Abstract
Background and Objectives:
Almost all living cells secret nano-sized structures enclosed by the lipid bilayer called extracellular vesicles (EVs) into their extracellular milieu. These EVs play important roles in several physiological processes as a cargo delivery system. In probiotics, EVs are the main communication tool with the host. The present study aimed to assess the effect of EVs originated from Lactobacillus rhamnosus GG on the Carcinoembryonic antigen (cea) gene expression and protein (CEA) synthesis in the SW480 and HT-29 cell lines.
Materials and Methods:
Different concentrations of Lactobacillus rhamnosus GG EVs were applied on the SW480 and HT-29 cell lines. The MTT assay, Real-Time PCR, and ELISA analysis methods were exploited to explore the cell viability and the expression level of the cea gene in comparison with the β-actin gene as the control.
Results:
The two concentrations of 80 and 100 μg/ml of Lactobacillus rhamnosus GG EVs considerably affected the anti-proliferation and increased the amount of both CEA mRNA and protein (p < 0.05).
Conclusion:
Our findings showed that EVs of Lactobacillus rhamnosus GG could induce the gene expression and protein synthesis of CEA. Also, they reduced the cell proliferation of HT29 and SW480. Thus, probiotics such as EVs of Lactobacillus rhamnosus GG could be useful for preventing colorectal cancer.
Keywords: Extracellular vesicles, Lactobacillus rhamnosus GG, Probiotic, Carcinoemberyonic antigen family
INTRODUCTION
Infectious microorganisms, especially Gram-negative bacteria, apply the carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) family members to attach to the intestine and colonize there. Bacterial agents such as Escherichia coli, Salmonella Typhimurium, and Haemophilus influenzae interact with the CEA protein expressed on the surface of intestinal epithelial cells via mannose-specific lectin (1) leading to the uptake of infectious agents and protection from diseases. The cea gene family from the immunoglobulin (Ig) superfamily has a remarkably varied array of highly glycosylated glycoproteins (2, 3). The cea product, CEA, is a glycoprotein with a size of 180-kDa that is highly presented on the surface of normal cells such as intestinal cells and numerous tumoral (e.g., colon cancer, stomach cancer, pancreas cancer, ovarian cancer, and lung cancer) (4). Previous phylogenetic analysis in humans shows that the CEA family includes 29 genes 21 of which are protein-coding located on chromosome 19 (region 19q13.2–19q13.4) and organized in contiguous clusters (5).
Intestinal bacteria located in the human digestive tract are responsible for digesting food into absorbable nutrients (6, 7). These bacteria are also in charge of preventing the localization of intestinal epithelium by pathogens (8). Therefore, probiotics as living microorganisms are vastly discussed that can help the health of the host if delivered in adequate amounts (9) by both anti-infective and anti-carcinogenic properties. They confer these protective effects through binding to, competitively inhibiting, and degrading the mutagens, boosting the host innate and adaptive immunity, inducing the beneficial gut microbe, and improving the metabolic activity (10).
Lactobacillus and Bifidobacterium species are among the beneficial probiotics present in the gut. Lactobacillus rhamnosus GG is reported to prevent infectious diseases by producing antimicrobial agents such as bacterial metabolites, prebiotics, lantibiotics, and extracellular vesicles (EVs) (9, 11). Generally, the Lactobacilli species are Gram-positive rod bacteria that are microaerophile or facultative anaerobe (9).
Most of the living cells release nano-sized subcellular structures enveloped by lipid bilayer membranes called extracellular vesicles with a typical diameter of 30–100 nm (12). Many different cell types secrete EVs to their extracellular environment such that they can be found in most biological fluids (13). EVs have proved to be physiologically active and able to exert various biological functions regardless of the presence of bacterial cells (14). EVs can regulate the recipient cell physiology and gene expression through initiating some cell signaling pathways and/or intercellular translocation of proteins, lipid molecules, and RNA cargo (15, 16). Today, we know that both Gram-negative and Gram-positive bacteria produce EVs from their outer membrane and peptidoglycan cell wall, respectively (17). EVs are also called outer-membrane vesicles (OMVs) in Gram-negative bacteria or membrane vesicles (MVs) in Gram-positive bacteria (18). Having thick cell walls, the exact process of generation and release of EVs by Gram-positive bacteria is a mystery yet (19). However, their effective interaction with cellular membranes and satisfactory ability to produce large aggregates at high concentrations have been shown in different studies (20, 21). In the present study, the EVs derived from L. rhamnosus GG are surveyed for their role in modifying the expression and production of the cea gene in colorectal cancer cells and their effect has been compared with that of L. rhamnosus GG alone.
MATERIALS AND METHODS
Bacterial culture and EVs isolation.
Lactobacillus rhamnosus GG, PTCC1637, was obtained from the Iranian Research Organization for Science and Technology (IROST), Tehran, Iran. The bacteria were cultured in Man Rogosa Sharp (MRS) broth at 37°C for 24 h. For isolation of EVs, 300 ml of condition medium was collected from the cultured L. rhamnosus GG. First, the collected medium was centrifuged for 10 min at 10000 g to eliminate the cell debris and dead cells. Then, the supernatant was passed from a 0.22 mm filter (GVS filter technology, UK), and concentrated by filtration using a Centricon Plus-70 (Millipore, MA, USA). Finally, the concentrate was ultra-centrifuged at 100000 g for 60 min and the protein amount was determined at 230 nm using Nanodrop 2000 spectrophotometer (Thermo-Scientific, US) in order to evaluate the concentration of EV.
Transmission electron microscopy.
In accordance with our previous work, the morphology and size of EVs were evaluated using a transmission electron microscope (TEM) (LEO906, Germany).
Cell culture.
Two types of human colon cancer cell lines (SW480 and HT 29) were provided from the Pasture Institute (Tehran, Iran). Both cell lines were cultured in high glucose DMEM culture media containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C and 5% CO2. All reagents for cell culture were provided by the Iranian Inocolon Institute.
Cell viability test.
MTT assay was applied to explore the role of EV in the viability of HT29 and SW480 cells. Briefly, 5000 cells were exposed to different EV concentrations, including 5, 10, 20, 40, 60, 80, 100, 150, and 200 μg/ml. Also, 1 × 106 CFU of L. rhamnosus GG and PBS were tested as negative controls. Incubation was performed for 24 hours. After removing the medium of wells, 100 μl of fresh DMEM containing 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml) (Sigma-Aldrich, Germany) was added to each one and incubated at 37°C and 5% CO2 for further 4 h. Afterward, the culture medium was substituted with 100 μl dimethyl sulfoxide (Sigma-Aldrich, Germany). ELISA reader (Biorad, USA) was used to record the optical density of each well at 570 nm.
Gene expression analysis.
Real-time PCR was used for evaluating the effect of EVs in changing the cea gene expression level. Briefly, 80, 100, 150, 200 μg/ml, 1 × 106 CFU of L. rhamnosus GG, and PBS were used to treat 5 × 105 W480 and HT29 cells for 24 h. Then, the RNX-Plus kit (Cinnagen, Iran) was used according to the manufacturer's protocol for mRNA purification. The concentration of mRNA was measured using the Nanodrop spectrophotometer. Also, mRNA integrity was investigated by electrophoresis on gel agarose 1%. The Bioneer kit (Takara, Japan), containing M-MLV reverse transcriptase and both random hexonucleotides and oligo dT primers, was used for synthesizing cDNA. In the following, further real-time PCR was performed using specific primers (as depicted in Table 1) and Corbett Rotor-Gene 6000 real-time PCR cycler (Qiagen Corbett, Hilden, Germany) to evaluate the relative expression of cea and β-actin genes. The PCR procedure contained 3 min initialization time at 94°C, 40 cycles for denaturation each of 30 s at 94°C, 30 s annealing time related to each gene, and final extension for 30 s at 72°C. Rest 2009 (Qiagen, USA) was used to calculate the relative expression.
Table 1.
The sequence of primer pairs.
| Gene name | Forward sequence | Reverse sequence | Annealing temperature (°C) |
|---|---|---|---|
| cea | AGGCCAATAACTCAGCCAGT | GGGTTTGGAGTTGTTGCTGG | 59 |
| β-actin | TCATGAAGATCCTCACCGAG | CCGACACGCTAAGACTGC | 56 |
ELISA analysis.
For determining the impact of EVs on the secretion of CEA protein, the ELISA assay was carried out using the Carcinoembryonic Antigen Human ELISA kit (Abcam, USA). In summary, 1 × 106 of each SW480 and HT-29 cells were exposed to four various concentrations including 80, 100, 150, and 200 μg/ml of EVs, 1 × 106 CFU of L. rhamnosus GG, and PBS for 24 h. Then, the culture medium of each well was collected and 100 μl from each culture medium was added to a well coated with specific human anti-CEA antibody. After incubation for 150 min, washing steps were performed according to the kit protocol, and 100 μl of 1× biotinylated CEA detection antibody was added to the wells and shaken for 1 h at room temperature. Then, 100 μl 1× HRP-streptavidin solution was added to each well and incubated for 45 min at room temperature. Next, 100 μl TMB substrate was poured into each well and after incubation for 30 min, the reaction was stopped with 50 μl stop solution and the optical density of each well was recorded at 450 nm using the ELISA reader. The quantity of each sample was calculated according to the standard curve prepared with standard solutions using different concentrations that were available within the kit.
Statistical analysis.
Results were analyzed with the nonparametric Mann-Whitney test using SPSS.11 software (SPSS, Chicago, IL, USA) considering p-value < 0.05 as statistically significant. Rest 2009 software (Qiagen, USA) was used to statistically analyze the relative gene expressions.
RESULTS
Transmission electron microscopy analysis.
Fig. 1 depicts the TEM image of the EVs derived from L. rhamnosus GG. EVs appear in the form of round shapes in a dark field with a diameter of 50–150 nm.
Fig. 1.
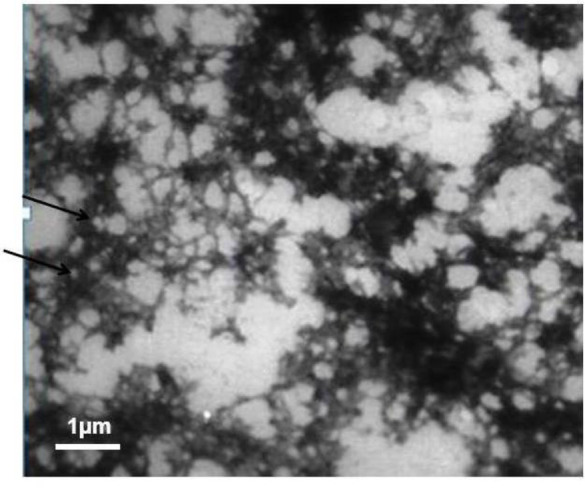
The electron microscopy image of the extracellular vesicle from Lactobacillus rhamenosus. Arrows show the example of vesicles.
Cytotoxicity impacts of extracellular vesicles from Lactobacillus rhamnosus GG.
MTT assay was implemented to evaluate the cytotoxic properties of EVs on the tested colorectal cancer cells. As shown in Figs. 2 and 3, among all studied concentrations, the values of 60, 80, 100, 150, and 200 μg/ml of EVs caused substantial inhibitory effects on both Sw480 and HT29 after 12 and 24 h incubation, respectively, in comparison with the negative control (p < 0.05). Whereas only the Sw480 cells were influenced by the significant cytotoxicity of EVs at concentrations of 10, 20, and 40 μg/ml (p < 0.05). The IC50 calculated for Sw480 cells and HT29 cells were 158.9 and 244.66 μg/ml, respectively. According to the present results, the growth of neither of tested cell lines was inhibited by L. rhamnosus GG.
Fig. 2.
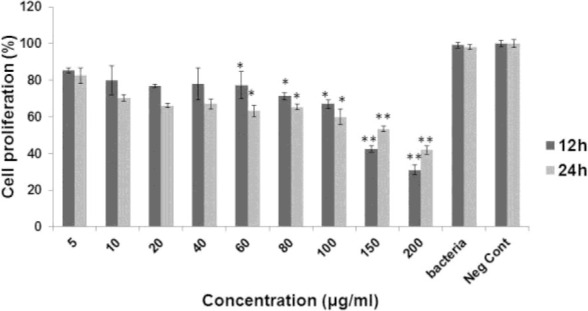
The viability percentage of Sw480 cells in different concentration of extracellular vesicle from Lactobacillus rhamenosus after 12 h and 24 h. * and ** indicate p value less than 0.05 and 0.1, respectively.
Fig. 3.
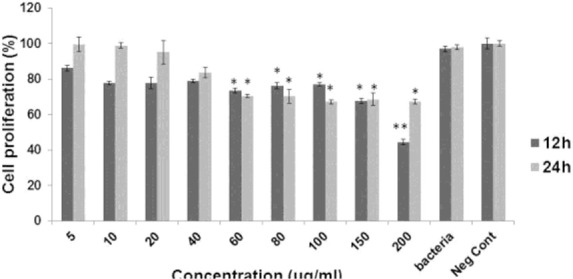
The viability percentage of HT-29 cells in different concentration of extracellular vesicle from Lactobacillus rhamenosus after 12 h and 24 h. * and ** indicate p value less than 0.05 and 0.1, respectively.
Gene expression analysis.
Real-time PCR was performed to determine the effect of probiotic EVs on the cea gene expression level. In this regard, four selected cytotoxic concentrations of EVs (80, 100, 150, and 200 μg/ml) were examined. The results obtained from the relative expression test showed a significant increase in the expression of cea gene after exposure to 150 and 200 μg/ml of EVs in SW480 cells (2.89- and 3.1-fold change, respectively) (Fig. 4) and HT29 cells (3.97- and 3.53-fold change, respectively) (Fig. 4) in comparison with the negative control (p < 0.05). On the other hand, treating cells with L. rhamnosus GG had no significant effect on the cea gene expression.
Fig. 4.
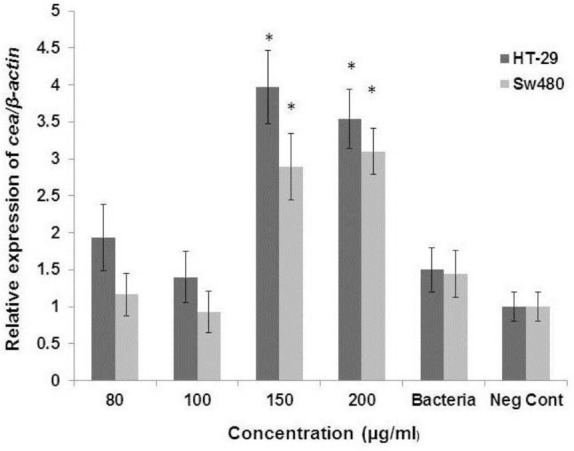
Ratio of cea gene expression mRNA after treating with different concentration of extracellular vesicle from Lactobacillus rhamenosus (80, 100, 150, and 200 μg/ml) in Sw480 cells and HT-29 * indicates p value less than 0.05.
ELISA analysis.
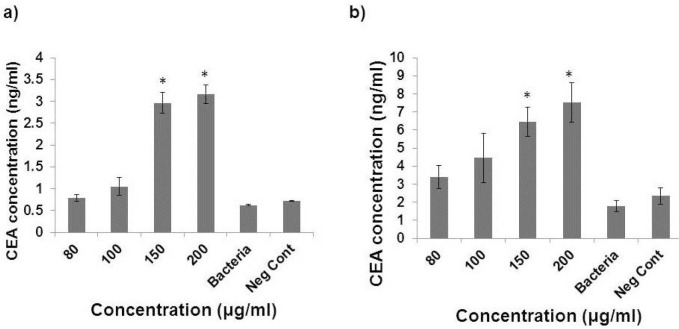
ELISA was used to investigate the effect of EVs on the CEA secretion. CEA concentration increased after exposure of SW480 cells to 150 and 200 μg/ml of EVs (2.8 and 3.01 ng/ml, respectively) compared to the negative control (0.7 ng/ml) (p < 0.05) (Fig. 5a). Also, a significant increase was observed in the amount of CEA in the culture medium of HT29 cells exposed to 150 and 200 μg/ml of EVs (6.43 and 7.5 ng/ml, respectively) compared to the negative control (2.34 ng/ml) (p < 0.05) (Fig. 5b). In contrast, direct treating with L. rhamnosus GG did not change the amount of CEA secretion from both cell lines.
Fig. 5.
CEA amount after treating with different concentration of extracellular vesicle from Lactobacillus rhamenosus (80, 100, 150, and 200 μg/ml) in Sw480 cells (a) and HT-29 (b). * indicates p value less than 0.05.
DISCUSSION
Previous studies have verified that some probiotic microorganisms can trigger cell signaling against cancer cells. These probiotics especially contain Lactobacillus and bifidobacterium species including L. casei, L. reutei, L. lactis, and L. rhamnosus. The beneficial effect of probiotic bacteria is the result of their direct and/or indirect interaction with cells via secreting bioactive components such as bacteriocins and polysaccharides (22). EV formation and their active function in delivering different elements have been frequently reported in Gram-negative bacteria and showed to be involved in their infection process. In contrast, EV formation is less studied and reported Gram-positive bacteria. Despite the differences in the cell structure of the Gram-positive and Gram-negative bacteria, there are many morphological similarities in the MVs from Gram-positive bacteria and OMVs from Gram-negative bacteria (18).
Either of pathogenic and non-pathogenic microorganisms interacting with the colonic mucosa bind to mucosal cells through the interconnection between host cell membrane receptors and specific bacterial adhesive agents. The host receptors called carcinoembryonic antigen-related cell adhesion molecules or CEACAMs are expressed on the apical membrane of the polarized colonic epithelial cells. A subfamily of these adhering molecules is Glycosylphosphatidy-linisotol (GPI) anchored CEACAMs that act as epithelial cellular receptors and interact with the adhesins expressed by some Gram-negative bacteria cells (1, 2). CEACAM receptors also contribute to the innate immune defence of host and are believed to exert a protective effect against microbial attack to the colon (23). Naghibalhosaini et al. have shown in their study that E. coli can significantly inhibit or decrease the CEA production in human colon-originated cell lines (LS-180 and HT29/219). They also highlighted that Caco-2 cells co-culture with E. coli leads to a considerable decrease the CEA production, however not statistically significant. They also showed a significant impact of lipid A treatment on decreasing the CEA production from cancer cells. They argued that E. coli and lipid A effect in a time- and dose-dependent manner, respectively (24). Another study by Sun et al. has reported the interaction between bacterial adhesins of Lactobacillus Plantarum strains and colonic epithelial cells through the d-mannosyl residue of CEA (25). However, its exact mechanism is not recognized yet.
To the best of the author’s knowledge, the present study is the first of its kind focusing on the increasing effect of EVs from Lactobacillus rhamnosus GG on CEA release from human cancer cells. The current results showed that EVs derived from L. rhamnosus can increase CEA secretion from HT29 and SW480 cancer cell lines in comparison with the control group. Our findings also showed that the proliferation of HT29 and SW480 was suppressed with 80 to 200 μg/ml concentrations of purified L. rhamnosus GG EVs. Furthermore, the effect of purified EVs from L. rhamnosus GG on the up-regulation of cea gene expression and its protein (CEA) production was notable in this study.
CONCLUSION
Overall, in the present study, probiotic EVs were isolated and their anti-proliferative effect on two colon cancer cell lines (HT29 and SW480) was investigated for the first time. Our findings showed that the gene expression within the recipient cells was altered by these probiotic-derived EVs. The increased expression of cea in cells was triggered following the exposure to 150 and 200 μg/ml of probiotic EVs. Finally, since the field of studying EVs from Gram-positive bacteria is young, many essential questions are still unanswered and understanding the exact mechanisms and features of these vesicles require further investigations.
REFERENCES
- 1.Baranov V, Hammarstrom S. Carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1), apically expressed on human colonic M cells, are potential receptors for microbial adhesion. Histochem Cell Biol 2004;121:83–89. [DOI] [PubMed] [Google Scholar]
- 2.Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 2006;18:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol 2010;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatakeyama K, Wakabayashi-Nakao K, Ohshima K, Sakura N, Yamaguchi K, Mochizuki T. Novel protein isoforms of carcinoembryonic antigen are secreted from pancreatic, gastric and colorectal cancer cells. BMC Res Notes 2013; 6: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizeq B, Zakaria Z, Ouhtit A. Towards understanding the mechanisms of actions ofcarcinoembryonic antigen-related cell adhesion molecule 6 incancer progression. Cancer Sci 2018; 109: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed) 2011;16:1768–1786. [DOI] [PubMed] [Google Scholar]
- 7.Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res 2009;56:1–15. [DOI] [PubMed] [Google Scholar]
- 8.O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol 2008;24:51–58. [DOI] [PubMed] [Google Scholar]
- 9.Khani S, Hosseini HM, Taheri M, Nourani MR, Imani Fooladi AA. Probiotics as an alternative strategy for prevention and treatment of human diseases: a review. Inflamm Allergy Drug Targets 2012;11:79–89. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 11.Doron S, Snydman DR, Gorbach SL. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am 2005;34:483–498. [DOI] [PubMed] [Google Scholar]
- 12.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012;64:676–705. [DOI] [PubMed] [Google Scholar]
- 13.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, Aindelis G, Tompoulidou E, Lamprianidou EE, et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of trail in colon carcinoma cells. PLoS One 2016;11(2):e0147960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011;71:3792–3801. [DOI] [PubMed] [Google Scholar]
- 16.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847–856. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini HM, Fooladi AA, Nourani MR, Ghanezadeh F. The role of exosomes in infectious diseases. Inflamm Allergy Drug Targets 2013;12:29–37. [DOI] [PubMed] [Google Scholar]
- 18.Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One 2011;6(11):e27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M. Potential roles of fungal extracellular vesicles during infection. mSphere 2016;1(4):e00099–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 2015;13:620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 2010;64:163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oelschlaeger TA. Mechanisms of probiotic actions - A review. Int J Med Microbiol 2010;300:57–62. [DOI] [PubMed] [Google Scholar]
- 23.Muenzner P, Rohde M, Kneitz S, Hauck CR. CEA-CAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol 2005;170:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naghibalhossaini F, Sayadi K, Jaberie H, Bazargani A, Eftekhar E, Hosseinzadeh M. Inhibition of CEA release from epithelial cells by lipid A of gram-negative bacteria. Cell Mol Biol Lett 2015;20:374–384. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Le GW, Shi YH, Su GW. Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett Appl Microbiol 2007;44:79–85. [DOI] [PubMed] [Google Scholar]