Abstract
Pharmacodynamic data on ciprofloxacin indicate that a target area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC ratio of ≥125 is necessary to achieve optimal bactericidal activity for the treatment of gram-negative pneumonia. The purpose of this prospective study was to (i) develop a pharmacokinetic (PK) model to be utilized for therapeutic drug monitoring (TDM) of ciprofloxacin and (ii) evaluate current ciprofloxacin dosing regimens for pneumonias in cystic fibrosis (CF) patients. Twelve adult CF patients received a single 400-mg dose of IV ciprofloxacin. Six blood samples were obtained over a 12-h interval. Serum drug concentrations were determined by high-pressure liquid chromotography and were fitted to one- and two-compartment models by using NPEM2. Ciprofloxacin MIC data for Pseudomonas aeruginosa were obtained from 1,213 CF patients enrolled in a large clinical trial. A Monte Carlo simulation was performed to estimate the fractional attainment of an AUC0–24/MIC ratio of ≥125. A two-compartment model best describes the serum drug concentration data. The mean fitted PK parameter values are volume of distribution in the central compartment, 0.29 liter/kg; volume of distribution at steady state, 1.1 liters/kg; total clearance, 0.34 liter/h/kg; distributional clearance, 0.89 liter/h/kg; half-life at α phase, 0.16 h; and half-life at β phase, 2.9 h. The overall fractional attainment of achieving an AUC0–24/MIC ratio of ≥125 against P. aeruginosa isolates with ciprofloxacin (400 mg every 12 h [q12h] and 8 qh) were 10 and 30%, respectively. A clinical breakpoint MIC of <0.5 μg/ml for susceptibility is suggested, based on an examination of the fractional attainment of the AUC0–24/MIC target at each MIC. The recommended doses of 400 mg q8h or q12h may be inadequate to treat an acute pulmonary exacerbation when given alone. The poor and variable AUC0–24/MIC ratios support the use of TDM to monitor and adjust the dosage to optimize the efficacy of ciprofloxacin therapy in these patients.
Acute pulmonary exacerbations requiring antibiotic and airway clearance therapy are the most frequent complication of cystic fibrosis (CF). Acute exacerbations occur as a result of a flare-up of the chronic infection within the lower airways. In this scenario, the therapeutic goals include reduction of bacterial density and improvement of pulmonary function and nutritional status (16). Therefore, the primary therapeutic approach is institution of antimicrobial therapy directed against the most commonly encountered pathogens, including Pseudomonas aeruginosa, Staphylococcus aureus, and Haemophilus influenzae.
Ciprofloxacin is a second-generation fluoroquinolone with a broad spectrum of activity. It possesses potent bactericidal activity against P. aeruginosa isolates from CF patients (1, 18). In addition, ciprofloxacin has consistently demonstrated synergistic activity with other antipseudomonal agents against multiple-drug-resistant P. aeruginosa isolates obtained from CF patients (18). With these attributes, it is not surprising that ciprofloxacin is frequently prescribed for the treatment of pulmonary exacerbations in CF patients.
The pharmacokinetics of ciprofloxacin have been extensively evaluated in stable CF patients (4, 9, 11, 14, 17, 20, 23). Results of these studies indicate no significant alterations in pharmacokinetics when patients are compared with normal healthy volunteers. In contrast, data on the pharmacokinetics of ciprofloxacin during acute pulmonary exacerbations are limited (2, 21). In addition, none of these studies performed compartmental pharmacokinetic analysis, which would permit dosage individualization for the patient. Studies performed in non-CF patients with nosocomially acquired lower respiratory tract infections demonstrated that optimal clinical and bacteriological outcomes are associated with achievement of specific threshold values of pharmacodynamic variables, such as the area under the concentration time curve from 0 to 24 h (AUC0–24)/MIC ratio and peak/MIC ratio (6, 15). Specifically, Forrest et al. demonstrated that optimal clinical and bacteriological responses in non-CF patients with lower respiratory tract infections are associated with the ability to achieve an AUC0–24/MIC ratio of at least 125 (6).
The purpose of this prospective study was to (i) develop a compartmental pharmacokinetic model to be utilized for therapeutic drug monitoring (TDM) and dosage adjustment of ciprofloxacin in adult CF patients and (ii) to evaluate the appropriateness of present ciprofloxacin dosing regimens for pneumonias in CF patients by using Monte Carlo simulation.
MATERIALS AND METHODS
Patients and samples.
Institutional review board approval was obtained to conduct this study. Twelve adult CF patients admitted for an acute pulmonary exacerbation were enrolled. Acute pulmonary exacerbation was diagnosed when the following signs and symptoms were present: an increase in cough and sputum production, significant weight loss from baseline, and decreased exercise tolerance (3). Inclusion criteria for this study were as follows: age of >18 years, most recent sputum culture being positive for P. aeruginosa, and admission for treatment of an acute pulmonary exacerbation. Patients were excluded if they were pregnant, attempting to conceive, or nursing an infant; had a history of seizure disorder or hypersensitivity reaction to any quinolone; or were currently receiving theophylline. Each patient received a single 400-mg dose of intravenous ciprofloxacin, which was infused over 1 h, in addition to the empiric antibiotic regimen prescribed for the acute pulmonary exacerbation.
Serial blood samples for determination of ciprofloxacin concentrations were obtained from an indwelling venous cannula before the dose and at the following times after the end of a 1-h infusion: 0 h and between 0.25 and 0.5 h, at 1.5 h, between 2.5 and 3 h, between 7 and 8 h, and between 10 and 12 h. Exact times were always noted. The catheter was flushed with 5 to 10 ml of 0.9% sodium chloride before and after each blood sample was collected in an evacuated tube. The first 3 ml of each blood sample was discarded. After sampling, the blood was allowed to clot on ice and directly centrifuged, and the serum was stored at −70°C in the freezer until analysis.
Analytic determination of ciprofloxacin concentrations.
The samples were processed and assayed by high-performance liquid chromatography by using methods derived from Nix et al. (13). The mobile phase consisted of ammonium phosphate buffer and acetonitrile, 74/26 (vol/vol), pH 2.77. Elutants were detected using fluorometric detection where excitation and emission wavelengths were set at 276 and 440 nm, respectively. The mobile phase flowed through an octadecylsilane column (C18, 5-μm particle size, 4.5-mm diameter, and 250-mm length; Alltech and Associates, Inc.) at a rate of 1 ml/min. The lower limit of detection of this assay was 0.04 μg/ml. The intra- and interday coefficients of variation for this assay ranged from 0.42 to 5.41% and 5.95 to 12.24%, respectively, with six standards from 0.04 to 4.0 μg/ml. The assay error variance and standard deviation were modeled with zero through second-order polynomials. The first-order polynomial equation was used for the final model: assay standard deviation = 0.0159 + 0.0609 × concentration.
Susceptibility.
Susceptibility data utilized in this study were based on data reported from a recent large clinical trial (19). The study reported susceptibility data for 1,213 isolates of P. aeruginosa obtained from 495 CF patients enrolled in clinical trials for evaluating the safety and efficacy of tobramycin solution for inhalation (19). Each isolate was tested against six agents, including ciprofloxacin. MICs were determined by serial twofold broth microdilution using media and test conditions in accordance with NCCLS recommended methodology (12). The MIC range of ciprofloxacin used against P. aeruginosa isolates was ≤0.5 to >4 μg/ml.
Pharmacokinetics and Monte Carlo simulation.
USC∗PACK (version 11.2; Laboratory of Applied Pharmacokinetics, University of Southern California School of Medicine, Los Angeles, California) was used to perform the pharmacokinetic analysis. Serum ciprofloxacin concentrations were fitted to one- and two-compartment models, using the NPEM2 program. Model discrimination was performed based on Akaike information criteria and an examination of log-likelihood values. Of the 12 patients enrolled, one received ciprofloxacin as part of his treatment regimen. For this individual, complete dosing histories and times of administration were input to the PASTRX module of USC∗PACK to account for preexisting drug and were included in the pharmacokinetic analysis. The concentrations were weighted according to the inverse of the assay error variance, using the first-order polynomial described above. The initial ranges for the pharmacokinetic parameters utilized in the IT2B analysis (the front end of NPEM2) were as follows: volume of distribution in the central compartment (V1) (0.3312 to 1.0488), the elimination rate constant (0.0423 to 0.6621), and the intercompartmental rate constants (0.37766 to 1.1959 and 0.36192 to 1.7671, respectively). These initial ranges were determined based on previously published pharmacokinetic data (7). The Bayesian posterior density determined within NPEM2 was utilized to estimate parameter values for each individual. The total clearance (CLT), distributional clearance (CLD), volume of distribution at steady state (Vss), and half-lives at α and βphases (t1/2α and t1/2β) were derived using standard methods (8).
Pharmacodynamic analysis was performed using Monte Carlo simulations (5). This method accounts for the variability in pharmacokinetic as well as MIC data to determine the probability of reaching a target AUC0–24/MIC ratio. An AUC0–24/MIC ratio of 125 was set as the target based on results from Forrest et al. (6). We also performed additional simulations using target AUC0–24/MIC ratios ranging from 75 to 175. The ADAPT II software was used for population simulations of 1,000 subjects receiving ciprofloxacin. The mean pharmacokinetic parameter values and the covariance matrix estimated from the NPEM2 analysis were embedded within the Subroutine Prior portion of ADAPT II. The parameter values were sampled from a log-normal distribution. Three simulations were performed to generate 1,000 clearance values each for dosages of 400 mg every 12 h (q12h), 400 mg q8h, and 600 mg q8h. Dividing the 24-h dose by the individual clearance value enabled determination of AUC values for each simulated patient.
Five additional simulations were performed in order to evaluate the impact of various clearance values on the attainment of the target AUC0–24/MIC ratio of 125. A volume of distribution of 2.5 liters/kg and clearance values of 0.2, 0.3, 0.4, 0.5, and 0.6 liter/kg/h were utilized in the simulations. All simulations were performed in ADAPT II using a one-compartment model and coefficients of variation of 33% for both parameters to compute AUC based on dose and clearance data.
Statistical analysis.
The fraction of simulated subjects achieving the target ciprofloxacin AUC0–24/MIC ratio of 125 was determined for each dose of ciprofloxacin (fractional target attainment) at each MIC. The overall response for P. aeruginosa to ciprofloxacin for each dose was determined from the sum of the products of the fractional distribution of MICs and the fractional target attainment at each MIC as previously described (5). For this study, a 90% probability of achieving an AUC0–24/MIC ratio greater than 125 was considered to be an acceptable clinical breakpoint. The response rates for additional target AUC0–24/MIC ratios (i.e., 75, 100, 125, 150, and 175) were also determined.
RESULTS
Patients.
Patient demographics are summarized in Table 1. All patients were treated with antibiotics for an acute pulmonary exacerbation. The most commonly prescribed antibiotic regimens consisted of either ceftazidime (n = 5) or piperacillin-tazobactam (n = 6) in combination with tobramycin. Eleven patients received a single 400-mg intravenous dose of ciprofloxacin for study purposes, while one patient received ciprofloxacin as part of his treatment regimen. The study patients had a mean age of 31 years and weight of 54.5 kg (86% of ideal weight for height). All patients had normal renal function, as determined by estimation of creatinine clearance using the method described by Jelliffe and Jelliffe (10). All patients' genotypes were known: two were ΔF508 homozygotes and six were ΔF508 heterozygotes, while four did not possess the ΔF508 mutation.
TABLE 1.
Patient characteristics
| Type of data | Age (yr) | Ht (in.) | Wt (kg) | Lean body mass (kg) | CLCRa (ml/min/1.73 m2) | Genotype (n)
|
||
|---|---|---|---|---|---|---|---|---|
| ΔF508/ΔF508 | ΔF508/other | Other/other | ||||||
| Mean ± SD | 31 ± 6.8 | 66 ± 3.7 | 54.5 ± 8.9 | 46.0 ± 7.4 | 115 ± 22.8 | 2 | 6 | 4 |
| Range | 22–42 | 59–74 | 41.5–72 | 33.1–60.4 | 86.3–150.4 | |||
CLCR, creatinine clearance. There were 10 male patients and 2 female patients.
MIC distributions.
The relative and cumulative frequency distributions are depicted in Fig. 1. The MICs at which 50 and 90% of P. aeruginosa isolates tested were inhibited by ciprofloxacin (MIC50 and MIC90) are 1.0 and 4.0 μg/ml, respectively.
FIG. 1.
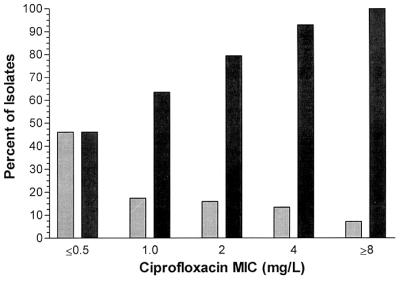
Ciprofloxacin susceptibility against CF clinical isolates of P. aeruginosa (n = 1,213). Cumulative percentage (■) of isolates at each MIC is given. (MIC50 = 1.0; MIC90 = 4.0). Also given is the relative percentage of isolates at each MIC ( ).
).
Pharmacokinetic analysis.
A two-compartment model best describes the serum drug concentration data. The results of the population pharmacokinetic analysis performed with measured serum ciprofloxacin concentrations are summarized in Table 2. The volume of distribution and CLT values are lower than previously described in patients with CF. However, the elimination half-life is consistent with previously reported values.
TABLE 2.
Population pharmacokinetic parameter values for ciprofloxacina
| Type of data | V1 (liters/kg) | Vss (liters/kg) | CLT (liters/h/kg) | CLD (liters/h/kg) | k10 (h−1) | t1/2α (h) | t1/2β (h) | AUC0–24 (mg · h/liter) for
|
|
|---|---|---|---|---|---|---|---|---|---|
| 400 mg q12h | 400 mg q8h | ||||||||
| Mean | 0.29 | 1.1 | 0.34 | 0.89 | 1.86 | 0.16 | 2.9 | 48.3 | 72.4 |
| SD | 0.20 | 0.38 | 0.12 | 0.81 | 1.29 | 0.13 | 0.52 | 17.3 | 26.0 |
k10, elimination rate constant.
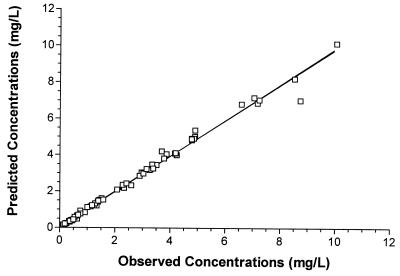
Using the median of the Bayesian posterior joint density of each individual enabled creation of a plot of measured versus predicted concentrations (Fig. 2). The r2 value of this regression was 0.99, indicating that the model provided an excellent fit to the data.
FIG. 2.
Predicted versus observed serum ciprofloxacin concentrations (n = 67). r2 = 0.99; P << 0.0001. The predicted concentrations were based on the parameter medians for the distribution of individual patients.
Attainment of pharmacodynamic target.
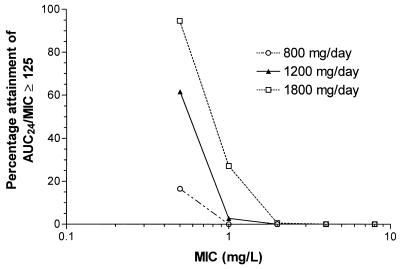
The fraction of simulated patients attaining an AUC0–24/MIC ratio of ≥125 for the three ciprofloxacin dosage regimens by MIC is shown in Fig. 3. Only 15% of patients would be expected to attain the target AUC0–24/MIC ratio at a MIC of 0.5 μg/ml using a standard dose of 400 mg q12h. A dose of 400 mg q8h increases the proportion achieving the target to 60%. However, a dosage of 600 mg q8h would be required in order to attain the target AUC0–24/MIC ratio in excess of 90% of subjects at a MIC of 0.5 μg/ml. These data indicate that the clinical breakpoint for ciprofloxacin against P. aeruginosa should be considered <0.5 μg/ml, using routine clinical doses (i.e., 400 mg q12h and q8h).
FIG. 3.
Fractional attainment of AUC0–24/MIC ratio of ≥125 at each MIC based on ciprofloxacin doses of 800, 1,200, and 1,800 mg/day (n = 1,000 simulations for each dose). AUC24, AUC0–24.
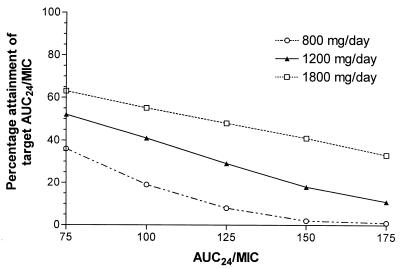
The fractional attainment of the target AUC0–24/MIC ratio as a function of specific AUC0–24/MIC ratios is depicted in Fig. 4. As expected, lower AUC0–24/MIC targets yield greater percentage achievement of the goal at each dose level. Routine clinical doses of 400 mg q12h and 400 mg q8h would be expected to achieve the target AUC0–24/MIC ratio of 125 in approximately 10 and 30% of patients, respectively.
FIG. 4.
Percentage achievement of the pharmacodynamic goal utilizing various AUC0–24/MIC target values. Simulations were based on ciprofloxacin doses of 800, 1,200, and 1,800 mg/day (n = 1,000 for each dose). AUC24, AUC0–24.
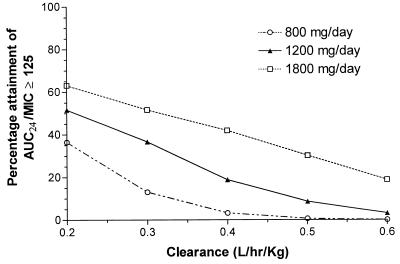
The effect of various clearance values on the fractional attainment of the target AUC0–24/MIC ratio is displayed in Fig. 5. Since AUC varies in inverse proportion to clearance, it is not surprising that changes in clearance alter the fractional attainment of the target AUC0–24/MIC.
FIG. 5.
Percentage achievement of AUC0–24/MIC ratio of ≥125 at various ciprofloxacin clearance values. Simulations were based on ciprofloxacin doses of 800, 1,200, and 1,800 mg/day (n = 1,000 for each clearance value). AUC24, AUC0–24.
DISCUSSION
The primary purpose of the present study was to develop a compartmental pharmacokinetic model to be used for intravenous ciprofloxacin in adult CF patients. There are presently no published studies involving intravenous ciprofloxacin in CF patients in which compartmental pharmacokinetic analysis was performed (2, 4, 21). We found that a two-compartment model best described the disposition of ciprofloxacin in our adult CF population. This is similar to the results of Forrest et al., who evaluated the pharmacokinetics of ciprofloxacin in acutely ill, non-CF patients (7).
Interestingly, the pharmacokinetic description of intravenous ciprofloxacin in this study differs from that found in previously published CF studies (2, 4, 21). For comparison purposes, the pharmacokinetic parameters from those studies, in addition to a study looking at acutely ill, non-CF patients, are summarized in Table 3. Our subjects exhibited a lower clearance and volume of distribution than did those in other studies. The CLT is roughly half, in comparison to the clearances obtained from CF patients in other studies (2, 4, 21). Differences between study populations that may account for this apparent discrepancy include age, degree of acute illness, and differences in genotypes. Patients in this study are on average older than the other study populations, with a mean age of 31 years, ranging from 22 to 42 years. In contrast, Christensson et al. and Davis et al. enrolled younger CF patients, ranging from 17 to 27 years old and 18 to 34 years old, respectively (2, 4). In another study, the patient population had a mean age of 16.75 or 20.67 years, depending on the intravenous dose administered (4). Since the patients in the present study were older and had received multiple courses of aminoglycosides, they could exhibit some changes in renal function, which may contribute to the decreased ciprofloxacin clearance observed, compared to the other CF studies.
TABLE 3.
Summary of intravenous ciprofloxacin pharmacokinetic studies in CF patients
| Source or reference | n | V1 (liters/kg) | Vss (liters/kg) | CLT (liters/kg/h) | t1/2 (h) |
|---|---|---|---|---|---|
| 4 | 12 | 0.42 ± 0.38 | 2.21 ± 0.89 | 0.51 ± 0.11b | 4.5 ± 1.9 |
| 21 | 9 | 0.61 ± 0.17 | 2.8 ± 1.42 | ||
| 2 | 14 | 2.71 ± 0.705 | 0.62 ± 0.10 | ||
| 7c | 74 | 0.69 ± 0.18 | 1.2a | 0.24 ± 0.11 | 6.5 ± 3.25 |
| Present Study | 12 | 0.29 ± 0.20 | 1.1 ± 0.38 | 0.34 ± 0.12 | 2.9 ± 0.52 |
Vss was calculated as V1 + Vp, where Vp = volume of the peripheral compartment.
In this study, CLT was expressed in liters per hour. For comparison purposes, the CLT was divided by the mean weight of the CF patients.
This study did not include CF patients, but these data were included for comparison.
The Vss of our population is somewhat smaller than that previously reported. The V1 is similar to that of the findings of Davis et al., which indicates that the difference is a reflection of a smaller peripheral volume of distribution. While there is no obvious explanation for this difference, it may be a result of variations of CF disease. Patients with different CF genotypes have various manifestations of the disease. Our population differs from the classical northern European population, which is comprised predominantly of individuals who are homozygous ΔF508. These individuals typically have pancreatic insufficiency and significant pulmonary disease. In contrast, only two of our patients were homozygous ΔF508. It is possible that patients with non-ΔF508 genotypes may exhibit ciprofloxacin pharmacokinetics that more closely approximate those of their age-appropriate healthy counterparts. Although specific genotypes were not reported in published literature to allow for comparison, differences in genotypes might explain some of the discrepancies in pharmacokinetic parameters noted in the present study.
Another possible explanation for the differences in the pharmacokinetic parameters could be a result of an interaction between piperacillin and ciprofloxacin. Both drugs are subject to tubular secretion, leading to competition for active transport. Due to the higher molar concentration of piperacillin, the renal clearance of ciprofloxacin is reduced to a greater extent. Strenkoski-Nix et al. noted a 25% reduction in the Vss and CLT of ciprofloxacin when given in combination with piperacillin to a group of 12 healthy volunteers studied under controlled conditions (22). In our study 6 of 12 patients received the combination of piperacillin and ciprofloxacin. An examination of their pharmacokinetic parameters demonstrated no significant difference in Vss between the patients receiving ciprofloxacin in combination with piperacillin and those receiving ciprofloxacin alone (mean ± standard error of the mean: 1.1 ± 0.2 and 1.1 ± 0.2; P = 0.94). In addition, no significant difference was noted in clearance between the patients receiving ciprofloxacin in combination with piperacillin and those receiving ciprofloxacin alone (mean ± standard error of the mean: 0.31 ± 0.05 and 0.37 ± 0.05; P = 0.44). It does not appear, therefore, that this interaction contributed significantly to the differences in pharmacokinetic parameters noted in the present investigation.
The secondary objective of this study was to evaluate the probability of achieving optimal pharmacodynamic activity of ciprofloxacin against P. aeruginosa isolates for patients with CF receiving presently recommended dosage regimens. Based on the susceptibility results, the activity of ciprofloxacin as reflected by MICs is less than reported previously, a MIC50 of 0.5 μg/ml versus 1 μg/ml (1). This decline in sensitivity may be a result of extensive use of antibiotics, in particular, ciprofloxacin within the CF population. Since ciprofloxacin is the only oral antipseudomonal agent presently available, it is prescribed frequently in the outpatient management of mild to moderate pulmonary exacerbations in CF patients. However, a simple comparison of MICs is an insufficient means of predicting in vivo pharmacodynamic activity because it does not account for variability in the pharmacokinetics of the drug.
An accurate and precise pharmacokinetic model in combination with specific pharmacodynamic goals in CF patients would enable dosage individualization to maximize clinical and microbiological outcomes while minimizing the risk of toxicity. Forrest et al. demonstrated that optimal clinical and bacteriological responses in non-CF patients with lower respiratory tract infections are associated with the ability to achieve an AUC0–24/MIC ratio of at least 125 (6). In the present study, the Monte Carlo analysis was performed with presently recommended ciprofloxacin dosages of 400 mg every q12h and 400 mg q8h and yielded suboptimal AUC0–24/MIC ratios. Only 10 and 30% of simulated patients achieved an AUC0–24/MIC ratio of 125 or greater against P. aeruginosa isolates at 400 mg q12h and 400 mg q8h, respectively. These results reflect the relatively high ciprofloxacin MICs for P. aeruginosa isolates in patients with CF. Even with a dose of 600 mg q8h, only 55% of patients would reach the goal AUC0–24/MIC ratio of 125. Thus, ciprofloxacin monotherapy should not be given empirically for the treatment of acute pulmonary exacerbations in patients with CF. If the ciprofloxacin MIC for an isolate is known to be ≤0.5 μg/ml then a dosage regimen of 600 mg q8h would achieve the targeted AUC0–24/MIC ratio in 95% of patients; however, clinical experience with doses exceeding 1,200 mg per day is currently limited. In contrast, only 15 and 60% of patients would achieve the targeted AUC0–24/MIC ratio when given ciprofloxacin doses of 400 mg q12h and 400 mg q8h, respectively.
Our study has two limitations. First, the target AUC0–24/MIC ratio of 125 used for our pharmacodynamic analysis was obtained in non-CF patients with lower respiratory tract infections (6). It is uncertain if this target AUC0–24/MIC ratio is also optimal in the CF population. Considering that eradication is rarely achieved in patients with CF due to the presence of bacterial biofilms, viscous airway secretions, divalent cations, and other inhibitors of antibacterial activity, the target AUC0–24/MIC value is likely to be greater than 125. Nonetheless, our conclusions regarding the inadequacy of standard dosage regimens of ciprofloxacin and the need for combination therapy in the treatment of P. aeruginosa infections in patients with CF remain valid. A second limitation is the need to prospectively validate the predictive performance of the pharmacokinetic model developed using an independent CF population.
In conclusion, the pharmacokinetics of ciprofloxacin in adult CF patients are well described using a two-compartment model. Based on our simulation studies, recommended doses of 400 mg every 8 to 12 h are often inadequate to treat an acute pulmonary exacerbation in patients with CF when given alone. Despite a decline in susceptibility, ciprofloxacin remains an important agent against P. aeruginosa due to its proven safety profile and excellent oral bioavailability. Antibiotic combinations which are likely to provide additive or synergistic activity (beta-lactams in combination with ciprofloxacin) are necessary to optimally treat this patient population. In addition, the inadequate and variable AUC0–24/MIC ratios support an individualized approach to dosage regimen design according to each patient's weight, renal function, and organism susceptibility. The use of TDM may well be necessary in order to monitor and optimize dosage to ensure optimal therapy.
ACKNOWLEDGMENTS
This study was supported in part by an unrestricted grant from the Bayer Corporation.
We acknowledge the assistance of the 6th-floor nursing staff at USC University Hospital.
REFERENCES
- 1.Ansorg R, Muller K, Wiora J. Comparison of inhibitory and bactericidal activity of antipseudomonal antibiotics against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Chemotherapy. 1990;36:222–229. doi: 10.1159/000238770. [DOI] [PubMed] [Google Scholar]
- 2.Christensson B, Nilsson-Ehle I, Ljungberg B, Linblad A, Malmborg A S, Hjelte L, Strandvik B. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother. 1992;36:2512–2517. doi: 10.1128/aac.36.11.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. Clinical practice guidelines for cystic fibrosis. Bethesda, Md: Cystic Fibrosis Foundation; 1997. [Google Scholar]
- 4.Davis R, Koup J, Williams-Warren J, Weber A, Heggen L, Stempel D, Smith A L. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrob Agents Chemother. 1987;31:915–919. doi: 10.1128/aac.31.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drusano G L, Preston S L, Hardalo C, Hare R, Banfield C, Andes D, Vesga O, Craig W A. Use of preclinical data for selection of a phase II/II dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother. 2001;45:13–22. doi: 10.1128/AAC.45.1.13-22.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrest A, Nix D, Ballow C, Goss T, Birmingham M, Schentag J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest A, Ballow C, Nix D, Birmingham M, Schentag J. Development of a population pharmacokinetic model and optimal sampling strategies for intravenous ciprofloxacin. Antimicrob Agents Chemother. 1993;37:1065–1072. doi: 10.1128/aac.37.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. Drugs and the pharmaceutical sciences. Vol. 15. New York, N.Y: Marcel Dekker Inc.; 1982. p. 482. [Google Scholar]
- 9.Goldfarb J, Wormser G, Inchiosa M, Guideri G, Diaz M, Gandhi R, Goltzman C, Mascia A. Single-dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. J Clin Pharmacol. 1986;26:222–226. doi: 10.1002/j.1552-4604.1986.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 10.Jelliffe R W, Jelliffe S M. A computer program for estimation of creatinine clearance from unstable serum creatinine levels, age, sex and weight. Math Biosci. 1972;14:17–24. [Google Scholar]
- 11.LeBel M, Bergeron M, Vallee F, Fiset C, Chasse G, Bigonesse P, Rivard G. Pharmacokinetics and pharmacodynamics of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother. 1986;30:260–266. doi: 10.1128/aac.30.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Methods for dilution of antimicrobial susceptibility testing for bacteria that grow aerobically. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Nix D, De Vito J, Schentag J. Liquid-chromatographic determination of ciprofloxacin in serum and urine. Clin Chem. 1985;31:684–686. [PubMed] [Google Scholar]
- 14.Pedersen S, Jensen T, Hvidberg E. Comparative pharmacokinetics of ciprofloxacin and ofloxacin in cystic fibrosis patients. J Antimicrob Chemother. 1987;20:575–583. doi: 10.1093/jac/20.4.575. [DOI] [PubMed] [Google Scholar]
- 15.Peloquin C, Cumbo T, Nix D, Sands M, Schentag J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med. 1989;149:2269–2273. [PubMed] [Google Scholar]
- 16.Ramsey B. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 17.Reed M, Stern R, Myers C, Yamashita T, Blumer J. Lack of unique ciprofloxacin pharmacokinetic characteristics in patients with cystic fibrosis. J Clin Pharmacol. 1988;28:691–699. doi: 10.1002/j.1552-4604.1988.tb03202.x. [DOI] [PubMed] [Google Scholar]
- 18.Saiman L, Mehar F, Niu W, Neu H, Shaw K, Miller G, Prince A. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin Infect Dis. 1996;23:532–537. doi: 10.1093/clinids/23.3.532. [DOI] [PubMed] [Google Scholar]
- 19.Shawar R M, MacLeod D L, Garber R L, Burns J, Stapp J, Clausen C, Tanaka S. Activities of tobramycin and six other antibiotics against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1999;43:2877–2880. doi: 10.1128/aac.43.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith M, White L, Bowyer H, Willis J, Hodson M, Batten J. Pharmacokinetics and sputum penetration of ciprofloxacin in patients with cystic fibrosis. Antimicrob Agents Chemother. 1986;30:614–616. doi: 10.1128/aac.30.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steen H J, Scott E M, Stevenson M I, Black A E, Redmond A O, Collier P S. Clinical and pharmacokinetic aspects of ciprofloxacin in the treatment of acute exacerbations of pseudomonas infection in cystic fibrosis patients. J Antimicrob Chemother. 1989;24:787–795. doi: 10.1093/jac/24.5.787. [DOI] [PubMed] [Google Scholar]
- 22.Strenkoski-Nix L C, Forrest A, Schentag J J, Nix D E. Pharmacodynamic interactions of ciprofloxacin, piperacillin, and piperacillin/tazobactam in healthy volunteers. J Clin Pharmacol. 1998;38:1063–1071. doi: 10.1177/009127009803801112. [DOI] [PubMed] [Google Scholar]
- 23.Stutman H, Shalit I, Marks M, Greenwood R, Chartrand S, Hilman B. Pharmacokinetics of two dosage regimens of ciprofloxacin during a two-week therapeutic trial in patients with cystic fibrosis. Am J Med. 1987;82:142–145. [PubMed] [Google Scholar]