Abstract
The effects of Klebsiella pneumoniae endotoxin on the biliary excretion and renal handling of rhodamine-123 were investigated in rats at different times after intraperitoneal injection (1 mg/kg of body weight). The typical substrates for P glycoprotein, i.e., cyclosporine, colchicine, and erythromycin, inhibited the biliary clearance of rhodamine-123, whereas a substrate for organic cation transporter, cimetidine, did not inhibit clearance, suggesting that rhodamine-123 is transported mainly by P glycoprotein. The biliary, renal, and tubular secretory clearances of rhodamine-123 and the glomerular filtration rate significantly decreased 6 h after injection of endotoxin but returned to control levels by 24 h. These results suggest that endotoxin-induced decreases in P-glycoprotein-mediated biliary excretion and renal handling of rhodamine-123 were probably due to impairment of P-glycoprotein-mediated transport ability. Pretreatment with pentoxifylline (50 mg/kg) significantly inhibited endotoxin-induced increases in tumor necrosis factor alpha (TNF-α) levels in plasma, which ameliorated the endotoxin-induced reduction of the biliary excretion of rhodamine-123. It is likely that endotoxin-induced impairment of the transport of rhodamine-123 is caused, in part, by overproduction of TNF-α. The effect of endotoxin on the expression of P-glycoprotein mRNA in liver and kidneys of rats was investigated by using a reverse transcriptase PCR. The expression of Mdr1a mRNA in both liver and kidney decreased 6 h after endotoxin injection and returned to control levels after 24 h, whereas the expression of Mdr1b mRNA in liver increased at both times and that in kidney decreased at 24 h. These findings suggest that K. pneumoniae endotoxin dramatically decreases P-glycoprotein-mediated biliary and renal excretion of rhodamine-123 probably by decreasing the expression of Mdr1a, which is likely due to increased plasma TNF-α levels.
Endotoxin, which is an active component of the cell wall of gram-negative bacteria, is well known to induce damage of numerous organs, including the liver and kidney. The liver and kidney have crucial functions for the elimination of endogenous and exogenous substances, which are converted to more hydrophilic compounds by cytochrome P450 and/or conjugating enzyme and then are excreted into the bile or urine. A series of studies suggested that Klebsiella pneumoniae endotoxin reduces biliary and renal excretion of various organic anionic drugs as a result of changes in the biliary and renal tubular secretory systems (9, 10, 21–24). We recently found that K. pneumoniae endotoxin reduces hepatic cytochrome P450-dependent drug-metabolizing enzymes 24 h after intraperitoneal injection in rats, due to the overproduction of nitric oxide in plasma (17, 26). However, less is known about the effect of endotoxin treatment on hepatobiliary and renal transport systems.
It is well known that hepatobiliary excretion of organic anionic drugs is mediated by a hepatocanalicular multispecific organic anion transporter (cMOAT), which belongs to the ATP-binding cassette transporter superfamily (14). Another known transporter, P glycoprotein, acts as an ATP-dependent efflux pump for various drugs, such as vinca alkaloid and anthracycline anticancer drugs, calcium blockers, immunosuppressive agents, and macrolide antibiotics (37, 38, 42), in normal tissues, such as the brain, liver, intestine and kidney (7, 12, 15, 31, 35). Interestingly, there is evidence that the substrate specificities of P glycoprotein, cytochrome P450 3A4 (CYP3A4), and cMOAT overlap, and these proteins are located at hepatocytes and have similar functions for removing various drugs from the body (16, 20, 29, 41). On the basis of these observations, K. pneumoniae endotoxin might modify the pharmacokinetics of drugs mediated by P glycoprotein. However, there is little information regarding the effect of K. pneumoniae endotoxin on P-glycoprotein-mediated biliary and renal transport systems and the expression of P-glycoprotein transport genes.
The aim of the present study was to clarify whether K. pneumoniae endotoxin modifies P-glycoprotein-mediated biliary and renal transport systems in rats. Rhodamine-123 was chosen as the model drug, since this compound is primarily excreted into the bile and urine in an unchanged form (18). In order to evaluate the contribution of P-glycoprotein-mediated transport to the hepatobiliary and renal excretion of rhodamine-123, we used reverse transcriptase PCR (RT-PCR) to measure the expression of mRNA of the multidrug resistance proteins 1a and 1b (Mdr1a and Mdr1b), which are members of the P-glycoprotein subfamily, isolated from the liver and kidney at different times after intraperitoneal injection of endotoxin.
MATERIALS AND METHODS
Materials.
Endotoxin was isolated from K. pneumoniae LEN-1 (O3:K1−), which was identical to that used in previous studies (10, 17, 26). Rhodamine-123 was purchased from Kanto Kagaku (Tokyo, Japan). Doxorubicin hydrochloride in the form of a commercial preparation for injection (adriamycin) was obtained from Kyowa Hakko Kogyo (Tokyo, Japan). Erythromycin lactobionic acid in the form of a commercial preparation for injection was purchased from Abbot Laboratories (North Chicago, Ill.). Cyclosporine was obtained from Novartis Pharmaceutical Co. Ltd. (Tokyo, Japan). Inulin and cimetidine were purchased from Nacalai Tesque (Kyoto, Japan). Colchicine was purchased from Wako Pure Chemical Industries (Osaka, Japan). Pentoxifylline was synthesized in our laboratory and was identical to that previously described (8). All other reagents are commercially available and were of analytical grade. For in vivo experiments, rhodamine-123 was dissolved in physiological saline or 4% mannitol solution containing 1% inulin in order to determine the glomerular filtration rate (GFR), and other drugs were dissolved in physiological saline before use.
Animals and experimental protocols.
Eight- to nine-week-old male Wistar rats weighing 280 to 300 g (Japan SLC, Hamamatsu, Japan) were used for all experiments. The rats were housed under controlled environmental conditions (temperature, 23 ± 1°C; humidity, 55% ± 5%) with a commercial food diet and water freely available. All animal experiments were carried out according to Nagoya University School of Medicine guidelines for the care and use of laboratory animals.
For rhodamine-123 clearance experiments, control rats and rats treated 6 or 24 h earlier with an intraperitoneal injection of K. pneumoniae endotoxin (1 mg/kg of body weight) were anesthetized by pentobarbital (25 mg/kg) and were cannulated with polyethylene tubes in the right jugular vein for drug administration, the left jugular artery for blood collection, and the femoral vein for drug infusion. The dose of endotoxin used in this study was chosen on the basis of previous studies (17, 26). The urinary bladder and bile duct were also cannulated for urine and bile collection. All experiments were done under pentobarbital anesthesia, and body temperature was maintained at 37°C with a heat lamp.
To elucidate the effect of endotoxin on steady-state biliary excretion and renal handling of rhodamine-123, the rats received a bolus intravenous injection of rhodamine-123 and inulin in a loading dose of 85 μg/kg and 10 mg/kg followed by constant-rate infusion (PHD 2000 infusion pump; Harvard, South Natick, Mass.) of a 4% mannitol solution delivering doses of 87 μg of rhodamine-123 and 20 mg of inulin per h at a rate of 2 ml of inulin per h until the end of the study. A 60-min infusion was found to result in a steady-state concentration of rhodamine-123. These dosages were calculated using pharmacokinetic parameters obtained from preliminary testing. Mannitol was used to obtain a sufficient and constant urine flow rate. After a 60-min infusion, bile and urine were collected in preweighed tubes at 20-min intervals for 60 min throughout the experiment. Blood samples were taken at the midpoint of the bile and urine collection periods. Plasma samples were obtained by centrifugation of the blood samples at 3,000 × g for 10 min. The volume of bile and urine samples was measured gravimetrically, with specific gravity assumed to be 1.0. All plasma, bile, and urine samples were stored at −40°C until analysis.
To elucidate the effects of cyclosporine (5 mg/kg), erythromycin (50 mg/kg), colchicine (5 mg/kg), and cimetidine (50 mg/kg) on the steady-state biliary excretion of rhodamine-123, each drug was administered intravenously 120 min after the start of the rhodamine-123 infusion. Blood and bile collection was carried out as described above.
To clarify the role of tumor necrosis factor alpha (TNF-α) in the endotoxin-induced decrease in P-glycoprotein-mediated biliary excretion, the protective effect of pentoxifylline, an inhibitor of TNF-α production, on the steady-state biliary excretion of rhodamine-123 was investigated. Pentoxifylline at a dose of 50 mg/kg was administered intraperitoneally to rats treated 1 h earlier with endotoxin or saline. The dosage of pentoxifylline was based on a previous study (2). Blood and bile collection was carried out as described above. To measure concentrations of TNF-α in plasma, blood samples were collected at designated intervals (0.5, 1, 2, 3, and 4 h) after endotoxin injection in rats pretreated with or without pentoxifylline. Concentrations of TNF-α in plasma were measured with the Biotrak rat TNF-α enzyme-linked immunosorbent assay system (Amersham International, Little Chalfont, United Kingdom).
Protein binding experiments.
Plasma protein binding is known to be a limiting factor in drug disposition, since only the unbound drug is distributed in the body and is subjected to liver and kidney excretion. To estimate the difference in the protein binding of rhodamine-123 among control rats and endotoxin-treated rats, the protein binding experiment was done by equilibrium dialysis using a cellulose membrane (Visking Sheet; Sanplatec Corp., Osaka, Japan) with the molecular mass cutoff set at 10 to 20 kDa. Under light anesthesia with ethyl ether, blood samples were obtained from the abdominal aortas of control rats (saline) and rats 6 or 24 h after injection of endotoxin, and plasma samples were immediately obtained by centrifugation. Four hundred microliters of a pH 7.4 phosphate-buffered saline solution containing 0.2 μg of rhodamine-123 per ml was dialyzed against an equal volume of plasma samples at 37°C for 12 h to attain equilibrium. The concentration of rhodamine-123 was chosen on the basis of data on concentrations in plasma obtained in in vivo experiments. Concentrations of rhodamine-123 on both sides of the membrane were measured by high-pressure liquid chromatography (HPLC).
mRNA analysis by RT-PCR.
Rat specific PCR primers against Mdr1a and Mdr1b were used for the analysis of mRNA levels of the respective transporter genes. Total RNA was isolated from frozen livers and kidneys of control rats and of treated rats 6, 12, or 24 h after injection with endotoxin using TRIzol reagent (Gibco Laboratories, Grand Island, N.Y.) according to the manufacturer's instructions. Single-stranded cDNA was synthesized from the isolated mRNA using RT (Superscript preamplification system; GIBCO BRL, Rockville, Md.). The RT mixture was amplified by PCR using Taq DNA polymerase (GIBCO BRL) in the presence of 0.2 μM concentrations of forward and reverse primers. Primers for rat Mdr1a were 5′-AGAAACAGAGGAGCGCCATT-3′ (forward) and 5′-GAATTCAACTTCAGGATCCG-3′ (reverse) (511 bp) (3), those for rat Mdr1b were 5′-ACTGAGCTTCGAGGTGAAGA-3′ (forward) and 5′-CAGAGCTGATGTCGCTTCAT-3′ (reverse) (451 bp) (32), and those for rat β-actin were 5′-TTCTACAATGAGCTGGGTGTGGC-3′ and 5′-CTC(A/G)TAGCTCTTCTCCAGGGAGGA-3′ (456 bp) (40).
Drug analysis.
Concentrations of rhodamine-123 in plasma, urine, bile, and dialysate were determined by HPLC after deproteination of the samples. The apparatus used for HPLC was a Shimadzu LC-6A system (Kyoto, Japan) equipped with a fluorescence detector (RF-535; Shimadzu) (excitation, 501 nm; emission, 524 nm) consisting of an LC-6A liquid pump and an SIL-6A autoinjector. The conditions were as follows: column, Cosmocil 5C18 (4.6 by 150 mm; Nacalai Tesque); mobile phase, 10 mM NH4H2PO4-citric acid buffer (pH 4.0)–acetonitrile (2:1, vol/vol); column temperature (OTC-6A; Shimadzu), 50°C; flow rate, 1.0 ml/min. Briefly, 50 μl of plasma, appropriately diluted urine and bile, and dialysate was vortexed with 300 μl of acetonitrile. After centrifugation at 6,000 × g for 5 min, 300 μl of the supernatant was added to 200 μl of acetonitrile-water (2:1, vol/vol) solution, and each sample was subjected to HPLC. This assay was shown to be linear for the concentrations studied (0.05 to 2 μg/ml), with a correlation coefficient of 0.999. The within-day and between-day coefficients of variation for this assay were less than 5%.
Inulin concentrations in plasma and urine were determined spectrophotometrically by the method of Dische and Borenfreund (6).
Pharmacokinetic analysis.
Pharmacokinetic parameters were calculated by the usual methods. The biliary clearance (CLbile) of rhodamine-123 during each bile collection period was calculated by dividing the biliary excretion rate by the steady-state concentration in plasma (CSS) determined for that collection period. The renal clearance (CLR) of rhodamine-123 and inulin was calculated by dividing the renal excretion rate for that collection period by the CSS determined for that collection period. GFR was represented as inulin clearance. The clearance ratio of rhodamine-123 was calculated as CLR/GFR. The tubular secretion clearance for unbound drug, which represents the net tubular secretion, was calculated as CLR/fU minus GFR, where fU represents the unbound fraction of drug. Each parameter was calculated by using the mean value of three points during 60 min.
Statistical analysis.
The experiments were performed at least three times, and the results are presented as means ± standard errors. Statistical comparisons were assessed by one-way analysis of variance. When F ratios were significant (P < 0.05), Scheffe's post hoc tests were done, and P values of <0.05 were considered statistically significant.
RESULTS
Effects of cyclosporine, erythromycin, colchicine, and cimetidine on biliary excretion of rhodamine-123.
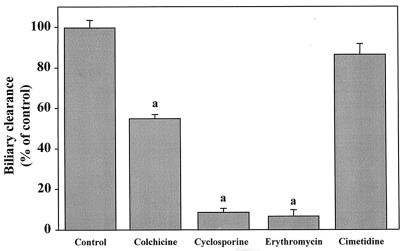
There are no in vivo data indicating whether rhodamine-123 is excreted into the bile by P glycoprotein, although rhodamine-123 is known to be secreted into the urine by P glycoprotein (18). To investigate whether the active biliary secretion of rhodamine-123 is mediated mainly by P glycoprotein, the effects of the well-known P-glycoprotein substrates cyclosporine, colchicine, and erythromycin and of cimetidine, a typical organic cation drug and P-glycoprotein inhibitor, on the biliary secretion of rhodamine-123 were investigated in normal rats. As shown in Fig. 1, cyclosporine, erythromycin, and colchicine significantly inhibited the biliary excretion of rhodamine-123, but cimetidine did not.
FIG. 1.
Inhibitory effects of cyclosporine, colchicine, erythromycin, and cimetidine on biliary excretion of rhodamine-123. Data are means plus standard errors. n = 15 (controls) or 3. a, Significantly different from control value (P < 0.05).
Effect of endotoxin on biliary excretion and renal handling of rhodamine-123.
Time-dependent effects of endotoxin on the biliary and renal excretion of rhodamine-123 under steady-state conditions in rats after intraperitoneal injection of endotoxin (1 mg/kg) are presented in Table 1. In the rats 24 h after injection of endotoxin, CLR of rhodamine-123 and GFR had a tendency to decrease (4.47 to 3.34 ml/min and 2.56 to 2.07 ml/min, respectively), although the differences failed to reach the 5% level of statistical significance. No change in CLbile of rhodamine-123 was observed. In contrast, significant reductions in the values of CLbile and biliary excretion rate of rhodamine-123 were observed in rats 6 h after injection with endotoxin (2.16 to 0.84 ml/min and 0.34 to 0.16 μg/min, respectively). The CLR of rhodamine-123 and GFR also significantly decreased in rats 6 h after endotoxin injection (4.47 to 1.79 ml/min and 2.56 to 1.66 ml/min, respectively). The CLR/GFR ratio of rhodamine-123 in control rats and rats 24 h after endotoxin injection was approximately 1.6, whereas that in rats after 6 h decreased to unity. Rhodamine-123 was relatively highly bound to plasma protein, and the unbound fraction in control rats was approximately 0.3, but endotoxin did not change the protein-binding behavior of rhodamine-123. To exclude the effect of plasma protein binding on the changes in the renal excretion of rhodamine-123, the net tubular secretion clearance for unbound drug (CLSU) was calculated as renal clearance for unbound rhodamine-123 (CLRU) minus GFR. No significant difference in CLSU was observed between control rats and rats 24 h after injection of endotoxin. However, the value in rats 6 h after injection of endotoxin dropped to approximately 35% of that in control rats (12.4 to 4.3 ml/min).
TABLE 1.
Effect of endotoxin on biliary excretion and renal handling of rhodamine-123 in ratsa
| Time (h) after endotoxin administration | CLbile (ml/min) | Biliary excretion rate (μg/min) | Bile flow rate (μl/min) | fu | CLR (ml/min) | GFR (ml/min) | CLRU/GFR | Net tubular secretion rate (ml/min) | Urine flow rate (μl/min) |
|---|---|---|---|---|---|---|---|---|---|
| 0 (control) | 2.16 ± 0.29 | 0.34 ± 0.05 | 19.8 ± 1.35 | 0.30 ± 0.01 | 4.47 ± 0.49 | 2.56 ± 0.18 | 5.82 ± 0.60 | 12.4 ± 1.53 | 29.2 ± 6.50 |
| 6 | 0.84 ± 0.09b | 0.16 ± 0.02b | 12.0 ± 1.31b | 0.31 ± 0.02 | 1.79 ± 0.23b | 1.66 ± 0.29c | 3.46 ± 0.51b | 4.26 ± 0.55b | 23.1 ± 1.56 |
| 24 | 1.95 ± 0.29 | 0.36 ± 0.07 | 19.1 ± 0.82 | 0.30 ± 0.02 | 3.34 ± 0.47 | 2.07 ± 0.09 | 5.38 ± 0.45 | 9.07 ± 1.51 | 19.6 ± 1.29 |
Values are means ± standard errors (n = 6).
Significantly different from control and 24-h values (P < 0.05).
Significantly different from control value (P < 0.05).
Role of TNF-α in endotoxin-induced decrease in biliary excretion of rhodamine-123.
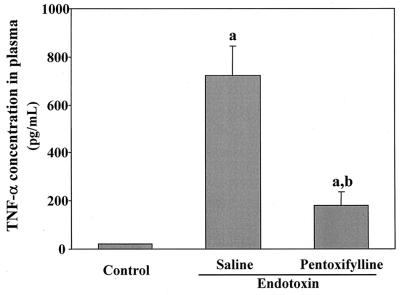
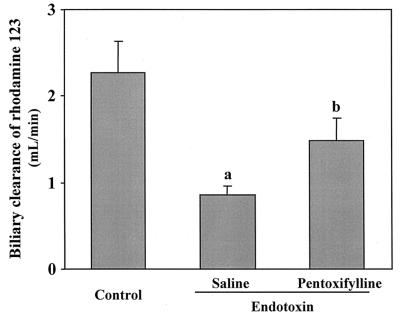
We expected that the area under the plasma concentration-time curve (AUC) of TNF-α, which represents the duration of exposure in the body, would be a more important factor than peak concentration. TNF-α in plasma reached a maximum approximately 2 h after endotoxin injection. Concentrations of TNF-α in plasma 2 h after injection of endotoxin and its AUC (AUC0–4) are represented in Fig. 2. The protective effect of pentoxifylline against endotoxin-induced increases in TNF-α levels is also demonstrated in Fig. 2. Pretreatment with pentoxifylline decreased the AUC0–4 of TNF-α to 50% (from 1,988 to 1,019 pg · h/ml). To investigate the contribution of TNF-α to endotoxin-induced reduction of the biliary secretion of rhodamine-123, pentoxifylline (50 mg/kg) was injected intraperitoneally 1 h before injection of endotoxin. Pretreatment with pentoxifylline significantly inhibited the effect of endotoxin on the biliary excretion of rhodamine-123 (Fig. 3).
FIG. 2.
Effect of pentoxifylline on endotoxin-induced increase in TNF-α concentrations in plasma in rats. The TNF-α concentration in plasma was measured 2 h after intraperitoneal injection of endotoxin (1 mg/kg). Data are means plus standard errors (n = 3). a, significantly different from control value (P < 0.05); b, significantly different from endotoxin value (P < 0.05).
FIG. 3.
Protective effect of pentoxifylline against endotoxin-induced decrease in the biliary clearance of rhodamine-123. Data are means plus standard errors (n = 3). a, significantly different from control value (P < 0.05); b, significantly different from endotoxin value (P < 0.05).
Effect of endotoxin on expression of P-glycoprotein mRNA in liver and kidney tissues by RT-PCR.
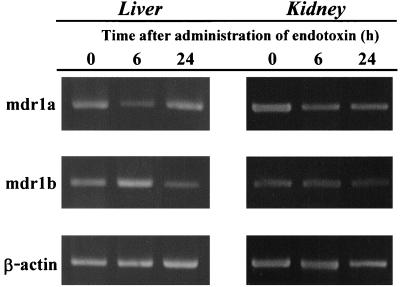
Time course changes in the expression of Mdr1a and Mdr1b mRNA as a function of endotoxin are shown in Fig. 4. By 6 h after injection of endotoxin, the expression of Mdr1a in both liver and kidney decreased. The expression of Mdr1a mRNA in the liver recovered after 24 h, but that in the kidney did not. The expression of Mdr1b RNA in the liver increased in rats treated 6 and 24 h earlier with endotoxin, but that in the kidney increased in rats after 24 h.
FIG. 4.
Effect of endotoxin on the expression of P-glycoprotein mRNA in rat tissues. RT-PCR was performed with total rat liver and kidney RNA at the indicated times after endotoxin injection (1 mg/kg). Each PCR product was separated on a 2% agarose gel.
DISCUSSION
The present study focused on the effect of K. pneumoniae endotoxin on P-glycoprotein-mediated biliary and renal excretion of the P-glycoprotein substrate rhodamine-123, which has been used as a marker for evaluating the role of P glycoprotein in anticancer drug-resistant cells and various normal tissues, such as small intestine, liver, kidney, and brain (4, 5, 18, 19, 28, 43). A series of studies reported that endotoxin-induced changes in the pharmacokinetics of drugs are not directly related to the histopathological changes in liver and kidney (17, 22, 25, 26). We also investigated the effect of K. pneumoniae endotoxin on the expression of Mdr1a and -1b mRNAs in liver and kidney tissues because P glycoprotein is located in the bile canalicular membrane of hepatocytes and brush border membranes of kidney-proximal tubules (7, 35).
It has been reported that rhodamine-123 is actively secreted into the urine by P glycoprotein and that glycerol-induced acute renal failure reduces P-glycoprotein-mediated renal tubular secretory clearance of rhodamine-123 by impairment of P glycoprotein (18). In the present experiments, no changes in the renal handling of rhodamine-123 were observed in rats treated 24 h earlier with endotoxin. However, the CLR of rhodamine-123 and GFR, which is represented as inulin clearance, significantly decreased in rats treated 6 h earlier with endotoxin. The clearance ratio (CLRU/GFR) for unbound rhodamine-123 in control rats was more than 5, indicating that rhodamine-123 is excreted into the urine by active tubular secretion. Endotoxin decreased the net tubular secretion of rhodamine-123 and the expression of Mdr1a in the kidney 6 h after injection, suggesting that P-glycoprotein-mediated renal secretory function in the brush border membrane of renal proximal tubular cells decreases by at least 6 h. The results of the present renal handling experiments might be supported by previous results indicating that endotoxin-induced reductions in GFR and organic anion transport ability are transient events (21, 22, 24).
To our knowledge, no data on whether the biliary excretion of rhodamine-123 is mediated by P glycoprotein are available. The present study using in vivo clearance experiments showed that the P-glycoprotein substrates cyclosporine, colchicine, and erythromycin significantly inhibited the biliary excretion of rhodamine-123, whereas cimetidine, which is not a P-glycoprotein substrate and is mediated by the organic cation transport system, did not. These results suggest that rhodamine-123 is mainly excreted into the bile by P glycoprotein and might be supported by a report that biliary excretion of P-glycoprotein substrates was decreased in Mdr1a knockout mice (39). In our experiments, significant reduction of P-glycoprotein-mediated biliary clearance of rhodamine-123 was observed in rats 6 h after endotoxin injection. Reduction of Mdr1a mRNA expression was also observed in the livers of rats treated 6 h earlier with endotoxin. These results suggest that P-glycoprotein-mediated biliary secretory function in the bile canalicular membrane of hepatocytes is impaired until 6 h after injection of endotoxin.
It is well known that endotoxin increases the levels of cytokines, including TNF-α, interleukin-1 (IL-1), IL-2, and IL-6, and that the elevation of these cytokines might play an important role in endotoxin-induced changes in certain transporter-mediated biliary excretion systems (11, 33). There is evidence that TNF-α induces up-regulation of transporter genes or MRP1 protein in human colon carcinoma cells and Mdr1 in rat hepatoma cells (1, 34). Nakamura et al. (27) reported that anti-IL-1 or anti-TNF-α antibody restores endotoxin-induced reduction in the expression of cMOAT/MRP2. It was previously proposed that endotoxin-induced suppression of hepatic cytochrome P450-dependent drug-metabolizing activity is caused by the synergistic action of more than one cytokine, for example, TNF-α and/or IL-1 (26). However, the roles of these cytokines and some mediators, such as nitric oxide and platelet-activating factor, in the physiological function of drug transporters remain unclear. We therefore focused on the role of TNF-α in endotoxin-induced decreases in the biliary clearance of rhodamine-123. We then investigated the effect of pentoxifylline, which inhibits the overproduction of TNF-α (2), on endotoxin-induced reduction of biliary clearance of rhodamine-123 and found that it restored this reduction while protecting the elevation of TNF-α levels in plasma. This suggests the possible involvement of TNF-α in decreased P-glycoprotein-mediated biliary transport.
The effects of endotoxin on the expression of mRNA of other transporters located in canalicular membranes of hepatocytes remain unclear, although it has been reported that endotoxin appears to induce the down-regulation of cMOAT/MRP2 mRNA in livers (30, 36, 40). It has also been reported that biliary excretion of P-glycoprotein substrates was decreased in Mdr1a knockout mice (39). Then, we attempted to investigate the effect of endotoxin on the expression of Mdr1a and -1b mRNA in both liver and kidney. RT-PCR analysis revealed that the expression of Mdr1a mRNA decreased in liver 6 h after endotoxin injection and returned to its control level 24 h after injection whereas Mdr1b mRNA levels increased. Vos et al. (40) have reported that Escherichia coli endotoxin (5 mg/kg) did not change the expression of Mdr1a mRNA in rat livers 6 h after intraperitoneal injection whereas Mdr1b mRNA levels increased. The discrepancy between their results and ours is not clear at present, although it might be a result of the different methods and the use of different endotoxins. Endotoxin-induced overexpression of Mdr1b may be supported by reports that TNF-α increases the expression of Mdr1b in rat hepatocytes (11) and that Mdr1b is overexpressed in Mdr1a knockout mice in compensation for efflux pump function of Mdr1a (31). On the basis of their reports and our results, it is possible that the overexpression of Mdr1b is caused by compensation for endotoxin-induced decrease in the expression of Mdr1a.
On the other hand, it has been suggested that several endogenous modulators of P glycoprotein exist in the human plasma (13). There is a possibility that the production of endogenous P-glycoprotein substrates in plasma of rats caused by endotoxin treatment is associated with endotoxin-induced decreases in P-glycoprotein-mediated biliary and renal excretion of rhodamine-123.
In conclusion, the present study is the first to use both in vivo clearance experiments and RT-PCR analysis to reveal that K. pneumoniae endotoxin decreases P-glycoprotein-mediated biliary and renal excretion of rhodamine-123 by decreasing the expression of Mdr1a. These results suggest that TNF-α may be at least one component associated with endotoxin-induced decrease in the biliary excretion of rhodamine-123. Further studies, however, are needed to clarify the role of TNF-α in reduced biliary and renal excretion of rhodamine-123 and in the level of P glycoprotein.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (13672417) from the Ministry of Education, Science, Sport, and Culture of Japan and by grants from the Imanaga Medical Foundation, the Heiwa Nakashima Foundation, and the Nakatomi Foundation.
REFERENCES
- 1.Chapekar M G, Huggett A C, Cheng C. Dexamethasone prevents the growth inhibitory effects of recombinant tumor necrosis factor in a rat hepatoma cell line Reuber-RC-3: an association with the changes in the messenger RNA levels for multidrug resistance gene. Biochem Biophys Res Commun. 1991;181:1524–1531. doi: 10.1016/0006-291x(91)92112-w. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y-L, Vraux V L, Giroud J-P, Chauvelot-Moachon L. Anti-tumor necrosis factor properties on non-peptide drugs in acute-phase responses. Eur J Pharmacol. 1994;271:319–327. doi: 10.1016/0014-2999(94)90789-7. [DOI] [PubMed] [Google Scholar]
- 3.Chianale J, Vollrath V, Wielandt A M, Miranda S, Gonzalez R, Fresno A M, Quintana C, Gonzalez S, Andrade L, Guzman S. Differences between nuclear run-off and mRNA levels for multidrug resistance gene expression in the cephalocaudal axis of the mouse intestine. Biochim Biophys Acta. 1995;1264:369–376. doi: 10.1016/0167-4781(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 4.Chin J E, Soffir R, Noonan K E, Choi K, Roninson L B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989;9:3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lange E C, de Bock G, Schinkel A H, de Boer A G, Breimer D D. BBB transport and P-glycoprotein functionality using mdr1a (-/-) and wild-type mice. Total brain versus microdialysis concentration profiles of rhodamine-123. Pharm Res. 1998;15:1657–1665. doi: 10.1023/a:1011988024295. [DOI] [PubMed] [Google Scholar]
- 6.Dische Z, Borenfreund E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951;192:583–587. [PubMed] [Google Scholar]
- 7.Fojo A T, Ueda K, Slamon D J, Poplack D G, Gottesman M M, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haghgoo S, Hasegawa T, Nadai M, Wang L, Ishigaki T, Miyamoto K, Nabeshima T. Brain distribution characteristics of xanthine derivatives and relation to their locomotor activity in mice. J Pharm Pharmacol. 1995;47:412–419. doi: 10.1111/j.2042-7158.1995.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 9.Haghgoo S, Hasegawa T, Nadai M, Wang L, Nabeshima T, Kato N. Effect of a bacterial lipopolysaccharide on biliary excretion of a β-lactam antibiotic, cefoperazone, in rats. Antimicrob Agents Chemother. 1995;39:2258–2261. doi: 10.1128/aac.39.10.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa T, Ohta M, Mori M, Nakashima I, Kato N, Morikawa K, Hanada T, Okuyama T. Structure of polysaccharide moiety of Klebsiella O3 lipopolysaccharide isolated from culture supernatant of decapsulated mutant (Klebsiella O3:K1−) Chem Pharm Bull. 1985;33:333–339. doi: 10.1248/cpb.33.333. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch-Ernst K I, Ziemann C, Foth H, Kozian D, Schmitz-Salue C, Kahl G F. Induction of mdr1b mRNA and P-glycoprotein expression by tumor necrosis factor alpha in primary rat hepatocyte cultures. J Cell Physiol. 1998;176:506–515. doi: 10.1002/(SICI)1097-4652(199809)176:3<506::AID-JCP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Hsing S, Gatmaitan Z, Arias I M. The function of Gp 170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology. 1992;102:879–885. doi: 10.1016/0016-5085(92)90173-v. [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa M, Yoshimura A, Furukawa T, Sumizawa T, Akiyama S. Modulators of the multidrug transporter, P-glycoprotein, exist in the human plasma. Biochem Biophys Res Commun. 1990;166:74–80. doi: 10.1016/0006-291x(90)91913-d. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am J Physiol. 1997;272:G16–G22. doi: 10.1152/ajpgi.1997.272.1.G16. [DOI] [PubMed] [Google Scholar]
- 15.Kamimoto Y, Gatmaitan Z, Hsu J, Arias I M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989;264:11693–11698. [PubMed] [Google Scholar]
- 16.Kiso S, Cai S H, Kitaichi K, Furui N, Takagi K, Takagi K, Nabeshima T, Hasegawa T. Inhibitory effect of erythromycin on P-glycoprotein-mediated biliary excretion of doxorubicin in rats. Anticancer Res. 2000;20:2827–2834. [PubMed] [Google Scholar]
- 17.Kitaichi K, Wang L, Takagi K, Iwase M, Shibata E, Nadai M, Takagi K, Hasegawa T. Decreased antipyrine clearance following endotoxin administration: in vivo evidence of the role of nitric oxide. Antimicrob Agents Chemother. 1999;43:2697–2701. doi: 10.1128/aac.43.11.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunihara M, Nagai J, Murakami T, Takano M. Renal excretion of rhodamine-123, a P-glycoprotein substrate, in rats with glycerol-induced acute renal failure. J Pharm Pharmacol. 1998;50:1161–1165. doi: 10.1111/j.2042-7158.1998.tb03328.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee J S, Paull K, Alvarez M, Hose C, Monks A, Grever M, Fojo A T, Bates S E. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol Pharmacol. 1994;46:627–638. [PubMed] [Google Scholar]
- 20.Mayer R, Kartenbeck J, Buchler M, Jedlitschky G, Leier I, Kuppler D. Expression of the MRP gene-encoded conjugate export pump in liver and its selective absence from the canalicular membrane in transport-deficient mutant hepatocytes. J Cell Biol. 1995;131:137–150. doi: 10.1083/jcb.131.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadai M, Hasegawa T, Kato K, Wang L, Nabeshima T, Kato N. Alterations in pharmacokinetics and protein binding behavior of cefazolin in endotoxemic rats. Antimicrob Agents Chemother. 1993;37:1781–1785. doi: 10.1128/aac.37.9.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadai M, Hasegawa T, Kato K, Wang L, Nabeshima T, Kato N. The disposition and renal handling of enprofylline in endotoxemic rats by bacterial lipopolysaccharide (LPS) Drug Metab Dispos. 1993;21:611–616. [PubMed] [Google Scholar]
- 23.Nadai M, Hasegawa T, Wang L, Haghgoo S, Nabeshima T, Kato N. Time-dependent changes in the pharmacokinetics and renal excretion of xanthine derivative enprofylline induced by bacterial endotoxin in rats. Biol Pharm Bull. 1995;18:1089–1093. doi: 10.1248/bpb.18.1089. [DOI] [PubMed] [Google Scholar]
- 24.Nadai M, Hasegawa T, Wang L, Haghgoo S, Okasaka T, Nabeshima T, Kato N. Alterations in renal uptake kinetics of the xanthine derivative enprofylline in endotoxaemic mice. J Pharm Pharmacol. 1996;48:744–748. doi: 10.1111/j.2042-7158.1996.tb03963.x. [DOI] [PubMed] [Google Scholar]
- 25.Nadai M, Matsuda I, Wang L, Itoh A, Naruhashi K, Nabeshima T, Asai M, Hasegawa T. Granulocyte colony-stimulating factor enhances endotoxin-induced decrease in biliary excretion of the antibiotic cefoperazone in rats. Antimicrob Agents Chemother. 1998;42:2178–2183. doi: 10.1128/aac.42.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadai M, Sekido T, Matsuda I, Wang L, Kitaichi K, Itoh A, Nabeshima T, Hasegawa T. Time-dependent effects of Klebsiella pneumoniae endotoxin on hepatic drug-metabolizing enzyme activity in rats. J Pharm Pharmacol. 1998;50:871–879. doi: 10.1111/j.2042-7158.1998.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura J, Nishida T, Hayashi K, Kawada N, Ueshima S, Sugiyama Y, Ito T, Sobue K, Matsuda H. Kupffer cell-mediated down regulation of rat hepatic CMOAT/MRP2 gene expression. Biochem Biophys Res Commun. 1999;255:143–149. doi: 10.1006/bbrc.1999.0160. [DOI] [PubMed] [Google Scholar]
- 28.Neyfakh A A, Serpinskaya A S, Chervonsky A V, Apasov S G, Kazarov A R. Multidrug-resistance phenotype of a subpopulation of T-lymphocytes without drug selection. Exp Cell Res. 1989;185:496–505. doi: 10.1016/0014-4827(89)90318-2. [DOI] [PubMed] [Google Scholar]
- 29.Oude Elferink R P, Meijer D K, Kuipers F, Jansen P L, Groen A K, Groothuis G M. Hepatobiliary secretion of organic compounds: molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995;1241:215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 30.Roelofsen H, Schoemaker B, Bakker C, Ottenhoff R, Jansen P L, Elferink R P. Impaired hepatocanalicular organic anion transport in endotoxemic rats. Am J Physiol. 1995;269:G427–G434. doi: 10.1152/ajpgi.1995.269.3.G427. [DOI] [PubMed] [Google Scholar]
- 31.Schinkel A H, Smit J J, van Tellingen O, Beijnen J H, Wagenaar E, van Deemter L, Mol C A, van der Valk M A, Robanus-Maandag E C, te Riele H P, Bermas A J M, Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 32.Silverman J A, Raunio H, Gant T W, Thorgeirsson S S. Cloning and characterization of a member of the rat multidrug resistance (mdr) gene family. Gene. 1991;106:229–236. doi: 10.1016/0378-1119(91)90203-n. [DOI] [PubMed] [Google Scholar]
- 33.Simpson K J, Lukacs N W, Colletti L, Strieter R M, Kunkel S L. Cytokines and the liver. J Hepatol. 1997;27:1120–1132. doi: 10.1016/s0168-8278(97)80160-2. [DOI] [PubMed] [Google Scholar]
- 34.Stein U, Walther W, Laurencot C M, Scheffer G L, Scheper R J, Shoemaker R H. Tumor necrosis factor-α and expression of the multidrug resistance-associated genes LRP and MRP. J Natl Cancer Inst. 1997;89:807–813. doi: 10.1093/jnci/89.11.807. [DOI] [PubMed] [Google Scholar]
- 35.Thiebaut F, Tsuruo T, Hamada H, Gottesman M M, Pastan I, Willingham M C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trauner M, Arrese M, Soroka C J, Ananthanarayanan M, Koeppel T A, Schlosser S F, Suchy F J, Keppler D, Boyer J L. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 37.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982;42:4730–4733. [PubMed] [Google Scholar]
- 38.Twentyman P R, Fox N E, White D J. Cyclosporin A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987;56:55–57. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Asperen J, van Tellingen O, Beijnen J H. The role of mdr1a P-glycoprotein in the biliary and intestinal secretion of doxorubicin and vinblastine in mice. Drug Metab Dispos. 2000;28:264–267. [PubMed] [Google Scholar]
- 40.Vos T A, Hooiveld G J, Koning H, Childs S, Meijer D K, Moshage H, Jansen P L, Muller M. Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology. 1998;28:1637–1644. doi: 10.1002/hep.510280625. [DOI] [PubMed] [Google Scholar]
- 41.Wacher V J, Wu C-Y, Benet L Z. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Kitaichi K, Cai S H, Takagi K, Takagi K, Sakai M, Yokogawa K, Miyamoto K-I, Hasegawa T. A reversal of anticancer drug resistance by macrolide antibiotics in vitro and in vivo. Clin Exp Pharmacol Physiol. 2000;27:587–593. doi: 10.1046/j.1440-1681.2000.03308.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Yang H, Miller D W, Elmquist W F. Effect of the P-glycoprotein inhibitor, cyclosporin A, on the distribution of rhodamine-123 to the brain: an in vivo microdialysis study in freely moving rats. Biochem Biophys Res Commun. 1995;211:719–726. doi: 10.1006/bbrc.1995.1872. [DOI] [PubMed] [Google Scholar]