Abstract
Extrapulmonary complications of different organ systems have been increasingly recognized in patients with severe or chronic Coronavirus Disease 2019 (COVID-19). However, limited information on the skeletal complications of COVID-19 is known, even though inflammatory diseases of the respiratory tract have been known to perturb bone metabolism and cause pathological bone loss. In this study, we characterize the effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on bone metabolism in an established golden Syrian hamster model for COVID-19. SARS-CoV-2 causes significant multifocal loss of bone trabeculae in the long bones and lumbar vertebrae of all infected hamsters. Moreover, we show that the bone loss is associated with SARS-CoV-2-induced cytokine dysregulation, as the circulating pro-inflammatory cytokines not only upregulate osteoclastic differentiation in bone tissues, but also trigger an amplified pro-inflammatory cascade in the skeletal tissues to augment their pro-osteoclastogenesis effect. Our findings suggest that pathological bone loss may be a neglected complication which warrants more extensive investigations during the long-term follow-up of COVID-19 patients. The benefits of potential prophylactic and therapeutic interventions against pathological bone loss should be further evaluated.
Subject terms: Viral infection, SARS-CoV-2
Although extrapulmonary complications of different organ systems are recognized in patients with severe COVID19 effects are less well studied. Here, Qiao et al. characterize the pathogenesis of SARS-CoV-2 on bone metabolism in Syrian hamster and find that bone loss is associated with virus-mediated cytokine dysregulation.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused nearly 346 million cases of Coronavirus Disease 2019 (COVID-19) and nearly 5.5 million deaths as of 23 January 2022 since the virus’ discovery in December 20191. Severe acute COVID-19 may be complicated by both pulmonary (pneumonia with acute respiratory distress syndrome and respiratory failure) and extrapulmonary manifestations, such as anosmia, ageusia, diarrhea, lymphopenia, and multi-organ dysfunction syndrome2–4. More recently, it has been increasingly recognized that some patients may develop long-term complications and persistent symptoms of COVID-19, such as fatigue, headache, dyspnea, anosmia, muscle weakness, low-grade fever, and cognitive dysfunction5–7. However, the full spectrum of clinical manifestations in the long-term post-acute sequelae of SARS-CoV-2 infection, or “long COVID”, remains incompletely understood. In particular, SARS-CoV-2-associated pathological changes in the skeletal system remain largely unknown.
Recently, a multi-center study showed that COVID-19 patients requiring intensive care had significantly lower bone mineral density (BMD) than those who were managed in non-intensive care settings8. Another clinical study found that the number of severe clinical incidence was significantly higher in patients with lower BMD compared to those with higher BMD, therefore vertebral BMD is a strong independent predictor of mortality in COVID-19 patients9. In addition, about 24% of long COVID patients reported bone ache or burning, with the symptoms lasting for up to 7 months after the onset of COVID-1910. Despite these emerging evidence on the long-term complications of COVID-19, very limited serial investigations have been conducted on skeletal system involvement in the post-recovery phase. This is not unexpected because in COVID-19, patients either succumb or recover from the acute phase. In patients who recover from COVID-19, the focus on follow-up is usually limited to the respiratory, cardiac, and neurological functions which are well reported in the literature, rather than skeletal pathologies which typically do not manifest in the acute phase and therefore may be neglected. Moreover, since severe COVID-19 is most often found in elderly patients and those with comorbidities, including those on chronic corticosteroid and immunosuppressive drugs, the disease-associated bone changes in these patients who may already have osteoporosis before the infection may not be appreciated.
The skeletal system undergoes continuous bone formation and degradation throughout life and this tightly regulated remodeling process can be disturbed by many factors, particularly metabolic alterations and hormonal changes11,12. An imbalance between bone formation and resorption can also result from various chronic inflammatory diseases, leading to systemic osteoporosis and increased fracture risk13,14. For instance, chronic pulmonary inflammation arising from chronic obstructive pulmonary disease (COPD), cystic fibrosis, and asthma were reported to induce systemic bone loss15,16. Indeed, it has been shown that the extent of local or systemic osteopenia is associated with the degree of inflammatory response and the inflammation-induced bone loss can continue after effective therapeutic intervention on the inflammatory disease13,17,18. Severe COVID-19 patients developed much higher serum concentrations of pro-inflammatory cytokines and chemokines (e.g., IL-1β, IFN-α, IL-1RA, and IL-8) than those with milder disease, indicating that the cytokine dysregulation was closely correlated with disease severity19–21. The pro-inflammatory cytokines are known to not only perpetuate the inflammation to impair lung function but also perturb bone metabolism, leading to bone resorption18,22. A recent radiological study on COVID-19 survivors with persisting symptoms for up to three months after discharge revealed that the inflammation in bone marrow persisted after recovery23. Additionally, reactive hemophagocytosis mediated by cytokine dysregulation-induced activation of macrophages was common in deceased COVID-19 patients24. Therefore, we hypothesize that, in addition to other reported extrapulmonary manifestations, the cytokine dysregulation in COVID-19 patients may also contribute to pathological changes in the skeletal system2.
In this work, we characterize the effects of SARS-CoV-2 infection on bone metabolism during the acute and post-recovery phases in our established golden Syrian hamster model which closely mimics human infection25. Moreover, we demonstrate that the inflammation-induced osteoclastic activation following the cytokine dysregulation plays an important role in pathological bone loss. The findings of this study highlight the need for optimizing clinical protocols for monitoring long-term complications of COVID-19 and finding novel treatment strategies for SARS-CoV-2-induced inflammatory osteopenia/osteoporosis.
Results
SARS-CoV-2 causes significant loss of bone trabeculae
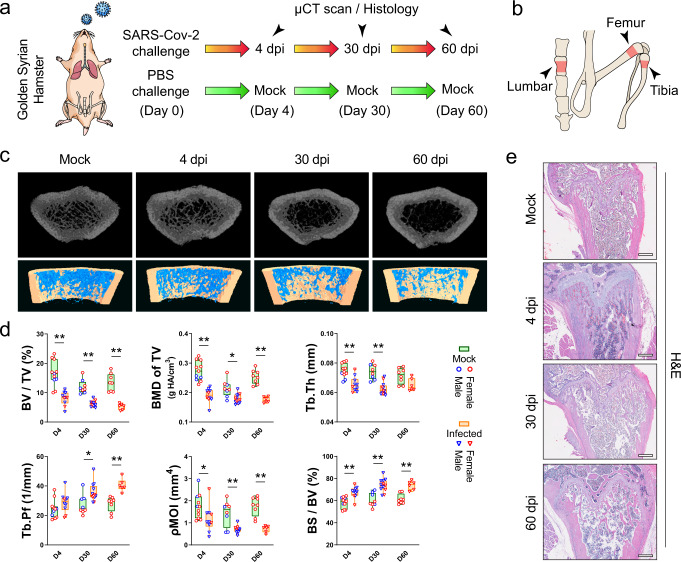
To investigate the in vivo effects of SARS-CoV-2 infection on bone metabolism, we utilized our established golden Syrian hamster model, which recapitulates the clinical, virological, immunological, and pathological features of COVID-19 in humans25 (Fig. 1a, b). In this hamster model, the most prominent disease manifestations were seen at about 4 days post-infection (dpi) and the hamsters generally recovered at about 7 to 10 dpi. As shown in our three-dimensional micro computerized tomography (μCT) scans, SARS-CoV-2-infected but not PBS-treated hamsters exhibited progressive loss of bone trabeculae at the distal metaphysis of femurs from the acute phase (4 dpi) to the post-recovery phase (30 dpi) and the chronic phase (60 dpi) of infection (Fig. 1c). A significant decrease in the trabecular bone volume fraction, bone mineral density, trabecular thickness, and the trabecular number could be evidenced at as early as 4 dpi (Fig. 1d and Supplementary Fig. 1a). There was also a significant decrease in the polar moment of inertia, though the cortical bone area and cortical thickness were barely affected (Supplementary Fig. 1b). On day 60, when the hamsters had recovered from the SARS-CoV-2 infection, the bone density was not restored. Instead, a gradual decrease of bone volume fraction, as well as progressive increases of trabecular pattern factor and the specific bone surface was detected (Fig. 1d). To verify our μCT scan findings, we examined the histological changes in the femurs of the hamsters (Fig. 1e and Supplementary Fig. 2a) and confirmed the reduction in bone trabeculae structures at the distal metaphysis of the femur was evident in the SARS-CoV-2-infected but not the mock-infected hamsters.
Fig. 1. SARS-CoV-2 induces bone loss in the golden Syrian hamster model.
a Golden Syrian hamsters were either treated with SARS-CoV-2 or PBS (Mock), followed by a μCT scan and histology analysis at 4, 30, and 60 days post-infection (dpi). b The regions of interest for μCT evaluation of bone density included the distal metaphysis of the femur, proximal metaphysis of tibia, and lumbar vertebrae. c Representative μCT images showing the reduction in trabecular bone volume in the femurs of the SARS-CoV-2-infected hamsters. d Corresponding measurements of trabecular bone volume fraction (BV/TV), bone mineral density (BMD of TV), trabecular thickness (Tb.Th), trabecular pattern factor (Tb.Pf), polar moment of inertia (pMOI), and specific bone surface (BS/BV). Mock: n = 11 (D4), 8 (D30), 8 (D60); Infected: n = 10 (D4), 12 (D30), 6 (D60). e Representative H&E staining images showing the cancellous bone structures in the femurs of the SARS-CoV-2-infected hamsters (n = 4, scale bars = 500 µm). Data were presented as box plots with whiskers from minima to maxima, the central line at the 50th percentile, and the ends of the box at the 25th and 75th percentiles. *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni’s multiple comparisons test.
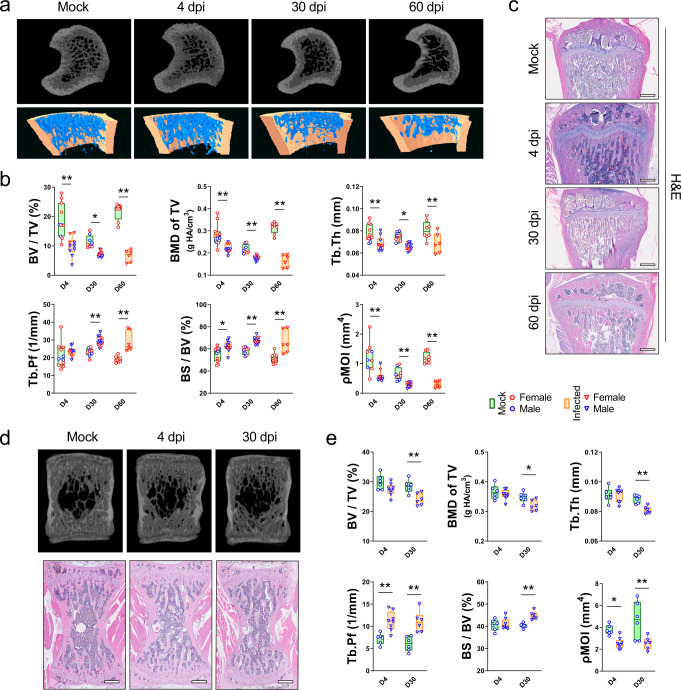
Next, we investigated whether similar changes were present in other sites. Both the μCT scan data and histological analysis showed that there was also a significant decrease in bone trabeculae at the proximal metaphysis of the tibia (Fig. 2a–c). The trabecular bone volume of SARS-CoV-2-infected hamsters at 60 dpi was less than 50% of that of the mock-infected hamsters (Fig. 2b). Corroboratively, there were significant decreases in bone mineral density, trabecular thickness, trabecular number, and polar moment of inertia after SARS-CoV-2 infection. Similar to our findings in the femur, the trabecular pattern factor, trabecular separation, and specific bone surface progressively decreased throughout the period of observation (Fig. 2b and Supplementary Fig. 1c). The same pattern of bone loss with a significant reduction in trabecular bone volume fraction, bone mineral density, trabecular thickness, trabecular number, and polar moment of inertia were observed also in the lumbar vertebrae at 30 dpi (Fig. 2d, e and Supplementary Fig. 1d). Meanwhile, a higher trabecular pattern factor and specific bone surface were detected after the infection. Overall, these findings showed that the SARS-CoV-2 infection causes a significant bone loss at different sites of a skeleton in the hamster model.
Fig. 2. Bone loss in the tibias and lumbar vertebrae after SARS-CoV-2 infection in the golden Syrian hamster model.
a Representative μCT image showing the reduction in trabecular bone volume in tibias of the SARS-CoV-2-infected hamsters. b Corresponding measurements of BV/TV, BMD of TV, Tb.Th, Tb.Pf, BS/BV, and pMOI. Mock: n = 11 (D4), 8 (D30), 8 (D60); Infected: n = 10 (D4), 12 (D30), 6 (D60). c Representative H&E staining images showing the cancellous bone structures in tibias of the SARS-CoV-2-infected hamsters (n = 4, scale bars = 500 µm). d Representative μCT and corresponding H&E staining images showing the bone loss in the lumbar vertebrae of the SARS-CoV-2-infected hamsters. e Corresponding measurements of BV/TV, BMD of TV, Tb.Th, Tb.Pf, BS/BV, and pMOI. Mock: n = 6 (D4), 6 (D30); Infected: n = 8 (D4), 6 (D30). Data were presented as box plots with whiskers from minima to maxima, the central line at the 50th percentile, and the ends of the box at the 25th and 75th percentiles. *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni’s multiple comparisons test.
SARS-CoV-2 activates osteoclastogenesis in hamsters
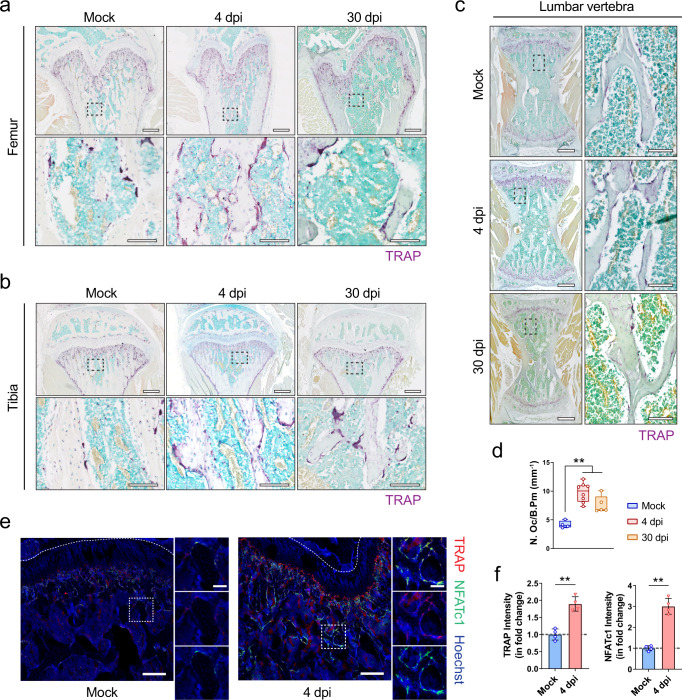
To provide mechanistic insights into the dysregulated bone metabolism in SARS-CoV-2-infected hamsters, we asked whether the bone loss is primarily caused by an alteration in bone resorption or in bone formation. Compared with mock-infected hamsters, a significantly higher number of tartrate-resistant acid phosphatase-positive (TRAP+) osteoclasts were found in the bone trabeculae at the distal metaphysis of the femur (Fig. 3a), the proximal metaphysis of the tibia (Fig. 3b), and the lumbar vertebrae (Fig. 3c) of SARS-CoV-2-infected hamsters. The number of TRAP+ osteoclasts at the distal metaphysis of the femur of the SARS-CoV-2-infected hamsters at 4 dpi and 30 dpi was almost double that of the mock-infected hamsters (Fig. 3d). Moreover, immunofluorescence staining demonstrated significantly more TRAP+ osteoclasts expressing nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) at the bone surface of SARS-CoV-2-infected hamsters (Fig. 3e). The increased intensities of TRAP and NFATc1 at the distal metaphysis of the femur at 4 dpi indicated that the osteoclastic activity was upregulated by SARS-CoV-2 infection (Fig. 3f). We also compared the expression of NFATc1, TRAP, cathepsin K, and receptor activator of NF-κB (RANK) in the bone tissues using Western blotting, which showed the upregulation of these osteoclastic markers after SARS-CoV-2 infection (Supplementary Fig. 2b, c). In contrast, alkaline phosphatase (ALP) staining (Supplementary Fig. 3a) and immunofluorescence staining for osteocalcin (Supplementary Fig. 3b) showed that there was no significant difference in the osteoblastic activities between the SARS-CoV-2-infected and mock-infected hamsters at 4 dpi.
Fig. 3. Osteoclastogenesis in the femurs and tibias of the hamsters after SARS-CoV-2 infection.
a–c Representative TRAP staining showing the increase in the number of TRAP+ osteoclasts at a the distal metaphysis of the femur (n = 4), b the proximal metaphysis of the tibia (n = 4), and c the lumbar vertebrae (n = 4) after the treatment with PBS (Mock) or SARS-CoV-2 infection (4 dpi). Lower images (scale bars = 100 µm) are high-resolution versions of the boxed regions in the upper images (scale bars = 500 µm). d Corresponding quantification of TRAP+ osteoclasts at the trabecular bone surface after treatment with PBS (Mock, n = 4) or SARS-CoV-2 infection (4 dpi, n = 8, 30 dpi, n = 5). e Representative immunofluorescence staining images and f the corresponding fluorescence intensity quantification showing the increase in the number of TRAP+ NFATc1+ osteoclasts at the distal metaphysis of the femur at 4 dpi (n = 4). Tile scans (scale bars = 200 µm) of the distal femoral metaphysis are shown along with high-magnification of the boxed regions (scale bars = 50 µm). Data were mean ± SD. ns: P > 0.05, *P < 0.05, **P < 0.01 by one-way ANOVA with Tukey’s post hoc test (d) or two-sided Student’s t-test (f).
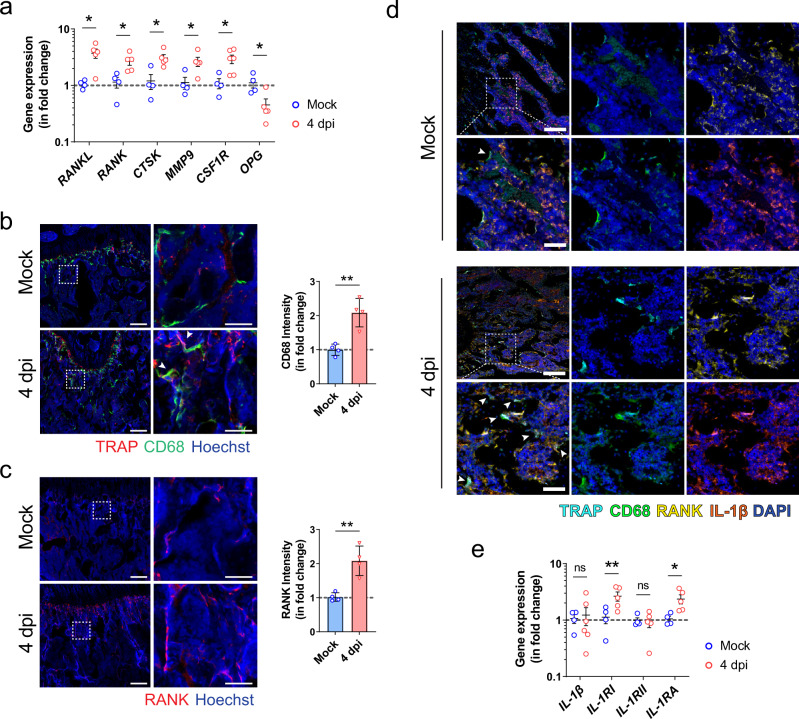
We next determined the expression of various osteoclastogenesis-related genes in the bone tissue of SARS-CoV-2-infected hamsters. Compared with mock-infected hamsters, the expression of receptor activator of nuclear factor-kappa B ligand (RANKL), which is essential for the osteoclastic differentiation, was tripled in SARS-CoV-2-infected hamsters (Fig. 4a). Moreover, several osteoclastic marker genes contribute to the formation and activity of osteoclasts, including receptor activator of NF-κB (RANK), cathepsin K (CTSK), matrix metallopeptidase 9 (MMP-9), and colony-stimulating factor 1 receptor (CSF1R), were significantly upregulated in the bone tissues after SARS-CoV-2 infection (Fig. 4a). The expression of osteoprotegerin (OPG), which acts as a decoy receptor for RANKL to inhibit RANK-RANKL mediated osteoclastogenesis and bone resorption, was correspondingly significantly downregulated after the infection (Fig. 4a). In addition to the increase in the number of TRAP+ osteoclasts, we also found that there were significantly more osteoclast progenitors, including CD68+ macrophages and RANK+ preosteoclasts, after SARS-CoV-2 infection (Fig. 4b, c). There were more multinuclear cells co-expressing TRAP and CD68 located at the bone trabeculae of SARS-CoV-2-infected hamsters (Fig. 4b). Using multiplex immunohistochemical (IHC) staining, we further identified the upregulated lineage of TRAP+RANK+CD68+ osteoclasts that expressed a higher level of interleukin-1 beta (IL-1β) in SARS-CoV-2-infected hamsters than mock-infected hamsters (Fig. 4d). Thus, to further elucidate the underlying mechanism, we then examined the expression of genes related to IL-1β signaling. Compared with the mock-infected hamsters, SARS-CoV-2-infected hamsters exhibited significant upregulation of interleukin-1 receptor type I (IL-1RI), but not interleukin-1 receptor type II (IL-1RII). Interestingly, the expression of IL-1β in the bone tissue was not significantly different between the two groups (Fig. 4e). Moreover, SARS-CoV-2 infection also induced a twofold increase in the expression of interleukin-1 receptor antagonist (IL-1RA) in bone tissue. Taken together, these findings indicate that SARS-CoV-2 infection leads to the activation of osteoclastic cascade resulting in the destruction of trabeculae structure in both long bones and axial skeleton.
Fig. 4. The inflammatory activation and osteoclastic differentiation of monocyte-macrophage lineage after SARS-CoV-2 infection.
a The expression of osteoclastogenesis-related genes in the bone tissue of the hamsters at day 4 after treatment with PBS (Mock, n = 4) or SARS-CoV-2 infection (4 dpi, n = 5). b Representative immunofluorescence staining images showing the increase in the number of CD68+ and TRAP+ osteoclasts (indicated by arrowhead) after SARS-CoV-2 infection (n = 4). Tile scans (scale bars = 200 µm) and high-magnification of the boxed regions (scale bars = 50 µm), as well as corresponding quantification for the fluorescence intensity of CD68, are shown. c Representative immunofluorescence staining images and the corresponding quantification showing the upregulation of RANK-expressing cells at the trabecular bone surface after SARS-CoV-2 infection. Tile scans (scale bars = 200 µm) and high-magnification of the boxed regions (scale bars = 50 µm) are shown. d Representative multicolor immunohistochemical staining for TRAP, CD68, RANK, and IL-1β was performed at the distal metaphysis of the femur on day 4 after treatment with PBS (Mock) or SARS-CoV-2 infection (4 dpi). DAPI was used for nuclear counterstaining. Tile scans (scale bars = 200 µm) and high-magnification of the boxed regions (scale bars = 50 µm) are shown. Arrowheads indicate osteoclasts on the bone surface (n = 3). e The expression of IL-1β signaling-related genes in bone tissue at day 4 after treatment with PBS (Mock, n = 4) or SARS-CoV-2 infection (4 dpi, n = 5). Data were mean ± SD. ns: P > 0.05, *P < 0.05, **P < 0.01 by two-sided Student’s t-test.
SARS-CoV-2 disturbs inflammatory microenvironment in skeleton system without direct infection
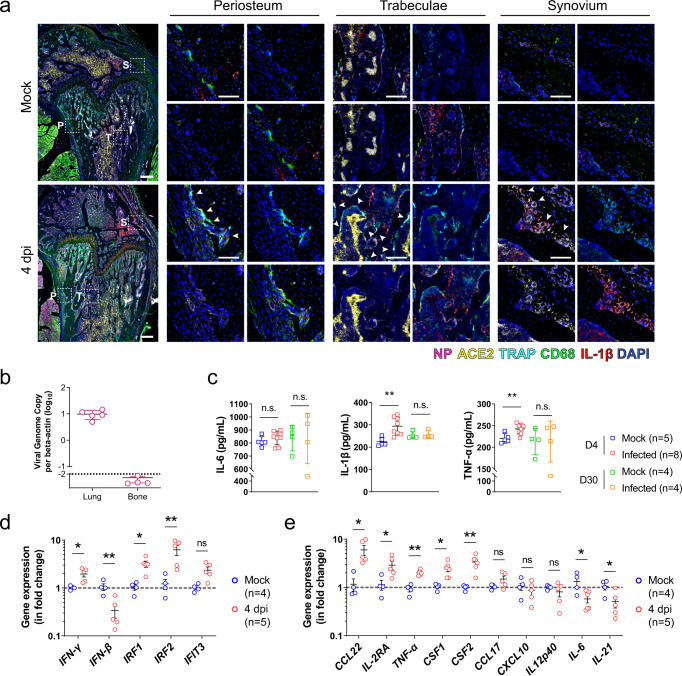
Having demonstrated the involvement of the local immune response in the pathological osteoclastogenesis in SARS-CoV-2 infection, we next asked whether the inflammatory bone loss was also caused by direct SARS-CoV-2 infection of the bone tissue. In SARS-CoV-2-infected hamsters, viral nucleocapsid protein (NP) in co-localized angiotensin-converting enzyme 2 (ACE2)-expressing cells were evident throughout the respiratory tract, from the nasal turbinate to the trachea and pulmonary alveoli at 4 dpi (Supplementary Fig. 4). CD68+ macrophages engulfing SARS-CoV-2-infected cells, which co-expressed viral NP and ACE2, were also observed, indicating the presence of an active immune response in these areas. In stark contrast, despite the presence of ACE2 in some of the immune cells residing in the bone tissue, viral NP was absent in the periosteum, bone trabeculae, and synovium of the femoral bone tissue in the SARS-CoV-2-infected hamsters (Fig. 5a). Moreover, viral RNA was not detected in the bone tissue (Fig. 5b). These findings indicated that the bone tissues were not directly infected by SARS-CoV-2. We then investigated whether osteoclastogenesis was associated with the virus-induced inflammatory response. Our ELISA results showed that at 4 dpi the mean serum IL-1β, tumor necrosis factor-alpha (TNF-α), and interleukin 6 (IL-6) protein levels were all higher in the SARS-CoV-2-infected hamsters than that of the mock-infected hamsters, with the levels of IL-1β and TNF-α reaching statistical significance (P < 0.01) (Fig. 5c).
Fig. 5. The absence of SARS-CoV-2 infection in bone tissues.
a Representative multicolor immunohistochemical staining for SARS-CoV-2 nucleocapsid protein (NP), angiotensin-converting enzyme 2 (ACE2), TRAP, CD68, and IL-1β was performed at the distal metaphysis of the femur after challenge with either PBS (Mock) or SARS-CoV-2 (4 dpi). Tile scans (scale bars = 200 µm) and high-magnification of the boxed regions (P periosteum, T trabeculae, S synovium; scale bars = 50 µm) are shown. Arrowheads indicate osteoclasts on the bone surface (n = 3). b Viral genome copies of the lung tissue (n = 5) and bone tissue (n = 4) harvested from SARS-CoV-2-infected hamsters. The viral genome copy was below the detection limit (dashed line) for all four bone samples. c The inflammatory cytokines, including IL-6, IL-1β, and TNF-α, in the serum of the hamsters treated with either PBS or SARS-CoV-2. d The expression of interferon signaling-related genes and e viral infection-associated inflammatory genes in bone tissue at day 4 after treatment with either PBS or SARS-CoV-2. Data were mean ± SD. ns: P > 0.05, *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni’s post hoc test (c) or two-sided Student’s t-test (d, e).
Interestingly, while direct SARS-CoV-2 infection of the bone tissue was absent, the expression of interferon-gamma (IFN-γ) and its downstream signals, including interferon regulatory factor 1 (IRF1) and interferon regulatory factor 2 (IRF2), were significantly upregulated in the bone tissue of SARS-CoV-2-infected hamsters at 4 dpi (Fig. 5d). The expression of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) was only marginally increased. Compared with the mock control, the inflammation-related genes upregulated in bone tissue in response to the respiratory infection of SARS-CoV-2 include C-C motif chemokine 22 (CCL22), interleukin-2 receptor antagonist (IL-2RA), TNF-α, colony-stimulating factor 1 (CSF1), and colony-stimulating factor 2 (CSF2). Nevertheless, the expressions of C-C motif chemokine 17 (CCL17), C-X-C motif chemokine ligand 10 (CXCL10), and interleukin 12 p40 (IL12p40) remained similar (Fig. 5d, e). Additionally, it is noteworthy that the infection in the respiratory system intriguingly downregulated the expression of interferon-beta (IFN-β), interleukin 21 (IL-21), and IL-6 in the bone tissue, which are vital for the clearance of virus infection.
SARS-CoV-2-induced inflammatory cytokines upregulate osteoclastogenesis
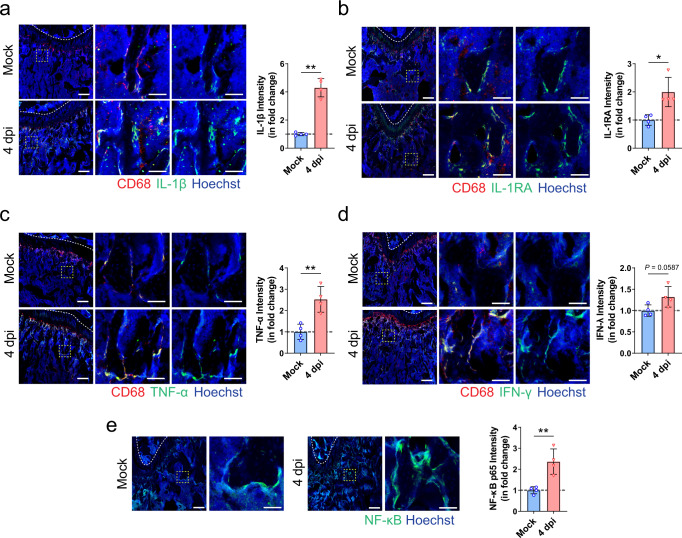
To address whether the bone loss subsequent to SARS-CoV-2 infection was caused by the pro-inflammatory cytokines originating from the respiratory system, we first compared the expression of various inflammatory cytokines in the bone tissue of SARS-CoV-2-infected and mock-infected hamsters. Using immunofluorescence staining, we showed that SARS-CoV-2 infection contributed to a significant increase in the levels of IL-1β, IL-1RA, and TNF-α, as well as a marginal increase in the level of IFN-γ in the bone tissue (Fig. 6a–d). These changes were confirmed by Western blotting which demonstrated a more than a sixfold increase of IL-1β, a sevenfold increase of TNF-α, and a threefold increase of IL-1RA in the bone tissue at 4 dpi in the SARS-CoV-2-infected hamsters compared to the mock-infected hamsters (Supplementary Fig. 5a, b). Additionally, immunofluorescence staining showed the co-expression of these inflammatory cytokines with CD68, the marker for bone resident macrophage (Fig. 6a–d). More importantly, we showed that the expression of NF-κB, the key transcription factor in inflammatory responses, was significantly higher at the bone surface of SARS-CoV-2-infected hamsters than that of the mock-infected hamsters (Fig. 6e). Semi-quantification by both immunofluorescence staining and Western blotting showed that the level of NF-κB in the bone tissue of SARS-CoV-2-infected hamsters was doubled that of the mock-infected hamsters (Fig. 6a and Supplementary Fig. 5a, b).
Fig. 6. Macrophage-mediated inflammatory response in bone tissues after SARS-CoV-2 infection.
a–d Representative immunofluorescence staining images and the corresponding quantification showing the increase in the expressions of a IL-1β, b IL-1RA, c TNF-α, and d IFN-γ, at the distal metaphysis of the femur after treatment with PBS (Mock, n = 4) or SARS-CoV-2 infection (4 dpi, n = 4). Tile scans (scale bars = 200 µm) and high-magnification of the boxed regions (scale bars = 50 µm) are shown. e Representative immunofluorescence staining images and the corresponding quantification showing the increase in NF-κB p65 expression at the distal metaphysis of the femur after treatment with PBS (Mock, n = 4) or SARS-CoV-2 infection (4 dpi, n = 4). Data were mean ± SD. *P < 0.05, **P < 0.01 by two-sided Student’s t-test.
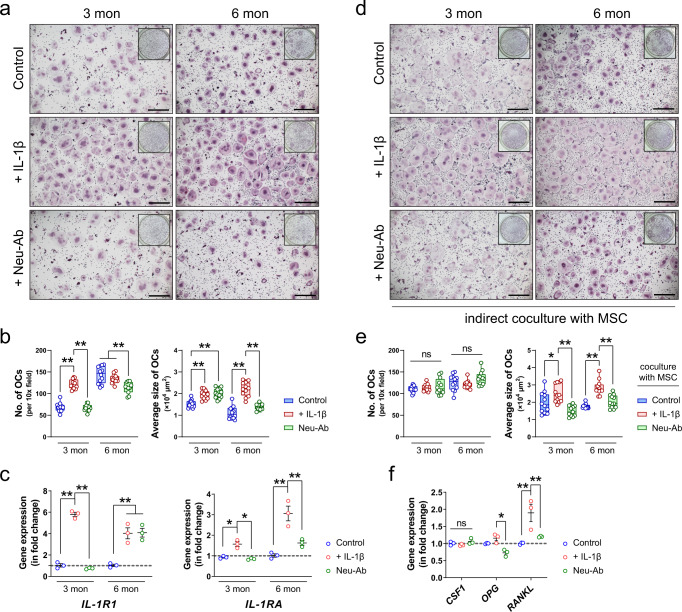
To further confirm the effects of these inflammatory cytokines on osteoclastic activities in bone tissue, we conducted a series of experiments using mouse bone marrow macrophages (BMMs) isolated from young (three-month-old) or matured adult (six-month-old) mice. First, we tested the response of murine BMMs to the stimulation of IL-1β as our previous experiment showed the activation of the IL-1β signaling cascade in SARS-CoV-2-infected hamsters (Fig. 4e). The presence of recombinant murine IL-1β contributed to a twofold increase in the number of TRAP+ multinuclear cells derived from BMM isolated from young mice (Fig. 7a, b). When added to BMMs isolated from matured adult mice, IL-1β led to a doubled size of fused osteoclasts (Fig. 7a, b). In contrast, the addition of IL-1β neutralizing antibody (Neu-Ab) not only reduced the number of BMM-derived osteoclasts but also resulted in a smaller size of osteoclasts differentiated from matured adult mouse BMMs. Meanwhile, the expression of IL-1R1 was increased by six times in the young BMMs and four times in the matured adult BMMs (Fig. 7c). However, the effect of Neu-Ab on the downregulation of IL-1R1 was only significant in BMMs from young mice, but not the ones from matured adult mice. Nevertheless, the gene expression of IL-1RA, which encodes interleukin-1 receptor antagonist, was significantly upregulated in both young and matured adult BMMs in response to the stimulation of IL-1β and downregulated after the addition of Neu-Ab.
Fig. 7. Inflammatory cytokine IL-1β promotes osteoclastogenesis.
a Representative microscopic images (scale bars = 500 µm) and b the corresponding quantifications (n = 12) showing the formation of TRAP+ multinuclear cells from bone marrow macrophage (BMM) isolated from young mice (3 months) and matured adult mice (6 months). Recombinant murine IL-1β or its neutralizing antibody (Neu-Ab) was added to the culture medium throughout the osteoclastic induction using M-CSF and RANKL. c The gene expression of IL-1R1 and IL-1RA in BMMs from young or matured adult mice with or without the presence of recombinant murine IL-1β or its neutralizing antibody (n = 3). d Representative microscopic images (scale bars = 500 µm) and e the correspond quantifications (n = 12) showing the formation of TRAP+ multinuclear cells when the BMMs were indirectly co-cultured with mesenchymal stem cells (MSCs) stimulated with recombinant murine IL-1β or its neutralizing antibody. f The gene expression of CSF1, OPG, and RANKL in MSC-treated with recombinant murine IL-1β or its neutralizing antibody (n = 3). Data were mean ± SD. ns: P > 0.05, *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni’s post hoc test (b, c, e) or one-way ANOVA with Tukey’s post hoc test (f).
Besides the direct effect of IL-1β on BMMs, we further tested whether IL-1β could promote osteoclastogenesis through the regulation of the osteoblast lineage. The indirect coculture of BMMs with mesenchymal stem cells (MSCs) was achieved using a transwell assay. IL-1β led to a significant increase in the size of BMM-derived osteoclasts after their coculture with IL-1β-treated MSCs, even though the number of TRAP+ multinuclear cells remained unchanged (Fig. 7d, e). Neu-Ab-treated MSCs, instead, decreased the average size of osteoclasts. This might be explained by the changes in the pro-osteoclastogenesis cytokines produced by MSCs because IL-1β contributed to a twofold increase in the expression of RANKL without changing the level of OPG in MSCs (Fig. 7f). Neither IL-1β nor its neutralizing antibody significantly regulated the expression of CSF1 in MSCs.
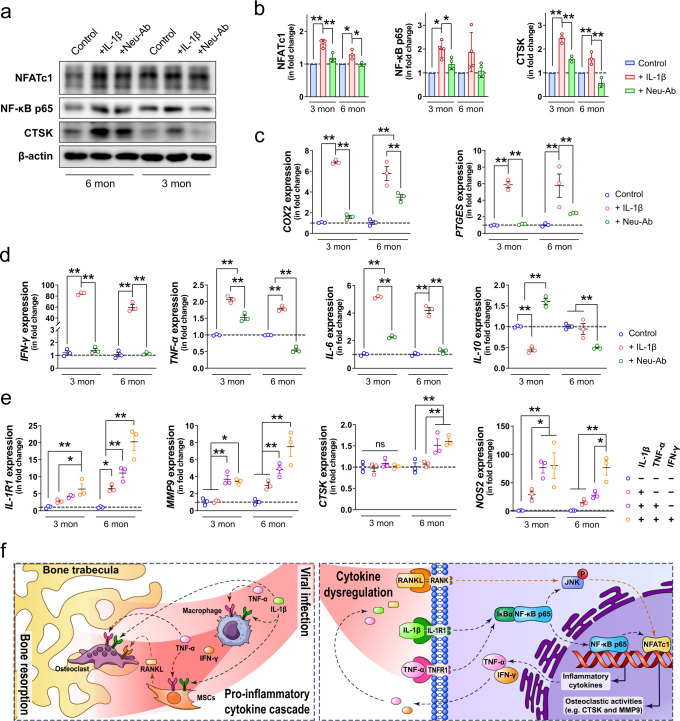
The effects of IL-1β and its neutralizing antibody on osteoclastogenesis were further confirmed using Western blotting. In both osteoclasts derived from BMM of young and matured adult mice, the expressions of NFATc1, NF-κB p65, and CTSK were significantly upregulated by recombinant murine IL-1β (Fig. 8a, b). Additionally, Neu-Ab suppressed the pro-osteoclastogenesis effects of IL-1β, as it downregulated the expression of NFATc1, NF-κB p65, and CTSK in BMM-derived osteoclasts from young and matured adult mice. Moreover, the Neu-Ab inhibited the IL-1β induced phosphorylation of c-Jun N-terminal kinase (JNK) (Supplementary Fig. 5c). Similar to the direct effect of IL-1β on osteoclastogenesis, IL-1β-treated MSCs also led to a significant upregulation in the expression of NFATc1 and the phosphorylation of JNK, which were both significantly downregulated by Neu-Ab-treated MSCs (Supplementary Fig. 6a). Although IL-1β-treated MSCs only contributed to a marginal increase in the expression of NF-κB p65 and CTSK in BMMs, Neu-Ab-treated MSCs significantly inhibited both of them (Supplementary Fig. 6a).
Fig. 8. Inflammatory cytokines exacerbate the pro-inflammatory response in bone marrow macrophages.
a, b Representative Western blots (a) and corresponding quantification (b) showing the expression of NFATc1 (n = 3), NF-κB p65 (n = 4), and CTSK (n = 3) in BMMs isolated from matured adults (6 months) or young (3 months) mice with or without the presence of recombinant murine IL-1β or its neutralizing antibody. c, d The gene expression of pain-associated cytokines (c) and other pro/anti-inflammatory cytokines (d) in BMMs from young or matured adult mice with or without the addition of recombinant murine IL-1β or its neutralizing antibody (n = 3). e The effects of various inflammatory cytokines on the gene expression of IL-1R1, MMP9, CTSK, and NOS2 (n = 3). f Schematic diagram showing SARS-CoV-2 infection-induced cytokine dysregulation leads to an amplified and broadened pro-inflammatory cascade in the skeletal tissue, resulting in pathological bone resorption. Data were mean ± SD. ns: P > 0.05, *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni’s post hoc test.
The pro-inflammatory cascade promotes pathological bone resorption
Since our results have verified the absence of SARS-CoV-2 infection in bone tissue, we hypothesized that the prominent inflammatory response in bone tissue was related to the circulating inflammatory cytokines that originate from the respiratory system after viral infection. Therefore, in addition to the pro-osteoclastogenesis effect of IL-1β, we also explored the immunomodulatory effects of IL-1β and its neutralizing antibody on BMMs isolated from young and matured adult mice. The recombinant murine IL-1β contributed to an approximately sixfold increase in the expression of cyclooxygenase-2 (COX2) and prostaglandin E synthase (PTGES) (Fig. 8b), which are both involved in the synthesis of prostaglandin E2 (PGE2). The presence of Neu-Ab inhibited the increase in the expression of COX2 and PTGES induced by IL-1β. The pro-inflammatory effect of IL-1β was also manifested by the significant changes in the expression of various inflammation-related genes. For instance, in BMMs isolated from young mice, IL-1β led to a more than 80-fold increase in IFN-γ expression and an around twofold increase in TNF-α expression, as well as a fivefold increase in IL-6 expression. In contrast, the expressions of IL-10 and IL-23 were significantly downregulated by IL-1β, with the expression of IL-22 remained unchanged (Fig. 8c and Supplementary Fig. 6b). In BMMs isolated from matured adult mice, IL-1β also contributed to a 60-fold increase in IFN-γ expression, an approximately 1.75-fold increase in TNF-α expression, and an around fourfold increase in IL-6 expression, and a less than 1.5-fold increase in IL-22 expression. However, the expression of IL-10 and IL-23 were not significantly altered by the addition of IL-1β (Fig. 8c and Supplementary Fig. 6b). In BMMs from both young and matured adult mice, the addition of Neu-Ab abolished the upregulation in the expression of IFN-γ, TNF-α, and IL-6 caused by IL-1β. In BMM from young mice, Neu-Ab resulted in significant upregulation in the expression of IL-10, IL-22, and IL-23 (Fig. 8c and Supplementary Fig. 6b).
We then asked whether the amplified and broadened pro-inflammatory cascade in bone following the SARS-CoV-2 infection-induced cytokine dysregulation synergistically promoted osteoclastogenesis. First, we showed that recombinant murine TNF-α and IFN-γ exaggerated the effects of IL-1β through the upregulation of IL-1R1 expression (Fig. 8d). This phenomenon was more prominent in BMMs isolated from matured adult mice, as IL-1β, TNF-α, and IFN-γ together led to an around 20-fold increase in the expression of IL-1R1, while IL-1β alone only upregulated IL-1R1 by fivefold. As a result, two major marker genes (i.e., MMP9 and CTSK) for osteoclastic activities were found to be further upregulated when the three inflammatory cytokines were administered in combination (Fig. 8d). In BMMs isolated from young mice, the addition of TNF-α and IFN-γ to IL-1β resulted in a fourfold increase in MMP9 expression, when IL-1β alone failed to induce higher MMP9 expression than the control. Meanwhile, in BMMs isolated from matured adult mice, the combination of IL-1β, TNF-α, and IFN-γ led to a more than sevenfold increase in the expression of MMP9 and a 1.5-fold increase in the expression of CTSK, while IL-1β alone did not significantly alter the expression of these two osteoclastic marker genes. Notably, the three inflammatory cytokines synergistically contributed to a more than 60-fold increase in the expression of Nitric Oxide Synthase 2 (NOS2), when IL-1β alone only upregulated NOS2 expression by less than 30 times. Together, these findings indicated that the pro-inflammatory cytokines synergistically contribute to pathological bone resorption.
Discussion
In addition to respiratory tract manifestations, extrapulmonary manifestations are also commonly reported in severe coronavirus infections such as COVID-19, SARS, and MERS4,26,27. Based on the phylogenetic similarities of SARS-CoV-2 and SARS-CoV, it has been postulated that the two beta coronaviruses may cause similar clinical features in infected patients. However, recent evidence has increasingly shown that there are more musculoskeletal sequelae associated with COVID-19 than SARS10,28. The most severe musculoskeletal complication in SARS patients was non-progressive avascular necrosis of the femoral head caused by high-dose steroid pulse therapy29. In contrast, musculoskeletal sequelae have been increasingly reported in COVID-19 patients including those who have recovered from the acute phase of the infection10,28. During the revision of this work, it was also reported elsewhere that SARS-CoV-2 infection can induce bone loss in a lethal human ACE2-transgenic mouse model30–32. Nevertheless, it would be important to investigate the long-term bone changes not only in severe COVID-19 cases but also in mild to moderate ones, because most patients recover from the severe inflammation after proper treatment. Thus, using our non-lethal hamster model represent mild to moderate human disease, we showed significant bone resorption happens at the acute inflammatory stage after SARS-CoV-2 infection. Moreover, in addition to a significantly increased number of RANK+ osteoclast precursors at this stage33, there were also more TRAP+ osteoclasts expressing NFATc1, which is known to serve as a master regulator for terminal differentiation of osteoclasts34. This implies that pathological bone destruction may happen quickly after the onset of COVID-19. More importantly, similar findings were evident in different bone tissues harvested from the hamsters, suggesting that the bone loss is not site-specific but systemic. Without proper intervention, the bone volume and bone mineral density were barely restored even after the viral load became undetectable at the post-recovery/chronic inflammatory phase. The presence of pathological bone loss may in turn complicate the rehabilitation of COVID-19 patients. For example, low bone mineral density is a known risk factor for vertebral fractures that may impair the respiratory function of COVID-19 patients in the rehabilitation phase35,36. It was recently reported that thoracic vertebral fractures occurred in 36% of COVID-19 patients and increased the patients’ need for noninvasive mechanical ventilation37.
Osteoporosis, which is characterized by a decrease in bone mass, microarchitectural bone disruption, and skeletal fragility leading to a higher fracture rate, has been extensively reported in critically ill patients38. The inflammatory cytokines are suggested to be one of the most vital mediators of the pathological bone loss in these diseases because they not only activate osteoclasts but also impede osteoblast function18. Cytokine dysregulation has been associated with various clinical manifestations of COVID-19, including some involving the musculoskeletal system, such as myalgia, sarcopenia, arthralgia, and arthritis39,40. Our findings in this study demonstrated that SARS-CoV-2-induced pathological bone resorption through a pro-inflammatory cascade instead of direct infection in the skeletal tissue. Indeed, the direct infection of SARS-CoV-2 in bone tissue is very unlikely because there is little to no expression of ACE2 or co-expression of TMPRSS2, which are both vital for viral entry, in the bone marrow41. Additionally, a recent study demonstrated that SARS-CoV-2 was not able to bind and infect hematopoietic stem and progenitor cells in bone marrow, from which BMMs were derived42. Consistent with our findings, it has been reported that while the viral load in the lung tissue of SARS-CoV-2-infected hamsters dropped from the peak level at 3 dpi to a significantly lower but still detectable level at 9 dpi, the viral load in bone marrow remained at the same level as mock control over the entire study period43. Multiplex quantification of immune mediators of SARS-CoV-2 peptide-stimulated cells from different organs also indicated that SARS-CoV-2–specific immune responses were primarily located in the lung or lung-associated lymph nodes instead of the bone marrow44. Although we measured a significant increase in serum level of IL-1β, the gene expression of IL-1β in bone tissue did not significantly change. This might indicate that the increase in IL-1β level in the bone tissue may be primarily attributed to the SARS-CoV-2-induced immune response in the respiratory system. After the inflammatory cytokines, (e.g., IL-1β and TNF-α) produced in the respiratory tract reached skeletal tissue via circulation, they quickly modulated the monocyte-macrophage lineage residing there to initiate additional pro-inflammatory cytokine cascade in the bone tissue. The cytokines produced in the skeletal system, including but not limited to IFN-γ, IL-6, and PGE2, not only contributed to osteoclastogenesis in several interdependent signaling pathways18 but also augmented the pro-osteoclastogenic actions of IL-1β and TNF-α by upregulating the expression of IL-1R1, which was implicated to primarily mediate pathological bone resorption45.
Among the various inflammatory factors that may be associated with osteoclastogenesis22, we identified IL-1β and TNF-α as the key mediators for SARS-CoV-2-induced bone loss in the hamster model. As two of the most significantly upregulated inflammatory cytokines in the serum samples of SARS-CoV-2-infected hamsters, IL-1β and TNF-α have been known as potent stimulators of bone resorption from as early as the 1980s46,47. They are both shown to be essential in RANKL-induced osteoclast formation through the activation of osteoclastogenesis-related signaling pathways such as the c-Jun N-terminal Kinase (JNK) signaling48,49. Besides their interdependent roles in mediating inflammatory osteopenia50, IL-1β and TNF-α were also found to synergistically interact with other pro-inflammatory cytokines to stimulate osteoclastic differentiation46,51,52. In this study, we found that the concurrence of three pro-inflammatory cytokines (i.e., IL-1β, TNF-α, and IFN-γ) in the bone tissue after SARS-CoV-2 infection is preferably upregulated IL-1R1, which is primarily expressed in pathologically activated osteoclasts responsible for inflammatory bone destruction53,54. In contrast, the expression of IL-1R2, which serves as a decoy receptor for IL-1β to negatively regulate IL-1β signaling, was not significantly altered. Consequently, these SARS-CoV-2 infection-induced pro-inflammatory cytokines dramatically upregulated the expression of MMP9 and CTSK, which are both known to play dominant roles in the degradation of extracellular matrix55. Additionally, we also found that the inflammatory cytokines elevated in COVID-19 (e.g., IL‐1β) were able to promote the formation of osteoclasts via regulating the production of RANKL from MSCs, as reported elsewhere56.
As we have demonstrated the SARS-CoV-2-induced bone loss in adult hamster, we further involved in vitro experiments using BMMs isolated from 3-month-old (young adult) or 6-month-old (matured adult) mice to test whether the inflammatory-mediated osteoclastogenesis is age-related. Importantly, we demonstrated that BMMs isolated from young adult mice were more responsive to the pro-osteoclastic stimulation of IL‐1β. This is clinically relevant because young patients generally have a stronger ability to adequately respond to viral infections with rapid production of a high level of pro-inflammatory cytokines57,58. This heightened pro-inflammatory response, together with the lower baseline pro-inflammatory state in young patients, makes them more susceptible to various syndromes related to immune dysregulation58,59. Meanwhile, we also found that the involvement of other pro-inflammatory cytokines, such as TNF-α and IFN-γ, contributed to a more prominent effect on promoting the osteoclastic activities in BMMs isolated from matured adult mice than the ones from young mice. This might explain why musculoskeletal symptoms are mostly seen in adult patients rather than children and the elderly60. Additionally, we noticed that the neutralizing antibody failed to block the late osteoclastic differentiation, especially in BMMs from young mice, as the size of osteoclasts and the expression of CTSK did not drop to the baseline level after the addition of the IL‐1β neutralizing antibody. These data suggest that multiple neutralizing antibodies targeting different pro-inflammatory cytokines may need to be considered for limiting IL‐1β-induced osteoclastogenesis. As demonstrated in our study that the initial stimulation of IL‐1β led to an amplified and broadened pro-inflammatory cascade, resulting in the production of several other cytokines favoring osteoclastogenesis, simultaneous block of IL‐1β and other pro-inflammatory cytokines would be required for preventing bone loss61.
Besides the immunomodulatory effect and the pro-osteoclastogenesis effect, the accumulation of various pro-inflammatory cytokines in the skeletal tissue can lead to several other long-term health concerns. For example, we showed that IL‐1β dramatically upregulated COX2 and PTGES, which both contribute to the production of PGE262. PGE2 is not only an inflammatory mediator involved in bone modeling but also a neuromodulator that can sensitize peripheral sensory neurons leading to inflammatory pain63. More than 20% of COVID-19 patients report lasting (from week 0 to week 28) bone ache or burning feeling10. Therefore, long-term monitoring of the inflammatory status of bone tissue after the recovery of the disease would be necessary. As a major indicator but not the sole driver in the pathology of COVID-1964, IL-6 is commonly found significantly increased in patients with exacerbating disease progression64. However, many studies have shown that the serum level of IL-6 would not be significantly upregulated in hamsters after SARS-CoV-2 infection65,66, because SARS-CoV-2 would not cause severe or even lethal disease in hamsters. Instead, the virus would induce a mild to moderate pathologic procedure resembling the clinical situation found in most COVID-19 patients. Therefore, our observation suggests that the inflammatory bone loss can happen in mild to moderate infection or post-recovery cases of COVID-19 patients. The inflammation in bone tissue can also alter the output of immune cells or cytokines from the bone marrow, which are supposed to participate in combating viral infection67. In our study, the significantly upregulated expression of IFN-γ and its signaling-associated genes (e.g., IRF1 and IRF2) in bone tissue indicate they might play an essential role in protecting bone tissue from virus infection68. However, it is also noteworthy that the expression of several anti-viral chemokines and cytokines, such as IFN-β69, IL-2170, and CCL1771, remained unchanged or even downregulated in the bone tissue. Thus, the suppression of the bone marrow-derived anti-viral factors may be one of the immune-evading mechanisms for SARS-CoV-2 that warrants further investigation.
In a recently published meta-analysis, Anakinra, a recombinant IL-1 receptor antagonist, was suggested as a safe, anti-inflammatory treatment option to reduce the mortality risk in patients admitted to hospital with moderate to severe COVID-19 pneumonia, especially in the presence of signs of hyperinflammation72. This suggests that adequate use of an anti-inflammatory treatment may be beneficial for COVID-19 during both the acute phase and the chronic phase involving long-term inflammatory complications. Therefore, evaluation of the effects of anti-inflammatory agents such as IL-1 receptor antagonists and IL-1 neutralizing antibodies on the prevention and/or treatment of SARS-CoV-2 infection-induced inflammatory bone loss should be included in future studies. In addition, there are several limitations to our study. First. due to the lack of commercialized detection assays for evaluating biomolecules of hamster at the protein level, we only tested the circulating levels of IL-1β, TNF-α, and IL-6, which are known as three of the most important pro-inflammatory cytokines of the innate immune response. Nevertheless, the striking similarities in the cytokine dysregulation between hamster model and human patients have been verified elsewhere using single-cell RNA sequencing73. Secondly, given the fact that the current hamster model mainly represents mild to moderate disease in human, additional investigations on the characteristics and treatment options for SARS-CoV-2-induced bone loss should be further conducted in more severe and/or lethal models.
In this study, we demonstrated the influence of SARS-CoV-2 infection on systemic bone loss during the acute and post-recovery/chronic phases. We revealed the pro-inflammatory cytokines derived from the respiratory system as the major mediators for pathological bone resorption. These pro-inflammatory cytokines disrupt the balance in bone metabolism and trigger another pro-inflammatory cascade in the skeletal tissue to further augment their pro-osteoclastogenesis effect (Fig. 8e). The findings in our study highlight the need to closely monitor COVID-19 patients’ bone density. The benefits of prophylactic or therapeutic interventions against the development of pathological bone loss in COVID-19 patients should be further evaluated in animal models and clinical trials.
Methods
Virus and biosafety
SARS-CoV-2 (strain HKU-001a, GenBank accession number: MT230904) was a clinical strain isolated from the nasopharyngeal aspirate specimen of a COVID-19 patient in Hong Kong74. The plaque-purified viral isolate was amplified by one additional passage in VeroE6 cells to make working stocks of the virus as described previously75. All experiments involving live SARS-CoV-2 followed the approved standard operating procedures of The University of Hong Kong (HKU) Biosafety Level-3 facility76,77.
Animal model
The animal experiments were approved by the HKU Committee on the Use of Live Animals in Teaching and Research. Briefly, 6–10-week-old male or female golden Syrian hamsters (Mesocricetus auratus) were obtained from the Chinese University of Hong Kong Laboratory Animal Service Center through the HKU Center for Comparative Medicine Research. The animals were kept in Biosafety Level-2 housing and given access to standard pellet feed and water ad libitum until a virus challenge in our Biosafety Level-3 animal facility. Each animal was intranasally treated with 105 PFU of SARS-CoV-2 in 50 µL of PBS under intraperitoneal ketamine (200 mg/kg) and xylazine (10 mg/kg) anesthesia at 0 dpi as we previously described78. Mock-infected animals were treated with 50 µL of PBS. Their blood, bone, and lung tissues were collected at sacrifice at 4, 30, and/or 60 dpi for µCT, virological, and histopathological analyses.
Micro-CT analysis
The PBS or virus-treated hamsters were sacrificed at 4, 30, and 60 dpi for micro-CT analysis following a standard guideline described previously79. Before being transferred from the biosafety Level-3 facility, the specimens were fixed in 4% paraformaldehyde for 48 h and 70% ethanol for 24 h to inactivate the pathogens. The bone specimens were scanned by a high-resolution micro-CT scanner (SkyScan 1076, Kontich, Belgium) at a resolution of 8.665 μm per pixel. The voltage of the scanning procedure was 88 kV with a 110-μA current. Two phantom-contained rods with a standard density of 0.25 and 0.75 g/cm3 were used for calibration of bone mineral density (BMD). Data reconstruction was done using the NRecon software (Skyscan Company), the image analysis was done using CTAn software (Skyscan Company), and the 3D model visualization was done using CTvox (Skyscan Company) and CTvol (Skyscan Company). The region of interest (ROI) contained 200 layers of images beginning from the distal metaphyseal growth plate of femurs or the proximal metaphysis growth plate of tibias (Fig. 1b). Trabecular bone parameters, including bone volume fraction (BV/TV), specific bone surface (BS/BV), bone mineral density (BMD of TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular pattern factor (Tb.Pf), trabecular separation (Tb.Sp), polar moment of inertia (ρMOI), and structure model index (SMI), as well as cortical bone parameters, including cortical bone area (Ct.Ar) and cortical bone thickness (Ct.Th) were measured from the µCT data.
Histological analysis
Histology and immunohistochemical staining were performed on both paraffin sections and cryosections. In brief, the bone specimens, after fixation in 4% PFA for 48 h, were decalcified with 12.5% ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich) for 4 weeks. For paraffin sections, the specimens were processed, embedded in paraffin, and cut into 5-µm-thick sections using a rotary microtome (RM215, Leica Microsystems, Germany). Haematoxylin and eosin (H&E) staining, TRAP staining (Sigma-Aldrich), and ALP staining (Sigma-Aldrich) were performed on selected sections from each sample following the manufacturer’s instructions. Images were captured using the Vectra Polaris Imaging System (Akoya Biosciences, USA). For immunostaining, the samples were dehydrated in 20% sucrose solution with 2% polyvinylpyrrolidone (PVP, Sigma-Aldrich) for 24 h and embedded in 8% gelatin (Sigma-Aldrich) supplemented with 20% sucrose and 2% PVP. Forty-μm-thick coronal-oriented sections of the femurs were obtained using a cryostat microtome. Immunostaining was performed using a standard protocol. Briefly, after blocking with 10% goat serum, the sections were incubated with primary antibodies to CD68 (Abcam, ab31630/ab125212), RANK (Abcam, ab13918), TRAP (Abcam, ab216025), osteocalcin (TAKARA, M186), IL-1β (Abcam, ab9722), IL-1RA (Abcam, ab124962), TNF-α (Abcam, ab9635), IFN-γ (Abcam, ab9657), NF-κB (CST, #8242), anti-NFATc1 (CST, #8032) overnight at 4 °C. Alexa-Fluor 488-conjugated and Alexa-Fluor 647-conjugated secondary antibodies (Thermo Fisher Scientific) were used for immunofluorescent staining, while the nuclei were counterstained with Hoechst 33342 (Thermo Fisher Scientific). Immunofluorescent images were captured using LSM 780 confocal microscopy (Zeiss, Germany).
Multiplex IHC analysis
Antigen retrieval and blocking were performed on the dewaxed slides using the Antigen retrieval reagent (pH 6.0) and Blocking/antibody diluent provided in the Opal Polaris Multicolor Manual IHC Detection Kit (Akoya Biosciences, USA), following the manufacturer’s instructions. In brief, the incubation of each primary antibody was done overnight at 4 °C. The primary antibodies used in this study included anti-CD68 (Abcam, ab31630, USA), anti-TRAP (Abcam, ab216025), anti-RANK (Abcam, ab13918), anti-IL-1β (Abcam, ab9722), anti-NP (Thermo Fisher, USA), anti-ACE2 (Thermo Fisher). Between each incubation of the primary antibody, tyramide signal amplification (TSA) visualization was performed using the Opal Polymer Horseradish peroxidase (HRP) secondary antibody and fluorophores: Opal 520, Opal 570, Opal 620, Opal 690, and DAPI (Akoya Biosciences, USA). The stained slides were imaged using the Vectra Polaris Imaging System (Akoya Biosciences, USA).
Cell culture
The mesenchymal stem cells (MSCs) and bone marrow macrophages (BMMs) were isolated from the long bones of 3-month-old or 6-month-old C57L6/J mice. In brief, the mice were euthanized with overdosage of intraperitoneal injection of Pentobarbital. After the removal of attached soft tissues using forceps and gauze, the dislocated femurs and tibias were dissected into pieces. The whole bone marrow cells were resuspended in a serum-free DMEM medium (Gibco, USA) using a vortex mixer, while the bone chips and debridement were removed by passing the mixture through a cell strainer. After centrifugation, the cell pellet was resuspended in a DMEM medium, supplemented with 10% FBS and 1% Penicillin-Streptomycin (complete DMEM medium), and cultured in culture flasks. After a 6-h incubation in a humidified incubator with 5% CO2 at 37 °C, the unattached cells were gently removed for the induction of BMMs. Meanwhile, the attached bone marrow cells were gently washed in PBS and further cultured as MSCs in a complete DMEM medium until they reached 80% confluence. The culture medium was refreshed every 2 days, and only passages 3–5 were used for the experiments. After the 3-day macrophage induction using a complete DMEM medium supplemented with 20 ng/ml of macrophage colony-stimulating factor (M-CSF), the BMMs became adherent for the osteoclastic differentiation using a complete DMEM supplemented with 50 ng/mL of receptor activator of nuclear factor-kappa-Β ligand (RANKL, R&D System, USA) and 20 ng/mL of M-CSF (R&D System, USA). The coculture of BMMs with MSCs was done using a transwell system (0.4 µm pore size, Corning Costar, USA). In brief, MSCs were seeded in the transwell inserts and cultured overnight with 5% CO2 at 37 °C for attachment. Afterward, the transwell inserts were placed into the lower chambers in which BMMs have been differentiated using a medium supplemented with M-CSF for three days as described previously. To mimic the challenge of the pro-inflammatory microenvironment in COVID-19, 1 ng/mL recombinant murine IL-1β (R&D system, USA) was supplemented in the medium. For the inhibition of IL-1β, 10 ng/mL IL-1β neutralizing antibody (Abcam, ab9722) was added to the cell culture. For studying the synergistic effects of pro-inflammatory cytokines on BMMs, the culture medium of BMMs was further supplemented with 1 ng/mL TNF-α (R&D system, USA) and 1 ng/mL IFN-γ (R&D system, USA).
Real-time quantitative polymerase chain reaction (RT-qPCR) assay
The total RNA from the bone specimens were isolated using Trizol reagent (Thermo Fisher, USA) following the manufacturer’s instructions. The total RNA from the cultured cells was extracted and purified using an RNeasy Plus kit (Qiagen, USA) following the manufacturer’s instructions. For the reverse transcript, complementary DNA was synthesized using a Takara RT Master Mix (Takara, Japan), following the manufacturer’s instructions. The primers used in the RT-qPCR assay were synthesized using Integrated DNA Technologies (IDT, Singapore), based on sequences designed using Primer-BLAST (National Center for Biotechnology Information, NCBI, Supplementary table 1) or retrieved from the Primer Bank (http://pga.mgh.harvard.edu/primerbank/, Supplementary table 2). The SYBR Green Premix Ex Taq (Takara, Japan) was used for the amplification and detection of cDNA targets on the LightCycler480 Real-time PCR system (Roche, USA). The mean cycle threshold (Ct) value of each target gene was normalized to the housekeeping genes (i.e., RPL18 or GAPDH). The results were shown in a fold change using the ∆∆Ct method.
Western blotting
The protein from homogenized bone tissue from hamster were isolated using Trizol reagent (Thermo Fisher, USA) following the manufacturer’s instructions. The protein from cultured BMM was harvested using RIPA Lysis and Extraction Buffer (Thermo Fisher, USA) containing a 1% Phosphatase Inhibitor Cocktail (Thermo Fisher, USA). The concentration of protein was measured using the BCA Protein Assay Kit (Thermo Fisher, USA). A total of 20 µg of protein from each sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to the polyvinylidene difluoride (PVDF) membrane (Merck Millipore, USA). Then, the membrane was blocked in 5% w/v bovine serum albumin (BSA, Sigma-Aldrich, USA) and incubated with blocking buffer-diluted primary antibodies overnight at 4 °C. The primary antibodies used include mouse anti-NFATc1 (Santa Cruz, USA), mouse anti-TRAP (Abcam), rabbit anti-Cathepsin K (Abcam), mouse anti-RANK (Abcam), rabbit anti-NF-κB p65 (CST), rabbit anti-IL-1β (Abcam), rabbit anti-IL-1RA (Abcam), rabbit anti-TNF-α (Abcam), rabbit anti-phospho-JNK (CST), rabbit anti-JNK (CST), rabbit anti-β-actin (Abcam). The protein bands were visualized by using HRP conjugated secondary antibodies and an enhanced chemiluminescence (ECL) substrate (Advansta, USA) and exposed under the Typhoon5 Biomolecular Imager 680 (GE Amersham, USA).
ELISA assay
The serum samples of the hamsters were collected at 4 dpi or 30 dpi for cytokine/chemokine analysis. The serum level of IL-1β, TNF-α, and IL-6 were detected using a specific ELISA kit (MyBiosource, USA) following the manufacturer’s instructions.
Statistical analysis
All data analyses were performed and illustrated using the Prism software (version 7, GraphPad, USA). The results were expressed as means ± standard deviation (SD). The exact sample size (n) for each experimental group was clearly shown as dot plots in the figures and indicated in the legends. For comparisons among multiple groups, a one-way or two-way analysis of variance (ANOVA) was used, followed by Tukey’s multiple-comparison post hoc test or Bonferroni’s post hoc test. The levels of significant difference among the groups were defined and noted as *P < 0.05 and **P < 0.01. The sample size was decided based on preliminary data, as well as on observed effect sizes.
Supplementary information
Acknowledgements
We sincerely thank the staff members at the Faculty Core Facility, Li Ka Shing Faculty of Medicine, The University of Hong Kong, the Centre for Comparative Medicine Research of The University of Hong Kong, and the Laboratory Animal Service Centre of The Chinese University of Hong Kong for their facilitation of the study. K.W.-K.Y. received funding from the National Key R&D Program of China (2018YFA0703100), Health and Medical Research Fund (19180712), Hong Kong Innovation Technology Fund (ITS/287/17 and ITS/405/18), Science and Technology Commission of Shanghai Municipality (18410760600), International Partnership Program of Chinese Academy of Sciences (GJHZ1850). K.W.-K.Y. and K.M.-C. C. received funding from General Research Fund (17207719, 17214516, and N_HKU725/16), HKU-SZH Fund for Shenzhen Key Medical Discipline (SZXK2020084), Shenzhen Science and Technology Funding (JSGG20180507183242702), Sanming Project of Medicine in Shenzhen, China (SZSM201612055), Guangdong Financial Fund for High-Caliber Hospital Construction (174-2018-XMZC-0001-03-2125/D-10). J.F.-W.C. received funding from Collaborative Research Fund (C7060-21GF) of the Research Grants Council, Hong Kong Special Administrative Region, the National Key R&D Program of China (2020YFA0707500 and 2020YFA0707504), Health and Medical Research Fund (20190572 and COVID190121), the University of Hong Kong Outstanding Young Researcher award, the University of Hong Kong Research Output Prize, and donations from Lo Ying Shek Chi Wai Foundation. K.-Y.Y. and J.F.-W.C. received funding from the Theme-Based Research Scheme (T11-709/21-N) of the Research Grants Council of HKSAR, Sanming Project of Medicine in Shenzhen, China (SZSM201911014), the High Level-Hospital Program of the Health Commission of Guangdong Province, Health@InnoHK of Innovation and Technology Commission, the Government of Hong Kong Special Administrative Region, the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for Department of Health of the HKSAR Government, the Major Science and Technology Program of Hainan Province (ZDKJ202003), the Hainan Talent Development Project (SRC200003), the research project of Hainan Academician Innovation Platform (YSPTZX202004), the Collaborative Project (EKPG22-01) of Guangzhou Laboratory, the Emergency COVID-19 Project (2021YFC0866100), Major Projects on Public Security, National Key Research and Development Program. K.-Y.Y. and J.F.-W.C. are also grateful for the donations of May Tam Mak Mei Yin, Richard Yu, and Carol Yu, the Shaw Foundation of Hong Kong, Michael Seak-Kan Tong, the Providence Foundation Limited (in memory of the late Lui Hac Minh); Lee Wan Keung Charity Foundation Limited, Hui Ming, Hui Hoy, and Chow Sin Lan Charity Fund Limited, Hong Kong Sanatorium & Hospital, Chan Yin Chuen Memorial Charitable Foundation, Tse Kam Ming Laurence, Marina Man-Wai Lee, the Hong Kong Hainan Commercial Association South China Microbiology Research Fund, the Jessie & George Ho Charitable Foundation, Perfect Shape Medical Limited, Kai Chong Tong, Foo Oi Foundation Limited, Betty Hing-Chu Lee, and Ping Cham So. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Source data
Author contributions
W.Q. performed the in vitro and in vivo tests, as well as analyzed and interpreted the data. H.E.L. and H.X. contributed to the in vitro tests and helped with the data analysis. V.K.-M.P. and C.C.-S.C. contributed to the infection of hamsters and the collection of specimens. H.C., S.Y., T.T.-T.Y., K.K.-H.C., J.O.-L.T., C.C.-Y.C., J.-P.C., and C.L. performed experiments and analyzed data. K.-Y.Y. and K.M.-C.C. provided funding and interpreted data. J.F.-W.C. and K.W.-K.Y. contributed to the study design, data interpretation, and supervision of the project. W.Q., J.F.-W.C., and K.W.-K.Y. wrote the manuscript, with input from all authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All relevant data that support the findings of this study are available within the Article and Supplementary Information or from the corresponding author upon reasonable request. Source Data file is provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/31/2022
A Correction to this paper has been published: 10.1038/s41467-022-30952-x
Contributor Information
Jasper Fuk-Woo Chan, Email: jfwchan@hku.hk.
Kelvin Wai-Kwok Yeung, Email: wkkyeung@hku.hk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-30195-w.
References
- 1.World Health, O. COVID-19 Weekly Epidemiological Update 76 edn (World Health Organization, Geneva, 2021).
- 2.Gupta A, et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 3.Chan JF, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To KK, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 2021;10:507–535. doi: 10.1080/22221751.2021.1898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottlors J, et al. Early extrapulmonary prognostic features in chest computed tomography in COVID-19 pneumonia: bone mineral density is a relevant predictor for the clinical outcome - A multicenter feasibility study. Bone. 2021;144:115790. doi: 10.1016/j.bone.2020.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahtabasi, M. et al. The prognostic value of vertebral bone density on chest CT in hospitalized COVID-19 patients. J. Clin. Densitom. 10.1016/j.jocd.2021.07.007 (2021). [DOI] [PMC free article] [PubMed]
- 10.Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine10.1016/j.eclinm.2021.101019 (2021). [DOI] [PMC free article] [PubMed]
- 11.Mosekilde L. Primary hyperparathyroidism and the skeleton. Clin. Endocrinol. 2008;69:1–19. doi: 10.1111/j.1365-2265.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel B, et al. Regulation of adult bone turnover by sex steroids. J. Cell Physiol. 2010;224:305–310. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundy GR. Osteoporosis and inflammation. Nutr. Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 14.Romas E, Gillespie MT. Inflammation-induced bone loss: can it be prevented? Rheum. Dis. Clin. North Am. 2006;32:759–773. doi: 10.1016/j.rdc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Dam TT, et al. Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporos. Int. 2010;21:1341–1349. doi: 10.1007/s00198-009-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shead EF, Haworth CS, Barker H, Bilton D, Compston JE. Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. J. Cyst. Fibros. 2010;9:93–98. doi: 10.1016/j.jcf.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Smolen JS, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann. Rheum. Dis. 2009;68:823–827. doi: 10.1136/ard.2008.090019. [DOI] [PubMed] [Google Scholar]
- 18.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Disco. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamopoulos IE. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018;30:59–64. doi: 10.1097/BOR.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sollini M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1460–1466. doi: 10.1007/s00259-020-05084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris CK, Hung YP, Nielsen GP, Stone JR, Ferry JA. Bone marrow and peripheral blood findings in patients infected by SARS-CoV-2. Am. J. Clin. Pathol. 2021;155:627–637. doi: 10.1093/ajcp/aqaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JF, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020;71:2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng VC, et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JF, et al. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramani, S. L. et al. Musculoskeletal involvement of COVID-19: review of imaging. Skeletal Radiol. 10.1007/s00256-021-03734-7 (2021). [DOI] [PMC free article] [PubMed]
- 29.Zhang P, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awosanya OD, et al. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone. 2021;154:116227. doi: 10.1016/j.bone.2021.116227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalloul, C. et al. Osteoclast-mediated bone loss observed in a SARS-CoV-2 mouse model. In ASBMR 2021 Annual Meeting (2021).
- 32.Blaine Christiansen, E. B. et al. Bone loss following SARS-CoV-2 infection in mice. In ASBMR 2021 Annual Meeting (2021).
- 33.Atkins GJ, et al. RANK expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J. Bone Miner. Res. 2006;21:1339–1349. doi: 10.1359/jbmr.060604. [DOI] [PubMed] [Google Scholar]
- 34.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe R, Shiraki M, Saito M, Okazaki R, Inoue D. Restrictive pulmonary dysfunction is associated with vertebral fractures and bone loss in elderly postmenopausal women. Osteoporos. Int. 2018;29:625–633. doi: 10.1007/s00198-017-4337-0. [DOI] [PubMed] [Google Scholar]
- 36.Kim B, et al. Risk of pneumonia after vertebral compression fracture in women with low bone density: a population-based study. Spine. 2018;43:E830–E835. doi: 10.1097/BRS.0000000000002536. [DOI] [PubMed] [Google Scholar]
- 37.di Filippo L, et al. Radiological thoracic vertebral fractures are highly prevalent in COVID-19 and predict disease outcomes. J. Clin. Endocrinol. Metab. 2021;106:e602–e614. doi: 10.1210/clinem/dgaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orford NR, Pasco JA, Kotowicz MA. Osteoporosis and the critically Ill patient. Crit. Care Clin. 2019;35:301–313. doi: 10.1016/j.ccc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Kirwan R, et al. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Disser NP, et al. Musculoskeletal consequences of COVID-19. J. Bone Jt. Surg. Am. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sungnak W, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huerga Encabo H, et al. Human erythroid progenitors are directly infected by SARS-CoV-2: implications for emerging erythropoiesis in severe COVID-19 patients. Stem Cell Rep. 2021;16:428–436. doi: 10.1016/j.stemcr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang SJ, et al. Characterization of virus replication, pathogenesis, and cytokine responses in Syrian hamsters inoculated with SARS-CoV-2. J. Inflamm. Res. 2021;14:3781–3795. doi: 10.2147/JIR.S323026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon MML, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021;6:eabl9105. doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trebec DP, et al. Increased expression of activating factors in large osteoclasts could explain their excessive activity in osteolytic diseases. J. Cell Biochem. 2007;101:205–220. doi: 10.1002/jcb.21171. [DOI] [PubMed] [Google Scholar]
- 46.Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J. Bone Min. Res. 1989;4:113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- 47.Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 48.Lam J, et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SK, Gardner AE, Kalinowski JF, Jastrzebski SL, Lorenzo JA. RANKL-stimulated osteoclast-like cell formation in vitro is partially dependent on endogenous interleukin-1 production. Bone. 2006;38:678–685. doi: 10.1016/j.bone.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Polzer K, et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann. Rheum. Dis. 2010;69:284–290. doi: 10.1136/ard.2008.104786. [DOI] [PubMed] [Google Scholar]
- 51.Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987;138:1464–1468. [PubMed] [Google Scholar]
- 52.Taki N, Tatro JM, Lowe R, Goldberg VM, Greenfield EM. Comparison of the roles of IL-1, IL-6, and TNFalpha in cell culture and murine models of aseptic loosening. Bone. 2007;40:1276–1283. doi: 10.1016/j.bone.2006.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiratori T, et al. IL-1beta induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of kindlin-3 and talin-1. J. Immunol. 2018;200:218–228. doi: 10.4049/jimmunol.1602035. [DOI] [PubMed] [Google Scholar]
- 54.Ruscitti P, et al. The role of IL-1beta in the bone loss during rheumatic diseases. Mediators Inflamm. 2015;2015:782382. doi: 10.1155/2015/782382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu, L. X. et al. Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases. Sci. Transl. Med. 12, eaaw6143 (2020). [DOI] [PMC free article] [PubMed]
- 56.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng PC, et al. Inflammatory cytokine profile in children with severe acute respiratory syndrome. Pediatrics. 2004;113:e7–e14. doi: 10.1542/peds.113.1.e7. [DOI] [PubMed] [Google Scholar]
- 58.Costagliola G, Spada E, Consolini R. Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19. Immun. Inflamm. Dis. 2021;9:331–339. doi: 10.1002/iid3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobbs CV, Khaitan A, Kirmse BM, Borkowsky W. COVID-19 in children: a review and parallels to other hyperinflammatory syndromes. Front. Pediatr. 2020;8:593455. doi: 10.3389/fped.2020.593455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Group, I. C. C. COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection49, 889–905 (2021). [DOI] [PMC free article] [PubMed]
- 61.Kimble RB, et al. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 62.Chen, L. H., Yang, G. R. & Grosser, T. Macrophage COX-2/mPGES-1/PGE(2) mediates inflammatory pain in peripheral tissues. FASEB J.31, 812.9 (2017).
- 63.Brazill JM, Beeve AT, Craft CS, Ivanusic JJ, Scheller EL. Nerves in bone: evolving concepts in pain and anabolism. J. Bone Min. Res. 2019;34:1393–1406. doi: 10.1002/jbmr.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Port JR, et al. SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters. Nat. Commun. 2021;12:4985. doi: 10.1038/s41467-021-25156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinoshita T, et al. Co-infection of SARS-CoV-2 and influenza virus causes more severe and prolonged pneumonia in hamsters. Sci. Rep. 2021;11:21259. doi: 10.1038/s41598-021-00809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mann, E. R. et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci. Immunol. 10.1126/sciimmunol.abd6197 (2020). [DOI] [PMC free article] [PubMed]
- 68.Galani IE, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22:32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 69.Hadjadj J, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powell TR, et al. The chemokine CCL17 is associated with protection against HIV-1 acquisition. J. Immunol. 2020;204:225–237. [Google Scholar]
- 72.Kyriazopoulou E, et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nouailles G, et al. Temporal omics analysis in Syrian hamsters unravel cellular effector responses to moderate COVID-19. Nat. Commun. 2021;12:4869. doi: 10.1038/s41467-021-25030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu H, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan, J. F. et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 10.1128/JCM.00310-20 (2020). [DOI] [PMC free article] [PubMed]
- 76.Chan JF, et al. Surgical mask partition reduces the risk of noncontact transmission in a golden Syrian hamster model for coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2139–2149. doi: 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan S, et al. Viruses harness YxxO motif to interact with host AP2M1 for replication: a vulnerable broad-spectrum antiviral target. Sci. Adv. 2020;6:eaba7910. doi: 10.1126/sciadv.aba7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan S, et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021;593:418–423. doi: 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- 79.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data that support the findings of this study are available within the Article and Supplementary Information or from the corresponding author upon reasonable request. Source Data file is provided with this paper.