Abstract
Background and Purpose
The expression of the 18-kDA mitochondrial translocator protein (TSPO) in the brain is an attractive target to study neuroinflammation. However, the binding properties of TSPO ligands are reportedly dependent on genetic polymorphism of the TSPO gene (rs6971). The objective of this study is to investigate the rs6971 gene polymorphism in the Korean population.
Methods
We performed genetic testing on 109 subjects including patients with mild cognitive impairment, Alzheimer’s disease (AD) dementia, non-AD dementia, and cognitively unimpaired participants. Magnetic resonance imaging scans and detailed neuropsychological tests were also performed, and 29 participants underwent 18F-DPA714 PET scans. Exon 4 of the TSPO gene containing the polymorphism rs6971 (Ala or Thr at position 147) was polymerase chain reaction amplified and sequenced using the Sanger method. The identified rs6971 genotype codes (C/C, C/T, or T/T) of the TSPO protein generated high-, mixed-, or low-affinity binding phenotypes (HABs, MABs, and LABs), respectively.
Results
We found that 96.3% of the study subjects were HAB (105 out of 109 subjects), and 3.7% of the subjects were MAB (4 out of 109 subjects). 18F-DPA-714 PET scans showed nonspecific binding to the thalamus and brainstem, and increased tracer uptake throughout the cortex in cognitively impaired patients. The participant with the MAB polymorphism had a higher DPA714 signal throughout the cortex.
Conclusions
The majority of Koreans are HAB (aprox. 96%). Therefore, the polymorphism of the rs6971 gene would have a smaller impact on the availability of second-generation TSPO PET tracers.
Keywords: TSPO Protein, Human; Single Nucleotide Polymorphism; Radioactive Tracer; Positron-Emission Tomography; Neurodegenerative Diseases; Neuroinflammatory Diseases
INTRODUCTION
Recent studies have revealed a significant contribution of the immune response in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD).1 Soluble oligomeric amyloid β (Aβ) and fibrils are recognized by receptors on the microglia, the main immune cells of the CNS, that induce the release of proinflammatory cytokines and chemokines, further promoting the accumulation of amyloid plaques.2
PET imaging targeting the 18 kD translocator protein (TSPO) has been used in clinical research to investigate the in vivo inflammatory response in several neurological diseases including stroke, multiple sclerosis and AD.3,4,5 TSPO is present in low concentrations under typical conditions in the normal brain, but its expression is greatly upregulated on the outer mitochondrial membrane of microglia in response to neuroinflammatory conditions, making it an attractive target for research.6
However, the first widely utilized TSPO ligand (11C-PK11195) showed a low signal-to-noise ratio due to poor brain permeability and marked nonspecific binding.7 Moreover, the use of 11C limited its availability to research centres with on-site cyclotrons. To overcome these problems, second-generation TSPO tracers have been developed.8
However, it is known that the binding properties of newer TSPO radioligands depend on the genetic polymorphism (rs6971) of the TSPO gene, resulting in groups with low, mixed, and high binding affinity.9 The aim of this study is to investigate the distribution of the rs6971 gene polymorphism in the Korean population and to determine its contribution to TSPO binding using the second-generation TSPO radioligand, 18F-DPA714.
MATERIALS AND METHODS
Participants
Cognitively impaired (CI) patients (n=75) and cognitively unimpaired (CU) participants (n=34) were enrolled prospectively after providing written informed consent for the study, which was approved by the Institutional Review Board of Gachon University Gil Medical Center. The CI group included patients with mild cognitive impairment (MCI; n=36), Alzheimer’s disease dementia (ADD; n=30) and non-AD dementia (n=9). The non-AD dementia group was composed of patients with frontotemporal lobar degeneration (FTLD; n=3), dementia of Lewy body (DLB; n=3), multiple system atrophy (MSA; n=1), chronic traumatic encephalopathy (CTE; n=1) and post-encephalitic dementia (n=1).
Patients with AD dementia met the probable AD criteria as proposed by the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association.10 Participants with MCI were categorized according to Petersen’s criteria for MCI.11 FTLD,12 DLB,13 MSA,14 CTE15 were diagnosed according to recently published guidelines. The old CU group (n=30) was composed of healthy volunteers with no underlying neurological or psychiatric diseases with a Clinical Dementia Rating score of 0 and normal cognitive function as determined by neuropsychological tests (defined as within 1.5 standard deviations of the normative mean adjusted for age and education level). We also included young CU volunteers (n=4, age 22–25) who were healthy volunteers with no underlying neurological or psychiatric diseases with a Clinical Dementia Rating score of 0 and normal cognitive function.
All participants completed a clinical interview and underwent a standardized neuropsychological examination, and brain magnetic resonance imaging (MRI). Individuals with structural lesions on brain MRI, such as territorial infarctions, intracranial hemorrhage, traumatic brain injury, hydrocephalus, severe white matter hyperintensity (WMH) or WMH associated with radiation, multiple sclerosis, or vasculitis were excluded. In order to exclude secondary causes of dementia, laboratory tests were performed to assess complete blood counts, vitamin B12, folate levels, thyroid function, metabolic profile and syphilis serology.
TSPO genotype
Blood samples were taken to determine TSPO genotypes. Exon 4 of the TSPO gene containing the polymorphism rs6971 (corresponding to Alanine [Ala] or Threonine [Thr] at position 147) as well as exon/intron junctions were amplified by polymerase chain reaction and sequenced using the Sanger method. Polymorphisms of rs6971 within the TSPO gene, which causes an Ala-to-Thr substitution, were categorized as high-affinity binders (HABs; C/C DNA polymorphism; Ala/Ala at position 147), mixed-affinity binders (MABs; C/T DNA polymorphism; Ala/Thr) or low-affinity binders (LABs; T/T DNA polymorphism; Thr/Thr).
MRI acquisition
3D T1-magnetization-prepared rapid gradient echo (repetition time: 1,810 ms, echo time: 2.91 ms, flip angle: 9°, pixel bandwidth: 340 Hz/pixel, matrix size: 512×512, field of view: 250 mm, number of excitations: 1, total acquisition time: 3 minutes 37 seconds, voxel size: 0.49×0.49×1.0 mm3) was acquired with a 3 T MRI scanner (Skyra, Siemens with a Siemens matrix coil). Images were analyzed using FreeSurfer 6.0 (www.surfer.nmr.mgh.harvard.edu) to define regions of interest (ROIs) in the native space for each subject and to support the correction of grey matter (GM) atrophy and white matter spillover for subsequent PET image analysis.
18F-DPA-714 PET data acquisition
Among the 109 participants, 29 underwent 18F-DPA-714 PET scans (4 young CU, 11 old CU, 1 MCI, 8 AD, and 5 non-AD). PET scans were acquired using a Siemens Biograph 6 PET/computed tomography scanner (Siemens, Knoxville, Tennessee, USA) with a list-mode emission acquisition. 18F-DPA-714 was synthesized and radiolabeled at Gachon University Neuroscience Research Institute. Twenty-five participants underwent a 30-minute emission scan starting 60 minutes after an intravenous injection of 185 MBq of 18F-DPA-714 (60–90 minutes). A low-dose computed tomography was performed prior to all scans for attenuation correction. The individual static images were reconstructed onto a 256×256×109 matrix with a voxel size of 1.3×1.3×1.5 mm3 using a 2D ordered-subset expectation maximization algorithm (8 iterations and 16 subsets), with corrections for physical effects.
PET quantification
Each 18F-DPA-714 image was coregistered with the corresponding T1 MRI image using FreeSurfer. Regional mean values of PET images were then extracted after region-based partial volume correction using the PETSurfer tool in FreeSurfer16,17 and were weight-averaged for predefined ROIs as described in the previous study.18 Regional standardized uptake value ratios (SUVRs) were calculated using cerebellar GM as the reference region for DPA images. SUVR images were also generated from PET images coregistered with the MRI images with voxel-based partial volume correction.16,17
Statistical analyses
Comparisons of demographic and clinical data among AD, MCI, CU, and non-AD dementia groups (Table 1) were conducted using one-way analysis of variance (ANOVA) with post-hoc tests with Bonferroni correction (p<0.05). Categorical variables were evaluated using the χ2 test.
Table 1. Baseline characteristics.
| Characteristics | Controls (n=34) | MCI (n=36) | ADD (n=30) | Non-ADD (n=9) | p value* | |
|---|---|---|---|---|---|---|
| Age (yr) | 62.0±14.9 | 69.3±8.4‡ | 70.1±11.8‡ | 71.2±6.8 | 0.016 | |
| Gender (% male) | 13 (38.2%) | 11 (30.6%) | 13 (43.3%) | 5 (55.6%) | 0.501† | |
| Education (yr) | 10.9±4.5 | 7.1±5.0‡ | 9.7±4.4§ | 7.5±5.5 | 0.007 | |
| K-MMSE | 29.1±1.0 | 25.8±3.1 | 16.8±6.2‡,§ | 19.6±5.0‡,§ | <0.001 | |
| CDR-SOB | 0.07±0.17 | 0.81±0.65 | 5.3±2.9‡,§ | 4.4±1.5‡,§ | <0.001 | |
| HAB | 33 (97.1%) | 35 (97.2%) | 28 (93.3%) | 9 (100%) | ||
| MAB | 1 (2.9%) | 1 (2.8%) | 2 (6.7%) | 0 | ||
| LAB | 0 | 0 | 0 | 0 | ||
Data are presented as mean ± standard deviation unless otherwise noted. CDR-SOB scored out of 18; K-MMSE scored out of 30.
MCI: mild cognitive impairment, ADD: Alzheimer’s disease dementia, K-MMSE: Korean version of Mini Mental State Examination, CDR-SOB: Clinical Dementia Rating-sum of boxes, HAB: high-affinity binder, MAB: mixed-affinity binder, LAB: low-affinity binder.
*One-way analysis of variance followed by post hoc test with Bonferroni correction (except for genderja); †χ2 test; ‡ p<0.05 versus NC; § p<0.05 versus MCI.
RESULTS
Subject characteristics
Thirty AD, 34 aMCI, 36 CU and 9 non-AD dementia participants were enrolled for a total of 109 participants (Table 1). The CU group was significantly younger and had more years of education than the MCI group. There was no significant gender difference among the AD and MCI patients, and the NC group. Compared to the CU group, the MCI and AD groups showed significantly impaired cognition based on MMSE and CDR scores.
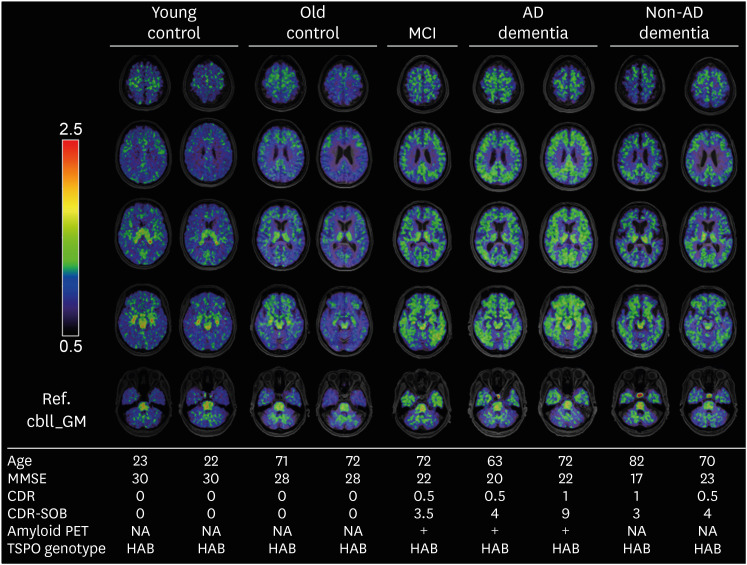
Among the 109 participants, 29 underwent 18F-DPA-714 PET scans (4 young CU, 11 old CU, 1 MCI, 8 AD, and 5 non-ADD). Representative SUVR images of 18F-DPA714 PET are presented in Fig. 1. Nonspecific binding to the thalamus and brainstem was prominent in controls as well as in CI participants. Increased uptake of DPA714 throughout the cortex was observed in prodromal AD (MCI) and ADD patients as well as in patients with non-ADD compared to CU individuals.
Fig. 1. Representative 18F-DPA714 PET standardized uptake value ratio images. Selected images of high affinity binders in the young control, old control, MCI, AD dementia, and non-AD dementia groups.
MCI: mild cognitive impairment, AD: Alzheimer’s disease, MMSE: Mini Mental State Examination, CDR-SOB: Clinical Dementia Rating-sum of boxes, NA: not applicable, TSPO: mitochondrial translocator protein, HAB: high-affinity binder.
TSPO polymorphism
We found that 96.3% of the study subjects were HAB (105 out of 109 subjects), while 3.7% were MAB (4 out of 100 subjects). None of the participants was a LAB.
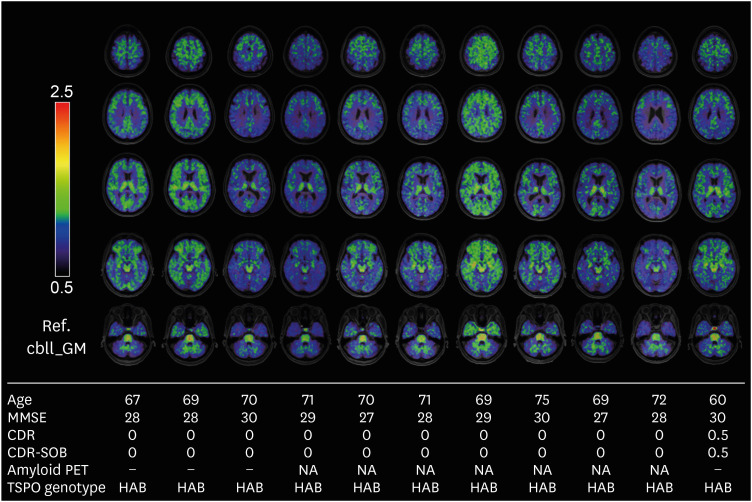
Among 29 participants who underwent DPA714 PET scans, only one of the participants in the old CU group was a MAB, while all the others were HAB. Figure 2 shows representative DPA714 PET images of 11 old CU participants, including the MAB polymorphism. There was an increase in the radiotracer uptake throughout the cortex in the MAB participant.
Fig. 2. 18F-DPA714 PET standardized uptake value ratio images of HAB and MAB polymorphisms. DPA714 PET images of ten representative HABs and one MAB in the old cognitively unimpaired group.
MMSE: Mini Mental State Examination, CDR-SOB: Clinical Dementia Rating-sum of boxes, NA: not applicable, TSPO: mitochondrial translocator protein, HAB: high-affinity binder, MAB: mixed-affinity binder.
DISCUSSION
Studies are revealing important links between neuroinflammation and neurodegenerative diseases, such as Alzheimer’s disease. However, several limitations of PET radioligands, including variable binding according to TSPO genotypes, need to be overcome in order to study this in vivo. In this study, we assessed the frequency of TSPO genotypes in a pool of South Koreans that took part in dementia studies at a single tertiary medical center.
The rs6971 missense polymorphism of the TSPO gene leads to three genotypes and an amino acid substitution from alanine to threonine: C/C (Ala/Ala), C/T (Ala/Thr) and T/T (Thr/Thr).9 Each genotype results in specific binding characteristics: high binding affinity (C/C), mixed binding affinity (C/T) and low binding affinity (T/T). This difference in ligand binding affinity may be caused by location of the TSPO polymorphism in the cholesterol-binding domain, which led to a significantly reduced affinity of cholesterol for the Thr substitution.19 This substitution has a greater effect on the binding of the second-generation TSPO tracers than the first-generation TSPO tracer, PK11195. Because of the poor binding of radioligands to the TSPO of LABs, PET results were difficult to distinguish from nonspecific binding, which limits the usefulness of secondary TSPO tracers in this group.8,20 In our study, we found that only a minority of subjects (4/109) had the LAB single nucleotide variation at SNP rs6971. Our findings were consistent with data from the Korean Genome Project (n=1,832) and the Korean population from the Korean Refence Genome Database (KRGDB, n=2,930) where 97.7% and 98.4%, respectively, had the alternate allele polymorphism.21 Similarly, the allele data obtained from the HapMap database (n=1,886) show that in East Asia, the frequency of the HAB genotype is 96.9%, MAB is 6.1%, and LAB is 0.1%.22 In contrast, other nations have a much lower percentage of HABs. According to the HapMap database, 69.2% of Americans are HABs, 28% MABs, and 2.8% LABs. In Africa, the percentage of HABs was 67.4%, MABs 29.4%, and LABs 3.2%. In Europe, the percentage of HABs was the lowest, with 45.7% HABs, 43.8% MABs, and 10.5% LABs. Other studies showed a similar distribution of rs6971 polymorphisms (Table 2). Due to the high percentage of high binding phenotypes in Korea and East Asia as a whole, the use of second-generation TSPO radioligands, such as 18F-DPA-714 is more favourable increasing the pool of subjects that can successfully utilize the tracer and reducing recruitment time for in vivo studies of the relationship between the immune response and neurodegenerative diseases.
Table 2. rs6971 polymorphism allele frequency.
| Variables | HAB (Ala/Ala) | MAB (Ala/Thr) | LAB (Thr/Thr) | Country |
|---|---|---|---|---|
| Current study | 105/109 (96%) | 4/109 (4%) | 0/109 (0%) | Korea |
| Hamelin et al.,23 2016 | 42/96 (43.8%) | 42/96 (43.8%) | 12/96 (12.5%) | France |
| Kreisl et al.,24 2013 | 9/27 (33.3) | 18/27 (66.7) | Not described | USA |
| Kim et al.,25 2018 | 22/36 (61.1) | 14/36 (38.9) | Not described | USA |
| Mizrahi et al.,26 2012 | 13/20 (65%) | 6/20 (30%) | 1/20 (5%) | Canada |
HAB: high-affinity binder, MAB: mixed-affinity binder, LAB: low-affinity binder.
In the present study, 18F-DPA-714 PET scans (n=29) revealed increased tracer retention in AD compared to NC subjects in the precuneus, mesial temporal cortex, entorhinal cortex, amygdala, hippocampus, fusiform gyrus, mid and inferior temporal regions. These data are consistent with previous studies showing increased TSPO binding in AD and prodromal AD.23,24 Since only one of the NC participants who underwent 18F-DPA-714 PET imaging in the current study was a MAB, no conclusions could be drawn regarding the binding affinity of the tracer in our population.
In this study, the frequency of HABs in Korea is approximately 96%. Although the second-generation TSPO PET tracers have the caveat that their binding affinities are susceptible to the genetic polymorphism (rs6971) of the TSPO gene, the low frequency of MABs and LABs in Korea, as in other East Asian populations, circumvents a major obstacle in their use. This could improve the availability of second-generation TSPO PET imaging in these populations.
Footnotes
Funding: This study was supported by a grant of the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No: HI14C1135).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Noh Y, Park HM.
- Data curation: Lee H, Noh Y, Kim WR, Seo HE, Park HM.
- Formal analysis: Lee H, Kim WR.
- Funding acquisition: Noh Y.
- Investigation: Lee H, Noh Y, Kim WR, Seo HE, Park HM.
- Methodology: Kim WR, Seo HE.
- Project administration: Noh Y.
- Resources: Kim WR, Seo HE.
- Software: Kim WR.
- Supervision: Noh Y, Park HM.
- Validation: Noh Y, Kim WR, Seo HE, Park HM.
- Visualization: Kim WR.
- Writing - original draft: Lee H.
- Writing - review & editing: Lee H, Noh Y, Kim WR, Seo HE, Park HM.
References
- 1.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagens MH, Golla SV, Wijburg MT, Yaqub M, Heijtel D, Steenwijk MD, et al. In vivo assessment of neuroinflammation in progressive multiple sclerosis: a proof of concept study with [18F]DPA714 PET. J Neuroinflammation. 2018;15:314. doi: 10.1186/s12974-018-1352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen DR, Matthews PM. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. Int Rev Neurobiol. 2011;101:19–39. doi: 10.1016/B978-0-12-387718-5.00002-X. [DOI] [PubMed] [Google Scholar]
- 5.Gulyas B, Toth M, Vas A, Shchukin E, Kostulas K, Hillert J, et al. Visualising neuroinflammation in post-stroke patients: a comparative PET study with the TSPO molecular imaging biomarkers [11C]PK11195 and [11C]vinpocetine. Curr Radiopharm. 2012;5:19–28. doi: 10.2174/1874471011205010019. [DOI] [PubMed] [Google Scholar]
- 6.Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- 7.Alam MM, Lee J, Lee SY. Recent progress in the development of TSPO PET ligands for neuroinflammation imaging in neurological diseases. Nucl Med Mol Imaging. 2017;51:283–296. doi: 10.1007/s13139-017-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivash L, O’Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57:165–168. doi: 10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]
- 9.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 12.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–236. doi: 10.1016/j.neuroimage.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, et al. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–343. doi: 10.1016/j.neuroimage.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JM, Lee SY, Seo S, Jeong HJ, Woo SH, Lee H, et al. Tau positron emission tomography using [18F]THK5351 and cerebral glucose hypometabolism in Alzheimer’s disease. Neurobiol Aging. 2017;59:210–219. doi: 10.1016/j.neurobiolaging.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Owen DR, Fan J, Campioli E, Venugopal S, Midzak A, Daly E, et al. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem J. 2017;474:3985–3999. doi: 10.1042/BCJ20170648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, et al. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans. 2015;43:586–592. doi: 10.1042/BST20150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International HapMap Consortium The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 23.Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 2016;139:1252–1264. doi: 10.1093/brain/aww017. [DOI] [PubMed] [Google Scholar]
- 24.Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, et al. Influence of alcoholism and cholesterol on TSPO binding in brain: PET [11C]PBR28 studies in humans and rodents. Neuropsychopharmacology. 2018;43:1832–1839. doi: 10.1038/s41386-018-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab. 2012;32:968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]