Abstract
OBJECTIVE:
To identify regional cerebral blood flow (rCBF) alterations in children and adolescents with congenital heart disease (CHD) in relation to neurocognitive outcomes using a non-biased data-driven approach.
METHODS:
This is a prospective, observational study of children and adolescents with CHD without brain injury and healthy controls using pseudo-continuous arterial spin labeling (pCASL) MRI. Quantitative rCBF was compared between participants with CHD and healthy controls using a voxel-wise data-driven method. Mediation analysis was then performed on a voxelwise basis, with the grouping variable as the independent variable, neurocognitive outcomes (from the NIH Toolbox Cognitive Battery) as the dependent variables, and rCBF as the mediator.
RESULTS:
After motion correction, a total of 80 studies were analyzable (27 for patients with CHD, 53 for controls). We found steeper age-related decline in rCBF among those with CHD compared to normal controls in the insula/ventromedial prefrontal regions (salience network) and the dorsal anterior cingulate and precuneus/posterior cingulate (default mode network), and posterior parietal/dorsolateral prefrontal (central executive network) (FWE-corrected p < 0.05). The reduced rCBF in the default mode/salience network was found to mediate poorer performance on an index of crystallized cognition from the NIH Toolbox Cognitive Battery in those with CHD compared to controls. In contrast, reduced rCBF in the central executive network/salience network mediated reduced deficits in fluid cognition among patients with CHD compared to controls.
CONCLUSION:
Regional cerebral blood flow alterations mediate domain-specific differences in cognitive performance in children and adolescents with CHD compared to healthy controls, independent of injury, and are likely related to brain and cognitive reserve mechanisms. Further research is needed to evaluate the potential of interventions in CHD targeting regional cerebral blood flow across lifespan.
Keywords: Arterial spin labeling, congenital heart disease, cerebral blood flow, crystalized cognition, fluid cognition
Graphical Abstract
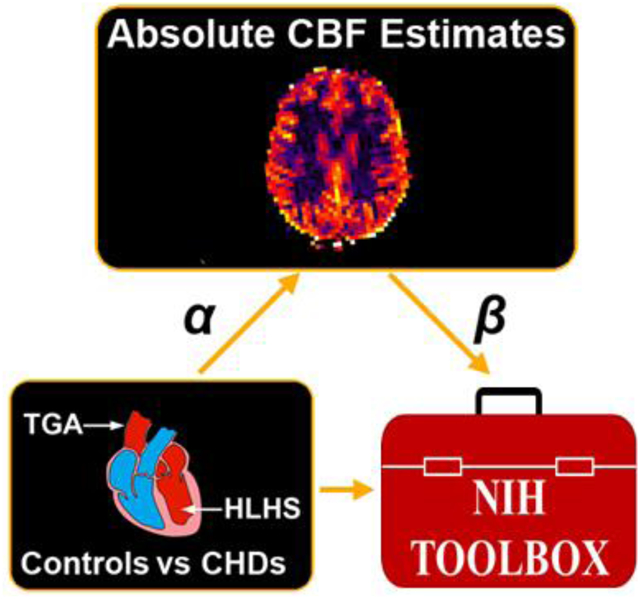
CENTRAL PICTURE LEGEND
Data-driven voxelwise CBF comparison of CHD vs controls vs mediation of NIH Toolbox scores
CENTRAL MESSAGE
Regional cerebral blood flow alterations mediate cognitive performance in children and adolescents with CHD compared to controls and are likely related to brain and cognitive reserve mechanisms.
INTRODUCTION
Children and adolescents with congenital heart disease (CHD) are known to be at greater risk of adverse neurocognitive outcomes across a range of domains including language, visuospatial skills, attention, and executive functions1. The precise neurophysiological underpinnings of these outcomes are not fully clear yet, and this constitute a very important clinical question relevant for tailoring interventions2.
Impaired substrate delivery in utero has long been thought to impact the developing brain in fetuses with CHD in the third trimester, resulting in downstream effects on post-natal brain development.3, including delayed maturation of cortical folding4 and white matter microstructure5, and a greater risk of white matter injury and/or metabolic deficits6. Impaired substrate delivery could also result from regional cerebral vascular abnormalities (possibly of genetic origin) in addition to decreased cardiac output. Recently, infants with CHD were found pre-operatively to have overall (global) reduced global and regional cerebral blood flow (rCBF)7 in a study utilizing arterial spin labeling (ASL) magnetic resonance imaging (MRI), a non-invasive MRI method of measuring CBF. In addition, post-operatively, reduced CBF has been correlated with increased acquired brain injury in single ventricle patients in the first few months of life.8 However, it is unknown if these CBF deficits persist into childhood and adolescents in CHD patients and if changes in CBF actually underlie neurocognitive deficits.
Thus, we performed an MRI study comparing children and adolescents with CHD to healthy controls using ASL to compare CBF on a voxelwise basis to investigate regionally specific CBF differences. Typically, these CBF measurements are averaged over several minutes and thus dynamic changes in CBF related to cardiac and respiratory cycles are averaged over. We also employed a novel mediation analysis (we refer the reader to the following review9) performed on a voxelwise basis to investigate whether rCBF differences in fact underlie differences in neurocognitive outcomes, as measured using the NIH Toolbox Cognitive Battery. We have previously used mediation analyses10 to show that brain structural connectivity differences mediate neurocognitive outcomes in adolescents with transposition of the great arteries.
METHODS
Participants:
143 children and adolescents aged 6 to 21 year old were recruited prospectively with the use of flyers/advertisement in office, social media and a University of Pittsburgh Clinical Translational Research Registry. Our study was approved by the institutional IRB and parental/patient consent was obtained. Of those, 82 received MR scanning, and data was analyzable from 80 participants (27 individuals with CHD, 53 controls); data from 2 participants were excluded due to excess motion. Demographic information is detailed in Table 1. Conventional images were screened for brain injury (focal infarction) by a pediatric neuroradiologist (AP) and no patients were excluded on this basis. Of the 80 with usable MR data, neurocognitive outcomes from the NIH Toolbox were available for 62 participants.
Table 1.
Demographic information on the cohort of children and adolescents with CHD and normal controls for which MR data was successfully acquired. RMS movement is translational motion in units of fraction of a voxel. Our cohort did include 1 CHD subject with microarray VUS and chromosomal duplication– 1.4 megabase loss at 3p14.1 on microarray, chromosome 16p11.2 duplication syndrome.
| Controls | CHD | p | |
|---|---|---|---|
| N | 53 (26M, 27F) | 27 (19M, 8F) | 0.069 |
| Age (Years) | 14.4 +/− 3.87 | 14.5 +/− 3.56 | 0.948 |
| RMS Movement | 0.105 +/− 0.110 | 0.086 +/− 0.071 | 0.414 |
MRI Scanning:
Data was acquired on a Siemens Skyra 3T system at UPMC Children’s Hospital of Pittsburgh. Pseudo-continuous ASL (pCASL) images11 were acquired using the following parameters: pulse duration = 500 μs, 1 ms between-pulse time, labeling duration = 1500 ms, post-inversion delay (PID) = 1000 ms, TR = 4000 ms, 4 mm3 resolution, 2D gradient-echo EPI, TE = 12 ms, 45 tagged images, SENSE factor = 2, 45 control images, total scan time = 6 minutes.
NIH Toolbox:
To assess their cognitive functioning, participants were administered the NIH Toolbox Cognition Battery12, 13. The battery offers a computerized and time-efficient assessment of multiple cognitive abilities that are considered to be important for day-to-day functioning across the lifespan. The battery generates three composite scores: a Crystallized Cognition Composite Score, a Fluid Cognition Composite Score, and a Cognitive Function Composite Score (which summarizes performance across the former two indices). Each of the composite scores and their underlying subtests has been shown to demonstrate strong test-retest reliability, robust developmental effects across childhood, and expectable correlations with “gold standard” measures of similar cognitive abilities. Scores are presented as age-corrected standard scores, with a mean of 100 and standard deviation of 15.
Crystallized cognition refers to the ability to retrieve and use information that has been acquired throughout a lifetime; crystallized abilities are dependent on experience such as education, change markedly throughout childhood, and are thought to be relatively robust in the face of acquired brain injury. On the NIH Toolbox, the Crystallized Cognition Composite Score summarizes performance on tests of receptive vocabulary (Picture Vocabulary Test) and reading accuracy (Oral Reading Recognition), knowledge and skills acquired throughout development.
By contrast, fluid cognition refers to the ability to store, process, and manipulate novel information. Fluid abilities are thought to be more dependent on biological influences than experience, improve rapidly in childhood and peak in early adulthood, and tend to be susceptible to acquired brain injuries. The Fluid Cognition Composite Score summarizes performance across five tests. It includes one test of learning and immediate memory (Picture Sequence Memory Test), one test of processing speed (Pattern Comparison Processing Speed Test), and three tests of executive functioning, assessing attention and inhibitory control (Flanker Inhibitory Control and Attention Test), cognitive flexibility and concept formation (Dimensional Change and Cart Sorting Test), and auditory and visual working memory (List Sorting Working Memory).
Regional CBF (rCBF) Estimation:
A diagram of the data analysis pipeline is given in Figure 1. To minimize possible effects of participant motion on the computed rCBF values, the raw ASL images from each participant were motion-corrected using an affine transformation14. Root-mean-square (across frames) translational motion was computed for each participant and saved for later analysis. Using in-house routines in Interactive Data Language (ID), average (control-tag) and control images were computed using a voxelwise general linear model (GLM), with motion and drift (linear and quadratic) parameters included as nuisance covariates. Fractional signal change maps ((control-tag)/control) were then computed. Maps were converted into absolute CBF estimates (in units of ml/100 g/min) using the two-compartment model15 and literature values16 for labeling efficiency, gray matter tissue T1, arterial T1, brain-blood partition coefficient, and tissue transit time.
Figure 1.
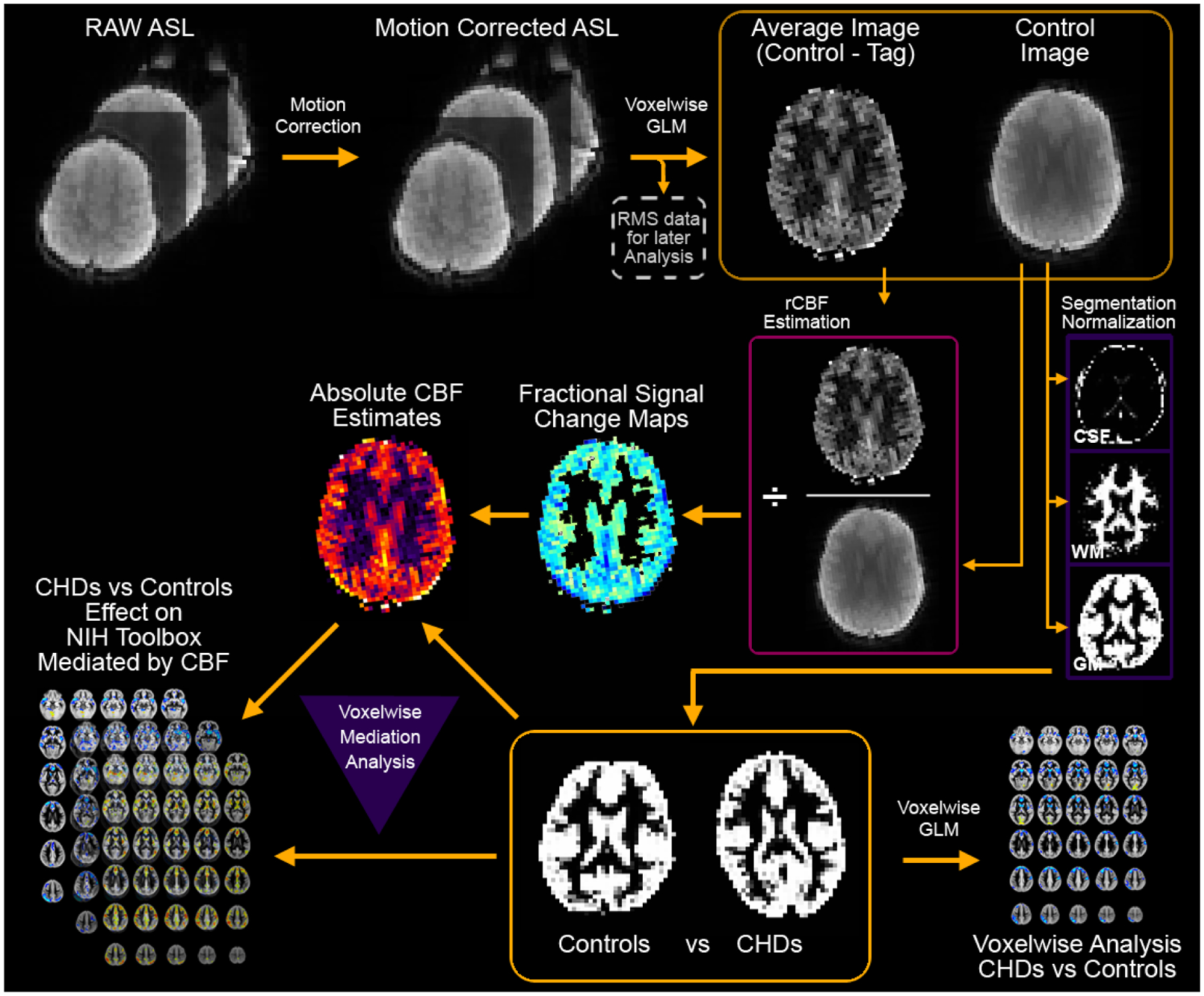
Flowchart detailing the quantitative data-driven analysis pipeline. The pipeline includes motion correction, absolute CBF estimation, gray matter segmentation, and spatial normalization to a study-specific GM template. Results are analyzed on a voxelwise basis either for comparison of CHD participants to normal controls or for mediation of NIH Toolbox composite scores, with inclusion (on a voxelwise basis) of GM probability.
Segmentation/Normalization:
The reference image for the motion coregistration was skull-stripped using FSL (fMRIB, Oxford, UK) and segmented in native space into GM, WM, and CSF using routines in SPM8 (Wellcome Dept. of Cognitive Neurology, London, UK). Using SPM8, the GM maps were spatially normalized into MNI space using the gray matter template in SPM8; a study-specific GM template was obtaining by averaging across participants and the spatial normalization repeated. The rCBF images were transformed into MNI space using the same transformation. (The CBF and GM maps were spatially filtered using a Gaussian filter with width σ = 3 mm.)
Voxelwise GLM:
Analyses were restricted to participants with rms motion < 1 voxel and to voxels with GM probability > 78%. A General Linear Model (GLM) was performed on a voxelwise basis with CHD status as the variable of interest and age, sex, maternal education, rms motion, and GM probability (different for each voxel) as covariates of no interest. This procedure minimizes the risk of spurious results resulting from spatial misregistration. Results were deemed significant at FWE-corrected p < 0.05, determined via Monte Carlo analysis17.
Mediation:
Briefly summarized, mediation analyses9 compare the strength of an effect (between an independent and a dependent variable) with and without the inclusion of a covariate (the mediator). If the strength of the effect decreases after inclusion of the covariate, the covariate is said to “mediate” the relationship between the independent and dependent variables, since at least some of the association is explained by it. On the other hand, if the strength of the effect increases, the covariate is said to “suppress” the relationship. Mediation analyses are frequently used, for instance, in psychology research to investigate whether environmental differences account for group differences in adjustment outcomes. Significant results from these analyses provide stronger effects of a mediator than simple correlations, since the null hypothesis of an epiphenomenon can be rejected (at a given confidence level). However, when interpreting results, it needs to be kept in mind that only a randomized controlled study can prove causality.
Thus, similar voxelwise analyses were performed with the grouping variable (patient with CHD vs. healthy control) as the independent variable, voxelwise CBF as the mediator, and NIH Toolbox Cognition Battery composite scores as the dependent variables (using the same covariates included as above for the GLM). Statistical significance at the voxel level was obtained via bootstrapping (5,000 samples)18 with bias-corrected and accelerated confidence intervals19. Bootstrapping the mediation analysis yields a p-value for the strength of the mediation. This p-value was converted into a Z-score (e.g. from the cdf of a Gaussian distribution) for the purposes of our voxel-wise analysis which accounts for the non-normal distribution of the parameter of interest (the product of two regression parameters). The individual “regression arms” of the mediation are that of CHD vs. CBF and rCBF vs. neurocognitive outcome controlling for CHD.
RESULTS
Participant demographics and socio-economic related information are detailed in Table 1 and Supplemental Table 1–3. There was a larger percentage of boys among participants with CHD as compared to healthy controls; however, this difference only trended towards significance (p = .088). Otherwise, the two groups of participants did not differ on any demographic variables. A comparison of performance on the three composite scores of the NIH Toolbox and the 7 individual subtests of the NIH Toolbox is reflected in Table 2. Participants with CHD tended to display worse cognitive outcomes than healthy controls; still, they generally performed within normal limits across subtests, similar to prior research with face-to-face measures of gross cognitive functioning20, 21. The distribution of heart lesions were as followed: left ventricular outflow tract obstruction (hypoplastic left heart syndrome, n= = 7; coarctation of the aorta, n= = 4; bicuspid aortic valve, n = 1; and Shone’s complex, n = 1); transposition of the great arteries (n= = 13); double outlet right ventricle, n= = 3; tetrology of Fallot, n = 3; ventricular or atrial septal defect, n = 10; double inlet left ventricle, n = 5; pulmonary atresiaatresia, n = 1; Ebstein’s anomaly, n= = 1.
Table 2.
Demographic information on the cohort of children and adolescents with CHD and normal controls for which MR data was successfully acquired and for which Maternal Education and NIH Toolbox data was available (age-corrected standard scores). RMS movement is translational motion in units of fraction of a voxel.
| Controls | CHD | p | |
|---|---|---|---|
| N | 39 (19M, 20F) | 23 (17M, 6F) | 0.052 |
| Age (years) | 13.2 +/− 3.24 | 13.5 +/− 2.83 | 0.777 |
| RMS Movement | 0.118 +/− 0.123 | 0.092 +/− 0.075 | 0.351 |
| Maternal Education | 5.41 +/− 1.82 | 5.00 +/− 1.73 | 0.386 |
| Crystallized Cognition Composite | 112.9 +/− 14.47 | 108.66 +/− 10.37 | 0.223 |
| Fluid Cognition Composite | 107.3 +/− 17.50 | 98.67 +/− 16.14 | 0.059 |
| Total Cognition Composite | 113.7 +/− 19.09 | 103.33 +/− 16.03 | 0.033 |
| Dimensional Change Card Sort | 104.1 +/− 13.48 | 95.03 +/− 17.32 | 0.025 |
| Flanker Inhibitory Control | 100.1 +/− 11.02 | 98.32 +/− 14.45 | 0.597 |
| List Sorting Working Memory | 105.6 +/− 12.09 | 107.40 +/− 13.67 | 0.603 |
| Picture Sequence Memory | 105.6 +/− 16.09 | 95.12 +/− 14.15 | 0.012 |
| Oral Reading Recognition | 116.0 +/− 16.48 | 109.38 +/− 13.49 | 0.109 |
| Picture Vocabulary | 107.1 +/− 11.93 | 105.21 +/− 10.21 | 0.533 |
| Pattern Comparison Processing | 103.9 +/− 19.35 | 98.74 +/− 21.57 | 0.337 |
Maternal education is coded according to the following rubric: 1 = less than HS diploma, 2 = HS diploma, 3 = some college, 4 = completed trade/vocational training, 5 = Associate’s degree, 6 = Bachelor’s degree, 7 = Master’s degree, 8 = Doctorate/professional degree. No significant gender differences were detected on any of the neuropsychologic scores (p > 0.05, all tests). Maternal educational attainment was significantly (p < 0.05) correlated with crystallized cognition (as well as oral reading and picture vocabulary).
We found regionally specific CBF differences in children and adolescents with CHD (Figure 2). Reduced rCBF (controlled for age, gender and maternal education) was seen in the following cortical regions: (1) fronto-medial cortex [ventromedial prefrontal (salience network) and dorsal anterior cingulate (anterior default network)] >fronto-lateral cortex [bilateral dorsolateral prefrontal (central executive network)]; (2) parietal-medial cortex [posterior cingulate and precuneus (posterior default mode network)] > parietal-lateral cortex [right inferior parietal lobule (central executive network)]; (3) subcortically, in the bilateral insula (salience network) and bilateral caudate. We also noted a significant age (independent variable) interaction by CHD diagnosis (moderator) on rCBF (dependent variable) in many of these cortical (dorsal anterior cingulate, dorsolateral prefrontal cortex, and posterior cingulate) and subcortical (cerebellum) regions (Figure 3A) (p<.05 FWE-family wise error corrected). From regions of interest analysis, we noted that rCBF decreased with increasing age in both groups, but the CHD group demonstrating a steeper decline in late adolescence in multiple cerebral regions compared to the control group (Figure 3B–3E). We also noted that single ventricle physiology patients within the CHD group demonstrated reduced areas of rCBF compared to biventricular patients (Supplemental Figure 1). No differences in rCBF were noted between acyanotic and cyanotic patients.
Figure 2.
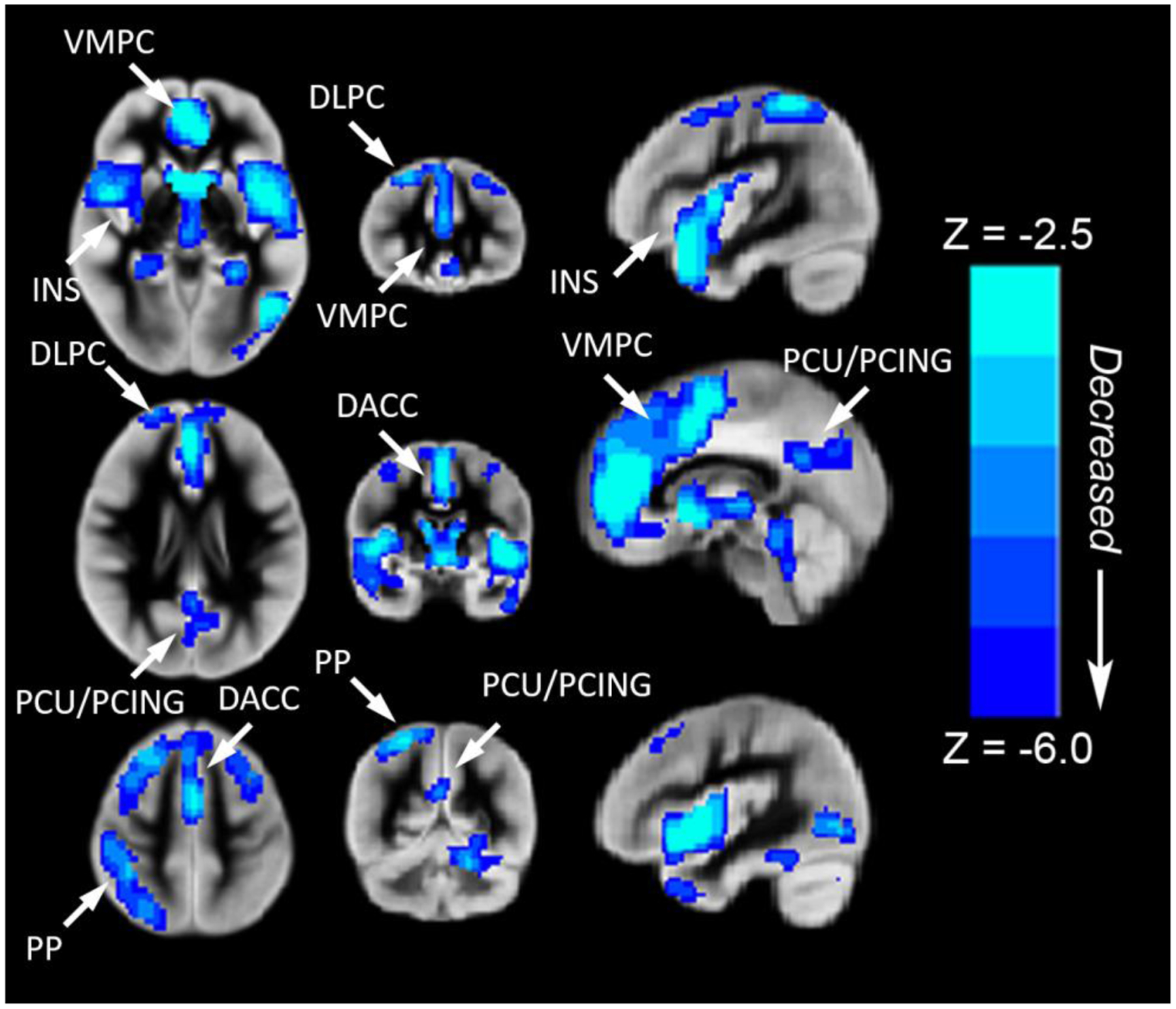
Regions with decreased rCBF (blue voxel color) in children and adolescents with CHD compared to normal controls (voxel-wise group comparison with results projected on one image). Reduced rCBF (controlled for age, gender and maternal education) was seen in the following cortical regions: (1) fronto-medial cortex [ventromedial prefrontal-VMPC (salience network) and dorsal anterior cingulate-DACC (anterior default network)] >fronto-lateral cortex [bilateral dorsolateral prefrontal-DLPC (central executive network)]; (2) parietal-medial cortex [posterior cingulate-PCING and precuneus-PCU (posterior default mode network)] > parietal-lateral cortex [right inferior parietal lobule (central executive network)]; (3) subcortically, in the bilateral insula-INS (salience network) and bilateral caudate. All regions significant at FWE-corrected p < 0.05. Abbreviations: Ventrolateral medial prefrontal cortex (VMPC), Insula (INS), Dorsolateral prefrontal cortex (DLPC), Posterior Cingulate (PCING), Precuneus (PCU), Dorsal Anterior Cingulate Cortex (DACC), Posterior Parietal (PP). Images in radiologic orientation.
Figure 3.
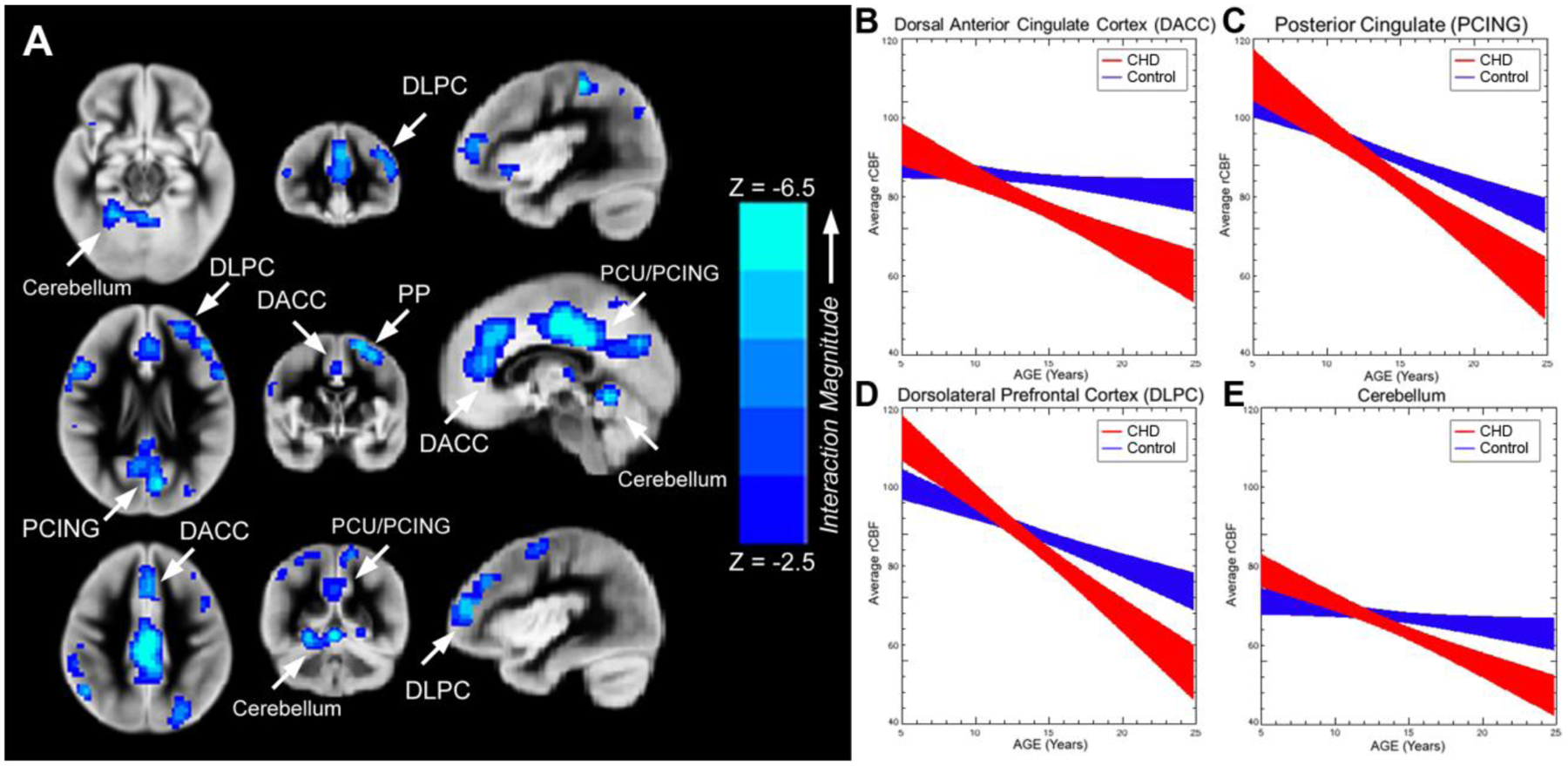
A-E. Areas of blue color (Figure 3A) indicate specific brain regions (p<.05 FWE corrected) that demonstrate significant age (independent variable) by CHD diagnosis (moderator) interaction on rCBF (dependent variable). As a post-hoc informative analysis, age trajectories (+/− std err) are provided for rCBF (ml/100g/min) for CHD patients and normal referents (Figure 3B-3E) from selected regions of interest (ROIs) including cortical (dorsal anterior cingulate, dorsolateral prefrontal cortex, and posterior cingulate) and subcortical (cerebellum). We noted that rCBF decreased with increasing age in both groups, but the CHD group demonstrated a steeper decline in late adolescence. Abbreviations: Ventrolateral medial prefrontal cortex (VMPC), Insula (INS), Dorsolateral prefrontal cortex (DLPC), Posterior Cingulate (PCING), Precuneus (PCU), Dorsal Anterior Cingulate Cortex (DACC), Posterior Parietal (PP). (Beta values for interaction parameters from the informative analysis: DACC, −3.04 (1.53); PCING, −3.16 (1.67); DLPC, −3.91 (1.69); Cerebellum, −2.93 (1.36))
We did not find any cerebral regions which mediated the total composite score. However, for the Crystallized Cognition composite (Figure 4), we found that rCBF predominately in the medial fronto-parietal regions [ventromedial prefrontal (salience network), dorsal anterior cingulate, and precuneus/posterior cingulate (default mode network)] mediated worse crystallized cognition among participants with CHD compared to healthy control. Most of these regions overlap with those found to have reduced CBF between CHD and controls (regression analysis representing the left side of mediation triangle -Supplemental Figure 2). Controlling for CHD status, rCBF is positively associated with crystallized cognition in these same regions (regression analysis -right side of the mediation triangle Supplemental Figure 2 and 3).
Figure 4.
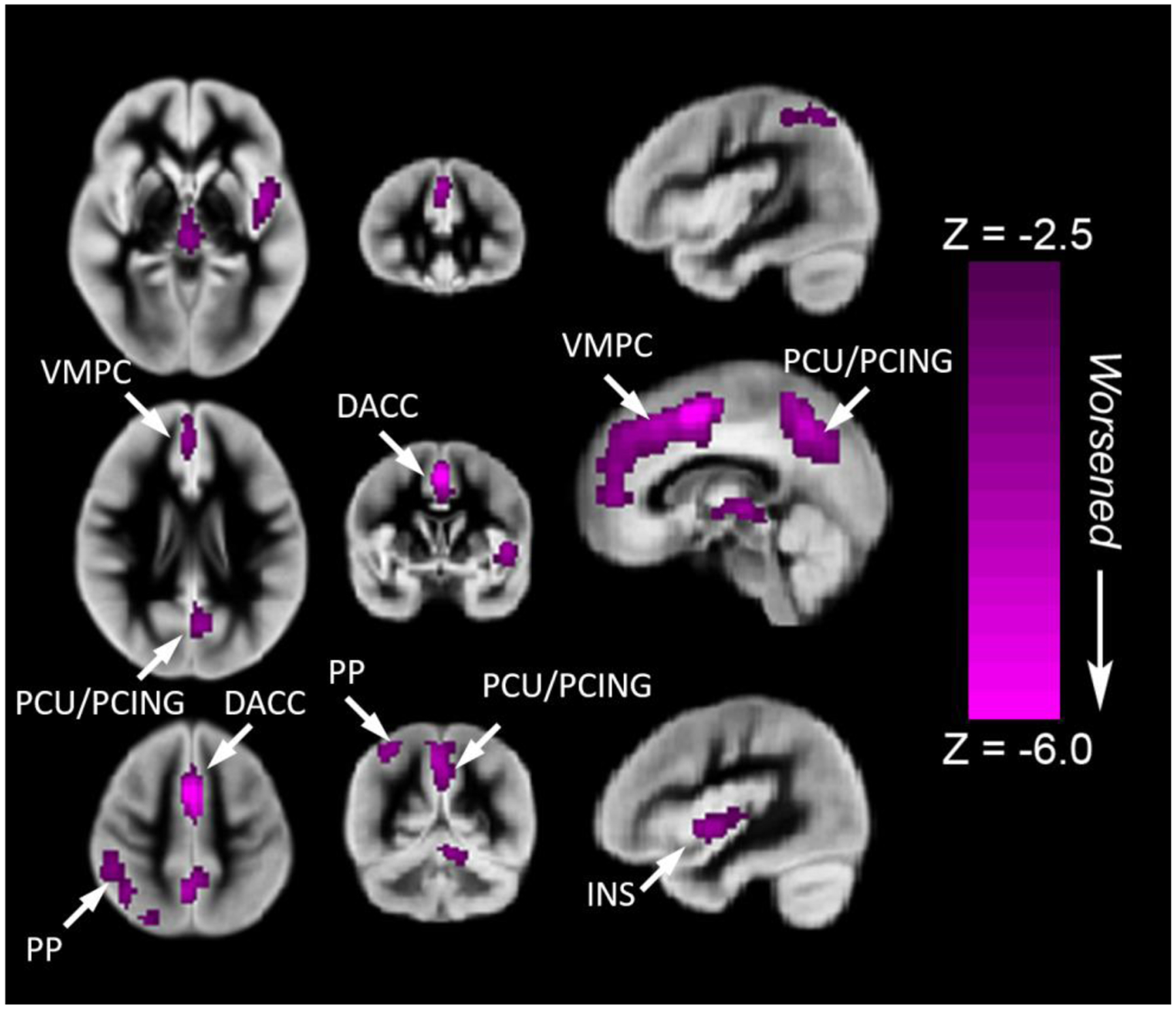
Regions where rCBF mediates improved Crystallized Cognition composite score of the NIH Toolbox Cognitive Battery in the medial fronto-parietal regions [ventromedial prefrontal (salience network), dorsal anterior cingulate, and precuneus/posterior cingulate (default mode network)] among participants with CHD compared to healthy control. All regions significant at FWE-corrected p < 0.05. Images in radiologic orientation. Abbreviations: Ventrolateral medial prefrontal cortex (VMPC), Insula (INS), Dorsolateral prefrontal cortex (DLPC), Posterior Cingulate (PCING), Precuneus (PCU), Dorsal Anterior Cingulate Cortex (DACC), Posterior Parietal (PP)
By contrast, we found selected lateral fronto-subcortical cerebral regions which mediated improved Fluid Cognition composite scores among participants with CHD compared to healthy controls (Figure 5). These areas include the left dorsolateral prefrontal cortex (superior frontal gyrus (Broca’s area -BA 8/9) or central executive network) and the insula (salience network) bilaterally and cerebellum. All regions overlap with those found to have reduced CBF between CHD and controls (regression analysis representing the left side of mediation triangle - Supplemental Figure 4); the only region of overlap with those that mediated improved crystallized cognition is the left insula (salience network). Controlling for CHD status, rCBF is negatively associated with crystallized cognition in some of these regions (regression analysis representing the right side of the mediation triangle in Supplemental Figures 4 with full neuroanatomic detail in Supplemental Figure 5).
Figure 5.
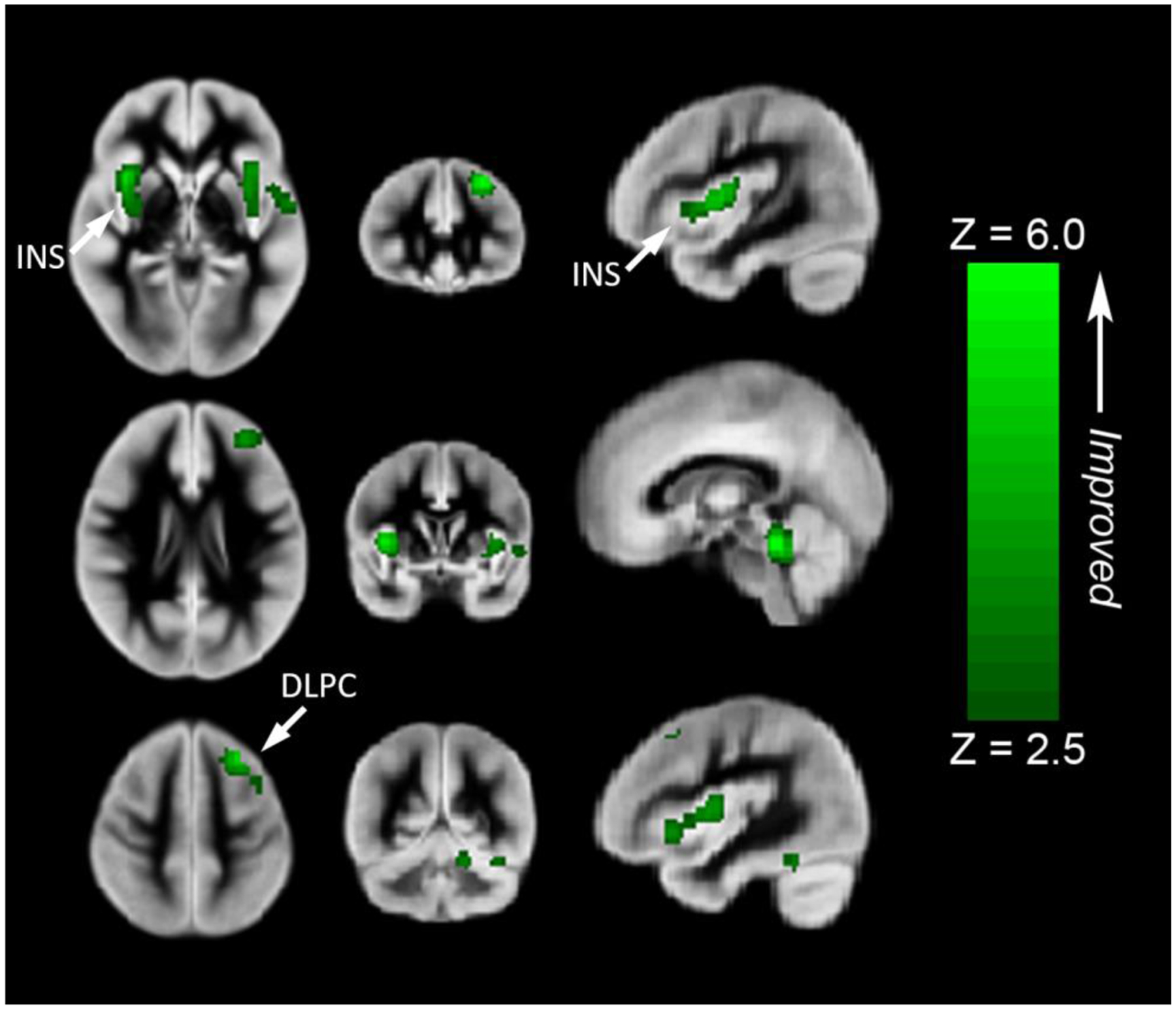
Regions where rCBF mediates worse Fluid Cognition composite score of the NIH Toolbox Cognitive Battery. These areas include the left dorsolateral prefrontal cortex (superior frontal gyrus (Broca’s area -BA 8/9) or central executive network) and the insula (salience network) bilaterally and cerebellum. All regions significant at FWE-corrected p < 0.05. Images in radiologic orientation. Abbreviations: Insula (INS), Dorsolateral prefrontal cortex (DLPC)
DISCUSSION
Our results in older children and adolescents with CHD suggest that regional cerebral blood flow disturbances play a key role in explaining cognition across the early lifespan before the transition to young adulthood. Reduced rCBF was noted in patients with CHD in selected regions of the brain that are known to overlap with cognitive neural networks that underlie multiple cognitive domains including executive functions and social-emotional functions (i.e.., default mode network, salience network and central-executive network). We also noted that single ventricle physiology patients within the CHD group demonstrated reduced areas of rCBF compared to biventricular patients in these same cognitive neural networks. Additionally, we detected a steeper rate of decline of rCBF with increasing age in multiple brain regions in patients with CHD compared to controls, absent major brain injury (focal infarcts), despite a slight increase in rCBF which has been noted in normal late adolescence (Figure 6). This similar decline in rCBF with age has been noted in aging populations including Alzheimer’s disease. In these populations, rCBF disturbances correlate with regional brain hypometabolism (brain structure/networks).22 Further research is needed to determine if these age-related cerebral perfusion declines in patients with CHD are related to microstructural acquired injury (i.e.., T2/FLAIR hyperintensities or multi-modal imaging biomarkers) or other factors such as changes in functional network structure or activity.
Figure 6.
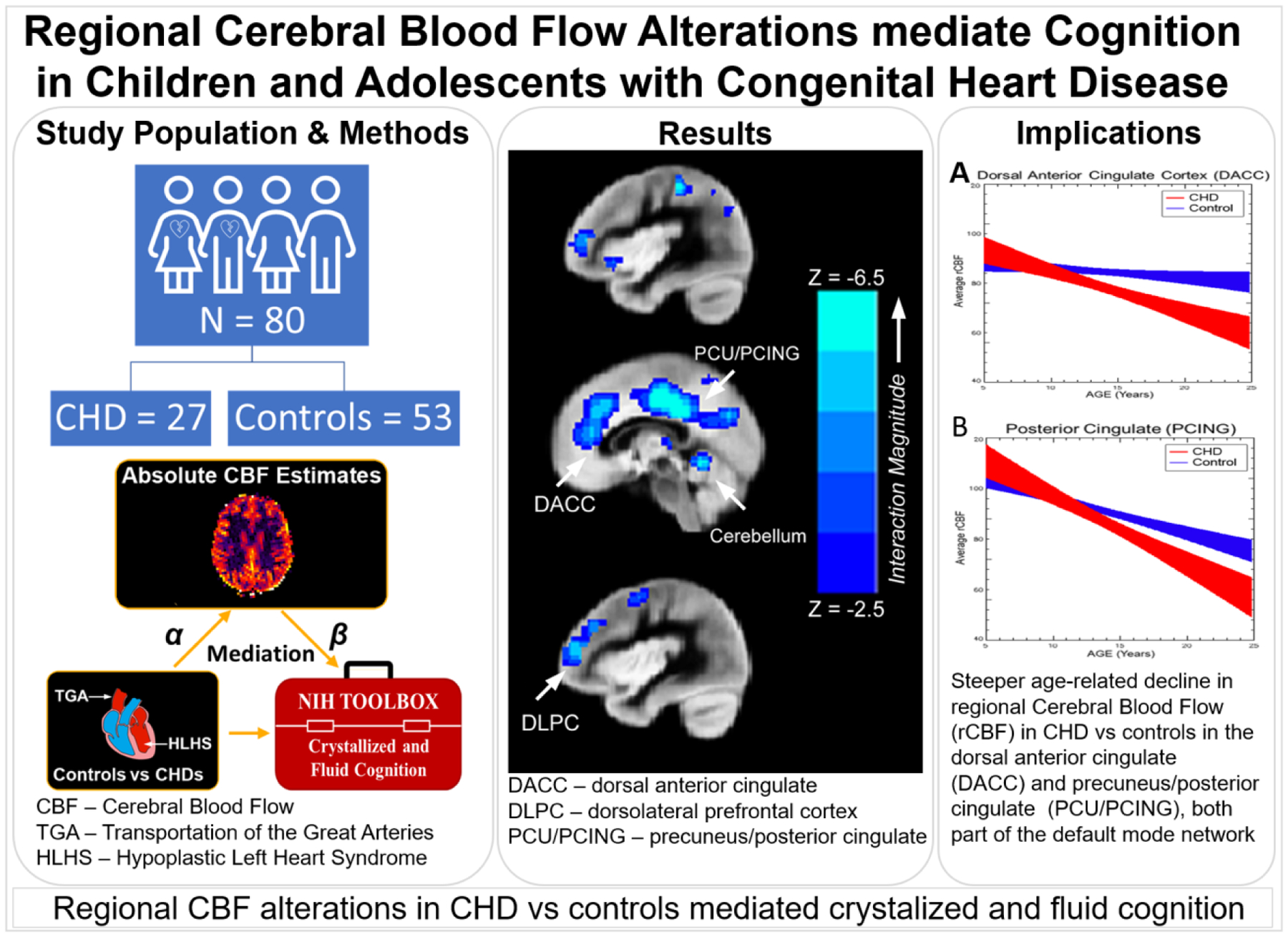
Graphical Abstract: Regional Cerebral Blood Flow Alterations mediated Cognition in Children and Adolescents with Congenital Heart Disease including Study Population and Methods: Voxelwise regional Cerebral Blood Flow measurements were compared between 27 CHD patients and 53 controls, and correlated with neurocognitive testing; Results: regional CBF alterations in CHD vs controls mediated crystalized and fluid cognition in known intrinsic cognitive network including the anterior default mode (dorsal anterior cingulate), posterior default mode (posteiror cingulate) and salience network (insula); Implications: Steeper age-related decline in regional Cerebral Blood Flow (rCBF) in CHD and controls were also noted in these structures. Taken together, regional cerebral vascular flow disturbances associated with CHD are an important component of neurocognitive deficits, and ultimately may impact brain/cognitive reserve across the lifesp
From a developmental perspective, our results agree with reduced CBF at earlier ages in individuals with CHD: a previous ASL-MRI study found reduced global CBF in CHD neonates pre-operatively7, while young children with single-ventricle CHD are at risk for development of aortic-to-pulmonary collaterals23 which contributes to reduced global CBF. Of note, we did not detect global disturbances in CBF in our single ventricle sub-analysis, only regional CBF disturbances. The vulnerability of the cerebral vascular system in CHD infants has been shown via recent studies showing impaired cerebral autoregulation in neonates with CHD pre-operatively24 and maintenance of global CBF in post-operative neonates with CHD25, indicating cerebral circulation fragility. Our results support that this vulnerability of the cerebral vascular system may persist through the developmental period and be relevant for neurocognitive outcome across the lifespan.
Our study also helps to further elucidate the relation between rCBF and cognitive function in individuals with CHD. It is known that rCBF correlates with cognitive function in healthy children26, indicating a likely link with neurocognitive outcome among those with CHD as well. Indeed, global CBF post-operatively has been correlated with neurodevelopmental outcomes in infants with CHD at 1 year of age27. In regards to our results, rCBF supplies the oxidative metabolic demand caused by increased neuronal activity due to performance of cognitive tasks; nevertheless, in a normal brain the vasculature vastly oversupplies the metabolic demand, and genetic factors could account for both cognitive and regional cerebral vascular differences.
We applied our statistical mediation analysis to the two global indices of cognition: crystallized cognition, on the one hand, and fluid cognition, on the other. Crystallized cognition involves the ability to retrieve and apply previously acquired knowledge28, while fluid cognition refers to the ability to store, process, manipulate new information, thereby solving novel problems29. Thus, crystallized cognition is modifiable over the course of the lifespan (dependent more on experience-dependent long-term potentiation and plasticity30), whereas fluid cognition is more innate in nature (dependent more on quantity of neurons and synapses).
Our mediation results implicate reduced rCBF in specific frontal, parietal, and subcortical regions (particularly the midline default mode network and the salience network) as underlying deficits in crystallized cognition in CHD (i.e., without these reductions, children and adolescents with CHD would have performed better on crystallized cognition). The Crystallized Cognition composite score comprises two language related tests of receptive word knowledge and reading, respectively. Particular aspects of cognitive function necessary for development of crystallized cognition are specifically relevant to the regions found with reduced rCBF. These findings suggest that CHD patients may have reduced CBF in brain regions that facilitating their education/experience dependent acquisition of skills and knowledge (including language), which is likely to impact their brain and cognitive reserve as they transition from adolescent to young adulthood.
The dorsal anterior cingulate and the insula have been identified as important for identifying the most relevant among competing stimuli in order to guide behavior31, and filtering out of irrelevant ones (comprising part of the “salience network”). The ventromedial prefrontal cortex, in conjunction with the precuneus/posterior cingulate have been identified as relevant for avoiding distractions (these regions comprising the “default mode network”), as a variety of studies have shown functional deactivation in these regions during performance of a demanding cognitive task, as well as correlation between cognitive performance and connectivity and connectivity to these regions, effects which are developmental in nature32. The posterior parietal cortex, together with the dorsolateral prefrontal cortex (comprising the “central executive network”) is relevant for executive function31, i.e. the proper marshalling of neuronal resources necessary for performance of a given task.
By contrast, reduced CBF in the insula bilaterally, as well as the dorsolateral prefrontal cortex (specifically, the left superior frontal gyrus), were found to suppress deficits in fluid cognition among those with CHD (i.e., without these reductions, children and adolescents with CHD would have performed worse on fluid cognition). Importantly, the Fluid Cognition composite score comprises a variety of subtests heavily reliant on executive function, working memory, monitoring/manipulation of information, and exclusion of irrelevant stimuli. Fluid cognition has been shown to be highly correlated with brain “network reconfiguration” upon performance of a cognitive task33 in the salience and central executive networks, meaning that in more intelligent individuals the functional configuration of the brain undergoes less modification, indicating a more efficient neural architecture. As such, a more efficient system would be expected to have less metabolic demand, thus corresponding with the reductions in rCBF seen in this study. This effect may be attenuated somewhat as individuals with a less efficient neural architecture may be able to compensate via a local increase in CBF to meet the increased metabolic demand.34 Future longitudinal studies are needed to determine if these findings may point to a form of brain and/or cognitive reserve/compensation, which is relevant to understanding the neural mechanism of cognitive deficits as patients with CHD transition to adulthood.
Our results thus suggest that the cerebral vascular system may constitute a promising target for intervention in pediatric patients with CHD to improve neurocognitive outcomes. Non-invasive behavioral interventions affect the developing brain and thus impact synaptic activity and may affect the vasculature through astrocytic mediation, as dynamic changes in astrocytes are mediated via synaptic activity, and astrocytes regulate vascular tone35.
Strengths of our approach include a robust method to account for participant motion and spatial misregistration, and the ability to perform both group analyses and mediations on a voxel wise basis. A limitation of our study is that we did not measure arterial/tissue transit times, as rCBF estimates are sensitive to misspecification of arterial/tissue transit times, although this sensitivity is reduced via use of a long PID. However, apparent differences in rCBF could thus, in theory, be the result of differences in transit times. Furthermore, our study is limited with a small sample size and we were not well powered to evaluate multiple different CHD subtypes or evaluate the relationship of brain injury to cerebral perfusion (including focal infarctions). The results of the analysis may also be subject to over-determination due to the small sample size and thus await independent replication.
CONCLUSION
We found steeper age-related decline in rCBF in children and adolescents with CHD compared to healthy controls using a voxel wise analysis procedure in multiple cerebral regions that are known to underlie intrinsic cognitive networks important for executive function and social cognition. Reduced rCBF was found to be regionally specific and primarily limited to the salience network (insula/ventromedial prefrontal region), the default mode network (dorsal anterior cingulate/precuneus/posterior cingulate), and frontal executive network (posterior parietal/dorsolateral prefrontal). The reduced rCBF in the default mode network was found to mediate poorer performance among children with CHD compared to controls on an index of crystallized cognition from the NIH Toolbox Cognitive Battery, which is likely to contribute to difficulty in acquisition of skills and knowledge over lifetime, ultimately impacting reserve mechanisms. In contrast, reduced rCBF in the salience network mediated reduced deficits in fluid cognition among children with CHD compared to controls, suggesting neural compensation is present, which can also impact reserve mechanisms. Together, these results suggest that regional cerebral vascular flow disturbances associated with CHD are an important component of neurocognitive deficits, and ultimately may impact brain/cognitive reserve across the lifespan.
Supplementary Material
Video Legend:
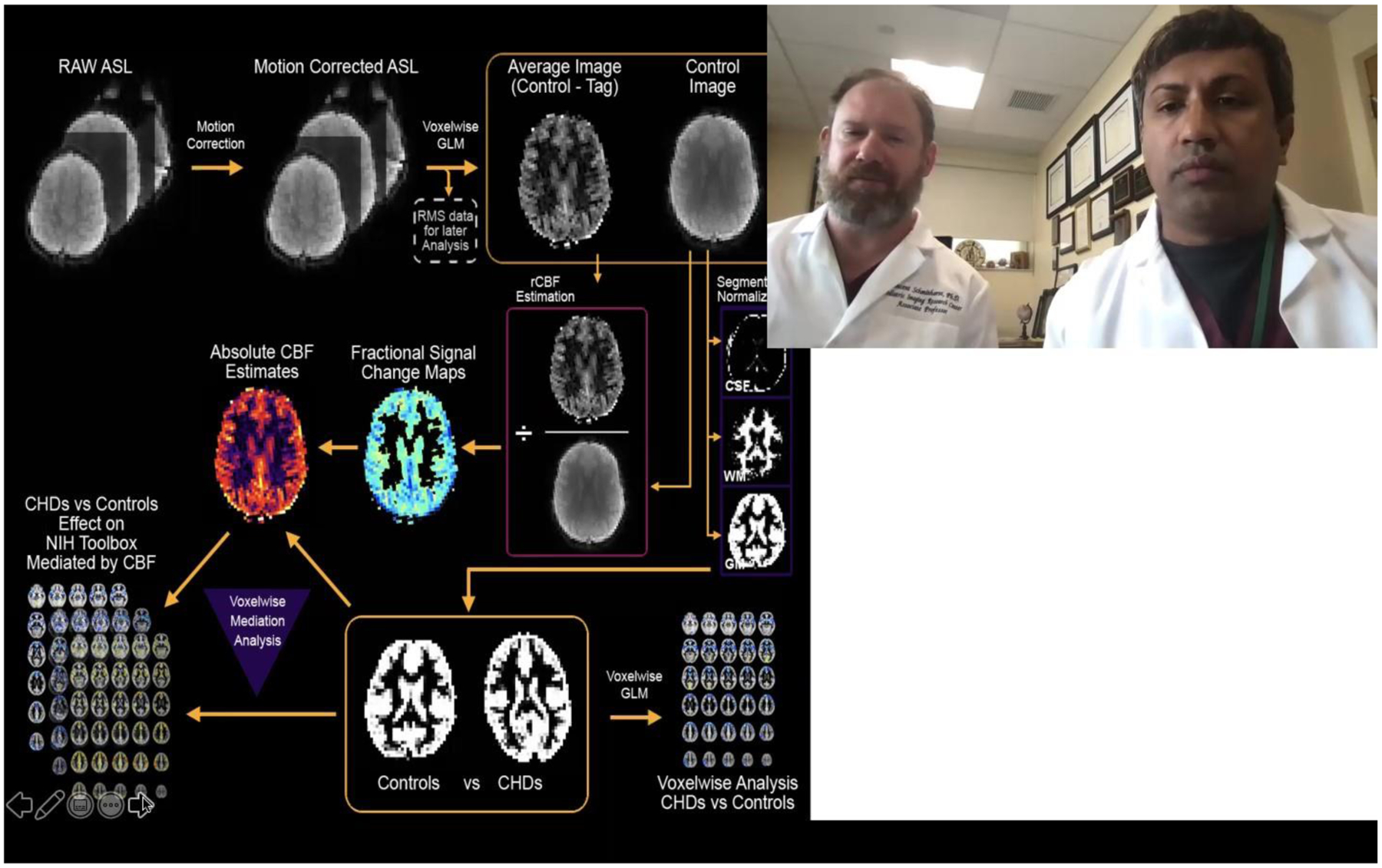
Dr. Panigrahy and Dr. Schmithorst explain the importance, main findings and relevance of this study.
PERSPECTIVE STATEMENT.
Children and adolescents with congenital heart disease (CHD) are at risk of neurocognitive deficits possibly related to regional cerebral blood flow (rCBF). We found steeper age-related decline in rCBF independent of injury in CHD patients vs. controls in cognitive related brain networks. Also, rCBF mediates cognitive performance in CHD patients vs. controls, independent of injury.
No conflict of interest to declare.
This work was supported by the Department of Defense (W81XWH-16-1-0613), the National Heart, Lung and Blood Institute (R01 HL152740-1, R01 HL128818-05), and the National Heart, Lung and Blood Institute with National Institute on Aging (R01HL128818-05 S1). IRB STUDY19040003 (previously PRO16040368) originally approved 08/09/2016, current approval 03/27/2020-03/23/2021.
GLOSSARY OF ABBREVIATIONS
- ASL
arterial spin labeling
- CBF
cerebral blood flow
- CHD
congenital heart disease
- CSF
cerebral spinal fluid
- DACC
Dorsal Anterior Cingulate Cortex
- DLPC
Dorsolateral prefrontal cortex
- DTI
diffusion tensor imaging
- FSL
FMRIB Software Library
- FWE
Family Wise Error
- GLM
general linear model
- GM
grey matter
- IDL
Interactive Data Language
- INS
Insula
- MNI
Montreal Neurological Institute
- PID
post-inversion delay
- pCASL
pseudo-continuous arterial spin labeling
- PCING
Posterior Cingulate (PCING)
- PCU
Precuneus
- PP
Posterior Parietal
- rCBF
regional cerebral blood flow
- SPM8
statistical parametric mapping maximum 8 regions
- WM
white matter
- VMPC
Ventrolateral medial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Our study was approved by the institutional IRB and parental/patient consent was obtained.
REFERENCES
- 1.Donofrio MT, Massaro AN. Impact of congenital heart disease on brain development and neurodevelopmental outcome. International journal of pediatrics. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wernovsky G, Licht DJ. Neurodevelopmental Outcomes in Children With Congenital Heart Disease-What Can We Impact? Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17:S232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of thoracic and cardiovascular surgery. 2009;137:529–536; discussion 536–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. The New England journal of medicine. 2007;357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 6.Dimitropoulos A, McQuillen PS, Sethi V, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaraj UD, Evangelou IE, Donofrio MT, et al. Impaired Global and Regional Cerebral Perfusion in Newborns with Complex Congenital Heart Disease. The Journal of pediatrics. 2015;167:1018–1024. [DOI] [PubMed] [Google Scholar]
- 8.Fogel MA, Li C, Elci OU, et al. Neurological injury and cerebral blood flow in single ventricles throughout staged surgical reconstruction. Circulation. 2017;135:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- 10.Panigrahy A, Schmithorst VJ, Wisnowski JL, et al. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage. Clinical 2015;7:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic resonance in medicine. 2008;60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub S, Bauer PJ, Zelazo PD, et al. I. NIH TOOLBOX COGNITION BATTERY (CB): INTRODUCTION AND PEDIATRIC DATA(). Monographs of the Society for Research in Child Development. 2013;78:1–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE transactions on image processing : a publication of the IEEE Signal Processing Society. 1998;7:27–41. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magnetic resonance in medicine. 2002;48:242–254. [DOI] [PubMed] [Google Scholar]
- 16.Schmithorst VJ, Hernandez-Garcia L, Vannest J, Rajagopal A, Lee G, Holland SK. Optimized simultaneous ASL and BOLD functional imaging of the whole brain. Journal of magnetic resonance imaging : JMRI. 2014;39:1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage. 1998;8:113–128. [DOI] [PubMed] [Google Scholar]
- 18.Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychological science. 2013;24:1918–1927. [DOI] [PubMed] [Google Scholar]
- 19.DiCiccio T, Efron B. Bootstrap confidence intervals. Statistical Science. 1996;13:189–228. [Google Scholar]
- 20.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. Journal of the International Neuropsychological Society : JINS. 2015;21:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstle M, Beebe DW, Drotar D, Cassedy A, Marino BS. Executive Functioning and School Performance among Pediatric Survivors of Complex Congenital Heart Disease. The Journal of pediatrics. 2016;173:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C-W, Hsu S-W, Chang Y-T, et al. Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer’s disease: A multiparametric neuroimaging study. Scientific reports. 2018;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogel MA, Li C, Wilson F, et al. Relationship of cerebral blood flow to aortic-to-pulmonary collateral/shunt flow in single ventricles. Heart. 2015;101:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Votava-Smith JK, Statile CJ, Taylor MD, et al. Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. The Journal of thoracic and cardiovascular surgery. 2017;154:1038–1044. [DOI] [PubMed] [Google Scholar]
- 25.Saiki H, Sugimoto M, Kuwata S, et al. Novel mechanisms for cerebral blood flow regulation in patients with congenital heart disease. American heart journal. 2016;172:152–159. [DOI] [PubMed] [Google Scholar]
- 26.Kilroy E, Liu CY, Yan L, et al. Relationships between Cerebral Blood Flow and IQ in Typically Developing Children and Adolescents. Journal of cognitive science. 2011;12:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng HH, Wypij D, Laussen PC, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. The Annals of thoracic surgery. 2014;98:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt E Let’s hear it for crystallized intelligence. Learn Individ Differ. 2000;12:123–129. [Google Scholar]
- 29.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nature reviews. Neuroscience 2004;5:471–482. [DOI] [PubMed] [Google Scholar]
- 30.Christoforou A, Espeseth T, Davies G, et al. GWAS-based pathway analysis differentiates between fluid and crystallized intelligence. Genes, brain, and behavior. 2014;13:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubia K Functional brain imaging across development. European child & adolescent psychiatry. 2013;22:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz DH, Cole MW. Higher Intelligence Is Associated with Less Task-Related Brain Network Reconfiguration. The Journal of Neuroscience. 2016;36:8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kübler A, Dixon V, Garavan H. Automaticity and Reestablishment of Executive Control—An fMRI Study. Journal of Cognitive Neuroscience. 2006;18:1331–1342. [DOI] [PubMed] [Google Scholar]
- 35.Juttukonda MR, Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. NeuroImage. 2019;187:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


