Abstract
Acute respiratory distress syndrome (ARDS) occurs in up to 10% of patients with respiratory failure admitted through the emergency department. Use of noninvasive respiratory support has proliferated in recent years; clinicians must understand the relative merits and risks of these technologies and know how to recognize signs of failure. The cornerstone of ARDS care of the mechanically ventilated patient is low-tidal volume ventilation based on ideal body weight. Adjunctive therapies, such as prone positioning and neuromuscular blockade, may have a role in the emergency department management of ARDS depending on patient and department characteristics.
Keywords: ARDS, Respiratory failure, Respiratory support, Mechanical ventilation, Noninvasive ventilation, High-flow nasal cannula
Key points
-
•
Acute respiratory distress syndrome (ARDS) occurs in up to 10% of patients with respiratory failure admitted through the emergency department.
-
•
Use of noninvasive respiratory support has proliferated in recent years; clinicians must understand the relative merits and risks of these technologies and know how to recognize signs of failure.
-
•
The cornerstone of ARDS care of the mechanically ventilated patient is low-tidal volume ventilation based on ideal body weight.
-
•
Adjunctive therapies, such as prone positioning and neuromuscular blockade, may have a role in the emergency department management of ARDS depending on patient and department characteristics.
-
•
Recognizing a patient with refractory disease is important to facilitate transfer to an expert center.
Background
Acute respiratory distress syndrome (ARDS) is characterized by inflammatory lung injury and carries a global mortality near 40%.1 It occurs in approximately 6% to 10% of patients with respiratory failure who are admitted through the emergency department.2 , 3 Even within the context of the intensive care unit (ICU), the diagnosis is missed in up to half of all patients meeting criteria for the disease.1 Early recognition is critical to ensure that evidence-based therapies can be implemented without delay.
The most common underlying causes for ARDS, by frequency, commonly encountered in the emergency department include the following: sepsis, pneumonia, aspiration, pancreatitis, blood transfusions, trauma, and burns.4 Although treating the underlying cause is a cornerstone of ARDS management, there are specific diagnostic and therapeutic considerations that must be pursued as early as possible. Not only are many interventions time sensitive but also the initial care choices in the emergency department often carry over to the ICU upon admission.5
Diagnosis
Criteria
ARDS is currently diagnosed using the Berlin definition (Table 1 ).6 In brief, it is characterized by an acute onset of respiratory illness (within 1 week) with bilateral opacities, which cannot completely be explained by hydrostatic pulmonary edema, with a partial pressure of oxygen to fraction of inspired oxygen ratio (Pao 2:Fio 2 ratio) of 300 mm of mercury or less. The current definition requires the application of at least 5 cm of water of positive airway pressure, delivered either via noninvasive positive pressure ventilation (NIV) or via invasive mechanical ventilation (IMV). Given the increase in the use of high-flow nasal cannula (HFNC) over the past decade, it has been proposed to include patients on HFNC in the definition,7 although currently the Berlin criteria remain the standard (see Table 1).
Table 1.
The Berlin definition of acute respiratory distress syndrome
| Timing | Within 1 wk of known insult, or new or worsening symptoms |
| Chest imaging | Bilateral opacities not fully explained by pleural effusions, lobar collapse, or nodules |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment is required |
| Oxygenation (measured on at least 5 cm H2O PEEP or CPAP) | |
| Mild | 200 mm Hg < Pao2:Fio2 ≤ 300 mm Hg |
| Moderate | 100 mm Hg < Pao2:Fio2 ≤ 200 mm Hg |
| Severe | 100 mm Hg ≤ Pao2:Fio2 |
Abbreviation: CPAP, continuous positive airway pressure.
Imaging
Radiograph is usually sufficient for chest imaging to evaluate a patient for ARDS. Although chest computed tomography (CT) is not necessarily required to diagnose ARDS, it may help better characterize the pulmonary parenchyma and possibly identify an underlying cause. Furthermore, contrasted CT scan could identify comorbid pulmonary emboli, which would likely alter therapy. Small series report concurrent pulmonary emboli to be 17% to 39% of the time in patients with COVID-19 ARDS.8 , 9
A more recent development in the diagnosis of ARDS includes the use of point-of-care ultrasound by the bedside clinician using a combination of both lung ultrasound and echocardiography. ARDS can be detected by lung ultrasonography through the recognition of a pulmonary interstitial pattern, which includes the following: B lines with inhomogeneous and gravity-independent distribution, spared areas, pleural line thickening with decreased lung sliding, and subpleural consolidations.10 Lung ultrasound performs reasonably well in terms of sensitivity and specificity (82.7% to 92.3% and 90.2% to 98.6%, respectively) in ARDS when compared with chest CT.11 It also provides the ability for real-time monitoring of changes to vent settings, such as positive end expiratory pressure (PEEP) titration,12, 13, 14 although this is not commonly used. Importantly, lung ultrasound would not be able to differentiate between well-aerated lung and overdistended lung, as both would result in an A-line pattern.
Focused cardiac ultrasound can also be used to establish the diagnosis (ie, rule out cardiogenic pulmonary edema), as well as monitor the effect of ARDS on the right ventricle. Right ventricular dysfunction can be seen in 22% to 27% of patients with ARDS.15 , 16 Right heart failure owing to increased pulmonary vascular resistance (acute cor pulmonale) is characterized by right-ventricle dilatation and septal dyskinesia on echocardiography.17 Moreover, a coexisting patent foramen ovale with shunt can occur in nearly 20% of patients with ARDS and may be responsible for a higher rate of refractory hypoxemia and an increased number of ventilator-dependent days.18 Discovering a shunt in the patient with ARDS may also alter therapy, whereby the pulmonary vascular resistance should be lowered as much as possible to decrease shunt fraction.
Management
Treatment of the underlying condition that led to ARDS is the primary management consideration. The remainder of care is largely supportive and centers on the careful application of respiratory assistance.
Noninvasive Respiratory Support
High-flow nasal cannula
HFNC has gained traction over the past decade, in large part to a multicenter trial showing decreased mortality compared with NIV and standard oxygen therapy in patients with acute hypoxemic respiratory failure19 as well as improved patient comfort compared with NIV and IMV. A clinical practice guideline has strongly endorsed HFNC over standard oxygen therapy for hypoxemic respiratory failure, as its use has been associated with reduction in intubation rates and escalation of respiratory support.20 Use can be considered in patients with ARDS if airway protective reflexes are intact and the patient is hemodynamically stable. Benefits include decreased respiratory effort, in part because of washout of anatomic dead space. Flow rate (in liters per minute) can be titrated to patient comfort, and administered Fio 2 (up to 1.0) can be adjusted based on oxygen saturation targets. Close monitoring of patients on HFNC is necessary, so patients may require admission to ICUs or intermediate care units depending on local resources. A metric used to predict HFNC failure is the ROX index ([oxygen saturation/Fio 2]/respiratory rate).21 A value of 4.88 or above from 2 to 12 hours after application of HFNC was associated with a lower risk of intubation. Values associated with HFNC failure at different time points are reported in Table 2 . It is important to note that this scoring system does not take work of breathing into account, and clinical assessment is needed to determine whether ongoing HFNC therapy is appropriate.
Table 2.
ROX score to predict high-flow nasal cannula failure
| Time Point, h | Likely Failure Value |
|---|---|
| 2 | 2.85 |
| 6 | 3.47 |
| 12 | 3.85 |
ROX score is calculated as the ratio of oxygen saturation/fraction of inspired oxygen over respiratory rate. A patient with oxygen saturation of 90% on fraction of inspired oxygen of 0.70 with a respiratory rate of 24 would have the value (90/0.7)/24 = 5.36.
Noninvasive ventilation
Noninvasive ventilation is frequently used in patients with ARDS, although its use remains controversial. The potential benefits include avoidance of ventilator-associated events and need for deep sedation, which often come with IMV. In addition, appropriately titrated end expiratory pressure could decrease injury related to vigorous spontaneous breathing.22 Potential harms include delayed (necessary) intubation, inability to control tidal volumes and monitor airway pressures, and inconsistent mask seal, which could lead to cyclic recruitment-derecruitment of lung units causing atelectrauma. A small randomized trial in patients with moderate to severe ARDS using a full-helmet interface showed reduction in rate of intubation as well as mortality when compared with standard facemask NIV.23 A study in patients with COVID-19 ARDS using helmet interface (compared with HFNC) did not show a mortality benefit, but did demonstrate a reduction in intubation rate as well as ventilator days.24 Patients in the helmet NIV group frequently required sedation to facilitate comfort. Helmet NIV must be used with a traditional ventilator, as it requires a dual limb circuit (inspiratory and expiratory limbs), and the settings must be adjusted carefully to avoid carbon dioxide rebreathing. Helmet NIV is not routinely available in most North American hospitals, requires familiarity with the technology, and would benefit from further study in pragmatic trials before widespread use.
Most patients receive NIV via facemask interface, with widespread use owing to its strong evidence base for hypercapnic respiratory failure and cardiogenic pulmonary edema. The data on its role in ARDS are conflicting. The RECOVERY-RS trial in patients with COVID ARDS demonstrated a decreased intubation rate, but no mortality benefit, in patients treated with NIV (continuous positive airway pressure, specifically) compared with standard oxygen therapy; the same benefit was not observed when comparing HFNC with standard oxygen therapy.25 A propensity-matched analysis of the LUNG-SAFE study demonstrated an increased risk of mortality of patients with moderate to severe ARDS who received NIV compared with those who received IMV only.26 High-quality, prospective randomized trials are needed to better define the role of NIV in ARDS.
Safety monitoring in NIV is essential, and the authors advocate for these patients to be admitted to ICUs when possible. Tidal volume monitoring plays an important role. Patients with moderate to severe hypoxemia and tidal volumes greater than 9.5 mL/kg of ideal body weight (IBW) despite attempts to lower the tidal volume by changing settings are expected to fail NIV (82% sensitivity, 87% specificity).27 The HACOR score (Heart rate, Acidosis, Consciousness, Oxygenation, Respiratory rate) predicts failure of NIV in patients with hypoxemic respiratory failure.28 A summary of the score and its value at different timepoints can be found at this referenced summary29; the score itself is listed in Table 3 . Score at 1 hour may also help differentiate patients who may be less likely to die if intubated early (within 12 hours of NIV initiation).28 HACOR scores will tend to improve in patients with NIV success and remain unchanged in patients with NIV failure. The presence of other organ failures may also predict NIV failure.30 In summary, if a patient with ARDS placed on NIV does not markedly improve, ongoing use in the individual should be reconsidered.
Table 3.
HACOR score to predict noninvasive ventilation failure
| Variables | Category | Assigned Points |
|---|---|---|
| Heart rate (beats/min) | ≤120 | 0 |
| >121 | 1 | |
| pH | ≥7.35 | 0 |
| 7.30–7.34 | 2 | |
| 7.25–7.29 | 3 | |
| <7.25 | 4 | |
| Glascow Coma Scale | 15 | 0 |
| 13–14 | 2 | |
| 11–12 | 5 | |
| ≤10 | 10 | |
| Pao2:Fio2 ratio | ≥201 | 0 |
| 176–200 | 2 | |
| 151–175 | 3 | |
| 126–150 | 4 | |
| 101–125 | 5 | |
| ≤100 | 6 | |
| Respiratory rate (breaths/min) | ≤30 | 0 |
| 31–35 | 1 | |
| 36–40 | 2 | |
| 41–45 | 3 | |
| ≥46 | 4 |
Awake prone positioning
Awake prone positioning became popular during the early portion of the COVID-19 pandemic, despite a lack of meaningful outcome data at that time. Subsequently, a multinational randomized “meta-trial” of 1121 patients compared HFNC and awake prone positioning with HFNC alone.31 There was a 6% absolute reduction in treatment failure (defined as intubation or death) in the prone position group, with no signal of harm detected. Patients who were able to prone for more than 8 hours per day had a lower rate of treatment failure compared with those who were in the prone position for less than 8 hours as well. Although prone, patients had improvement in ROX score as well as its components (SpO2:Fio 2 ratio and respiratory rate). This treatment is easy to implement and could be instituted while still in the emergency department, assuming the patient is an otherwise suitable candidate for HFNC and can self-prone without difficulty or excess discomfort. Less is known about the combination of NIV and awake prone positioning.
Invasive mechanical ventilation management
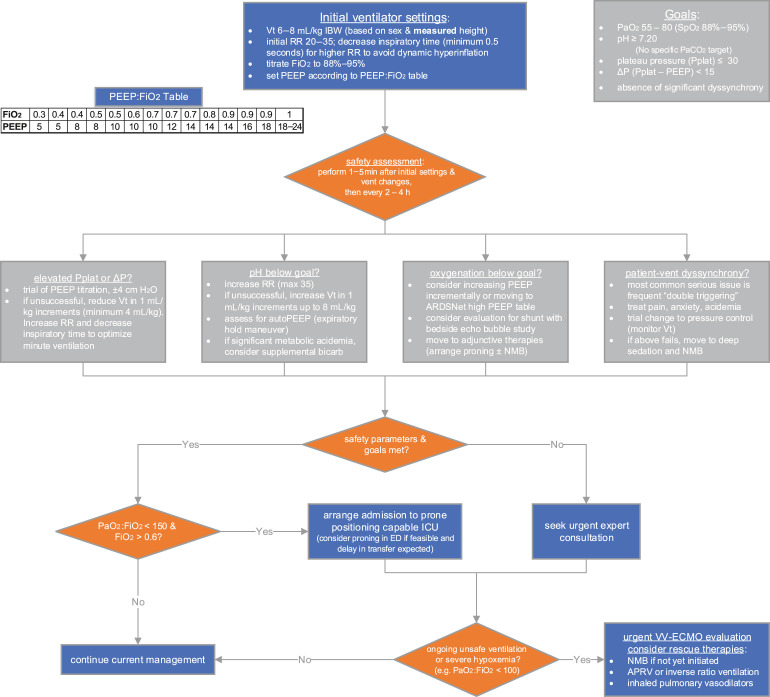
Fig. 1 provides a comprehensive management algorithm. The supporting information for this algorithm is described in the following sections.
Fig. 1.
Proposed management algorithm for ARDS in the emergency department. ΔP, driving pressure; ED, emergency department; max, maximum; Paco2, partial pressure of arterial carbon dioxide; Pplat, plateau pressure; RR, respiratory rate; Vt, tidal volume.
Safety parameters
Limiting harm from mechanical ventilation is the highest-priority action. Details on tidal volume and PEEP selection will be detailed subsequently. In general, oxygenation goals include partial pressure of arterial oxygen (Pao 2) target between 55 and 80 mm Hg or pulse oximetry (SpO2) values from 88% to 95%. Permissive hypercapnia is generally well tolerated and may actually be protective.32 Various lower limits for pH have been described (anywhere from 7.15 to 7.25), although no specific threshold is supported by strong evidence. Plateau pressure, a surrogate for compliance of the respiratory system, should be kept less than 30 cm H2O.33 This value can be obtained by initiating a brief (0.5 second) inspiratory pause on the ventilator, during passive ventilation. Observational data also suggest decreased mortality with the driving pressure (the difference between plateau pressure and PEEP) kept lower than 13 to 15 cm H2O.34, 35, 36 Plateau pressure should be rechecked periodically and after each ventilator adjustment; changes to plateau and driving pressures can be observed in as short as 1 to 5 minutes after adjustments are made,37 although longer time periods are required to see improvements from slow recruitment. More information on these targets and how to reach them is included in Fig. 1.
Low-tidal-volume ventilation
Despite more than 2 decades of knowledge that lower tidal volumes are associated with lower mortality,33 implementation of low-tidal volume ventilation (LTVV) is inconsistent,1 even in expert centers and with higher severity of disease.38 Several studies have demonstrated that protocolized care in ARDS is associated with better adherence to LTVV, as well as improved outcomes.39 , 40 In fact, similar findings have been demonstrated for protocol-driven ventilator management in the emergency department. LOV-ED, a before-after implementation study, showed a 48% increase in lung-protective ventilation after protocol implementation.5 Importantly, this protocol also improved adherence to lung protective ventilation of the same patients in the ICU, suggesting a “carry-over” effect. Prompt initiation of LTVV is important, as observational data suggest even early application of higher tidal volumes is associated with higher mortality.41 It must be emphasized that tidal volume targets should be set based on ideal rather than actual body weight, as patients with obesity are more likely to receive higher tidal volumes.42 For patients with ARDS, the goal tidal volume range falls between 4 and 8 mL/kg of IBW (starting at 6 mL/kg for most patients).43
Positive end expiratory pressure titration
The goal of PEEP titration is to achieve alveolar recruitment without overdistention, thereby homogenizing the alveoli as much as possible. No approach to the titration of PEEP has proven superior to any other in terms of patient-centered outcomes.44 , 45 Patients with more severe hypoxemia may have lower mortalities when a higher PEEP strategy is used.46 In practice, PEEP may be set lower than appropriate, as even patients with severe hypoxemia receive only 10 cm H2O PEEP on average.1 Some clinicians may be hesitant to increase PEEP if hemodynamic instability is present, although it is reasonable to still perform a brief trial of PEEP increase to see if it is tolerable and safe by hemodynamics and plateau pressures. Often, changes in oxygenation are used to assess if PEEP titration was helpful, although a “positive” response may be better reflected by a decrease in driving pressure.34 , 47 More data are needed to assess strategies of PEEP titration. In general it is reasonable to use the ARDSnet low PEEP table as a starting point (see Fig. 1), but to consider the high PEEP table if hypoxemia is more severe.48 It is essential to monitor plateau and driving pressures, as well as hemodynamics, while titrating PEEP.
Prone position ventilation
The physiologic benefits of prone positioning have been discussed in detail elsewhere,49 but can be summarized briefly as improving dorsal lung recruitment, overall ventilation/perfusion matching, and homogenizing stress and strain throughout the lung. There may also be hemodynamic benefits as well, because prone positioning tends to offload the right ventricle through a combination of mechanisms.50 Prone positioning is consistently associated with improved mortality in patients with moderate to severe ARDS,51 , 52 especially when paired with LTVV.53 The largest trial showing mortality benefit, PROSEVA, enrolled patients with moderate to severe ARDS after 12 to 24 hours of optimized mechanical ventilation,52 which calls into question whether this strategy is needed in patients while still in the emergency department. It may become necessary if long delays in transfer to the ICU or another facility are anticipated, although familiarity with the process is important, as the mortality reduction in PROSEVA was approximately 16%. A process for performing prone positioning in the emergency department has been described elsewhere.54
Neuromuscular blockade
The use of neuromuscular blockade (NMB) in patients with moderate to severe ARDS is associated with improved oxygenation, decreased ventilator-associated lung injury, and improved mortality at 28 days, without increasing the incidence of neuromuscular weakness.55 The most recent multicenter trial of NMB in ARDS (ROSE, data from which are included in Ref.55), did not demonstrate a mortality benefit compared with a control group, which only used light sedation, so routine use of NMB has been called into question. NMB use does require deep sedation, which is associated with increased mortality even after accounting for severity of illness, so this strategy must be used cautiously.56 Optimal usage would likely be in a patient with moderate to severe ARDS who is also having significant ventilator dyssynchrony not amenable to ventilator adjustments. Bolus and/or infusions of NMB agents should be used for the shortest duration possible. In the emergency department, it may be more practical to use intermittent boluses depending on length of stay. It is also important to note that depth of sedation does not correlate with respiratory drive, so deep sedation without NMB may not be a viable strategy to mitigate dyssynchrony.57
Fluid management
A conservative (compared with liberal) fluid strategy in patients with ARDS is associated with fewer days of mechanical ventilation.58 Patients with ARDS may present with clinical signs of hypoperfusion as well as frank shock. Despite this fact, clinicians should exercise caution with fluid administration, ideally doing so in a targeted fashion (ie, with formal preload responsiveness assessment), if at all.
Corticosteroids
The use of corticosteroids in ARDS remains controversial, owing to decades of conflicting data. Their use has increased after positive clinical trial results for dexamethasone in patients with COVID-19 pneumonia, demonstrating decreased days of mechanical ventilation as well as mortality,59 as well as a subsequent meta-analysis with compatible results.60 Similar findings were seen in patients with moderate to severe ARDS, prepandemic, treated with dexamethasone 20 mg for 5 days followed by 10 mg for 5 days, in an open-label, multicenter trial.61 A meta-analysis that included the aforementioned trial also concluded corticosteroids are associated with decreased mortality and days of mechanical ventilation, without increasing risk of hospital-acquired infections.62 The decision to initiate corticosteroids (and at what dose) will likely depend on local/institutional factors, and multidisciplinary collaboration is encouraged. It is reasonable to consider steroids earlier in patients with higher severity of illness.
Inhaled pulmonary vasodilators
Inhaled pulmonary vasodilators have been used in ARDS with the intent of reversing hypoxemic vasoconstriction in ventilated lung units. This effect could also lead to decreased pulmonary vascular resistance, and therefore, right ventricular afterload. Unfortunately, these medications have not been shown to have any patient-centered outcome benefit in trials or high-quality observational studies. Nitric oxide may improve oxygenation without any benefit on mortality and potentially increases the risk of acute kidney injury.63 In addition, nitric oxide is very expensive in the United States. Inhaled prostaglandins, such as epoprostenol, similarly have been shown to improve oxygenation and lower pulmonary artery pressures, without mortality benefit.64 As such, these medications should be used sparingly (if at all) and could be considered in the following situations: (1) rescue of refractory hypoxemia, (2) coexistent right ventricular dysfunction, and/or (3) coexistent intracardiac shunt.
Airway pressure release ventilation
Airway pressure release ventilation (APRV) is an alternative mode of ventilation that leverages inverse ratio ventilation (inspiratory time greater than expiratory time) and allows spontaneous breathing. Trial data on APRV are sparse, and its use is most supported by a single-center study that demonstrated improved compliance, oxygenation, decreased days of mechanical ventilation, and sedation.65 The use of APRV cannot be routinely recommended over conventional modes, but could be considered in centers with sufficient expertise to manage the mode, as well as in cases of refractory hypoxemia, especially if the patient is not a candidate for extracorporeal life support.
Extracorporeal life support
Extracorporeal carbon dioxide removal (ECCO2R) is a low-flow form of venovenous support that has been studied in ARDS. An advantage over venovenous extracorporeal membrane oxygenation (VV-ECMO) is the smaller cannula size (15.5 French, slightly larger than a hemodialysis catheter), although it does not provide any oxygenation support. The most recent trial compared usual care with a strategy of using ECCO2R to achieve ultraprotective tidal volumes (as low as 3 mL/kg IBW). No mortality benefit was seen, and patients in the intervention group required more sedation and NMB and had longer duration of mechanical ventilation.66 Currently, the use of ECCO2R is not approved by the Food and Drug Administration and can only be used in the context of a clinical trial or under Emergency Use Authorization.
VV-ECMO has been studied in 2 large multicenter trials, CESAR67 and EOLIA,68 with somewhat conflicting results. However, an individual patient data meta-analysis of the 2 trials concluded that VV-ECMO reduces 90-day mortality in patients with severe ARDS (relative risk, 0.75; 95% confidence interval, 0.60–0.94).69 Notably, the median Pao 2:Fio 2 of patients at enrollment in both treatment and control groups was just under 80. VV-ECMO carries significant cost, is invasive, and is associated with increased risk of morbidity owing to cannulation and anticoagulation. Both trials were done in expert centers, an important consideration with evaluating external validity.
Approach to refractory hypoxemia
No standard definition for refractory hypoxemia exists; some studies have used a Pao 2:Fio 2 ratio of less than 60, whereas Fio 2 is set at 1.0.70 Furthermore, refractory ARDS may be better captured by inclusion of other factors, such as severe respiratory acidemia, unsafe airway pressures (plateau and driving pressure), and right ventricular dysfunction, rather than solely by hypoxemia.
Most concerning is the poor utilization of evidence-based therapies in patients with moderate to severe ARDS. A recent multicenter observational study demonstrated that less than one-third of patients received lung protective ventilation (defined as tidal volume ≤6.5 mL/kg IBW and plateau pressure <30 cm H2O).38 PEEP is usually lower than recommended targets in these patients as well. Interventions with low quality of evidence, such as inhaled pulmonary vasodilators, are more frequently implemented than prone positioning.38 , 70
The primary considerations for treating a patient with refractory/severe ARDS is sequential implementation of evidence-based therapies, as in Fig. 1. Patients who are referred to expert centers may have improved outcomes,67 so early consultation is recommended. After optimization of tidal volume and PEEP, early prone positioning and NMB should be initiated. If these interventions do not achieve safe airway pressure and gas exchange parameters, consultation with a VV-ECMO center is advised. If the patient is deemed not a candidate for VV-ECMO, alternative modes of ventilation, such as APRV, can be considered, as well as inhaled pulmonary vasodilators.
Summary
ARDS occurs commonly in patients with respiratory failure in the emergency department. Lung protective ventilation strategies as well as early prone position ventilation have the largest impact on mortality. Protocolized care for ARDS, in the emergency department or ICU, is associated with increased adherence to lung protective ventilation and improved outcomes. Other interventions, such as NMB and VV-ECMO, can be considered in a sequential fashion if airway pressure and gas exchange targets cannot be achieved. In challenging or refractory cases, early expert consultation is advised.
Clinics care points
-
•
Evidence based management of ARDS for an intubated patient relies on accurate administration of low tidal volume ventilation based on ideal body weight.
-
•
There is no “best” strategy for PEEP titration. The PEEP:FiO2 table is a reasonable place to start.
Acknowledgments
Disclosure
The authors have no financial conflicts of interest.
Footnotes
ICMJE Statement: Both A. Gragossian and M.T. Siuba drafted, revised, and approved the final version of this article. No funding was received for this work.
References
- 1.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, Patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Fuller B.M., Mohr N.M., Dettmer M., et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med Off J Soc Acad Emerg Med. 2013;20(7):659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller B.M., Mohr N.M., Miller C.N., et al. Mechanical Ventilation and ARDS in the ED: A multicenter, observational, prospective, cross-sectional study. Chest. 2015;148(2):365–374. doi: 10.1378/chest.14-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenfeld G.D., Caldwell E., Peabody E., et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Fuller B.M., Ferguson I.T., Mohr N.M., et al. Lung-protective ventilation initiated in the emergency department (LOV-ED): a quasi-experimental, before-after trial. Ann Emerg Med. 2017;70(3):406–418. doi: 10.1016/j.annemergmed.2017.01.013. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Matthay M.A., Thompson B.T., Ware L.B. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;9(8):933–936. doi: 10.1016/S2213-2600(21)00105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soumagne T., Winiszewski H., Besch G., et al. Pulmonary embolism among critically ill patients with ARDS due to COVID-19. Respir Med Res. 2020;78:100789. doi: 10.1016/j.resmer.2020.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contou D., Pajot O., Cally R., et al. Pulmonary embolism or thrombosis in ARDS COVID-19 patients: a French monocenter retrospective study. PLoS ONE. 2020;15(8):e0238413. doi: 10.1371/journal.pone.0238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copetti R., Soldati G., Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6(1):16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiumello D., Umbrello M., Sferrazza Papa G.F., et al. Global and regional diagnostic accuracy of lung ultrasound compared to CT in patients with acute respiratory distress syndrome. Crit Care Med. 2019;47(11):1599–1606. doi: 10.1097/CCM.0000000000003971. [DOI] [PubMed] [Google Scholar]
- 12.Chiumello D., Cressoni M., Carlesso E., et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med. 2014;42(2):252–264. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 13.Algieri I., Mongodi S., Chiumello D., et al. CT scan and ultrasound comparative assessment of PEEP-induced lung aeration changes in ARDS. Crit Care. 2014;18(1):P285. [Google Scholar]
- 14.Bouhemad B., Brisson H., Le-Guen M., et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183(3):341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 15.Osman D., Monnet X., Castelain V., et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35(1):69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 16.Mekontso Dessap A., Boissier F., Charron C., et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. Available at: https://dx.doi.org.ccmain.ohionet.org/10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 17.Boissier F., Katsahian S., Razazi K., et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 18.Mekontso Dessap A., Boissier F., Leon R., et al. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med. 2010;38(9):1786–1792. doi: 10.1097/CCM.0b013e3181eaa9c8. [DOI] [PubMed] [Google Scholar]
- 19.Frat J.P., Thille A.W., Mercat A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 20.Rochwerg B., Einav S., Chaudhuri D., et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roca O., Caralt B., Messika J., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 22.Morais C.C.A., Koyama Y., Yoshida T., et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med. 2018;197(10):1285–1296. doi: 10.1164/rccm.201706-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel B.K., Wolfe K.S., Pohlman A.S., et al. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome. JAMA. 2016;315(22):2435. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial | critical care medicine | JAMA | JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/2778088 Available at: Accessed November 6, 2021. [DOI] [PMC free article] [PubMed]
- 25.An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19 | medRxiv. https://www.medrxiv.org/content/10.1101/2021.08.02.21261379v1.full Available at: Accessed November 6, 2021.
- 26.Bellani G., Laffey J.G., Pham T., et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 27.Carteaux G., Millán-Guilarte T., De Prost N., et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44(2):282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 28.Duan J., Han X., Bai L., et al. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43(2) doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 29.Predicting NIV failure in hypoxemic patients: the HACOR score. ESICM Published; 2017. https://www.esicm.org/article-review-icm-hacor-score-feb-2017/ Available at: Accessed November 6, 2021. [Google Scholar]
- 30.Rodríguez A., Ferri C., Martin-Loeches I., et al. Risk factors for noninvasive ventilation failure in critically Ill subjects with confirmed influenza infection. Respir Care. 2017;62(10):1307–1315. doi: 10.4187/respcare.05481. [DOI] [PubMed] [Google Scholar]
- 31.Ehrmann S., Li J., Ibarra-Estrada M., et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;0(0) doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Croinin D., Ni Chonghaile M., Higgins B., et al. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9(1):51–59. doi: 10.1186/cc2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acute Respiratory Distress Syndrome Network, Brower R.G., Matthay M.A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 34.Amato M.B.P., Meade M.O., Slutsky A.S., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 35.Guérin C., Papazian L., Reignier J., et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care. 2016;20(1):384. doi: 10.1186/s13054-016-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goligher E.C., Costa E.L.V., Yarnell C.J., et al. Effect of lowering tidal volume on mortality in ARDS varies with respiratory system elastance. Am J Respir Crit Care Med. 2021;13 doi: 10.1164/rccm.202009-3536OC. [DOI] [PubMed] [Google Scholar]
- 37.Sahetya S.K., Hager D.N., Stephens R.S., et al. PEEP titration to minimize driving pressure in subjects with ARDS: a prospective physiological study. Respir Care. 2020;65(5):583–589. doi: 10.4187/respcare.07102. [DOI] [PubMed] [Google Scholar]
- 38.Qadir N., Bartz R.R., Cooter M.L., et al. Variation in early management practices in moderate-to-severe ARDS in the United States: the severe ARDS: generating evidence study. Chest. 2021;160(4):1304–1315. doi: 10.1016/j.chest.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duggal A., Panitchote A., Siuba M., et al. Implementation of Protocolized Care in ARDS Improves Outcomes. Respir Care. 2020 doi: 10.4187/respcare.07999. Published online. [DOI] [PubMed] [Google Scholar]
- 40.Gallo de Moraes A., Holets S.R., Tescher A.N., et al. The Clinical effect of an early, protocolized approach to mechanical ventilation for severe and refractory hypoxemia. Respir Care. 2020;65(4):413–419. doi: 10.4187/respcare.07243. [DOI] [PubMed] [Google Scholar]
- 41.Needham D.M., Yang T., Dinglas V.D., et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. a prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalra S.S., Siuba M., Panitchote A., et al. Higher class of obesity is associated with delivery of higher tidal volumes in subjects with ARDS. Respir Care. 2020;65(10):1519–1526. doi: 10.4187/respcare.07110. [DOI] [PubMed] [Google Scholar]
- 43.NHLBI ARDS Network | Tools. http://ardsnet.org/tools.shtml Available at: Accessed November 7, 2021.
- 44.Yi H., Li X., Mao Z., et al. Higher PEEP versus lower PEEP strategies for patients in ICU without acute respiratory distress syndrome: a systematic review and meta-analysis. J Crit Care. 2022;67:72–78. doi: 10.1016/j.jcrc.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Walkey A.J., Del Sorbo L., Hodgson C.L., et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S297–S303. doi: 10.1513/AnnalsATS.201704-338OT. [DOI] [PubMed] [Google Scholar]
- 46.Briel M., Meade M., Mercat A., et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome. JAMA. 2010;303(9):865. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 47.Yehya N., Hodgson C.L., Amato M.B.P., et al. Response to ventilator adjustments for predicting acute respiratory distress syndrome mortality. driving pressure versus oxygenation. Ann Am Thorac Soc. 2020;18(5):857–864. doi: 10.1513/AnnalsATS.202007-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan E., Del Sorbo L., Goligher E.C., et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 49.Gattinoni L., Taccone P., Carlesso E., et al. Prone position in acute respiratory distress syndrome. rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 50.Paternot A., Repessé X., Vieillard-Baron A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care. 2016;61(10):1391–1396. doi: 10.4187/respcare.04943. [DOI] [PubMed] [Google Scholar]
- 51.Munshi L., Del Sorbo L., Adhikari N.K.J., et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 52.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 53.Sud S., Friedrich J.O., Adhikari N.K., et al. Comparative effectiveness of protective ventilation strategies for moderate and severe ARDS: network meta-analysis. Am J Respir Crit Care Med. 2021;6 doi: 10.1164/rccm.202008-3039OC. [DOI] [PubMed] [Google Scholar]
- 54.McGurk K., Riveros T., Johnson N., et al. A primer on proning in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(6):1703–1708. doi: 10.1002/emp2.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torbic H., Krishnan S., Harnegie M.P., et al. Neuromuscular blocking agents for ARDS: a systematic review and meta-analysis. Respir Care. 2021;66(1):120–128. doi: 10.4187/respcare.07849. [DOI] [PubMed] [Google Scholar]
- 56.Stephens R.J., Dettmer M.R., Roberts B.W., et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018;46(3):471–479. doi: 10.1097/CCM.0000000000002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dzierba A.L., Khalil A.M., Derry K.L., et al. Discordance between respiratory drive and sedation depth in critically Ill patients receiving mechanical ventilation. Crit Care Med. 2021;27 doi: 10.1097/CCM.0000000000005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann H.P., Wheeler A.P., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 59.Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 62.Zayed Y., Barbarawi M., Ismail E., et al. Use of glucocorticoids in patients with acute respiratory distress syndrome: a meta-analysis and trial sequential analysis. J Intensive Care. 2020;8(1):43. doi: 10.1186/s40560-020-00464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karam O., Gebistorf F., Wetterslev J., et al. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a Cochrane Systematic Review with trial sequential analysis. Anaesthesia. 2017;72(1):106–117. doi: 10.1111/anae.13628. [DOI] [PubMed] [Google Scholar]
- 64.Fuller B.M., Mohr N.M., Skrupky L., et al. The use of inhaled prostaglandins in patients with ARDS. Chest. 2015;147(6):1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y., Jin X., Lv Y., et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43(11):1648–1659. doi: 10.1007/s00134-017-4912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNamee J.J., Gillies M.A., Barrett N.A., et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the rest randomized clinical trial. JAMA. 2021;326(11):1013–1023. doi: 10.1001/jama.2021.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 68.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 69.Combes A., Peek G.J., Hajage D., et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46(11):2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duan E.H., Adhikari N.K.J., D’Aragon F., et al. Management of acute respiratory distress syndrome and refractory hypoxemia. a multicenter observational study. Ann Am Thorac Soc. 2017;14(12):1818–1826. doi: 10.1513/AnnalsATS.201612-1042OC. [DOI] [PubMed] [Google Scholar]