steady-state AUC during one dosing period (AUCτ) were calculated (time zero to 24 h postdose for atorvastatin and simvastatin and time zero to 12 h postdose for nelfinavir and AG1402). The geometric mean and its associated 95% CI of the Cmax were calculated for atorvastatin, simvastatin, and nelfinavir. The ratio and its associated 90% CI of the geometric mean of the AUCτ and Cmax of atorvastatin and simvastatin in the presence and absence of nelfinavir were also calculated. Analysis of variance was used to compare logarithmically transformed parameter values for atorvastatin and simvastatin in the absence and presence of nelfinavir, with the exception of Tmax, which was analyzed by a nonparametric method. Pharmacokinetic parameters for nelfinavir and AG1402 were compared to results from previous studies with healthy volunteers who had taken nelfinavir (1,250 mg BID) for at least 10 days. Mean baseline values and percent changes in total cholesterol, LDL, and triglycerides on days 14 and 28 were calculated for each group.
RESULTS
Demographic and baseline characteristics.
The 32 enrolled subjects consisted of 12 Caucasians (six males and six females), 15 African Americans (eight males and seven females), and five Hispanics (two males and three females). One Caucasian male in treatment group 1 (atorvastatin and nelfinavir) developed a rash, requested withdrawal from the study, and was not included in the pharmacokinetic analysis. The mean (standard deviation [SD]) ages of the atorvastatin and simvastatin groups were 31.5 (5.0) and 38.3 (9.7) years, respectively. The mean (SD) body weights of the atorvastatin and simvastatin groups were 74 (12) and 72 (9) kg, respectively.
Pharmacokinetics.
Mean (SD) trough atorvastatin equivalent concentrations on days 1, 5, 10, 14, 20, 25, and 28 were 0 (0.1), 1.5 (0.9), 2.4 (2.2), 2.0 (1.5), 2.2 (1.8), 3.7 (6.2), and 3.3 (3.3) ng-eq/ml, respectively. Mean (SD) trough simvastatin equivalent concentrations on days 1, 5, 10, 14, 20, 25, and 28 were 0 (0), 0.4 (0.3), 0.3 (0.3), 0.3 (0.4), 2.5 (2.9), 3.3 (8.7), and 2.3 (2.7) ng-eq/ml. These results indicated that the steady state of atorvastatin and simvastatin was reached on day 5 in period 1 and between days 20 to 25 in period 2. Mean trough concentrations of nelfinavir on days 15, 20, 25, and 28 were 0 (0), 2.4 (1.9), 3.0 (2.3), and 3.4 (2.0) ng-eq/ml, indicating that the steady state of nelfinavir was reached between days 20 and 35.
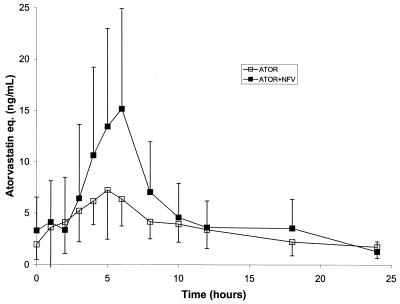
The mean (SD) concentration-time profiles of atorvastatin in the absence and presence of nelfinavir are presented in Fig. 1. Following the 14 days of atorvastatin dosing at 10 mg QD, the geometric mean AUCτ and Cmax for atorvastatin equivalents (sum of atorvastatin acid and active metabolites) were 77 ng-eq·h/ml and 7.4 ng-eq/ml, respectively (Table 1). After the addition of nelfinavir at 1,250 mg BID to the atorvastatin regimen, the geometric mean steady-state AUCτ and Cmax for atorvastatin equivalents were 134 ng-eq·h/ml and 16.4 ng-eq/ml, respectively (Table 1). These results represent increases of 74% in the AUCτ and 122% in the Cmax after the addition of nelfinavir.
FIG. 1.
Mean (SD) plasma concentration-time profiles of atorvastatin (ATOR) in the absence and presence of nelfinavir (NFV). eq., equivalents.
TABLE 1.
Pharmacokinetic parameters of atorvastatin and simvastatina
| Treatment | Cmax (ng-eq/ml) | Tmax (h) | AUC0–24 (ng-eq · h/ml) |
|---|---|---|---|
| Atorvastatin | 7.4 (5.8–9.4) | 5.0 (1.0–6.0) | 77 (64–93) |
| Atorvastatin + nelfinavir | 16.4 (12.9–20.9) | 5.0 (3.0–8.0) | 134 (112–161) |
| Ratio | 2.22 (1.68–2.93) | 1.74 (1.41–2.16) | |
| Simvastatin | 7.4 (5.8–9.4) | 3.0 (1.0–6.0) | 42 (35–50) |
| Simvastatin + nelfinavir | 45.7 (36.0–57.9) | 4.0 (2.0–6.0) | 255 (214–304) |
| Ratio | 6.17 (4.67–8.15) | 6.05 (4.93–7.43) |
Atorvastatin or simvastatin was measured as the sum of all HMG-CoA inhibitors, including statin acid and its active metabolites. Data are the geometric mean (95% CI) Cmax and AUC from time zero to 24 h. (AUC0–24) and the median (range) Tmax.
The ratios of Cmax and AUC0–24 are geometric mean ratios (90% CIs).
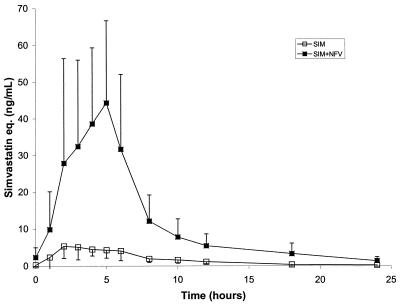
The mean (SD) concentration-time profiles of simvastatin in the absence and presence of nelfinavir are presented in Fig. 2. Following the 14 days of simvastatin dosing at 20 mg QD, the geometric mean AUCτ and Cmax for simvastatin equivalents (sum of simvastatin acid and active metabolites) were 42 ng-eq·h/ml and 7.4 ng-eq/ml, respectively (Table 1). After the addition of nelfinavir at 1,250 mg BID to the simvastatin regimen, the geometric mean AUCτ and Cmax for simvastatin equivalents were 255 ng-eq·h/ml and 45.7 ng-eq/ml, respectively (Table 1). These results represent increases of 505% in the AUCτ and 517% in the Cmax of simvastatin after the addition of nelfinavir.
FIG. 2.
Mean (SD) plasma concentration-time profiles of simvastatin (SIM) in the absence and presence of nelfinavir (NFV). eq., equivalents.
The geometric means of the AUCτ (time zero to 12 h postdose) and Cmax of nelfinavir in the presence of atorvastatin were 44 (95% CI, 35 to 54) μg·h/ml and 5.7 (95% CI, 4.8 to 6.8) μg/ml, respectively. The geometric mean of the AUCτ and Cmax of nelfinavir in the presence of simvastatin were 38 (range, 31 to 47) μg·h/ml and 5.1 (95% CI, 4.3 to 6.1) μg/ml, respectively. The median Tmax values of nelfinavir in the presence of atorvastatin and simvastatin were 5.0 (range, 2.0 to 8.0) and 5.0 (range, 2.0 to 6.0) h, respectively. The geometric mean ratios of the AG1402 AUCτ to the nelfinavir AUCτ were 0.35 (95% CI, 0.25 to 0.48) and 0.31 (95% CI, 0.22 to 0.42) in the presence of atorvastatin and simvastatin, respectively. There was no difference in the AUCτ, the Cmax, the Tmax, or the ratio of the AG1402 AUCτ to the nelfinavir AUCτ between these two groups (P > 0.05).
Safety.
Nelfinavir in combination with atorvastatin or simvastatin was well tolerated in this study. There were no serious adverse events. Twenty-seven subjects reported 64 adverse events. Thirty-eight (reported by 22 subjects) of the 64 adverse events were considered treatment-related adverse events, most related to nelfinavir treatment. The most frequently reported adverse event was diarrhea (reported by 17 subjects [53%]). Two grade 3 treatment-related adverse events (rash and migraine headache) were reported. Subject 3 had a severe rash on day 23 and withdrew from the study. Subject 3 was treated with Benadryl and prednisone orally plus Kenalog intramuscularly. The rash resolved after 13 days. Subject 14 experienced a severe migraine headache, which was treated with Tylenol and resolved after 2 days. Seven subjects received medications other than the study drugs. None of the medications were expected to have drug interactions with the study drugs.
Fasting cholesterol and LDL levels were significantly reduced in both arms on days 14 and 28 (Table 2). Triglyceride levels were very variable, with the standard deviations typically greater than the mean values. Thus, the change from the baseline could not be assessed with confidence. Other metabolic parameters, including glucose, insulin, and C-peptide, were generally within the normal range and showed no consistent change from the baseline. No consistently elevated creatine phosphokinase (CPK) values, an early indicator of rhabdomyolysis, were observed. None of the elevated CPK values were greater than threefold higher than the upper limit of the normal range. Four subjects had four CPK values between two- and threefold higher than the upper limit of the normal range. Three of these high CPK values were baseline values, suggesting that these high CPK values were not treatment related. CPK values tended to stay the same or become lower during the study period.
TABLE 2.
Percent changes in lipid levels from baselinea
| Treatment | Total cholesterol | LDL | Triglycerides |
|---|---|---|---|
| Atorvastatin | −29 ± 9b | −39 ± 12b | −30 ± 26b |
| Atorvastatin + nelfinavir | −18 ± 10b | −28 ± 15b | 21 ± 42b |
| Simvastatin | −25 ± 6b | −36 ± 7b | −16 ± 33b |
| Simvastatin + nelfinavir | −16 ± 11b | −24 ± 14b | 7 ± 45 |
Data are the mean percentage ± SD.
Significantly different from the baseline value (P < 0.05).
DISCUSSION
HIV-positive patients usually take multiple medications, and therefore, drug interaction between these medications is an important issue for antiretroviral therapies. Many of these drugs can interact with each other in drug absorption, distribution, metabolism, and elimination. To achieve optimal dosing regimens for HIV-positive patients, it is important to understand the drug interaction between the medications. In this study, we investigated the interaction between nelfinavir and two HMG-CoA reductase inhibitors, simvastatin and atorvastatin, because of the potential for drug interaction and the potential of the coadministration of these drugs. Both simvastatin and atorvastatin are extensively metabolized by humans, and a number of active and inactive metabolites are produced. The active metabolites account for most of the activity of atorvastatin and simvastatin. The enzyme inhibition assay used in this study measured the total active HMG-CoA reductase inhibitors.
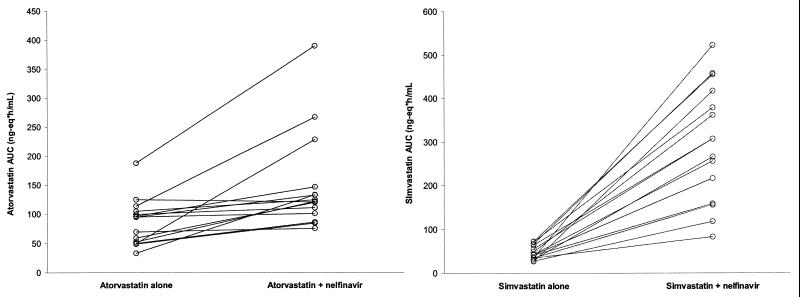
The results of this study indicate that administration of 1,250 mg of nelfinavir BID increases the steady-state simvastatin equivalent concentrations extensively and increases the steady-state atorvastatin equivalent concentrations moderately. The geometric mean AUCτ and Cmax of simvastatin-associated HMG-CoA inhibitors increased 505 and 517%, respectively, with the coadministration of nelfinavir. All of the subjects in the simvastatin arm showed an increase in the simvastatin equivalent AUCτ in the presence of nelfinavir, with a range of 145 to 1,524% (Fig. 3), suggesting a consistent inhibitory effect of nelfinavir on simvastatin metabolism. Two subjects had a greater than 10-fold increase in the simvastatin AUC, and they also had the greatest nelfinavir AUCs (78 and 104 μg·h/ml, respectively), suggesting that the magnitude of the increase in simvastatin levels was related to the levels of nelfinavir in plasma. Linear regression of the simvastatin AUC ratio (the ratio of the AUC of simvastatin plus nelfinavir to that of simvastatin alone) versus the nelfinavir AUC in the individual subjects also demonstrated a high correlation between the simvastatin AUC ratio and the nelfinavir AUC with an R2 value of 0.81. Other factors, such as the simvastatin AUC in the absence of nelfinavir, gender, and race, were not related to the simvastatin AUC ratio. These results suggested that the nelfinavir level was a major determinant of the change in simvastatin levels. In contrast, the geometric mean AUCτ and Cmax of atorvastatin-associated HMG-CoA inhibitors increased only 74 and 122%, respectively, with the coadministration of nelfinavir. Fourteen of the 15 subjects in the atorvastatin arm showed an increase in the AUCτ of atorvastatin equivalents in the presence of nelfinavir, with a range of −3 to 361% (Fig. 3), suggesting a fairly consistent inhibitory effect of nelfinavir on atorvastatin metabolism. Two subjects had a greater-than-threefold increase in atorvastatin. However, the AUCs of nelfinavir (42 and 60 μg·h/ml, respectively) in these two subjects were not particularly high, suggesting that the magnitude of the increase in atorvastatin levels was not related to the levels of nelfinavir in plasma. Factors such as the nelfinavir AUC, the atorvastatin AUC in the absence of nelfinavir, gender, and race were not related to the atorvastatin AUC ratio. The difference in the inhibitory effects of nelfinavir on simvastatin and atorvastatin was not unexpected. Simvastatin appears to be very sensitive to CYP3A4 inhibitors. Itraconazole, a classical CYP3A4 inhibitor, also increased the AUC and Cmax of simvastatin equivalents markedly, whereas itraconazole had only a moderate effect on atorvastatin equivalents (16, 17). This difference may be explained by the different roles of CYP3A4 in their biotransformation (3).
FIG. 3.
(Left) Comparative plot of atorvastatin AUCτ values in the absence and presence of nelfinavir. (Right) Comparative plot of simvastatin AUCτ values in the absence and presence of nelfinavir
The interaction between nelfinavir and simvastatin or atorvastatin is likely due mainly to the inhibition of CYP3A4 by nelfinavir since the in vitro and in vivo drug metabolism of these compounds is well established. Inhibition of CYP3A4 by nelfinavir could occur at the gastrointestinal wall, which would have a major effect on the bioavailability of simvastatin and atorvastatin, and/or at the liver, which would have an effect on the bioavailability and elimination rate of simvastatin and atorvastatin. In addition, a number of in vitro (4, 10, 23, 24) and in vivo (5, 10) studies have suggested that nelfinavir, atorvastatin, and simvastatin may also be substrates/inhibitors of the efflux pump P-glycoprotein. Inhibition of P-glycoprotein by nelfinavir may increase the oral absorption of atorvastatin and simvastatin and contribute to the observed clinical interaction. However, it is difficult to distinguish these mechanisms from this study, as it was designed to detect pharmacokinetic changes and not mechanisms of interaction.
The active site of action of HMG-CoA reductase inhibitors is the liver. The concentrations of orally administered drugs in the liver tended to be much higher than their concentrations in plasma, especially during the absorption period. The increase in the systemic concentrations of atorvastatin and simvastatin caused by the coadministration of nelfinavir may not reflect the change in drug concentrations in the liver. It was interesting to observe similar cholesterol-lowering effects of atorvastatin and simvastatin in the presence and absence of nelfinavir (Table 2) despite a significant increase in the systemic exposure of atorvastatin and simvastatin. These results may suggest different effects of nelfinavir on the systemic and liver pharmacokinetics of atorvastatin and simvastatin.
No significant CPK elevation or rhabdomyolysis was observed in this small, short-term study. The safety results of this study, while reassuring, are difficult to extrapolate to chronic coadministration of atorvastatin or simvastatin with nelfinavir in HIV-positive patients. The greater-than-500% increase in the simvastatin concentration in the presence of nelfinavir was quite extensive and may increase the risk of skeletal muscle damage. Therefore, it is recommended that simvastatin not be coadministered with nelfinavir. Nelfinavir had a more moderate effect on atorvastatin concentrations. The recommended doses of atorvastatin are 10 to 80 mg/day. Thus, the 10-mg dose of atorvastatin should be started and titrated with caution to achieve the desired effect when coadministered with nelfinavir.
In previous studies, the AUCτ and Cmax of nelfinavir after administration of 1,250 mg BID to healthy volunteers ranged from 25 to 40 μg·h/ml and from 4 to 6 μg/ml (Agouron database), respectively, and the ratio of the AG1402 AUCτ to the nelfinavir AUCτ ranged from 0.2 to 0.4. The pharmacokinetic parameters of nelfinavir and AG1402 in this study were similar to those in a previous studies with healthy volunteers, suggesting that atorvastatin and simvastatin did not alter the pharmacokinetics of nelfinavir and its active metabolite. These results were not unexpected, as atorvastatin and simvastatin are weak CYP3A4 inhibitors and their concentrations were much lower than those of nelfinavir and AG1402.
In conclusion, a clinical dose of nelfinavir (1,250 mg BID) increased systemic exposure to simvastatin approximately 500% and is not recommended for long-term coadministration with simvastatin. Nelfinavir also increased systemic exposure to atorvastatin moderately and should be coadministered with atorvastatin with caution.
ACKNOWLEDGMENTS
We thank Gary Hutton, Statistical Associates, Inc., for excellent statistical analysis of the data and the Pfizer Atorvastatin Team for valuable suggestions for the study.
REFERENCES
- 1.Arnadottir M, Eriksson L O, Thysell H, Karkas J D. Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron. 1993;65:410–413. doi: 10.1159/000187521. [DOI] [PubMed] [Google Scholar]
- 2.Ault A. FDA warns of potential protease-inhibitor link to hyperglycaemia. Lancet. 1997;349:1819. doi: 10.1016/S0140-6736(05)61703-5. [DOI] [PubMed] [Google Scholar]
- 3.Bertz R J, Granneman G R. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bogman K, Peyer A K, Torok M, Kusters E, Drewe J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br J Pharmacol. 2001;132:1183–1192. doi: 10.1038/sj.bjp.0703920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd R A, Stern R H, Stewart B H, Wu X, Reyner E L, Zegarac E A, Randinitis E, Whitfield L. Atorvastatin coadministration may increase digoxin concentrations: inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40:91–98. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV-1 infection in 1998: updated recommendations of the International AIDS Society—USA Panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 7.Cilla D D, Whifield L R, Gibson D M, Sedman A J, Posvar E. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy volunteers. Clin Pharmacol Ther. 1996;60:687–695. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- 8.Eastone J A, Decker C F. New-onset diabetes mellitus associated with use of protease inhibitor. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00017. [DOI] [PubMed] [Google Scholar]
- 9.Gulick R M, Mellors J W, Havlir, D. D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine and lamivudine in adults with human immunodeficiency virus and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 10.Kim R B, Fromm M F, Wandel C, Leake B, Wood A J J, Roden D M. The drug transporter P-glycoprotein limits oral absorption and brain entrance of HIV protease inhibitors. J Clin Investig. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliem V, Wanner C, Eisenhauer, T. T, Olbricht C J, Doll R, Boddaert M, O'Grady P, Krekler M, Mangold B, Christians U. Comparison of pravastatin and lovastatin in renal transplant patients receiving cyclosporine. Transplant Proc. 1996;28:3126–3128. [PubMed] [Google Scholar]
- 12.Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Clin Pharmacokinet. 1997;32:403–425. doi: 10.2165/00003088-199732050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Miller K D, Jone E, Yanovski J A, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 14.Mocroft A, Vella S, Benfield T L, Chiesi A, Miller V, Gargalianos P, d'Arminio Monforte A, Yust I, Bruun J N, Phillips A N, Lundgren J D. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 15.Murillas J, Martin T, Ramos A, Portero J L. Atorvastatin for protease inhibitor-related hyperlipidaemia. AIDS. 1999;13:1424–1425. doi: 10.1097/00002030-199907300-00030. [DOI] [PubMed] [Google Scholar]
- 16.Neuvonen P J, Jalava K M. Itraconazole drastically increases plasma concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1996;60:54–61. doi: 10.1016/S0009-9236(96)90167-8. [DOI] [PubMed] [Google Scholar]
- 17.Neuvonen P J, Kantola T, Kivisto K T. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther. 1998;63:332–341. doi: 10.1016/S0009-9236(98)90165-5. [DOI] [PubMed] [Google Scholar]
- 18.Norman D J, Illingwoth D R, Munson J, Housenpud J. Myolysis and acute renal failure in a heart-transplant patient receiving lovastatin. N Engl J Med. 1988;318:46–47. doi: 10.1056/NEJM198801073180110. [DOI] [PubMed] [Google Scholar]
- 19.Palella F J, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 20.Segaert M F, De Soete C, Vandewiele I, Verbanck J. Drug interaction-induced rhabdomyolysis. Nephrol Dial Transplant. 1996;11:1846–1847. [PubMed] [Google Scholar]
- 21.Shaw A J, McLean K A, Evans B A. Disorder of fat distribution in HIV infection. Int J STD AIDS. 1998;9:595–599. doi: 10.1258/0956462981921189. [DOI] [PubMed] [Google Scholar]
- 22.Torres R A, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531–1532. doi: 10.1056/nejm199705223362118. [DOI] [PubMed] [Google Scholar]
- 23.Wang E, Casciano C N, Clement R P, Johnson W W. HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharmacol Sci. 2001;18:800–806. doi: 10.1023/a:1011036428972. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Whitfield L R, Stewart B H. Atorvastatin transport in Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharmacol Sci. 2000;17:209–215. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K F, Wu E, Patick A K, Kerr B, Zorbas M, Lankford A, Kobayashi T, Maeda Y, Shetty B, Webber S. Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma, and antiviral activities. Antimicrob Agents Chemother. 2001;45:1086–1093. doi: 10.1128/AAC.45.4.1086-1093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]