Abstract
We conducted a phase I-II study of the safety, tolerance, and plasma pharmacokinetics of liposomal amphotericin B (L-AMB; AmBisome) in order to determine its maximally tolerated dosage (MTD) in patients with infections due to Aspergillus spp. and other filamentous fungi. Dosage cohorts consisted of 7.5, 10.0, 12.5, and 15.0 mg/kg of body weight/day; a total of 44 patients were enrolled, of which 21 had a proven or probable infection (13 aspergillosis, 5 zygomycosis, 3 fusariosis). The MTD of L-AMB was at least 15 mg/kg/day. Infusion-related reactions of fever occurred in 8 (19%) and chills and/or rigors occurred in 5 (12%) of 43 patients. Three patients developed a syndrome of substernal chest tightness, dyspnea, and flank pain, which was relieved by diphenhydramine. Serum creatinine increased two times above baseline in 32% of the patients, but this was not dose related. Hepatotoxicity developed in one patient. Steady-state plasma pharmacokinetics were achieved by day 7. The maximum concentration of drug in plasma (Cmax) of L-AMB in the dosage cohorts of 7.5, 10.0, 12.5, and 15.0 mg/kg/day changed to 76, 120, 116, and 105 μg/ml, respectively, and the mean area under the concentration-time curve at 24 h (AUC24) changed to 692, 1,062, 860, and 554 μg · h/ml, respectively, while mean CL changed to 23, 18, 16, and 25 ml/h/kg, respectively. These data indicate that L-AMB follows dose-related changes in disposition processing (e.g., clearance) at dosages of ≥7.5 mg/kg/day. Because several extremely ill patients had early death, success was determined for both the modified intent-to-treat and evaluable (7 days of therapy) populations. Response rates (defined as complete response and partial response) were similar for proven and probable infections. Response and stabilization, respectively, were achieved in 36 and 16% of the patients in the modified intent-to-treat population (n = 43) and in 52 and 13% of the patients in the 7-day evaluable population (n = 31). These findings indicate that L-AMB at dosages as high as 15 mg/kg/day follows nonlinear saturation-like kinetics, is well tolerated, and can provide effective therapy for aspergillosis and other filamentous fungal infections.
Cancer treatment and bone marrow transplantation/stem cell transplantation (BMT/SCT) regimens involve intensive cytotoxic chemotherapy, which often leads to prolonged myelosuppression and increased risk for opportunistic mycoses (5, 30, 31, 33, 39). Graft versus host disease (GVHD) following BMT/SCT may require immunosuppression that also increases the risk of invasive fungal infections. Despite empirical use of amphotericin B and the prophylactic use of antifungal triazoles, invasive fungal infections due to resistant Candida spp., Aspergillus spp., Fusarium spp., and zygomycetes, as well as other fungal pathogens, continue to emerge (6, 10, 11, 40). Infections due to these organisms may be unresponsive even to maximal tolerated doses of conventional deoxycholate amphotericin B (DAMB; 1.0 to 1.5 mg/kg of body weight/day). Moreover, when responses do occur, treatment may be accompanied by frequent dose-limiting nephrotoxicity (14, 43).
Liposomal amphotericin B (L-AMB; AmBisome) was found in preclinical studies to be as effective or more effective but less nephrotoxic than conventional amphotericin B in the treatment of experimental invasive pulmonary aspergillosis (13, 15, 37). Consistent with these findings are the results of four open label clinical trials which also demonstrated antifungal efficacy in immunocompromised patients (28, 29, 35) and a randomized trial in patients with invasive fungal infections complicating hematologic malignancies that found superior efficacy and safety (23). Toxicity has been minimal despite much higher dosages of amphotericin B being delivered than can be safely administered when given as DAMB. The reduced toxicity of this formulation thus, allows the administration of much higher doses of amphotericin B than that of the deoxycholate product (13, 18, 19, 27, 44).
Preclinical and clinical data demonstrate a dose-response relationship of the class of amphotericin B compounds against invasive fungal infections (4, 13, 17, 41, 42). An earlier study found that L-AMB had no dose-limiting toxicity between 1.0 and 7.5 mg/kg/day (45). As invasive fungal infections in immunocompromised patients may be unresponsive to approved dosages of L-AMB (3 to 5 mg/kg/day), clinicians will often increase the dosage of L-AMB to ≥7.5 mg/kg/day.
Little is known, however, about the safety, tolerance, and plasma pharmacokinetics of amphotericin B at these higher dosages of L-AMB. Moreover, the maximum tolerated dose (MTD) of L-AMB also is unknown. We therefore investigated the safety, tolerance, plasma pharmacokinetics, and MTD of L-AMB in an open label, multicenter, and sequential dose escalation study of the treatment of invasive fungal infections.
MATERIALS AND METHODS
Study design.
This study was designed as an open-label, multicenter, sequential dose escalation trial for the assessment of the safety, tolerance, plasma pharmacokinetics, and MTD of intravenous L-AMB. L-AMB was administered at four high dosage levels in a population of immunocompromised adults requiring antifungal therapy for invasive fungal infections. The study drug consisted of intravenous L-AMB (AmBisome; Fujisawa Healthcare, Inc. USA). Doses of L-AMB were calculated and expressed as the amount of amphotericin B administered. For example, a dose of 50 mg of L-AMB refers to the administration of an amount of L-AMB that contains 50 mg of amphotericin B. Patients were eligible for study if (i) they were between the ages of 18 and 80 years and were undergoing bone marrow transplantation, were receiving active chemotherapy for neoplastic disease or immunosuppressive therapy for solid organ transplantation or aplastic anemia, or were infected with human immunodeficiency virus (HIV) and (ii) they had evidence of an invasive mycosis due to a filamentous fungus.
Dosage cohorts received 7.5, 10.0, 12.5, or 15.0 mg/kg/day of amphotericin B as L-AMB administered once daily as a 2-h infusion. A minimum of six patients was enrolled at each dosage level, and 12 additional patients were enrolled at the MTD. An additional seven patients were permitted for enrollment to allow for inevaluability. No forms of amphotericin B other than that of the study drug were allowed during the clinical trial. If patients required conventional amphotericin B, administration of the study drug was discontinued and standard therapy was administered. Informed consent was obtained from the patient or legally authorized representative prior to entry. Patients declining enrollment into the study received DAMB or other lipid formulations of amphotericin B.
Patients were not eligible for enrollment into study if (i) there was clinical and laboratory evidence of veno-occlusive disease in BMT/SCT recipients, (ii) there was moderate or severe liver disease, as defined by aspartate aminotransferase (AST) or alanine aminotransferase (ALT) at >10 times the upper limit of normal (ULN), total bilirubin at >5 times ULN, or serum alkaline phosphatase at >10 times ULN, (iii) serum creatinine was >3 times ULN for age, (iv) hypokalemia was <3.0 mEq/liter, and (v) they had a history of anaphylaxis attributed to AMB. Provided that adverse events did not indicate a health risk to the patient, the administration of the study drug continued for a period of at least 7 days to a maximum of 100 days, fewer if the investigator judged that L-AMB was no longer required or beneficial.
Patients with proven invasive fungal infection were defined as those with chest radiographic or computed tomography (CT) scan evidence of new pulmonary infiltrates compatible with fungal pneumonia, plus a lower respiratory tract culture and/or histology which identified Aspergillus, Fusarium, or other filamentous fungal species by (i) transthoracic needle aspiration, (ii) bronchoalveolar lavage or brushings, or (iii) hyphae observed by direct exam from a biopsy demonstrating characteristic invasive fungal elements. Patients with probable invasive fungal infection had clinical evidence of pneumonia and characteristic findings on chest X rays or CT scans (nodules, wedge-shaped or cavitating lesions, “halo sign,” or progression of lesions from infiltrates to cavitary or crescent lesions) plus a bronchoalveolar lavage that was negative for other agents known to cause the observed pneumonic process. Patients with possible invasive fungal infection were those with persistent fever and neutropenia who also had pulmonary infiltrates or sinus opacifications.
Monitoring of safety and tolerance.
In order to monitor the safety and tolerance of L-AMB infusions, patients were closely observed for side effects during the administration of study drug. A previously validated bedside data extraction sheet was utilized by the nursing staff to record serial vital signs during and after infusion, as well as signs and symptoms of infusion-related toxicity (41, 45, 50). This data extraction sheet then became a source document for reporting infusion-related toxicity. Pulse and blood pressure were monitored immediately before, at 15 and 30 min, and at the end of the infusion. Between doses, temperature and vital signs were recorded every 4 h during waking hours. Signs, symptoms, and reported side effects associated with drug infusion or occurring at any time during the study period were documented and assessed for a relationship to the study drug.
The following laboratory examinations for assessment of safety were performed every other day and on the last day of dosing: hemoglobin, hematocrit, total white count with differential, platelet count, prothrombin time, partial thromboplastin time, BUN and serum creatinine, calcium, potassium, sodium, AST, ALT, alkaline phosphatase, total bilirubin, magnesium, complete urinalysis, and blood cultures obtained from two separate sites while the patient remained febrile. Prior to escalation to the next dosage cohort, six patients were required to have completed therapy with no associated grade 3 or 4 toxicity attributable to the study drug. The National Cancer Institute Common Toxicity Criteria were employed as the toxicity scale in this clinical trial (http://ctep.info.nih.gov/ctc3/ctc.htm). Escalation to the next higher dosage level was permitted after mutual agreement between the investigators and the Fujisawa Clinical Monitor that the above criteria for safety had been met was reached. No intrapatient dosage escalation occurred. A patient assigned to a particular dosage continued to receive that dosage throughout the study.
Pharmacokinetic sampling.
Pharmacokinetic sampling was performed on days 1 and 7 and on the last day of study drug. Two-milliliter venous blood samples were drawn at each time point. The blood was centrifuged and the plasma fraction was stored at −70°C until analysis. Samples were stored for a median of 4 months before analysis. First dose and day 7 pharmacokinetic sample collection times were as follows: prior to dosing, at the end of 60 min of infusion, at 65, 75, 90, and 120 min, and then at 3, 4, 6, 8, 12, 18, and 24 h postinfusion. Daily trough samples (immediately prior to the next dose) were subsequently obtained on days 3, 4, 5, and 6. Last-dose pharmacokinetic sample time points were then obtained prior to dose, at the end of 60 min of infusion, at 65, 75, 90, and 120 min, and then at 3, 4, 6, 8, 12, 18, and 24 h postinfusion, followed by wash-out samples 3, 7, and 14 days after the last dose of L-AMB.
Analytical methods for L-AMB assay.
Concentrations of amphotericin B were determined by a validated high-performance liquid chromatography assay (3). Following methanol deproteinization, amphotericin B and the internal standard, 3-nitrophenol, were separated by reversed-phase chromatography and detected by UV absorbance at 406 nm. The high-performance liquid chromatography system consisted of a Bio-Rad autosampler (Bio-Rad, Hercules, Calif.), Spectra-Physics Model 250 pump (Thermo Separations, San Jose, Calif.), Waters Model 441 UV-VIS detector (Waters Corp, Milford, Mass.), column heater, Supelcosil ABZ + Plus column (3-μm-diameter particle size, 150 by 4.6 mm [inner diameter]; Supelco, Bellefonte, Pa.), and a Keystone CL8 guard column (Western Analytical, Murrieta, Calif.). Data were collected and integrated on a VG Multichrom Data System for VAX/VMS. The lower limit of quantitation of the assay was 0.1 μg/ml. Two overlapping standard curves were used: 0.05 to 20 μg/ml and 0.5 to 200 μg/ml. The unweighted correlation coefficient was 0.998 for both curves, with an interday and intraday coefficient of variation of 1.8 to 11.2% and 6.9 to 10.1%, respectively.
Pharmacokinetic calculations.
The pharmacokinetic profile of amphotericin B following L-AMB administration was determined by noncompartmental analysis. The terminal elimination half-life (t1/2) was obtained from plasma data in the postdistribution phase. The elimination rate constant k was defined as 0.693/t1/2. The area under the concentration-time curve from 0 to 24 h (AUC0-24) and area under the first moment of the concentration-time curve from 0 to 24 h (AUMC0-24) were calculated using the linear trapezoidal method. The AUC0-∞ was determined as follows: AUC0-24 + AUC24-∞, with AUC24-∞ extrapolated from Ct/k, where Ct was the last measured concentration. The AUMC0-∞ was calculated similarly, with AUMC24-∞ extrapolated from (Ct × t)/k + Ct/k2. The mean residence time (MRT) was calculated as AUMCinf/AUCinf. Total body clearance (CL) was calculated as dose/AUC0-∞. The volume of distribution (V) was calculated as follows: V = CL/k. The volume of distribution at steady state (Vss) was calculated as follows: Vss = (dose)(AUMC0-∞)/(AUC0-∞)2 − (dose)(infusion time)/2 × AUC0-∞.
In order to further elucidate the disposition profile of L-AMB, a population pharmacokinetic analysis was conducted using the mixed effect computer program NONMEM. Screening of the data indicated that a two-compartment structural model with a zero-order constant infusion input (ADVAN3) was appropriate for these data. The model was reparameterized using TRANS4. The population analysis was conducted in the absence of potential covariates (base model). Covariate factors were added to each of the pharmacokinetic parameters and tested for statistical significance. Significance was determined by measuring the decrease in the minimum value of the objective function (MVOF), which equals 2 log (100-fold likelihood) of the data. A decrease in the MVOF corresponding to a P value of <0.05 was considered to be significant. Fixed effects tested included the type of bone marrow transplant patient (1 = autologous, 2 = allogeneic, 0 = none), infection status (1 = possible, 2 = probable, 3 = definite), outcome status (3 = success [complete response, particle response, or stability], 2 = failure, 1 = not evaluable), and immediate prior exposure to amphotericin B (1 = amphotericin B deoxycholate, 2 = amphotericin B lipid complex). Two fixed effects demonstrated a significant effect on the central compartment volume of distribution (V1) and two on clearance (CL) as shown:
TVV1 = Θ1 + Θ6 × ASES + Θ8 × INFC
V1 = TVV1 × eη1
TVCL = Θ2 + Θ5 × BMT + Θ7 × CONM
CL = TVCL × e(1+η2)
TVV2 = Θ3
V2 = TVV2 × eη3
TVQ = Θ4
Q = TVQ × eη4
k = CL/V1
k12 = Q/V1
k21 = Q/V2
Yij = Fij + Eij
where ASES is the outcome status, INFC is the infection status, BMT is the type of bone marrow transplant, CONM is the immediate prior exposure to amphotericin B product, TVV1 is the population volume of the central compartment, V1 is the individual volume of the central compartment, TVCL is the population clearance, CL is the individual clearance, TVV2 is the population volume of distribution for the peripheral compartment, V2 is the individual volume of distribution for peripheral compartment, TVQ is population intercompartment clearance, Q is the individual intercompartment clearance, k is the individual elimination rate constant from the central compartment, k12 is the individual transfer rate constant from the central to peripheral compartment, k21 is the individual transfer rate constant from the peripheral to central compartment, ηn is the random interindividual effect term, Θn is the regression parameter, Yij is jth observed concentration in the ith individual, Fij is the model-predicted jth individual, and Eij is the residual interindividual error term.
Monitoring and assessment of efficacy.
Efficacy was assessed by clinical response, radiological response, mycological response, and survival. Serial blood cultures, urine cultures, and chest radiographs were performed for all patients. Blood was cultured using lysis centrifugation (Wampole) and BacTAlert (Organon Teknika) systems. Serial chest CT scans were performed for monitoring therapeutic response of invasive fungal infections of the respiratory tract and sinuses.
Success or failure was defined as the primary antifungal efficacy endpoint. Investigators (T.J.W., J.L.G., P.P., and E.J.A.) classified the therapy for each patient as a success or failure. Success was defined as the disappearance of all signs and symptoms of the treated fungal infection (complete response) or continuous improvement and clinical evolution compatible with responding disease (partial response). Stabilization was defined as no progression or resolution of infection on study drug. Failure was defined as (i) discontinuation of study drug due to toxicity, (ii) death attributed to the fungal infection as a primary or contributing cause, or (iii) progressive infection while on therapy or within 1 month of last therapy. Progressive infection was defined as continuing fever, persistent positive blood cultures or progression of pulmonary infiltrates, sinus opacifications, or physical findings, such as cutaneous lesions.
Statistical analysis.
All patients who received at least one dose of study drug (modified intent to treat population) were included in the safety and efficacy analyses. A separate analysis of response was assessed for patients who received at least 7 days of therapy. This MTD study was not powered to detect differences in efficacy across dosage groups. Comparisons of the mean pharmacokinetic values between the first versus last doses and between different dose levels of L-AMB were performed using two-tailed unpaired Student's t test. A P value of ≤0.05 was considered to be statistically significant.
RESULTS
Study patient population.
A total of 44 patients enrolled in the study and received at least three doses of L-AMB (Table 1). These patients had a mean age of 43.2 years (range, 19 to 70 years). Thirty-five patients were male and nine were female. A total of 29 (66%) patients underwent BMT/SCT; 20 received allogeneic and 9 received autologous transplants. Nine (21%) had graft versus host disease (GVHD). The remaining 15 patients received antineoplastic chemotherapy, underwent solid organ transplantation, or had aplastic anemia or HIV infection. Consistent with the seriously ill condition of many of the patients enrolled into this study, approximately one-half of all patients had baseline elevation of serum creatinine, AST, ALT, or bilirubin. Proven fungal infections were documented for 21 patients, probable fungal infection was documented for 10, and possible fungal infection was documented for 13. The type of invasive fungal infection and the sites of these infections are summarized in Table 2. Invasive pulmonary aspergillosis was the predominant cause of infection, followed by three cases of zygomycosis and two cases of fusariosis. Patients may have had more than one site of infection.
TABLE 1.
Patient demographics
| Parameter | Total | No. of patients in dosage group:
|
|||
|---|---|---|---|---|---|
| 7.5 mg/kg | 10 mg/kg | 12.5 mg/kg | 15 mg/kg | ||
| No. of patients | 44 | 8 | 10 | 7 | 19 |
| Age (yr) mean ± SD | 43.2 ± 11.7 | 40.4 ± 9.5 | 39.8 ± 10.9 | 47.6 ± 12.6 | 44.6 ± 12.7 |
| Range (yr) | 19–70 | 19–49 | 19–57 | 30–67 | 20–70 |
| No. of Male/female | 35/9 | 8/0 | 9/1 | 5/2 | 13/6 |
| BMT/SCTa | 29 | 6 | 5 | 5 | 13 |
| Non-BMT | 15 | 2 | 5 | 2 | 6 |
| Antineoplastic therapy | 8 | 2 | 2 | 1 | 3 |
| Solid organ transplant | 2 | 0 | 0 | 0 | 2 |
| Aplastic anemia | 2 | 0 | 1 | 0 | 1 |
| HIV infection | 2 | 0 | 1 | 1 | 0 |
| Baseline ANC of <500/μl | 19 | 4 | 2 | 4 | 9 |
| Above baseline | |||||
| SCr | 22 | 4 | 6 | 3 | 9 |
| ALT | 20 | 4 | 6 | 2 | 8 |
| AST | 14 | 5 | 3 | 1 | 5 |
| Total bilirubin | 21 | 5 | 3 | 3 | 10 |
| Alkaline phosphatase | 23 | 3 | 7 | 2 | 11 |
| Type of fungal infection | |||||
| Proven | 21 | 4 | 6 | 4 | 7 |
| Probable | 10 | 4 | 1 | 1 | 4 |
| Possible | 13 | 0 | 3 | 2 | 8 |
Twenty had allogeneic transplants and nine had autologous transplants. Nine patients with allogeneic transplants had GVHD.
ANC, absolute neutrophil count.
SCr baseline, >1.2 mg/dl; ALT baseline, >35 IU/liter; AST baseline, >35 IU/liter; total bilirubin baseline, >130 IU/liter.
TABLE 2.
Baseline fungal infections and sites
| Organism | Site | Total [n (%)] | 7.5 mg/kg [n (%)] | 10 mg/kg [n (%)] | 12.5 mg/kg [n (%)] | 15 mg/kg [n (%)] |
|---|---|---|---|---|---|---|
| Aspergillus | Lungs | 31 (70.5) | 5 (62.5) | 7 (70.0) | 4 (57.1) | 15 (78.9) |
| Sinuses | 3 (6.8) | 1 (10.0) | 1 (14.3) | 1 (5.3) | ||
| Othera | 3 (6.8) | 2 (20.0) | 1 (5.3) | |||
| Skin | 2 (4.5) | 1 (12.5) | 1 (10.0) | |||
| Zygomycete | Skin | 2 (4.5) | 1 (14.3) | 1 (5.3) | ||
| Lungs | 1 (2.3) | 1 (12.5) | ||||
| Sinuses | 1 (2.3) | 1 (14.3) | ||||
| Blood | 1 (2.3) | 1 (5.3) | ||||
| Abscessb | 1 (2.3) | 1 (5.3) | ||||
| Fusarium | Skin | 1 (2.3) | 1 (12.5) | |||
| Blood | 1 (2.3) | 1 (12.5) | ||||
| Paronychia | 1 (2.3) | 1 (12.5) |
Duration of administration of L-AMB.
The number of infusions of L-AMB ranged from 1 to 83, with an actual cumulative dose ranging from 13.6 to 972.1 mg/kg (Table 3). The duration of the day 1 infusion ranged from 1 to 2.6 h. All patients stopped therapy within the protocol-specified maximum of 100 days. The median day for administration of the last study dose ranged from day 9 (15-mg/kg group) to day 24 (7.5-mg/kg group). A total of 9 (20%) patients discontinued taking the study drug due to an adverse event. These included elevated serum creatinine, renal failure, acute renal failure, pancreatitis, hyperbilirubinemia, hypotension associated with infusion, relapse of primary malignancy, cardiorespiratory failure, and multisystem organ failure; the latter three adverse events were attributed to underlying diseases and not to the study drug. Discontinuation was unrelated to dosage group, occurring in 38% of patients in the 7.5-mg/kg group, 10% in the 10-mg/kg group, 14% in the 12.5-mg/kg group, and 21% in the 15-mg/kg group.
TABLE 3.
Duration of infusion of L-AMB
| Parameter | Total | No. of patients in dosage group:
|
|||
|---|---|---|---|---|---|
| 7.5 mg/kg | 10 mg/kg | 12.5 mg/kg | 15 mg/kg | ||
| No. of patients | 44 | 8 | 10 | 7 | 19 |
| No. of daily infusions | |||||
| Mean ± SD | 18.5 ± 18.5 | 23.9 ± 16.4 | 22.9 ± 20.0 | 28.1 ± 30.6 | 10.4 ± 8.5 |
| Median | 11 | 23 | 16 | 10 | 9 |
| Range | 1–83 | 2–54 | 3–58 | 3–83 | 1–31 |
| Duration of infusion (h) | |||||
| Mean ± SD | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.2 |
| Median | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Range | 1.0–2.6 | 1.9–2.6 | 1.8–2.1 | 2.0–2.3 | 1.0–2.3 |
| Discontinuation of L-AMB due to adverse event [n (%)] | 9 (20) | 3 (37) | 1 (10) | 1 (14) | 4 (21) |
Infusion-related reactions.
Safety and tolerance were monitored prospectively with particular attention to infusion-related reactions and to nephrotoxicity. Premedications were not administered per protocol for infusion-related reactions prior to the day 1 study drug infusion. Any adverse events with onset during or within 1 h of completion of the study drug infusion were recorded as infusion-related reactions.
The overall frequency of infusion-related reactions on day 1 was summarized by dosage group in Table 4. A total of 16 (36%) patients experienced an infusion-related reaction on day 1. Infusion-related reactions appeared to be more common in the 7.5-mg/kg group (5 out of 8 [62%]) than in the other dose groups (20, 29, and 37%). Chills and rigors were observed in only 2 of the 36 patients receiving ≥10-mg/kg doses of L-AMB per day. Premedications for prevention of infusion-related reactions at some point after day 1 of infusions were administered in 37 (84%) of 44 cases. There was no dose dependency toward the use of premedications.
TABLE 4.
Safety and tolerance of high dosage L-AMB
| Parametera | Totals | No. (%) of patients in dosage group:
|
|||
|---|---|---|---|---|---|
| 7.5 mg/kg | 10 mg/kg | 12.5 mg/kg | 15 mg/kg | ||
| No. of patients | 44 | 8 | 10 | 7 | 19 |
| IRRs on day 1 | 16 (36) | 5 (62) | 2 (20) | 2 (29) | 7 (37) |
| Fever | 8 (18) | 2 (25) | 0 (0) | 1 (14) | 5 (26) |
| Chills/rigors | 1 (2) | 3 (38) | 0 (0) | 1 (14) | 1 (5) |
| Vomiting | 1 (2) | 0 (0) | 1 (10) | 0 (0) | 0 (0) |
| Other reactionsc | 6 (14) | 3 (38) | 1 (10) | 1 (14) | 1 (5) |
| Ratio of no. of IRRs to no. of infusions | 0.14 | 0.23 | 0.24 | 0.10 | 0.07 |
| Laboratory featuresd | |||||
| Nephrotoxicity (≥1.5× SCr) | 22 (50) | 3 (38) | 5 (50) | 4 (57) | 10 (53) |
| Nephrotoxicity (≥2.0× SCr) | 14 (32) | 2 (25) | 3 (30) | 3 (43) | 6 (32) |
| Hypokalemia (<3.0 mM) | 17 (39) | 2 (25) | 3 (30) | 5 (71) | 7 (37) |
| Hypokalemia (≤2.5 mM) | 9 (20) | 0 (0) | 0 (0) | 2 (29) | 7 (37) |
| Hepatotoxicity | 1 (2) | 0 (0) | 1 (10) | 0 (0) | 0 (0) |
| Anemia | 24 (54) | 5 (62) | 6 (60) | 5 (71) | 8 (42) |
| No. of deaths | 24 (54) | 3 | 4 | 4 | 13 |
| Causes of death | |||||
| Acute renal failure | 1 (2) | 0 | 0 | 1b | 0 |
| Cardiorespiratory failure | 7 (16) | 0 | 2 | 2 | 3 |
| Fungal infection | 4 (9) | 1 | 0 | 0 | 3 |
| Multiorgan failure | 2 (5) | 2 | 0 | 0 | 0 |
| Neoplastic disease | 7 (16) | 0 | 2 | 1 | 4 |
| Other | 3 (7) | 0 | 0 | 0 | 3 |
IRRs, infusion-related reactions.
Considered by the investigator to have a possible relationship to study drug.
Two patients receiving 7.5 mg/kg/day and one patient receiving 10 mg/kg/day on first infusion had substernal chest tightness and dyspnea within the first 5 min of infusion. Symptoms were relieved by intravenous diphenhydramine, and infusion continued without recurrence. Diphenhydramine subsequently was used for the prevention of these acute infusion-related reactions in the same three patients, with no recurrence of symptoms.
Nephrotoxicity (1.5× SCr), 1.5 times serum creatinine baseline and >1.2 mg/dl; nephrotoxicity (2× SCr), 2 times creatinine baseline and 1.2 mg/dl; hypokalemia (≤2.5 mM), potassium level of ≤2.5 mmol/liter; hypokalemia (<3.0 mM), potassium level of <3 mmol/liter; hepatotoxicity, AST (SGOT) or ALT (AGPT) value of >10 times ULN when baseline was <2 times ULN, >15 times ULN when baseline was 2 to 5 times ULN, or 20 times ULN when baseline was 5 to 10 times ULN; anemia, hemoglobin level of ≤8 g/dl.
Fever and chills were the two more common infusion-related reactions after day 1, each occurring in 23% (10 out of 44) of the patients. No consistent dose-related trend with respect to the occurrence of any infusion-related reaction was observed. Fever and chills abated with subsequent infusions.
Although individual patients may have experienced an infusion reaction on day 1 or beyond, the total number of infusion-related reactions occurring over the course at all infusions was 133 out of 815 (16.3%). The frequency of the total number of infusion-related reactions for total number of infusions was 23, 24, 10, and 7% for the 7.5-, 10-, 12.5-, and 15-mg/kg dose groups, respectively. Cardiorespiratory events occurred with relatively low frequency across the dosage groups despite the large amounts of L-AMB being administered. Nevertheless, infusion-related cardiopulmonary events, including chest pain (3 cases), dyspnea (3 cases), flushing (1 case), hypoxia (1 case), hypotension (1 case), and myalgias (1 case) did occur during L-AMB administration. Specifically, two patients receiving 7.5 mg/kg/day and one patient receiving 10 mg/kg/day on first infusion had substernal chest tightness and dyspnea within the first 5 min of infusion. Symptoms were relieved by intravenous diphenhydramine and by stopping the infusion. Upon resuming infusion of L-AMB, symptoms did not recur. Diphenhydramine at 25 to 50 mg/kg/day was given before subsequent doses for prevention of these acute infusion-related reactions in the same three patients, with no recurrence of symptoms.
None of the infusion-related reactions appeared to be dosage dependent. Indeed, the ratio of the number of infusion-related reactions to the number of infusions administered was higher in the 7.5-mg/kg (0.23) and 10-mg/kg (0.24) dosage groups than in the 12.5-mg/kg (0.10) or 15-mg/kg (0.07) groups.
Laboratory parameters.
The frequency of abnormal values for laboratory parameters of renal and hepatic function is presented in Table 4. One-half (22 of 44 [50%]) of patients had serum creatinine values that were ≥1.5 times greater than the baseline and >1.2 mg/dl at some time during the study. Fourteen (32%) had a value ≥2 times greater than the baseline. Excluding one patient, peak creatinine levels in serum ranged from 0.9 to 5.8 mg/dl during the study. The one patient who was HIV positive with sinus zygomycosis due to Rhizopus sp. was enrolled in the 12.5-mg/kg dosage cohort and received L-AMB over the course of 99 days. The level of creatinine in serum ranged between 1.4 and 2.3 mg/dl for 94 days. However, the creatinine level in serum increased abruptly to 6.4 mg/dl in one patient on day 100 and L-AMB was discontinued. No patients required hemodialysis.
No significant dosage-dependent trends were observed for changes in the creatinine level in serum. However, there were more cases of severe hypokalemia (≤2.5 mEq/liter) in those patients receiving >10 mg/kg/day in comparison to those receiving ≤10 mg/kg/day (0% versus [9 out of 26] 35%; P = 0.006). Hypomagnesemia was observed in eight cases but did not show a relation to dosage.
Only one patient (in the 10-mg/kg group) fulfilled criteria for hepatotoxicity. More than half the patients (24 out of 44 [55%]) were anemic at sometime during the study.
Overall adverse events.
All patients experienced at least one adverse event. The more common adverse events included fever (21 out of 44 [48%]), increased creatinine level (20 out of 44 [46%]), hypokalemia (17 out of 44 [39%]), chills (21 out of 44 [32%]), and abdominal pain (11 out of 44 [25%]). No obvious dose relationship with respect to the overall frequency of adverse events was observed.
Pharmacokinetics.
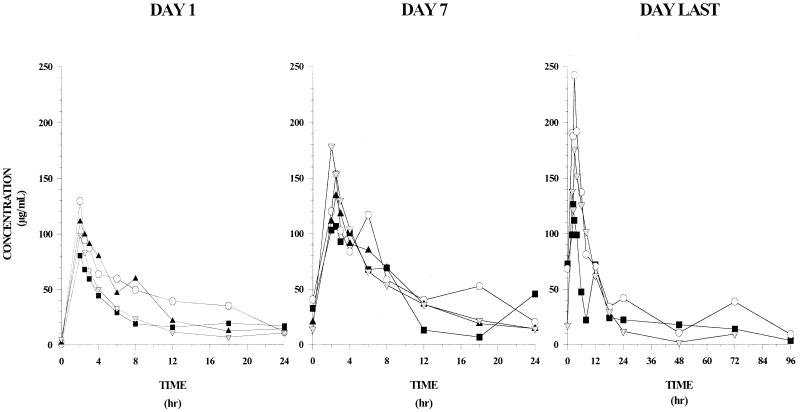
The pharmacokinetic profile of L-AMB administered at dosages of 7.5, 10, 12.5, or 15 mg/kg/day as a 2-h infusion is summarized in Fig. 1 and the noncompartmental parameters in Table 5. The interpatient concentration-time data were highly variable within a dosage group. The mean (± standard deviation [SD]) AUC24s after the initial dose were 692 ± 834, 1,062 ± 971, 860 ± 390, and 554 ± 174 for the respective ascending doses.
FIG. 1.
Plasma concentration-time profiles of L-AMB at doses of 7.5 mg/kg (▪), 10.0 mg/kg (○), 12.5 mg/kg (▴), and 15.0 mg/kg (▾) on day 1 (left panel), day 7 (middle panel), and last day (right panel) of infusion.
TABLE 5.
Noncompartmental pharmacokinetic parameters of high-dose L-AMB
| Dosage (mg/kg/day) | Day | n | Cmax (μg/ml) | AUC24 (μg · h/ml) | AUC∞ (μg · h/ml) | t1/2 (h) | CL (ml/h/kg) | V (liter/kg) | Vss (liter/kg) | MRT (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 7.5 | 1 | 8 | 75.9 ± 58.4 | 692 ± 834 | 815 ± 1068 | 6.8 ± 1.9 | 23 ± 14 | 0.22 ± 0.18 | 0.24 ± 0.18 | 10.7 ± 2.6 |
| 7 | 6 | 115.1 ± 104.9 | 1,333 ± 2,153 | 1,670 ± 2,868 | 6.0 ± 0.8 | 15 ± 11 | 0.14 ± 0.10 | 0.14 ± 0.11 | 8.7 ± 1.2 | |
| La | 4 | 144.3 ± 61.6 | 1,286 ± 973 | 1,498 ± 1,040 | 6.5 ± 3.4 | 11 ± 13 | 0.08 ± 0.08 | 0.18 ± 0.12 | 11.9 ± 4.4 | |
| 10 | 1 | 7 | 119.6 ± 69.8 | 1,062 ± 971 | 1,188 ± 1,058 | 8.0 ± 1.5 | 18 ± 19 | 0.23 ± 0.24 | 0.22 ± 0.23 | 12.3 ± 1.8 |
| 7 | 6 | 164.7 ± 119.7 | 1,919 ± 2,056 | 2,156 ± 2,221 | 8.4 ± 2.6 | 12 ± 12 | 0.16 ± 0.17 | 0.14 ± 0.14 | 12.8 ± 3.0 | |
| L | 4 | 208.9 ± 47.7 | 1,944 ± 592 | 2,431 ± 942 | 10.5 ± 6.6 | 5 ± 3 | 0.05 ± 0.03 | 0.06 ± 0.03 | 14.3 ± 7.1 | |
| 12.5 | 1 | 7 | 116.3 ± 47.8 | 860 ± 390 | 902 ± 450 | 7.1 ± 3.5 | 16 ± 6 | 0.18 ± 0.13 | 0.16 ± 0.07 | 9.8 ± 1.1 |
| 7 | 5 | 147.4 ± 69.2 | 1168 ± 911 | 1,292 ± 1,010 | 8.2 ± 2.5 | 13 ± 7 | 0.16 ± 0.10 | 0.13 ± 0.08 | 9.8 ± 1.1 | |
| L | 1 | 754.8 | 13,919 | 46,558 | 48 | 0.3 | 0.07 | 0.09 | —b | |
| 15 | 1 | 11 | 105.1 ± 30.9 | 554 ± 174 | 685 ± 252 | 9.0 ± 3.1 | 25 ± 8 | 0.33 ± 0.12 | 0.23 ± 0.09 | 9.1 ± 2.8 |
| 7 | 6 | 178.6 ± 49.0 | 1,152 ± 617 | 1,355 ± 693 | 9.0 ± 0.9 | 14 ± 7 | 0.18 ± 0.09 | 0.14 ± 0.06 | 10.2 ± 1.6 | |
| L | 2 | 231 | 2,168 | 2,300 | 8.5 | 9 | 0.14 | 0.12 | 10.9 |
L, last.
—, Insufficient data to determine MRT.
A nonlinear relationship between dosage and the plasma pharmacokinetic parameters of AUC24, AUC∞, and Cmax was evident. These parameters reached maximum values following administration of 10 mg/kg/day and declined at 12.5 and 15 mg/kg/day, suggesting a possible change in the elimination mechanism at high amphotericin B concentrations. The mean t1/2 ranged from 6 to 10.5 h and was similar across groups. The mean clearance on day 1 was 18 to 25 ml/h/kg, which decreased within each group, ranging from 5 to 11 ml/h/kg by day 7. Although interpatient trough concentrations in all groups were highly variable, intrapatient troughs indicated no amphotericin B accumulation in blood over the course of the study.
A population model evaluation using a two-compartment structural model with zero order input incorporating physiologic and demographic covariates was conducted and was found to be the most appropriate for these data. Evaluation of the population-based pharmacokinetic model resulted in the following parameter values:
TVV1 = 65.1 + 6.34 × ASES − 12.2 × INFC
V1 = TVV1 × e(6.14)
TVCL = 0.339 − 0.0992 × BMT + 0.266 × CONM
CL = TVCL × e(1 + 0.658)
V2 = 53.4 × e(2.44)
Q = 2.99 × e(30.9)
The infection status of the patient had a relatively large negative influence on V1, and the bone marrow transplant status had relatively small (although statistically significant) influence on CL. No statistically significant effect was seen on V2 or Q with any of the covariates evaluated. As observed by evaluation of the model diagnostics, there was a tendency to underestimate the plasma concentrations at the higher concentration values, indicating that other (unknown) factors not included in this model affected the disposition of amphotericin B following L-AMB administration and that these were necessary to more adequately characterize the disposition of amphotericin B at higher concentrations in plasma.
Overall success.
End-of-treatment response rates for all patients are summarized by dosage group in Table 6. Approximately one-half of the patients (23 out of 44 [52%]) had a response (complete or partial) or stabilization of infection. A somewhat greater response and stabilization rate (20 out of 31 [64%]) was observed in patients administered at least 7 doses of study drug (efficacy evaluable population). Response rates were higher in the first three dosage groups. The 15-mg/kg dosage group consisted of more patients who were either nonevaluable or had higher failure rates due to a relatively high number of patients with advanced disease at study entry. Response rates for patients with baseline definite or probable infection were similar to those of the overall response rate.
TABLE 6.
Efficacy of high-dose L-AMB
| Parameter | Total | No. (%) of patients in dosage group:
|
|||
|---|---|---|---|---|---|
| 7.5 mg/kg | 10 mg/kg | 12.5 mg/kg | 15 mg/kg | ||
| Modified intent-to-treat population | |||||
| No. of patients | 44 | 8 | 10 | 7 | 19 |
| No. not evaluable | 7 (16) | 0 | 1 (10) | 0 | 6 (32) |
| Response rate | 16 (36) | 5 (63) | 3 (30) | 4 (57) | 4 (21) |
| Complete response | 10 | 3 | 2 | 3 | 2 |
| Partial response | 6 | 2 | 1 | 1 | 2 |
| Stabilization | 7 (16) | 1 (12) | 4 (40) | 0 | 2 (10) |
| Failure | 14 (32) | 2 (25) | 2 (20) | 3 (43) | 7 (37) |
| Modified intent-to-treat population in study for ≥7 days | |||||
| No. of patients | 31 | 7 | 7 | 5 | 12 |
| No. not evaluable | 2 (6) | 0 | 0 | 0 | 2 (17) |
| Response rate | 16 (52) | 5 (71) | 3 (43) | 4 (80) | 4 (33) |
| Complete response | 10 | 3 | 2 | 3 | 2 |
| Partial response | 6 | 2 | 1 | 1 | 2 |
| Stabilization | 4 (13) | 0 | 3 (43) | 0 | 1 (8) |
| Failure | 9 (29) | 2 (29) | 1 (14) | 1 (20) | 5 (42) |
Thirty-six patients (82%) were alive at the end of treatment. Two patients in each dosage group died during the treatment period. At 2 months posttreatment, 20 (46%) patients were still alive. In four patients (two in the 7.5-mg/kg group and two in the 15-mg/kg group), death was related to fungal infection. As the study was not powered to detect differences in efficacy across dosage groups, no relationship between response and dose can be concluded.
DISCUSSION
This study of the safety, tolerance, and plasma pharmacokinetics of L-AMB found no demonstrable dose-limiting nephrotoxicity or infusion-related toxicity over a dosage range from 7.5 to 15.0 mg/kg/day. Hypokalemia developed more frequently at dosages >10 mg/kg/day than at dosages of ≤10 mg/kg/day. Overall infusion-related toxicity was relatively low; however, an idiosyncratic non-dose-dependent syndrome of acute dyspnea and chest pain was observed in three patients. This could be ameliorated by diphenhydramine administration. There was a distinctly nonlinear profile of the plasma pharmacokinetics of L-AMB over the dosage range of 7.5 to 15.0 mg/kg/day. Furthermore, Cmax and AUC achieved an upper limit at 10 mg/kg/day. Further dosage increases did not result in an increase in Cmax or AUC. Although this MTD study was not statistically powered for assessment of dose-dependent efficacy, L-AMB was effective in the treatment of severe infection in many of these critically ill patients.
The nephrotoxicity of amphotericin B may be understood in terms of its effects on glomerular and tubular functions. As evidenced by serial creatinine concentrations in serum, there was remarkably little glomerular nephrotoxicity at relatively high dosages from 7.5 to 15.0 mg/kg/day. This preservation of glomerular function by lipid formulations of amphotericin B is related to several possible mechanisms. Lipid formulations of amphotericin B have a high affinity for binding to high-density lipoproteins (HDL) (46–49). Binding to HDL promotes uptake in the reticuloendothelial system (RES), which has a relatively high level of expression of HDL receptors. By comparison, conventional deoxycholate amphotericin B has a higher affinity for low-density lipoproteins (LDL). Receptors for LDL are more highly expressed on glomerular endothelial cells and may contribute to the relatively high concentration of conventional amphotericin B in kidneys in comparison to those of L-AMB (22, 37). The preferential uptake of L-AMB by hepatic and splenic macrophages and correspondingly reduced concentration in the kidney further contributed to reduced nephrotoxicity and an improved therapeutic index in comparison to deoxycholate amphotericin B. Another potential mechanism contributing to decreased nephrotoxicity of lipid formulations of amphotericin B is the preferential selected diffusion of the amphotericin B molecule to fungal cell membranes versus mammalian cell membranes (1, 2, 26). The release of amphotericin B molecules from the lipid vehicle via fungal phospholipases onto the fungal cell membrane may further enhance the host-pathogen specificity of lipid formulations of amphotericin B (32).
Although there was no evidence of dose-dependent glomerular dysfunction in this study of high-dose L-AMB, there was a significant difference in the frequency of hypokalemia in patients receiving higher dosages of L-AMB than in those receiving lower dosages. These findings suggest that the renal tubular epithelium may be more sensitive to high dosages of L-AMB then cells mediating glomerular filtration. Hypokalemia developed in patients who were already undergoing close monitoring and routine supplementation of potassium. Thus, the use of high-dose L-AMB at dosages greater than 10 mg/kg/day warrants a particularly meticulous approach to monitoring of potassium levels in serum and may require preemptive administration of higher daily potassium supplementation than would routinely be administered without lipid formulation of amphotericin B.
Infusion-related toxicity was prospectively monitored using a bedside data acquisition sheet. Of the 815 infusions administered, a total of 133 (16.3%) was associated with infusion-related reactions. The relative infrequency of infusion-related reactions is consistent with earlier observations of L-AMB (41, 45, 50). However, this current study also found the development of an idiosyncratic reaction associated with severe substernal chest discomfort, dyspnea, and flank and/or abdominal pain. Developing within the first 1 to 2 min of infusion of L-AMB, these events were not dependent upon dosage or rate of infusion. This acute infusion-related toxicity of L-AMB was managed by the discontinuation of the infusion and the intravenous administration of 25 to 50 mg of diphenhydramine. The symptom complex of dyspnea and flank and abdominal pain resolved rapidly with these interventions. Following resolution of these symptoms, the infusion was resumed with no subsequent infusion-related toxicity. Subsequent infusions were preceded by the administration of diphenhydramine as 25 to 50 mg of intravenous fluid for prevention of infusion-related toxicity. This syndrome, which has been observed previously with L-AMB and other lipid formulations of amphotericin B (24), was recently reviewed by Johnson et al. (20). The mechanism of this infusion-related toxicity is not well understood but may be mediated by histamine. Given the small volume of compound that is infused, the absolute amount of liposomal amphotericin B does not appear to contribute to this toxicity. Instead, the reaction appears to be idiosyncratic and perhaps related to several interactions between the lipid material and host factors. Unlike the reactions of conventional amphotericin B that are mediated by tumor necrosis factor alpha (TNF-α) (7, 25), this distinctive syndrome of L-AMB appears to be more histamine mediated. Szebeni et al. report that some liposomes may activate complement and release C5a, resulting in a liposome-induced pulmonary hypertension with a complement-induced pseudoallergic reaction (36).
Our present study and other reports underscore the importance of being vigilant of this idiosyncratic acute liposome-infusion-related syndrome. Nonetheless, this study found that these infusion-related reactions of L-AMB are not dose dependent and that the MTD is ≥15 mg/kg/day. While the higher frequency of infusion-related toxicity could be related to higher peak levels in the plasma of the 7.5- and 10.0-mg/kg cohorts, only a small fraction of the total dosage was infused by the time the idiosyncratic symptoms began.
The plasma pharmacokinetics of L-AMB over the range of dosages from 7.5 to 15 mg/kg/day was clearly nonlinear. The Cmax and AUC appeared to reach maximum levels at 10 mg/kg/day. This nonlinear disposition of L-AMB suggests that new clearance mechanisms are induced or activated at levels exceeding 10 mg/kg/day. Amphotericin B is eliminated from the circulation by the RES, biliary tract, and urinary tract (21, 38). Elimination of L-AMB from the circulation also is dependent on the RES (2, 22, 34, 38). Higher concentrations of L-AMB may induce a concentration-driven clearance mechanism for amphotericin B, as observed for the renal elimination of carprofen (8) and reticuloendothelial elimination of hemoglobin (9) or amphotericin B lipid complex (44). These clearance mechanisms may be related to enhanced uptake by the RES, perhaps mediated by low-affinity receptors for lipids that permit clearance of these liposomes at relatively high plasma concentrations. Such enhanced uptake by the RES would explain high concentrations of amphotericin B in the liver, spleen, and bone marrow. The decreasing variability of pharmacokinetic parameters at the higher dosage range also may be related to the induction of a clearance mechanism (e.g., low-affinity reticuloendothelial receptors) that may enhance a more uniform interpatient clearance. That patients in the two higher dosage groups had greater mortality may also have contributed to the dosage dependency in the model.
The therapeutic implications of a nonlinear dosage-dependent distribution are important for treatment of fungal infections in non-RES tissues. For example, this study of the plasma pharmacokinetics of high-dosage L-AMB suggests that 10 mg/kg would be the upper dosage limit for treatment of fungal infections of the central nervous system (CNS). Based upon earlier studies in the treatment of experimental Candida meningoencephalitis, Cmax and AUC were the principal pharmacodynamic determinants of antifungal efficacy of amphotericin B and its lipid formulation (16). There was a direct relationship between peak concentrations in plasma, AUCs, and cerebral tissue concentrations, which in turn correlated directly with greater antifungal efficacy. The greatest antifungal efficacy in the CNS was observed with deoxycholate amphotericin B and L-AMB, which also exhibited the highest peak plasma concentrations, AUCs, and cerebral tissue concentrations. As a logical extension of these findings, the higher dosages of L-AMB beyond 10 mg/kg would be unlikely to impact on fungal infection of the central nervous system, if CNS penetration is based on Cmax and AUC are not increased with a corresponding increase in dosage. By comparison, an infection of RES tissues, such as the liver and spleen, as in chronic disseminated candidiasis may benefit from dosages exceeding 10 mg/kg/day. Liposomal amphotericin at such high dosages would likely be deposited in RES tissues.
The implications for such nonlinear plasma pharmacokinetics on treatment of fungal infections of the lungs remain to be determined. Earlier work demonstrated a dosage-dependent relationship between the dosage of L-AMB and parameters of efficacy (13). Such dosage-dependent relationships for liposomal formulations of amphotericin B also exist for other lipid formulations (4, 42). Further experimental investigation of antifungal pharmacodynamics of these higher dosages is clearly warranted.
This study was designed for determination of the MTD in patients with invasive fungal infections and not the optimal therapeutic dosage for treatment of these mycoses. A study designed to assess the optimal therapeutic dosage would require approximately 200 patients per dosage group in order to be sufficiently powered. Comparison of the overall response rate of 46% in this study with those of other clinical trials with L-AMB must be undertaken cautiously. Differences in underlying immunosuppression, patterns of infection, and etiologic organisms preclude statistical comparisons across studies. This problem is exemplified with two studies demonstrating response rates ranging from 39% (12) to 56% (11) for invasive aspergillosis. A case-matched study minimally and a randomized trial ideally permits sufficient certainty for comparison of responses in these complex infections. Nonetheless, one can infer that L-AMB is effective in producing complete and partial responses in approximately 40% of seriously ill patients with invasive fungal infections due to Aspergillus species and other molds. Whether these higher dosages of 7.5 to 15 mg/kg/day are more efficacious then the Food and Drug Administration-approved dosage of 5 mg/kg/day remains to be determined through carefully designed comparative trials. In the absence of such clinical trials, however, a pragmatic approach for patients who are critically ill with invasive fungal infections progressing through L-AMB at 5 mg/kg/day would be to increase the dosage of L-AMB to 7.5 or 10 mg/kg/day. Whether exceeding 10 mg/kg/day for an individual patient is therapeutically beneficial may depend upon the site of infection. For infection progressing in the CNS, it is not clear that further increment of the dosage beyond 10 mg/kg/day would have benefits. Instead, using an alternative agent such as an investigative antifungal triazole or using a combination of L-AMB with an echinocandin would be potential alternative therapeutic approaches for such patients. Certainly, for patients whose invasive fungal infections are progressing in the lungs, liver, spleen, or other non-CNS site while receiving 10 mg/kg/day of L-AMB, raising the dosage to 12.5 or 15.0 mg/kg/day is a rational therapeutic alternative.
REFERENCES
- 1.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14(Suppl. 5):S3–S7. [PubMed] [Google Scholar]
- 2.Adler-Moore J P, Proffitt R T. Development, characterization, efficacy, and mode of action of AmBisome, a unilamellar formulation of amphotericin B. J Liposome Res. 1993;3:429–450. [Google Scholar]
- 3.Alak A, Moy S, Bekersky I. A high-performance liquid chromatographic assay for the determination of Amphotericin B serum concentrations after the administration of AmBisome, a liposomal Amphotericin B formulation. Ther Drug Monit. 1996;18:604–609. doi: 10.1097/00007691-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Allende M C, Lee J W, Francis P, Garrett K, Bacher J, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion (ABCD) in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl.)1:S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 6.Anaissie E, Bodey G P, Kantarjian H, Ro J, Vartivarian S E, Hopfer R, Hoy J, Rolston K. New spectrum of fungal infections in patients with cancer. Rev Infect Dis. 1989;11:369–378. doi: 10.1093/clinids/11.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Arning M, Kliche K O, Heer-Sonderhoff A H, Wehmeier A. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;38:459–465. doi: 10.1111/j.1439-0507.1995.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 8.Bekersky I, Colburn W A. Renal clearance of carprofen in the isolated perfused rat kidney. Drug Metab Dispos. 1981;9:25–29. [PubMed] [Google Scholar]
- 9.Boswell G, Rodkey W G. Pharmacokinetics of hemoglobin infusion. In: Bolin R B, Geyer R P, Nemo G J, editors. Advances in blood substitute research. New York, N.Y: Alan R. Liss Inc.; 1983. pp. 139–147. [PubMed] [Google Scholar]
- 10.Boutati E I, Anaissie E J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 11.Denning D W, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, Kibbler C, Kcrmery V, Offner F, Cordonnier C, Jehn U, Ellis M, Collette L, Sylvester R. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group J Infect. 1998;37:173–180. doi: 10.1016/s0163-4453(98)80173-4. [DOI] [PubMed] [Google Scholar]
- 12.Fleming R V, Kantarjian H M, Husni R, Rolston K, Lim J, Raad I, Pierce S, Cortes J, Estey E. Comparison of amphotericin B lipid complex (ABLC) vs. ambisome in the treatment of suspected or documented fungal infections in patients with leukemia. Leuk Lymphoma. 2001;40:511–520. doi: 10.3109/10428190109097650. [DOI] [PubMed] [Google Scholar]
- 13.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar lavage D-mannitol and galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 14.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 15.Gondal J A, Swartz R P, Rahman A. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1989;33:1544–1548. doi: 10.1128/aac.33.9.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll A H, Giri N, Petraitis V, Petraitiene R, Candelario M, Bacher J S, Piscitelli S C, Walsh T J. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000;182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 17.Groll A, Piscitelli S, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 18.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22:S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann V, Kahny B, Debus A, Wackholz K, Jehn U. Pharmacokinetics of liposomal amphotericin B (AmBisome) versus other lipid-based formulations. Bone Marrow Transplant. 1994;14(Suppl.):S8–S9. [PubMed] [Google Scholar]
- 20.Johnson M D, Drew R H, Perfect J R. Chest discomfort associated with liposomal amphotericin B: report of three cases and review of the literature. Pharmacotherapy. 1998;18:1053–1061. [PubMed] [Google Scholar]
- 21.Lawrence R M, Hoeprich P D, Jagdis F A, Monji N, Huston A C, Schaffner C P. Distribution of doubly radiolabelled amphotericin B methyl ester and amphotericin B in the non-human primate Maccaca mulatta. J Antimicrob Chemother. 1980;6:241–249. doi: 10.1093/jac/6.2.241. [DOI] [PubMed] [Google Scholar]
- 22.Lee J W, Navarro E, Francis P, McManus E, Schaufele R, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leenders A C, Daenen S, Jansen R L, Hop W C, Lowenberg B, Wijermans P W, Cornelissen J, Herbrecht R, van der Lelie H, Hoogsteden H C, Verbrugh H A, deMarie S. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103:205–212. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 24.Levine S J, Walsh T J, Martinez A, Eichacker P, Lopez-Berestein G, Natanson C. Hypoxemia, pulmonary hypertension, and depression of cardiac output as sequelae of liposomal amphotericin B infusion. Ann Int Med. 1991;114:664–666. doi: 10.7326/0003-4819-114-8-664. [DOI] [PubMed] [Google Scholar]
- 25.Louie A, Baltch A L, Franke M A, Smith R P, Gordon M A. Comparative capacity of four antifungal agents to stimulate murine macrophages to produce tumour necrosis factor alpha: an effect that is attenuated by pentoxifylline, liposomal vesicles, and dexamethasone. J Antimicrob Chemother. 1994;34:975–987. doi: 10.1093/jac/34.6.975. [DOI] [PubMed] [Google Scholar]
- 26.Mehta T, Lopez-Berestein G, Hopfer R, Mills K, Juliano R L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochem Biophys Acta. 1984;770:230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- 27.Meunier F, Prentice H G, Ringden O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J Antimicrob Chemother. 1991;28(Suppl. B):83–91. doi: 10.1093/jac/28.suppl_b.83. [DOI] [PubMed] [Google Scholar]
- 28.Mills W, Chopra R, Linch D C, Goldstone A H. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-center experience of 133 episodes in 116 patients. Br J Haematol. 1994;86:754–760. doi: 10.1111/j.1365-2141.1994.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 29.Ng T T C, Denning D W. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections: evaluation of United Kingdom compassionate use data. Arch Intern Med. 1995;155:1093–1098. [PubMed] [Google Scholar]
- 30.Pannuti C, Gingrich R, Pfaller M A, Kao C, Wenzel R P. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer. 1992;69:2653–2662. doi: 10.1002/1097-0142(19920601)69:11<2653::aid-cncr2820691106>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Patterson T F, Kirkpatrick W R, White M, Hiemenz J W, Wingard J R, Dupont B, Rinaldi M G, Stevens D A, Graybill J R. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Perkins W R, Minchey S R, Boni L T, Swenson C E, Popescu M C, Pasternack R F, Janoff A S. Amphotericin B phospholipid interactions responsible for reduced mammalian cell toxicity. Biochem Biophys Acta. 1992;1107:271–282. doi: 10.1016/0005-2736(92)90414-h. [DOI] [PubMed] [Google Scholar]
- 33.Pittet D, Wenzel R P. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 34.Proffitt R T, Satorius A, Chiang S M, Sullivan L, Adler-Moore J P. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991;28(Suppl. B):49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- 35.Ringden O, Meunier F, Tollemar J, Ricci P, Tura S, Kuse E, Viviani M A, Gorin N C, Klastersky J, Fenaux P. Efficacy of amphotericin B encapsulated in liposome (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother. 1991;28(Suppl. B):73–82. doi: 10.1093/jac/28.suppl_b.73. [DOI] [PubMed] [Google Scholar]
- 36.Szebeni J, Baranyi L, Savay S, Bodo M, Morse D S, Basta M, Stahl G L, Bunger R, Alving C R. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 37.Van Etten E W M, Otte-Lambillion M, van Vianen W, ten Kate M T, Bakker-Woudenberg I A J M. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother. 1995;35:509–519. doi: 10.1093/jac/35.4.509. [DOI] [PubMed] [Google Scholar]
- 38.van Etten E W, Snijders S V, van Vianen W, Bakker-Woudenberg I A. Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic mice. Antimicrob Agents Chemother. 1998;42:2431–2433. doi: 10.1128/aac.42.9.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald A, Leisenring W, van Burik J A, Bowden R A. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 40.Walsh T J. Emerging fungal pathogens: evolving challenges to immunocompromised patients. In: Scheld W M, Armstrong D, Hughes J, editors. Emerging infections. Washington, D.C.: ASM Press; 1998. pp. 221–232. [DOI] [PubMed] [Google Scholar]
- 41.Walsh T J, Finberg R, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg R N, Dummer S, Schuster M, Holcenberg J S, Dismukes W E for the National Institute of Allergy and Infectious Diseases Mycoses Study Group. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 42.Walsh T J, Garrett K, Feuerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo P A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography: a novel non-invasive method for measuring responses of organism-mediated tissue injury. Antimicrob Agents Chemother. 1995;39:1065–1069. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh T J, Hiemenz J, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 44.Walsh T J, Jackson A J, Lee J W, Amantea M, Sein T, Bacher J, Zech L. Dose-dependent pharmacokinetics of amphotericin B lipid complex in rabbits. Antimicrob Agents Chemother. 2000;44:2068–2076. doi: 10.1128/aac.44.8.2068-2076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh T J, Yeldandi V, McEvoy M, Gonzalez C, Chanock S J, Freifeld A, Seibel N I, Jarosinski P, Boswell G, Bekersky I, Alak A, Buell D, Barret J, Wilson W. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother. 1998;42:2391–2398. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasan K M, Grossie V B, Jr, Lopez-Berestein G. Concentrations in serum and distribution in tissue of free and liposomal amphotericin B in rats during continuous intralipid infusion. Antimicrob Agents Chemother. 1994;38:2224–2226. doi: 10.1128/aac.38.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasan K M, Kennedy A L, Cassidy S M, Ramaswamy M, Holtorf L, Chou J W, Pritchard P H. Pharmacokinetics, distribution in serum lipoproteins and tissues, and renal toxicities of amphotericin B and amphotericin B lipid complex in a hypercholesterolemic rabbit model: single-dose studies. Antimicrob Agents Chemother. 1998;42:3146–3152. doi: 10.1128/aac.42.12.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasan K M, Morton R E, Rosenblum M G, Lopez-Berestein G. Decreased toxicity of liposomal amphotericin B due to association of amphotericin B with high-density lipoproteins: role of lipid transfer protein. J Pharm Sci. 1994;83:1006–1010. doi: 10.1002/jps.2600830716. [DOI] [PubMed] [Google Scholar]
- 49.Wasan K M, Rosenblum M G, Cheung L, Lopez-Berestein G. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob Agents Chemother. 1994;38:223–227. doi: 10.1128/aac.38.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wingard J R, White M H, Anaissie E, Raffalli J, Goodman J, Arrieta A. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. Clin Infect Dis. 2000;31:1155–1163. doi: 10.1086/317451. [DOI] [PubMed] [Google Scholar]