Abstract
We conducted a retrospective review of medical records of patients with croup seen during the coronavirus disease 2019 pandemic. Approximately 50% underwent testing for severe acute respiratory syndrome coronavirus 2. During the Delta wave, 2.8% of those tested were positive for severe acute respiratory syndrome coronavirus 2; this increased to 48.2% during the Omicron wave, demonstrating a strong correlation between the Omicron variant and croup.
Keywords: pediatric emergency medicine, pediatric infectious disease, croup, COVID-19, SARS-CoV-2, laryngotracheobronchitis
Abbreviations: COVID-19, Coronavirus disease 2019; ED, Emergency department; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Croup is a common childhood upper respiratory disease, usually associated with parainfluenza or other endemic viruses, including seasonal coronaviruses.1 The infection results in varying degrees of inspiratory stridor, hoarse voice, barky cough, and respiratory distress, up to and including complete airway obstruction. Sporadic case reports have theorized an association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and croup.2, 3, 4, 5, 6, 7, 8, 9, 10 Ex vivo studies have shown that the Omicron variant (B.1.1.529) of SARS-CoV-2 replicates more rapidly in higher airways than previous lineages, suggesting the possibility of an increased risk for croup.11 As the Omicron variant surged in our community and the number of coronavirus disease 2019 (COVID-19) cases we saw in our emergency department (ED) increased significantly, our clinical observation was that there seemed to be a surge of cases of croup. We therefore conducted this study to compare rates of croup among children in our ED due to the Delta variant (B.1.617.2) compared with the Omicron variant. We merged regional epidemiologic data with our clinical data to evaluate whether there was indeed a high rate of croup as a manifestation of the Omicron variant.
Methods
High-level data were extracted retrospectively from the electronic medical records of children seen in the ED at Seattle Children's Hospital, an academic, quaternary-care hospital with more than 50 000 annual ED visits. Inclusion criteria were patients who were assigned a diagnosis containing the word “croup” during either May 30, 2021, to November 30, 2021, a time period correlating with predominance of the SARS-CoV-2 Delta variant (B.1.617.2), or during December 1, 2021, to January 15, 2022, corresponding to the initial phase of the Omicron variant surge. Extracted data fields included patient age, SARS-CoV-2 testing status and results, disposition (admission) decision, and medications administered (specifically racemic epinephrine). A query was also performed to assess for return visits to the ED within 72 hours, as well as for admissions on those repeat visits.
Qualitative SARS-CoV-2 polymerase chain reaction testing from nasopharyngeal or mid-turbinate swabs were obtained at the discretion of the treating clinician, as were interventions including racemic epinephrine and hospitalization. Contemporaneous publicly available local data on the proportion of SARS-CoV-2 samples in surrounding King County, Washington, with spike gene target failure on TaqPath (ThermoFisher Scientific/Applied Biosystems) polymerase chain reaction assays was used as a proxy for the proportion of infections caused by the Delta and Omicron SARS-CoV-2 variants.12 This study was approved by the Seattle Children's Hospital institutional review board.
Results
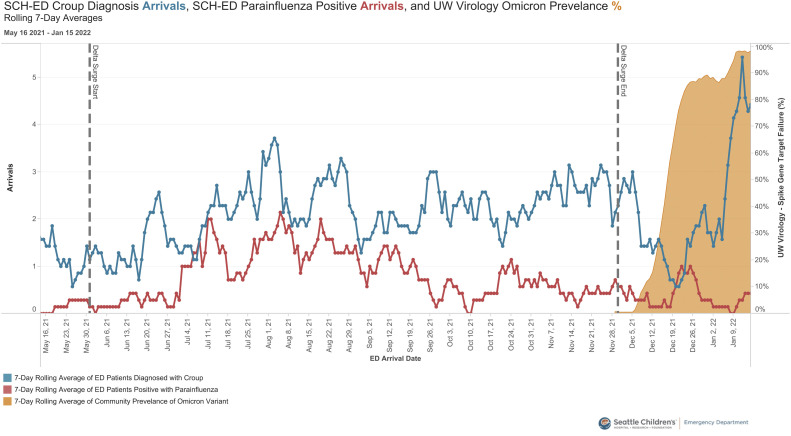
A total of 401 patients were diagnosed with croup during the Delta surge and 107 patients were diagnosed with croup during the Omicron surge. Patients with croup who presented during the Omicron surge were more likely to test positive for COVID-19 (48.2% vs 2.8%, P < .0001, OR 31.8) (Table ). The rate of viral respiratory testing remained similar during both time periods. Children with a clinical diagnosis of croup during the Omicron surge were more likely to be administered racemic epinephrine as part of their care (21.5% vs 13.0%, P = .032). There were no differences in presenting age, rate of admission, rate of return to the ED within 72 hours, or admission among those who returned within 72 hours. Using the local community prevalence of spike gene target failure as a proxy for the Omicron variant, we found that the incidence of croup doubled compared with the rate in previous months, whereas at the same time the number of cases of parainfluenza virus identified decreased (Figure ).
Table.
Characteristics of patients with croup during 2 different phases of the COVID-19 pandemic
| Characteristics | COVID-19 phase |
P value‡ | OR (95% CI) | |
|---|---|---|---|---|
| Delta surge∗ N = 401 |
Omicron surge† N = 107 |
|||
| Age, y, median (IQR) | 2.33 (1.42, 3.83) | 2.25 (1.08, 3.62) | .29 | |
| SARS-CoV-2 status, No. (%) | ||||
| Detected | 6 (2.8%) | 27 (48.2%) | <.0001 | 31.8 (12.7-80.2) |
| Not detected | 205 (97.2%) | 29 (51.8%) | ||
| Not tested | 190 | 51 | ||
| Received racemic epinephrine, No. (%) | 52 (13.0%) | 23 (21.5%) | .032 | 1.84 (1.06-3.18) |
| Admitted to hospital, No. (%) | 15 (3.7%) | 4 (3.7%) | >.99 | 1.00 (0.35-2.85) |
| 72-h return to ED, No. (%) | 12 (3.0%) | 6 (5.6%) | .23 | 1.93 (0.72-5.06) |
| 72-h return admission, No. (%) | 3 (25.0%) | 1 (16.7%) | >.99 | 0.60 (0.04-5.19) |
Time period when Delta variant was locally predominant: May 30, 2021, to November 30, 2021.
Time period when Omicron variant was locally predominant: December 1, 2021, to January 15, 2022.
Wilcoxon rank sum test for age; Fisher exact test for other variables.
Figure.
Number of patients diagnosed with croup, number of patients diagnosed with parainfluenza, and community prevalence of spike gene target failure for SARS-CoV-2 (7-day rolling averages).
Discussion
We identified a sharp rise in cases of croup seen in our pediatric ED in parallel with the replacement of the SARS-CoV-2 Delta variant by Omicron as the dominant variant in our community. A similar surge was seen by colleagues on the other side of the country, confirming this observation is more than just local to our practice.13 This increase was not temporally associated with the circulation of parainfluenza virus, and indeed Omicron replaced parainfluenza as the predominant virus associated with croup during this time. Viral testing is not routinely recommended for patients with croup; testing that was performed was for a wide variety of patients and indications, but we do believe our data are a reasonable proxy for local population prevalence. The end of the study period coincided with a decrease in hospital testing capacity and thus we were not able to see if this trend continued beyond January 15, 2022, with as much precision.
As a pragmatic retrospective data analysis, complete microbiologic data are not available, and this is a limitation of this study. Testing for respiratory pathogens, including SARS-CoV-2, was up to clinician discretion in the context of active patient care. To minimize this bias, we censored data collection in mid-January 2022, the first time that testing supplies became limited. With regards to possible sample bias, we note that SARS-CoV-2 testing rates were consistent across the Delta and Omicron waves, at 52.6% and 52.3% of patients with croup being tested, respectively.
Patients with croup during the Omicron surge were more likely to receive racemic epinephrine, suggesting a more severe initial clinical presentation. Based on admission rates and return visits, there are not enough data to alter current management after ED discharge.
As a common reason for ED visits, croup places a significant resource burden on health care systems. It appropriately generates fear and anxiety among children and their caretakers. As has been seen repeatedly during the COVID-19 pandemic, the protean clinical manifestations of SARS-CoV-2 have frequently humbled health care providers, with croup now needing to be recognized as a common presentation of the Omicron variant, and possibly future variants as well. With the recent rise in the BA.2, BA2.12.1, and subsequent Omicron subvariants,14 this observation deserves further investigation, ideally through multisite observations from geographically dispersed settings.
Acknowledgments
We thank Pavitra Roychoudhury, PhD, Department of Laboratory Medicine and Pathology, University of Washington, for her help in accessing local SARS-CoV-2 surveillance data.
Footnotes
The authors declare no conflicts of interest.
Supplementary Data
References
- 1.Sung J.Y., Lee H.J., Eun B.W., Kim S.H., Lee S.Y., Lee J.Y., et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. 2010;29:822–826. doi: 10.1097/INF.0b013e3181e7c18d. [DOI] [PubMed] [Google Scholar]
- 2.Brackel C.L.H., Rutjes N.W., Kuijpers T.W., Terheggen-Lagro S.W.J. SARS-CoV-2 and croup, a rare relationship or coincidence? Am J Emerg Med. 2021;49:410–411. doi: 10.1016/j.ajem.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur R., Schulz S., Fuji N., Pichichero M. COVID-19 pandemic impact on respiratory infectious diseases in primary care practice in children. Front Pediatr. 2021;9:722483. doi: 10.3389/fped.2021.722483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim C.C., Saniasiaya J., Kulasegarah J. Croup and COVID-19 in a child: a case report and literature review. BMJ Case Rep. 2021;14:e244769. doi: 10.1136/bcr-2021-244769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson K., Patel J., Collier C., Chan S.B. SARS-CoV-2 and croup, not a rare coincidence. Am J Emerg Med. 2021 doi: 10.1016/j.ajem.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitstick C.E., Rodriguez K.M., Smith A.C., Herman H.K., Hays J.F., Nash C.B. A curious case of croup: laryngotracheitis caused by COVID-19. Pediatrics. 2021;147 doi: 10.1542/peds.2020-012179. e2020012179. [DOI] [PubMed] [Google Scholar]
- 7.Tsoi K., Chan K.C., Chan L., Mok G., Li A.M., Lam H.S. A child with SARS-CoV-2-induced croup. Pediatr Pulmonol. 2021;56:2377–2378. doi: 10.1002/ppul.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venn A.M.R., Schmidt J.M., Mullan P.C. Pediatric croup with COVID-19. Am J Emerg Med. 2021;43:287. doi: 10.1016/j.ajem.2020.09.034. e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuccarelli A.M., Leonard C.G., Hampton S.M. Laryngotracheobronchitis, croup, an unusual presentation of SARS-CoV-2. Ulster Med J. 2022;91:57–58. [PMC free article] [PubMed] [Google Scholar]
- 10.Murata Y., Tomari K., Matsuoka T. Children with croup and SARS-CoV-2 infection during the large outbreak of Omicron. Pediatr Infect Dis J. 2022;41:e249. doi: 10.1097/INF.0000000000003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 12.University of Washington Department of Laboratory Medicine UW Virology COVID-19 dashboard. https://depts.washington.edu/labmed/covid19/#sequencing-information
- 13.Brewster R.C.L., Parsons C., Laird-Gion J., Hilker S., Irwin M., Sommerschield A., et al. COVID-19-associated croup in children. Pediatrics. 2022;149 doi: 10.1542/peds.2022-056492. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention CDC COVID data tracker: variant proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.