Abstract
We investigated the in vitro activity of various antimicrobial combinations against carbapenem-resistant Acinetobacter baumannii (CRAB) isolates. The in vitro activity of six two-drug combinations against CRAB isolates collected from the blood samples of patients with bloodstream infection was evaluated using the checkerboard method and time-kill assay [0.5 ×, 1 ×, and 2 × minimum inhibitory concentration (MIC)] to identify potential synergistic and bactericidal two-drug combinations against CRAB isolates. The effects of meropenem, colistin, tigecycline, rifampin, and ceftolozane/tazobactam combinations were investigated. All 10 CRAB isolates in our study produced the OXA-58-type and OXA-23-type carbapenem-hydrolyzing oxacillinases. The colistin-ceftolozane/tazobactam combination showed synergistic effects in both the time-kill assay (using an antibiotic concentration of 1 × MIC) and the checkerboard method. It also showed bactericidal effects in the time-kill assay. For all 10 CRAB isolates, time-kill curves showed synergistic bactericidal activity of the colistin-ceftolozane/tazobactam combination at 0.5 × MIC. Overall, there was substantial discordance of synergistic activity between the checkerboard microdilution and time-kill assays (with a concordance of 31.7%). Our study demonstrated that two-drug combinations of colistin and ceftolozane/tazobactam could be useful treatment alternatives for CRAB infections. The effects of these antibiotic combinations should be evaluated using in vivo experimental models.
Subject terms: Drug discovery, Microbiology, Medical research
Introduction
Carbapenem-resistant Acinetobacter baumannii (CRAB), a leading nosocomial pathogen, poses a global threat to public health1. This pathogen is resistant to most clinically available antibiotics, and other treatment options are extremely limited. As a result, infection-related mortality has increased2–4. Furthermore, the spread of CRAB in a hospital environment makes infection control difficult. These bacteria can colonize various body parts of hospitalized patients and survive for a long time on various surfaces in hospital facilities5,6.
In the Republic of Korea, the carbapenem resistance rate of A. baumannii isolated from patients hospitalized in intensive care units was 90%7,8. According to the Korean arm of the Global Antimicrobial Resistance Surveillance System, CRAB is the most common multidrug-resistant pathogen causing bloodstream infections in intensive care units, with an incidence of 6.3 cases per 10,000 patient-days9.
The World Health Organization has designated CRAB as a pathogen of critical priority in the global priority list of multidrug-resistant bacteria and has urged the development of new antibiotics10. Despite relentless attempts to improve therapeutic approaches, there is no new promising antibiotic that can suitably control CRAB infections11. Currently, only a few antibiotics of uncertain efficacy, such as colistin and tigecycline, are available for treating CRAB infections. The reduced antibiotic susceptibility of CRAB, unfavorable pharmacokinetic properties, unclear optimal dosing, and potential adverse effects are all barriers to the clinical use of existing drugs such as colistin and tigecycline.
Given the increasing multidrug-resistance rates and the lack of effective antibiotics, combination therapy should be considered as an alternative interim strategy for effectively managing CRAB infections. Antimicrobial combination therapy may broaden the spectrum of activity, minimize the development of antimicrobial resistance, and synergistically inactivate microorganisms. Proposed mechanisms for synergistic antibacterial effects include enhanced bioavailability, inhibitor suppression, sequential blockade, mutual stabilization, parallel pathway inhibition, and regulation modulation12. However, evaluating the in vitro activity of antimicrobial combinations in clinical microbiology laboratories is challenging, as the experimental process is labor-intensive, time-consuming, and requires specialized skills. Furthermore, several methods available to evaluate the in vitro activity of antimicrobial combinations for CRAB isolates do not always show consistent results13–15. Nevertheless, several studies have addressed the therapeutic potential of combination therapy against CRAB infections16–20.
The purpose of this study was to investigate the in vitro activity of antimicrobial combinations of meropenem, colistin, tigecycline, rifampin, and ceftolozane/tazobactam against CRAB isolates producing OXA-23 carbapenemases.
Methods
Study population
A total of 158 clinical isolates of A. baumannii were collected from nonduplicate patients with CRAB bacteremia in a 1,048-bed tertiary care hospital in Seoul, Republic of Korea from April 2018 to January 2020. Ten clinical isolates of CRAB exhibiting resistance to imipenem, meropenem, and ertapenem were randomly selected for the study21.
The study protocol was approved prior to study initiation by the Institutional Review Board of Korea University Anam Hospital [No. 2020AN0157]. The study was performed in accordance with the ethical principles outlined in the Declaration of Helsinki. Informed consent was obtained from all subjects involved in this study.
Bacterial isolates and antimicrobial susceptibility testing
The identification and antimicrobial susceptibility testing of A. baumannii strains were initially performed using the MicroScan Pos Combo Panel Type 6 automated system (Baxter Diagnostics, West Sacramento, CA, USA) in a clinical microbiology laboratory. The identity of the A. baumannii strains was confirmed using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany).
The minimum inhibitory concentration (MIC) was determined for 15 antimicrobial agents using the broth microdilution method. Cation-adjusted Mueller–Hinton II broth (CA-MHB) (Becton Dicknson & Co., Sparks, MD, USA) was used, and geometric twofold serial dilutions were performed according to the CLSI recommendations21,22 for the following antimicrobial agents: meropenem (Yuhan Co., Seoul, Korea), ertapenem (MSD-Chibret, France), colistin (SteriMax Inc., ON, Canada), amikacin (Shinpoong Co., Seoul, Korea), tigecycline (Pfizer Pharmaceuticals, Seoul, Korea), ceftolozane/tazobactam (Wyeth, Madison, NJ, USA), piperacillin/tazobactam (Chong Kun Dang Pharmaceutical Co., Seoul, Korea), ampicillin/sulbactam (Keun-Hwa Pharmaceutical Co., Seoul, Korea), ceftazidime (Hanmi Co., Seoul, Korea), cefepime (Boryung Co., Seoul, Korea), aztreonam (Crystal Lifesciences, Cheonju, Korea), minocycline (SK Chemical Co., Seongnam, Korea), fosfomycin (Pharmbio Co., Chungju, Korea), rifampin (Yuhan Co., Seoul, Korea), and ciprofloxacin (Bayer AG, Leverkusen, Germany). Independent experiments were performed in duplicate. The MIC results were interpreted according to the CLSI breakpoint criteria21. Because the CLSI guidelines did not include breakpoint criteria for tigecycline, we used the criteria of the United States Food and Drug Administration for Enterobacteriaceae for tigecycline (susceptibility, 2 mg/L; resistance, 8 mg/L)23. For interpretation of colistin and rifampin susceptibilities, we used the breakpoints proposed by Gales et al. (resistance, 4 mg/L) and Hogg et al. (resistance, 2 mg/L), respectively24,25. Pseudomonas aeruginosa (ATCC 27853) was used as a quality control isolate.
All CRAB isolates from blood cultures were stored in brain heart infusion broth (Becton Dickinson & Co., Sparks, MD, USA) containing 20% glycerol and frozen at − 70 °C until February 2020. Then the isolates were thawed, and primary and secondary cultures were inoculated in 5% sheep blood agar for the experiments.
Detection of carbapenem resistance determinants
For all CRAB isolates, Ambler class B metallo-β-lactamase genes (blaIMP-1, blaIMP-2, blaVIM-1, and blaVIM-2) and Ambler class D OXA-type carbapenemases-encoding genes (blaoxa-23, blaoxa-24, blaoxa-51, and blaoxa-58) were detected using simplex or multiplex polymerase chain reaction (PCR) techniques. The primers used in this study are shown in Table 1.
Table 1.
Polymerase chain reaction (PCR) primers, conditions, and product size for the detection of carbapenemases among Acinetobacter baumannii strains.
| Target gene | Primers (5′ → 3′) | Annealing temperature for multiplex PCR (°C) | Product size (bp) |
|---|---|---|---|
| blaIMP-1 |
ACCGCAGCAGAGTCTTTGCC ACAACCAGTTTTGCCTTACC |
55 | 587 |
| blaIMP-2 |
GTTTTATGTGTATGCTTCC AGCCTGTTCCCATGTAC |
55 | 678 |
| blaVIM-1 |
GGGAGCCGAGTGGTGAGT GGCACAACCACCGTATAG |
55 | 519 |
| blaVIM-2 |
ATGTTCAAACTTTTGAGTAAG CTACTCAACGACTGAGCG |
55 | 801 |
| blaoxa-23 |
GATCGGATTGGAGAACCAGA ATTTCTGACCGCATTTCCAT |
52 | 501 |
| blaoxa-24 |
GGTTAGTTGGCCCCCTTAAA AGTTGAGCGAAAAGGGGATT |
52 | 246 |
| blaoxa-51 |
TAATGCTTTGATCGGCCTTG TGGATTGCACTTCATCTTGG |
52 | 353 |
| blaoxa-58 |
AAGTATTGGGGCTTGTGCTG CCCCTCTGCGCTCTACATAC |
52 | 599 |
Each reaction mixture (20 µL) contained 1 µL of genomic DNA, 10 pmol of each primer, 1 U of Taq DNA polymerase, 0.25 mM dNTP, 10 mM Tris–HCl (pH 9.0), 40 mM KCl, and 1.5 mM MgCl2. PCR conditions for amplifying the Ambler class B metallo-β-lactamase genes were as follows: 94 °C for 5 min, followed by 30 cycles of 94 °C for 45 s, annealing at the temperature specified for each set of primers for 1 min, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min (Table 1). PCR conditions for amplifying the Ambler class D OXA-type carbapenemase-encoding genes were as follows: 94 °C for 5 min, and then, 30 cycles of 94 °C for 30 s, annealing at the temperature specified for each set of primers for 40 s and 72 °C for 50 s, followed by a final extension at 72 °C for 7 min (Table 1).
Checkerboard assays for synergy testing
The synergistic activities of various two-drug combinations against the 10 CRAB isolates were evaluated using the checkerboard assay, which was conducted in 96-well microtiter plates (Corning Inc., Kennebunk, ME, USA). The 10 isolates selected for in vitro synergy tests were chosen randomly without any specific criteria. Ceftolozane/tazobactam was recently approved, and data relating to it is limited. In addition to ceftolozane/tazobactam, we selected antibiotics for in vitro synergic tests based on a review of the literature on antibiotic combinations likely to be especially powerful in a clinical environment. In brief, panels of 96-well microtiter plates were prepared based on the MIC of each antibiotic, as determined using broth microdilution. Dilution intervals were determined to be 2–32 times higher than and 1/8–1/64 times lower than the MIC values obtained from the preliminary analysis. The antibiotic stock solutions were diluted with CA-MHB, and the concentrations of the upper left parts of the plates were set to 0. The plate rows contained 100 µL of the two-fold serial dilutions of the first antibiotic in each well, and the plate columns contained 100 µL of the two-fold serial dilutions of the second antibiotic. Test concentration ranges for each antibiotic in the experimental combinations were as follows: colistin, 0–128 mg/L; meropenem, 0–128 mg/L; tigecycline, 0–8 mg/L; ceftolozane/tazobactam, 0–128 mg/L; and rifampin, 0–256 mg/L.
The A. baumannii inoculum consisted of two-fold diluted 0.5 McFarland turbidity standard (100 µL) prepared using CA-MHB. The final inoculum concentration was 5 × 105 CFU/mL in each well. Except for the sterility control well, all the wells were inoculated and then incubated at 37 °C for 18–20 h. At the stationary phase, the wells were diluted to an OD of 600 nm, as measured with an absorbance microplate reader (SpectraMax Plus 384, Molecular Devices, Inc). The MIC was visually determined by identifying the wells in the microtiter plate that had the lowest drug concentrations and no visible growth. The fractional inhibitory concentration index (FICI) was calculated using the formula below.
FICI = [(MIC of drug A in combination)/(MIC of drug A alone)] + [(MIC of drug B in combination)/(MIC of drug B alone)].
Interpretation of the FICI was as follows: FICI ≤ 0.5, synergistic; 0.5 < FICI ≤ 1, additive; 1 < FICI ≤ 4, indifferent; and FICI > 4, antagonistic26.
Time-kill assay for synergy testing
In addition to the checkerboard assay, time-kill assays were conducted using the 10 A. baumannii isolates. In brief, tubes containing freshly prepared CA-MH broth supplemented with antibiotics, alone and in combination, were inoculated with CRAB isolates at a concentration of 104 CFU/mL. The final volume of the suspensions in the tubes was 10 mL (in each tube); the tubes were incubated at 37 °C in a shaking incubator (200 rpm) in ambient air.
Then, 100-µL aliquots were obtained from each tube at 0, 2, 4, 8, 12, and 24 h of incubation and serially diluted in saline for the determination of viable counts. Diluted samples (10 µL) were plated on CA-MHA plates using a spreader (SPL Life Science Co.) and incubated at 37 °C for 18–24 h, and then, the number of colonies was counted. The antibiotic carry-over effect was minimized by washing the aliquots in sterile phosphate-buffered saline (PBS) and then centrifuging them for 5 min at 1,300 rpm before a tenfold serial dilution in sterile PBS. The initial bacterial density of the original sample was calculated based on the dilution factor. The lower limit of detection for the colony counts was 2 log10 CFU/mL. The concentrations of the antibiotics used were 0.5 × MIC, 1 × MIC, and 2 × MIC alone or in combination.
The bactericidal activity of single antibiotics or combinations was defined as a decrease of ≥ 3 log10 in 24 h compared with the number of viable cells at the initial time point27. A synergistic effect was defined as a decrease of ≥ 2 log10 CFU/mL within 24 h when the antibiotics in combinations were compared with the most active individual drug at different time points. An increase of > 2 log10 was considered to indicate antagonism. Indifference was defined as any outcome that did not meet the criteria for either synergy or antagonism28.
Ethics committee approval
The study protocol was approved before study initiation by the Institutional Review Board of Korea University Anam Hospital [No. 2020AN0157].
Results
Characteristics of A. baumannii clinical isolates
The clinical isolates involved in this study are listed in Table 2. Out of 10 patients with CRAB bacteremia, 50% had in-hospital mortality. All 10 CRAB isolates carried the OXA-58-type and OXA-23-type carbapenem-hydrolyzing oxacillinases but did not harbor class B metallo-carbapenemases or other class D carbapenemases. Ten clinical isolates were resistant to meropenem, with MICs of 64 mg/L. Among them, the susceptibility rate associated with each antibiotic was as follows: tigecycline 90%, minocycline 100%, rifampin 30%, colistin 10%, ceftolozane/tazobactam 0%, ciprofloxacin 0%, fosfomycin 0%, aztreonam 0%, ceftazidime 0%, ampicillin/sulbactam 0%, piperacillin/tazobactam 0%, and amikacin 0% (Table 2).
Table 2.
Clinical and microbiological characteristics of Acinetobacter baumannii isolates.
| Isolate no | Patient information | Carbapenemase genes | MICs of antimicrobial agents (mg/L)* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Clinical specimen | In-hospital mortality | blaoxa-51 | blaoxa-23 | Me | C | T | C/T | R | Cip | F | Mi | Az | Cef | A/S | P/T | Am | |
| ATCC27853 | − | − | − | − | − | − | 0.25 | 2 | 4 | 0.5 | 64 | 0.125 | 4 | 16 | 8 | 2 | > 128 | 4 | 1 |
| CRAB 19 | 90 | M | Blood | No | + | + | 64 | 2 | 0.5 | 128 | 4 | 16 | > 128 | 0.25 | 128 | > 128 | 64 | > 128 | 128 |
| CRAB 32 | 66 | F | Blood | Yes | + | + | 64 | 8 | 0.5 | 16 | 4 | 32 | 128 | 0.25 | 64 | 128 | 64 | > 128 | > 128 |
| CRAB 33 | 95 | F | Blood | Yes | + | + | 64 | 8 | 0.5 | 32 | 4 | 64 | 128 | 0.25 | 64 | 128 | 64 | > 128 | > 128 |
| CRAB 34 | 81 | F | Blood | No | + | + | 64 | 8 | 0.5 | 64 | > 128 | 64 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 35 | 75 | F | Blood | No | + | + | 64 | 4 | 0.5 | 64 | > 128 | 64 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 36 | 57 | F | Blood | No | + | + | 64 | 8 | 0.5 | 64 | > 128 | 128 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 37 | 66 | M | Blood | No | + | + | 64 | 8 | 0.5 | 32 | > 128 | 64 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 38 | 81 | F | Blood | Yes | + | + | 64 | 8 | 0.5 | 64 | > 128 | 64 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 39 | 68 | M | Blood | Yes | + | + | 64 | 4 | 1 | 32 | > 128 | 64 | 128 | 0.25 | 128 | > 128 | 64 | > 128 | > 128 |
| CRAB 40 | 89 | M | Blood | Yes | + | + | 64 | 8 | 4 | 32 | > 128 | 128 | 64 | 0.5 | 64 | > 128 | 64 | > 128 | > 128 |
Am amikacin, A/S ampicillin/sulbactam, Az aztreonam, Cef ceftazidime, CRAB carbapenem-resistant Acinetobacter baumannii, Cip ciprofloxacin, C colistin, C/T ceftolozane/tazobactam, F female, F fosfomycin, M male, Me meropenem, Mi minocycline, MIC minimum inhibitory concentration, P/T piperacillin-tazobactam, R rifampin, T tigecycline.
*Bold values, non-susceptible; white cell, susceptible.
Checkerboard assay against A. baumannii clinical isolates
The results of the checkerboard assay used to measure the in vitro synergism and MIC values of the individual antibiotic combinations against the 10 CRAB isolates are summarized in Table 3. The checkerboard assay showed that in vitro synergistic activity (∑FICI ≤ 0.5) against CRAB isolates was the highest for the meropenem-tigecycline combination (90%), followed by the meropenem-ceftolozane/tazobactam (70%), meropenem-rifampin (70%), colistin-ceftolozane/tazobactam (60%), colistin-tigecycline (30%), and meropenem-colistin (30%) combinations (Table 3). All combinations displayed synergism to a certain extent.
Table 3.
Results of checkerboard assay for two-drug combinations against clinical isolates of Acinetobacter baumannii.
| Clinical isolates | MIC of a single agent (mg/L) | MIC in combination (mg/L) | FICI | Result of checkerboard assay | ||
|---|---|---|---|---|---|---|
| Drug A | Drug B | Drug A | Drug B | |||
| Meropenem (A) + colistin (B) | ||||||
| CRAB 19 | 64 | 2 | 4 | 0.25 | 0.19 | Synergistic |
| CRAB 32 | 64 | 8 | 16 | 0.5 | 0.31 | Synergistic |
| CRAB 33 | 64 | 8 | 0.125 | 0.25 | 0.03 | Synergistic |
| CRAB 34 | 64 | 8 | 32 | 0.25 | 0.53 | Additive |
| CRAB 35 | 64 | 8 | 32 | 0.25 | 0.56 | Additive |
| CRAB 36 | 64 | 8 | 32 | 0.5 | 0.56 | Additive |
| CRAB 37 | 64 | 8 | 32 | 0.25 | 0.53 | Additive |
| CRAB 38 | 64 | 8 | 32 | 0.25 | 0.53 | Additive |
| CRAB 39 | 64 | 4 | 32 | 0.25 | 0.56 | Additive |
| CRAB 40 | 64 | 8 | 32 | 0.25 | 0.53 | Additive |
| Meropenem (A) + tigecycline (B) | ||||||
| CRAB 19 | 64 | 0.5 | 0.125 | 0.03 | 0.06 | Synergistic |
| CRAB 32 | 64 | 0.5 | 8 | 0.125 | 0.38 | Synergistic |
| CRAB 33 | 64 | 0.5 | 32 | 0.5 | 1.5 | Indifferent |
| CRAB 34 | 64 | 0.5 | 16 | 0.125 | 0.5 | Synergistic |
| CRAB 35 | 64 | 0.5 | 16 | 0.125 | 0.5 | Synergistic |
| CRAB 36 | 64 | 0.5 | 0.125 | 0.125 | 0.25 | Synergistic |
| CRAB 37 | 64 | 0.5 | 0.125 | 0.125 | 0.25 | Synergistic |
| CRAB 38 | 64 | 0.5 | 16 | 0.125 | 0.5 | Synergistic |
| CRAB 39 | 64 | 1 | 16 | 0.125 | 0.38 | Synergistic |
| CRAB 40 | 64 | 4 | 8 | 0.125 | 0.16 | Synergistic |
| Meropenem (A) + rifampin (B) | ||||||
| CRAB 19 | 64 | 4 | 32 | 0.25 | 0.56 | Additive |
| CRAB 32 | 64 | 4 | 32 | 4 | 1.5 | Indifferent |
| CRAB 33 | 64 | 4 | 16 | 2 | 0.75 | Additive |
| CRAB 34 | 64 | 256 | 4 | 8 | 0.09 | Synergistic |
| CRAB 35 | 64 | 256 | 16 | 4 | 0.27 | Synergistic |
| CRAB 36 | 64 | 256 | 16 | 8 | 0.28 | Synergistic |
| CRAB 37 | 64 | 256 | 16 | 4 | 0.27 | Synergistic |
| CRAB 38 | 64 | 256 | 16 | 4 | 0.27 | Synergistic |
| CRAB 39 | 64 | 256 | 16 | 4 | 0.27 | Synergistic |
| CRAB 40 | 64 | 256 | 8 | 8 | 0.16 | Synergistic |
| Meropenem (A) + ceftolozane/tazobactam (B) | ||||||
| CRAB 19 | 64 | 128 | 32 | 16 | 0.63 | Additive |
| CRAB 32 | 64 | 16 | 8 | 4 | 0.38 | Synergistic |
| CRAB 33 | 64 | 32 | 8 | 16 | 0.63 | Additive |
| CRAB 34 | 64 | 64 | 8 | 4 | 0.19 | Synergistic |
| CRAB 35 | 64 | 64 | 2 | 2 | 0.06 | Synergistic |
| CRAB 36 | 64 | 64 | 32 | 32 | 1 | Additive |
| CRAB 37 | 64 | 32 | 4 | 2 | 0.13 | Synergistic |
| CRAB 38 | 64 | 64 | 16 | 2 | 0.28 | Synergistic |
| CRAB 39 | 64 | 32 | 2 | 4 | 0.16 | Synergistic |
| CRAB 40 | 64 | 32 | 8 | 8 | 0.38 | Synergistic |
| Colistin (A) + tigecycline (B) | ||||||
| CRAB 19 | 2 | 0.5 | 0.006 | 0.03 | 0.06 | Synergistic |
| CRAB 32 | 8 | 0.5 | 4 | 0.25 | 1 | Additive |
| CRAB 33 | 8 | 0.5 | 0.5 | 0.25 | 0.56 | Additive |
| CRAB 34 | 8 | 0.5 | 8 | 0.5 | 2 | Indifferent |
| CRAB 35 | 8 | 0.5 | 4 | 0.25 | 1.5 | Indifferent |
| CRAB 36 | 8 | 0.5 | 2 | 0.125 | 0.5 | Synergistic |
| CRAB 37 | 8 | 0.5 | 8 | 0.5 | 2 | Indifferent |
| CRAB 38 | 8 | 0.5 | 8 | 0.5 | 2 | Indifferent |
| CRAB 39 | 4 | 1 | 4 | 1 | 2 | Indifferent |
| CRAB 40 | 8 | 4 | 0.125 | 1 | 0.27 | Synergistic |
| Colistin (A) + ceftolozane/tazobactam (B) | ||||||
| CRAB 19 | 2 | 128 | 2 | 128 | 2 | Indifferent |
| CRAB 32 | 8 | 16 | 4 | 8 | 1 | Additive |
| CRAB 33 | 8 | 32 | 0.125 | 2 | 0.08 | Synergistic |
| CRAB 34 | 8 | 64 | 0.5 | 8 | 0.19 | Synergistic |
| CRAB 35 | 8 | 64 | 0.25 | 16 | 0.31 | Synergistic |
| CRAB 36 | 8 | 64 | 8 | 64 | 2 | Indifferent |
| CRAB 37 | 8 | 32 | 1 | 16 | 0.63 | Additive |
| CRAB 38 | 8 | 64 | 2 | 2 | 0.28 | Synergistic |
| CRAB 39 | 4 | 32 | 0.25 | 4 | 0.19 | Synergistic |
| CRAB 40 | 8 | 32 | 0.125 | 8 | 0.27 | Synergistic |
CRAB carbapenem-resistant Acinetobacter baumannii, FICI fractional inhibitory concentration index, MIC minimum inhibitory concentration.
Notably, we did not observe antagonistic interactions in our study. The MICs of the antibiotic combinations were lower than the MICs of the antibiotics used by themselves.
Time-kill assay against A. baumannii clinical isolates
All 10 CRAB isolates were also evaluated using the time-kill assay. When evaluating the bactericidal effects of antibiotic monotherapy on the 10 CRAB isolates, antibiotic concentrations of 0.5 × MIC, 1 × MIC, and 2 × MIC were used. Bactericidal activity was noted for meropenem (0.5 × MIC, 0%, 0/10; 1 × MIC, 40%, 4/10; 2 × MIC, 70%, 7/10), ceftolozane/tazobactam (0.5 × MIC, 0%, 0/10; 1 × MIC, 0%, 0/10; 2 × MIC, 70%, 7/10), colistin (0.5 × MIC, 0%, 0/10; 1 × MIC, 50%, 5/10; 2 × MIC, 70%, 7/10), rifampin (0.5 × MIC, 0%, 0/10; 1 × MIC, 10%, 1/10; 2 × MIC, 40%, 4/10), and tigecycline (0.5 × MIC, 10%, 1/10; 1 × MIC, 10%, 1/10; 2 × MIC, 40%, 4/10) at 24 h (Supplementary file).
For the time-kill assay performed using an antibiotic concentration of 1 × MIC, in vitro synergistic and bactericidal effects against the 10 CRAB isolates were most frequently observed for the meropenem-colistin (40% and 100%, respectively), colistin-ceftolozane/tazobactam (50% and 100%, respectively), and colistin-tigecycline (40% and 100%, respectively) combinations. These effects were seen less often for the meropenem-tigecycline (50% and 50%, respectively), meropenem-ceftolozane/tazobactam (50% and 50%, respectively), and meropenem-rifampin (20% and 30%, respectively) combinations at 24 h (Table 4).
Table 4.
Results of time-kill assay and bactericidal activity of two-drug combinations (1 × MIC) against each Acinetobacter baumannii isolate.
| Isolate | Time-kill assay | Bactericidal activity (Timepoint, hours; 2/4/6/8/12/24) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Me + T | Me + C | Me + R | Me + C/T | C + T | C + C/T | Me + T | Me + C | Me + R | Me + C/T | C + T | C + C/T | |
| CRAB 19 | I | I | I | I | I | I | N/N/N/N/B | N/N/B/B/B | N/N/B/B/B | N/N/N/B/B | N/B/B/B/B | N/N/B/B/B |
| CRAB 32 | I | Syn | I | Syn | I | Syn | N/N/N/B/B | N/N/B/B/B | N/N/N/N/N | N/N/N/N/B | N/N/N/B/B | N/N/B/B/B |
| CRAB 33 | Syn | Syn | I | Syn | Syn | Syn | N/N/N/N/N | N/N/N/B/B | N/N/N/N/N | N/N/N/N/N | N/N/N/N/B | N/N/N/B/B |
| CRAB 34 | Syn | I | Syn | Syn | I | I | N/N/N/N/B | N/N/N/B/B | N/N/N/B/B | N/N/N/N/B | N/B/B/B/B | B/N/B/B/B |
| CRAB 35 | Syn | Syn | I | I | Syn | Syn | N/N/N/N/N | N/N/N/B/B | N/N/N/N/N | N/N/N/N/N | N/N/N/N/B | N/N/N/B/B |
| CRAB 36 | Syn | I | I | Syn | I | I | N/N/N/B/B | N/N/N/B/B | N/N/N/N/N | N/N/N/N/B | N/N/B/B/B | N/N/N/B/B |
| CRAB 37 | Syn | Syn | Syn | Syn | Syn | Syn | N/N/N/B/B | N/N/N/B/B | N/N/N/B/B | N/N/N/N/B | N/B/B/B/B | N/N/N/B/B |
| CRAB 38 | An | I | An | An | I | I | N/N/N/N/N | N/N/N/B/B | N/N/N/N/N | N/N/N/N/N | N/N/N/N/B | N/N/N/B/B |
| CRAB 39 | An | I | An | An | Syn | Syn | N/N/N/N/N | N/N/N/B/B | N/N/N/N/N | N/N/N/N/N | N/N/N/B/B | N/N/N/B/B |
| CRAB 40 | I | I | An | An | I | I | N/N/N/N/N | N/N/N/B/B | N/N/N/N/N | N/N/N/N/N | N/N/N/B/B | N/N/N/B/B |
An antagonism, B bactericidal, C colistin, C/T ceftolozane/tazobactam, I indifference, Me meropenem, N non-bactericidal, R rifampin, Syn synergy, T tigecycline, MIC minimum inhibitory concentration.
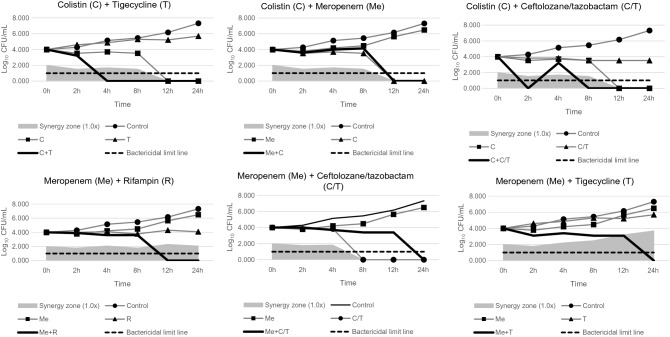
For the colistin-ceftolozane/tazobactam combination (both at levels of 1 × MIC), the 10 CRAB isolates yielded synergy rates of 60% after 12 h and 50% after 24 h (Table 5). For the meropenem-colistin combination (at 1 × MIC), the 10 CRAB isolates showed synergistic rates of 50% in 12 h and 40% in 24 h, respectively (Table 5). For the meropenem-ceftolozane/tazobactam combination (at 1 × MIC), the 10 CRAB isolates showed synergistic rates of 20% within 12 h and 50% within 24 h (Table 5). For the meropenem-tigecycline combination (at 1 × MIC), the 10 CRAB isolates showed synergistic rates of 40% within 12 h and 50% within 24 h (Table 5). However, the meropenem-rifampin combinations (at 1 × MIC) showed synergistic rates of only 20% at 8 h, 12 h, and 24 h. The colistin-tigecycline combinations (at 1 × MIC) also showed synergic rates of at most 40% within 24 h (Table 5). Overall, doubling the antibiotic concentration did not improve synergy rates for six combinations of antibiotics (Table 5). The synergistic inhibitory activity for the other antibiotic combinations was sustained for 24 h, except for the CRAB 34 isolates. These isolates demonstrated regrowth at 4 h for the colistin-ceftolozane/tazobactam combination (both at a concentration of 1 × MIC) (Fig. 1).
Table 5.
Results of time-kill assays and the bactericidal effect of two-drug combinations against 10 Acinetobacter baumannii isolates.
| Bactericidal effect (A) | Synergy effect (B) | Both A and B | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h (%) | 4 h (%) | 8 h (%) | 12 h (%) | 24 h (%) | 0 h | 2 h (%) | 4 h (%) | 8 h (%) | 12 h (%) | 24 h (%) | 0 h | 2 h (%) | 4 h (%) | 8 h (%) | 12 h (%) | 24 h (%) | |
| Me + T | ||||||||||||||||||
| 0.5x | 20 | 20 | 20 | 10 | ||||||||||||||
| 1.0x | 30 | 50 | 40 | 50 | 20 | 30 | ||||||||||||
| 2.0x | 10 | 10 | 10 | 40 | 100 | 10 | 10 | 10 | 30 | 10 | 10 | 10 | 30 | |||||
| Me + C | ||||||||||||||||||
| 0.5x | 10 | 20 | 10 | 40 | 10 | 20 | ||||||||||||
| 1.0x | 20 | 100 | 100 | 20 | 50 | 40 | 20 | 50 | 40 | |||||||||
| 2.0x | 30 | 100 | 100 | 10 | 20 | 20 | 10 | 20 | 20 | |||||||||
| Me + R | ||||||||||||||||||
| 0.5x | 10 | |||||||||||||||||
| 1.0x | 10 | 30 | 30 | 20 | 20 | 20 | 10 | 20 | 20 | |||||||||
| 2.0x | 10 | 20 | 30 | 90 | 10 | 10 | 20 | 10 | 10 | 20 | ||||||||
| Me + C/T | ||||||||||||||||||
| 0.5x | 10 | 10 | 30 | 10 | ||||||||||||||
| 1.0x | 10 | 50 | 10 | 20 | 50 | 10 | 40 | |||||||||||
| 2.0x | 10 | 50 | 70 | 10 | 10 | 10 | 10 | |||||||||||
| C + T | ||||||||||||||||||
| 0.5x | 30 | 60 | 100 | 60 | 70 | 90 | 30 | 60 | 90 | |||||||||
| 1.0x | 30 | 40 | 70 | 100 | 30 | 40 | 40 | 40 | 30 | 40 | 30 | 40 | ||||||
| 2.0x | 10 | 20 | 70 | 100 | 100 | 10 | 30 | 20 | 20 | 10 | 20 | 20 | 20 | |||||
| C + C/T | ||||||||||||||||||
| 0.5x | 20 | 50 | 100 | 10 | 10 | 50 | 80 | 100 | 20 | 50 | 100 | |||||||
| 1.0x | 10 | 30 | 100 | 100 | 20 | 10 | 40 | 60 | 50 | 10 | 30 | 60 | 50 | |||||
| 2.0x | 40 | 50 | 100 | 100 | 20 | 10 | 30 | 30 | 20 | 10 | 30 | 30 | ||||||
The percentage values indicate the ratio of isolates showing bactericidal or synergistic effects among all 10 CRAB isolates.
C colistin, C/T ceftolozane/tazobactam, Me meropenem, R, rifampin, T tigecycline.
Figure 1.
Time-kill curves of Acinetobacter baumannii isolates (CRAB 34) at 1 × minimum inhibitory concentration (MIC) for six combinations of antibiotics.
At concentrations of 1 × and 2 × MIC, combinations of colistin-ceftolozane/tazobactam (1 × MIC, 100%, 10/10; 2 × MIC, 100%, 10/10), colistin-tigecycline (1 × MIC, 100%, 10/10; 2 × MIC, 100%, 10/10), and meropenem-colistin (1 × MIC, 100%, 10/10; 2 × MIC, 100%, 10/10) usually showed bactericidal activity against the 10 CRAB isolates (Table 5).
In the time-kill assay tests that used antibiotic concentrations of 1 × MIC, the combinations that showed both bactericidal activity and a synergistic effect at 24 h were as follows: colistin-ceftolozane/tazobactam (50%), colistin-tigecycline (40%), meropenem-colistin (40%), meropenem-ceftolozane/tazobactam (40%), meropenem-tigecycline (30%), and meropenem-rifampin (20%) (Table 6). For over half of the 10 clinical isolates, the antibiotic combinations that showed bactericidal activity and a synergistic effect at the same time was colistin-ceftolozane/tazobactam (60% and 50% at 12 h and 24 h after inoculation, respectively) (Table 5).
Table 6.
Comparison of checkerboard assays and time-kill assays associated with the bactericidal activity of two-drug combinations (1 × MIC) against each Acinetobacter baumannii isolate.
| Isolate | Checkerboard assay results | Time-kill assay results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Me + T | Me + C | Me + R | Me + C/T | C + T | C + C/T | Me + T | Me + C | Me + R | Me + C/T | C + T | C + C/T | |
| CRAB 19 | Syn | Syn | Additive | Additive | Syn | I | I./B | I./B | I./B | I./B | I./B | I./B |
| CRAB 32 | Syn | Syn | I | Syn | Additive | Additive | I./B | Syn./B | I./N | Syn./B | I./B | Syn./B |
| CRAB 33 | I | Syn | Additive | Additive | Additive | Syn | Syn./N | Syn./B | I./N | Syn./N | Syn./B | Syn./B |
| CRAB 34 | Syn | Additive | Syn | Syn | I | Syn | Syn./B | I./B | Syn./B | Syn./B | I./B | I./B |
| CRAB 35 | Syn | Additive | Syn | Syn | I | Syn | Syn./N | Syn./B | I./N | I./N | Syn./B | Syn./B |
| CRAB 36 | Syn | Additive | Syn | Additive | Syn | I | Syn./B | I./B | I./N | Syn./B | I./B | I./B |
| CRAB 37 | Syn | Additive | Syn | Syn | I | Additive | Syn./B | Syn./B | Syn./B | Syn./B | Syn./B | Syn./B |
| CRAB 38 | Syn | Additive | Syn | Syn | I | Syn | An./N | I./B | An./N | An./N | I./B | I./B |
| CRAB 39 | Syn | Additive | Syn | Syn | I | Syn | An./N | I./B | An./N | An./N | Syn./B | Syn./B |
| CRAB 40 | Syn | Additive | Syn | Syn | Syn | Syn | I./N | I./B | An./N | An./N | I./B | I./B |
An antagonism, B bactericidal, C colistin, C/T ceftolozane/tazobactam, I indifference, Me meropenem, N non-bactericidal, R rifampin, Syn synergy, T tigecycline, MIC minimum inhibitory concentration.
*Bold values indicate that the results of the time-kill assay were consistent with the results of the checkerboard assays.
Regarding the synergistic effect of antibiotic combinations, 31.7% (19/60) of the results of the time-kill assay were consistent with the checkerboard results (Table 6). The combinations that simultaneously showed bactericidal and synergistic effects at 24 h in both the checkerboard and time-kill assays were as follows: colistin-ceftolozane/tazobactam (30%), meropenem-tigecycline (30%), meropenem-ceftolozane/tazobactam (30%), meropenem-colistin (20%), and meropenem-rifampin (20%) (Table 6).
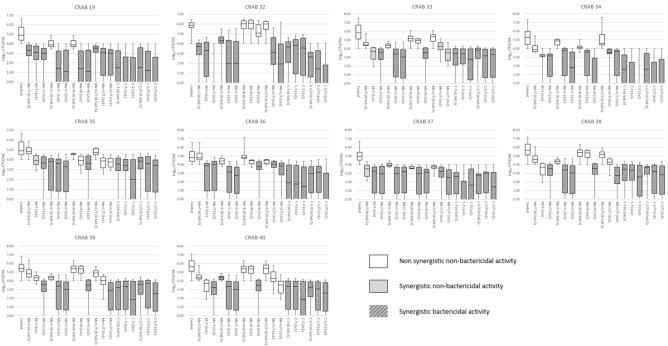
Figure 2 shows the time-kill curves of six different combinations of antibiotics at concentrations of 0.5 ×, 1 ×, 2 × MIC against the CRAB isolates. In the time-kill assay, antagonism was observed for the meropenem-rifampin (30%), meropenem-ceftolozane/tazobactam (30%), and meropenem-tigecycline (20%) combinations (Table 4).
Figure 2.
Time-kill curves of the 10 Acinetobacter baumannii isolates for six combinations of antibiotics with the different concentrations. C, colistin; C/T, ceftolozane/tazobactam; Me meropenem, R rifampin, T tigecycline.
Discussion
Our study was performed to determine which antibiotic combinations could be suitable options for treating CRAB infections. To the best of our knowledge, this is the first study to evaluate the in vitro synergistic activity of ceftolozane/tazobactam and other antibiotics against CRAB isolates. Our findings showed that the combination of colistin and ceftolozane/tazobactam had in vitro synergistic and bactericidal effects against OXA-23-type carbapenemase-producing CRAB isolates.
All 10 CRAB isolates in our study carried the OXA-58-type and OXA-23-type carbapenem-hydrolyzing oxacillinases. A majority of the CRAB isolates in the Republic of Korea have been shown to carry blaoxa-2329. All 10 CRAB isolates had an MIC of 64 mg/L for meropenem, and 90% of the CRAB isolates had an MIC of ≤ 1 mg/L for tigecycline. Particularly, MICs for colistin ranged from 2 to 8 mg/L. Notably, using an antibiotic concentration of 1 × MIC for the time-kill assay in our study failed to produce a stable bactericidal effect when antibiotic monotherapy was used against the 10 CRAB isolates. In this scenario, a combination antibiotic therapy could be the best way to treat CRAB infections. In addition, it is likely that any differences in susceptibility to each antibiotic would also alter the effects of any antibiotic combination.
The colistin-ceftolozane/tazobactam combination demonstrated a synergistic effect in both the time-kill assay and the checkerboard method, and it showed a bactericidal effect in the time-kill assay. In contrast, the meropenem-tigecycline, meropenem-ceftolozane/tazobactam, and meropenem-rifampin combinations showed antagonistic effects for some CRAB isolates in the time-kill assay.
Ceftolozane/tazobactam, a novel beta-lactam/beta-lactamase inhibitor, has demonstrated potent in vitro activity against Pseudomonas aeruginosa, including carbapenem-resistant isolates, except for class B carbapenemase producers. However, its activity against A. baumannii isolates is poor30. In our study, the susceptibility of CRAB isolates to ceftolozane/tazobactam when used as a single antibiotic was poor, with a MIC range of 16–128 mg/L. This finding is in accordance with previous results31. However, our findings from the time-kill assay (1 × MIC) identified the potential of the colistin and ceftolozane/tazobactam combination to induce synergistic interaction against all 10 CRAB isolates at 24 h. All 10 CRAB isolates in our study carried Ambler class D carbapenemases. Further studies are needed to assess the antimicrobial effect of regimens containing ceftolozane/tazobactam on CRAB isolates that exhibit different mechanisms of carbapenem resistance.
The time-kill curves for all 10 CRAB isolates showed that the colistin-ceftolozane/tazobactam combination exhibited significant synergistic bactericidal activity at 0.5 × MIC (Fig. 2). This finding has promising implications for using lower doses of colistin in treatment, thereby reducing its potential nephrotoxic effect.
We found that for the meropenem-tigecycline combination, in vitro synergistic activities took place in 90% and 50% of the 10 CRAB isolates in the checkerboard and time-kill assays, respectively. However, in vitro antagonistic activities were found in 20% of the 10 CRAB isolates in terms of the time-kill assay. A previous meta-analysis revealed a synergistic rate of 24.5% and 20.0% for CRAB isolates using the checkerboard (36 studies) and the time-kill (35 studies) methods, respectively19. It is possible that the high susceptibility rate (90%) of the CRAB isolates to tigecycline in our study contributed to these results. A recent clinical study reported that the colistin-tigecycline combination was associated with a higher mortality rate when the MIC of tigecycline was > 2 mg/L, which was achieved through the combination of a carbapenem and colistin32. Therefore, it is important to know the best way to select from existing antibiotic treatment regimens based on a strain’s antibiotic resistance phenotype (hospital antibiogram). This information can be used to achieve an improved clinical outcome. However, non-colistin-based combination regimens may play an important role in the treatment of CRAB infections, especially for those who are concerned about its nephrotoxic side effects and the emergence of hetero-resistance or resistance to colistin in CRAB isolates.
In our study, we incubated CRAB in the presence of antibiotics for 24 h, and the regrowth phenomenon was observed in the time-kill assay of the CRAB 34 isolate at 4 h after inoculation when the colistin-ceftolozane/tazobactam combination (with both at a concentration of 1 × MIC) was evaluated (Fig. 1). Although most of the time-kill analyses incubated bacteria in the presence of antibiotics for 24 h, incubation for 48 h may be better because it would allow improved detection of the regrowth phenomenon, owing to the selective amplification of the resistant subpopulation33. A previous study reported the regrowth phenomenon to be commonly detected in time-kill assays using colistin, despite the in vitro antimicrobial activity of colistin against the CRAB isolates16.
In our study, the meropenem-colistin combination showed 30% and 40% synergistic activities in the checkerboard and time-kill assays, respectively. The effectiveness of the meropenem-colistin combination remains an area of active research for the treatment of CRAB infections. A previous study that used time-kill assays to investigate the effects of different antibiotic combinations against 12 CRAB isolates at 5 × 105 CFU/mL found that the meropenem-colistin combination was the most synergistic combination34. The checkerboard and time-kill assays showed synergistic effects of 60–73.3% and 60–96.1%, respectively19,35–37. In a previous meta-analysis, the synergistic rates shown by time-kill methods were significantly higher than those obtained using the checkerboard method, and our results are similar20,38. Overall, there is great discordance between the checkerboard microdilution and time-kill assays, and our study showed a concordance of 31.7%. In contrast, a previous meta-analysis showed a higher synergistic rate for CRAB isolates in a combination of meropenem and colistin, compared to a combination of imipenem and colistin18,20.
The present study has several limitations. First, the results do not apply to CRAB isolates that produce metallo-beta-lactamase. Notably, the CRAB isolates used in our study are highly resistant to meropenem, and they might have responded differently to the various antibiotic combinations if their MICs for meropenem were lower. Second, this study assessed the response of a small number of CRAB isolates using the checkerboard and time-kill assays. The MIC values of colistin in many of the CRAB isolates were remarkably high because only a few isolates were used to study the in vitro synergistic and bactericidal activities of the experimental antibiotic combinations. However, it is meaningful to collect isolates from patients with CRAB bacteremia in a clinical setting. Third, although antimicrobial susceptibility tests were performed in duplicate, this was not true for all the in vitro synergic tests. However, a strength of our in vitro synergy tests was that they tested a variety of antibacterial agents at different concentrations for their effect on 10 CRAB strains isolated in clinical practice. Finally, we acknowledge that in vitro studies do not always produce similar results in clinical practice; therefore, caution is required when applying these results in such a context.
Conclusions
In conclusion, the present study demonstrated that the combination of colistin and ceftolozane/tazobactam may be a promising alternative to colistin alone for treating CRAB infections. However, the benefits of these antibiotic combinations should be validated using in vivo experimental models.
Supplementary Information
Abbreviations
- Am
Amikacin
- An
Antagonism
- A/S
Ampicillin/sulbactam
- Az
Aztreonam
- B
Bactericidal
- C
Colistin
- CA-MHB
Cation-adjusted Mueller–Hinton II broth
- Cef
Ceftazidime
- Cip
Ciprofloxacin
- CLSI
Clinical and Laboratory Standards Institute
- CRAB
Carbapenem-resistant Acinetobacter baumannii
- C/T
Ceftolozane/tazobactam
- F
Female
- FICI
Fractional inhibitory concentration index
- F
Fosfomycin
- I
Indifference
- M
Male
- Me
Meropenem
- Mi
Minocycline
- MIC
Minimum inhibitory concentration
- N
Non-bactericidal
- PCR
Polymerase chain reaction
- P/T
Piperacillin-tazobactam
- R
Rifampin
- Syn
Synergy
- T
Tigecycline
Author contributions
Y.K.Y. conceived, designed, and performed the study. H.S.Y., M.-G.L., and Y.G.J. contributed to the experiments and data analysis. HJL and JWS assisted us in obtaining clinical isolates. Y.K.Y. and Y.G.J. wrote the manuscript.
Funding
This study was partly supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number: HI20C0384) and by the National Research Foundation of Korea (NRF) Grant, funded by the Korean Government (Ministry of Science and ICT) (No. NRF-2019R1F1A1051267). The funding sources had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11464-6.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: No longer a controversial issue. Crit. Care. 2007;11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemos EV, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2014;20:416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 4.Sheng WH, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int. J. Infect. Dis. 2010;14:e764–e769. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 5.Ayats J, et al. Epidemiological significance of cutaneous, pharyngeal, and digestive tract colonization by multiresistant Acinetobacter baumannii in ICU patients. J. Hosp. Infect. 1997;37:287–295. doi: 10.1016/s0195-6701(97)90145-6. [DOI] [PubMed] [Google Scholar]
- 6.Shamsizadeh Z, et al. Detection of antibiotic resistant Acinetobacter baumannii in various hospital environments: Potential sources for transmission of Acinetobacter infections. Environ. Health Prev. Med. 2017;22:44. doi: 10.1186/s12199-017-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EJ, et al. Korean national healthcare-associated infections surveillance system, Intensive Care Unit module report: Summary of data from July 2017 through June 2018. Korean J. Healthc. Assoc. Infect. Control Prev. 2019;24:69–80. doi: 10.14192/kjicp.2019.24.2.69. [DOI] [Google Scholar]
- 8.Kim D, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: Analysis of Korean antimicrobial resistance monitoring system (KARMS) data from 2013 to 2015. Ann. Lab. Med. 2017;37:231–239. doi: 10.3343/alm.2017.37.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: First one-year report from Kor-GLASS. Euro Surveill. 2018;23:1800047. doi: 10.2807/1560-7917.ES.2018.23.42.1800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed (2017). https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
- 11.Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019;25:951–957. doi: 10.1016/j.cmi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan GJ, Delgado NN, Maharjan R, Cain AK. How antibiotics work together: Molecular mechanisms behind combination therapy. Curr. Opin. Microbiol. 2020;57:31–40. doi: 10.1016/j.mib.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Tan TY, et al. In vitro antibiotic synergy in extensively drug-resistant Acinetobacter baumannii: The effect of testing by time-kill, checkerboard, and Etest methods. Antimicrob. Agents Chemother. 2011;55:436–438. doi: 10.1128/AAC.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonapace CR, White RL, Friedrich LV, Bosso JA. Evaluation of antibiotic synergy against Acinetobacter baumannii: A comparison with Etest, time-kill, and checkerboard methods. Diagn. Microbiol. Infect. Dis. 2000;38:43–50. doi: 10.1016/s0732-8893(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 15.Pankey GA, Ashcraft DS. The detection of synergy between meropenem and polymyxin B against meropenem-resistant Acinetobacter baumannii using Etest and time-kill assay. Diagn. Microbiol. Infect. Dis. 2009;63:228–232. doi: 10.1016/j.diagmicrobio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Ni W, et al. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2015;45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yang X, Chen L, Duan X, Jiang Z. In vitro activity of various antibiotics in combination with tigecycline against Acinetobacter baumannii: A systematic review and meta-analysis. Microb. Drug Resist. 2017;23:982–993. doi: 10.1089/mdr.2016.0279. [DOI] [PubMed] [Google Scholar]
- 18.March GA, Bratos MA. A meta-analysis of in vitro antibiotic synergy against Acinetobacter baumannii. J. Microbiol. Methods. 2015;119:31–36. doi: 10.1016/j.mimet.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, He X, Li J. Synergy effect of meropenem-based combinations against Acinetobacter baumannii: A systematic review and meta-analysis. Infect. Drug Resist. 2018;11:1083–1095. doi: 10.2147/IDR.S172137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zusman O, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob. Agents Chemother. 2013;57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 26th edn. CLSI supplement M100S (2016).
- 22.Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, M07–11th edn, (ed. Weinstein, M. P.) (CLSI, 2018).
- 23.Pharmaceuticals W. Tygacil (Tigecycline) for Injection [Package Insert] Wyeth Pharmaceuticals Inc.; 2005. [Google Scholar]
- 24.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0 (2022). https://www.eucast.org/clinical_breakpoints/.
- 25.Interpretation of MICs and Zone Diameters. Version 10.0 (2020). Available online.
- 26.Hogg GM, Barr JG, Webb CH. In-vitro activity of the combination of colistin and rifampicin against multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 1998;41:494–495. doi: 10.1093/jac/41.4.494. [DOI] [PubMed] [Google Scholar]
- 27.Tan TY, Ng LS, Tan E, Huang G. In vitro effect of minocycline and colistin combinations on imipenem-resistant Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 2007;60:421–423. doi: 10.1093/jac/dkm178. [DOI] [PubMed] [Google Scholar]
- 28.Principe L, et al. In vitro activity of doripenem in combination with various antimicrobials against multidrug-resistant Acinetobacter baumannii: Possible options for the treatment of complicated infection. Microb. Drug Resist. 2013;19:407–414. doi: 10.1089/mdr.2012.0250. [DOI] [PubMed] [Google Scholar]
- 29.Park GC, et al. In vitro interactions of antibiotic combinations of colistin, tigecycline, and doripenem against extensively drug-resistant and multidrug-resistant Acinetobacter baumannii. Ann. Lab. Med. 2016;36:124–130. doi: 10.3343/alm.2016.36.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun SH, et al. Clonal change of carbapenem-resistant Acinetobacter baumannii isolates in a Korean hospital. Infect. Genet. Evol. 2021;93:104935. doi: 10.1016/j.meegid.2021.104935. [DOI] [PubMed] [Google Scholar]
- 31.van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation β-lactam/β-lactamase inhibitor combinations. Clin. Infect. Dis. 2016;63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsueh SC, et al. In vitro activities of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J. Antimicrob. Chemother. 2019;74:380–386. doi: 10.1093/jac/dky425. [DOI] [PubMed] [Google Scholar]
- 33.Garnacho-Montero J, et al. Acinetobacter baumannii in critically ill patients: Molecular epidemiology, clinical features and predictors of mortality. Enferm. Infecc. Microbiol. Clin. 2016;34:551–558. doi: 10.1016/j.eimc.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Tam VH, Schilling AN, Nikolaou M. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J. Antimicrob. Chemother. 2005;55:699–706. doi: 10.1093/jac/dki086. [DOI] [PubMed] [Google Scholar]
- 35.Sheng WH, et al. Comparative in vitro antimicrobial susceptibilities and synergistic activities of antimicrobial combinations against carbapenem-resistant Acinetobacter species: Acinetobacter baumannii versus Acinetobacter genospecies 3 and 13TU. Diagn. Microbiol. Infect. Dis. 2011;70:380–386. doi: 10.1016/j.diagmicrobio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Timurkaynak F, et al. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int. J. Antimicrob. Agents. 2006;27:224–228. doi: 10.1016/j.ijantimicag.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Pongpech P, et al. Antibacterial activity of carbapenem-based combinations against multidrug-resistant Acinetobacter baumannii. J. Med. Assoc. Thail. 2010;93:161–171. [PubMed] [Google Scholar]
- 38.Pankuch GA, Lin G, Seifert H, Appelbaum PC. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008;52:333–336. doi: 10.1128/AAC.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.