Abstract
Macrolide resistance has been demonstrated in group B streptococcus (GBS), but there is limited information regarding mechanisms of resistance and their prevalence. We determined these in GBS obtained from neonatal blood cultures and vaginal swabs from pregnant women. Of 178 isolates from cases of neonatal GBS sepsis collected from 1995 to 1998, 8 and 4.5% were resistant to erythromycin and clindamycin, respectively, and one isolate showed intermediate penicillin resistance (MIC, 0.25 μg/ml). Of 101 consecutive vaginal or rectal/vaginal isolates collected in 1999, 18 and 8% were resistant to erythromycin and clindamycin, respectively. Tetracycline resistance was high (>80%) among both groups of isolates. Of 32 erythromycin-resistant isolates, 28 possessed the erm methylase gene (7 ermB and 21 ermTR/ermA) and 4 harbored the mefA gene; one isolate harbored both genes. One isolate which was susceptible to erythromycin but resistant to clindamycin (MIC, 4 μg/ml) was found to have the linB gene, previously identified only in Enterococcus faecium. The mreA gene was found in all the erythromycin-resistant strains as well as in 10 erythromycin-susceptible strains. The rate of erythromycin resistance increased from 5% in 1995–96 to 13% in 1998–99, which coincided with an increase in macrolide usage during that time.
Group B streptococcus (GBS), although a normal commensal of the gastrointestinal and genitourinary tracts, is capable of causing invasive infections in neonates, pregnant women, and persons with underlying medical conditions (26). Between 15 and 35% of pregnant women are asymptomatic carriers of GBS, and in the early 1990s 0.2 to 0.8% of neonates had GBS bacteremia (26). With the advent of consensus guidelines for intrapartum antibiotic prophylaxis in the United States, early-onset disease decreased by 65% from 1993 to 1998 (25). Further declines are predicted if compliance with prophylaxis can be improved (6). However, the increased use of antimicrobials for prophylaxis has raised concerns regarding the emergence of resistance. In addition, increased resistance may have an impact on the efficacy of prophylaxis itself.
Penicillin is the drug of choice for prophylaxis and treatment of GBS disease, and so far, resistance to this agent has not been reported (1, 2, 10, 16, 20, 30, 31). However, macrolides are the recommended second-line agents and the first alternative in mothers with a penicillin allergy. Allergy to penicillin has been reported in 12% of pregnant women in the United States (22). Several studies have reported increasing resistance of GBS to macrolides. The SENTRY surveillance study found that 25 and 14% of neonatal bloodstream isolates in the United States and Canada, respectively, were resistant to erythromycin, with 7% resistant to clindamycin (2). In a U.S. study of vaginal isolates, prevalence of erythromycin resistance rose from 1% from 1980 to 1993 to 18% in 1997–98 (19). Resistance rates have also been found to vary with geographical location. High rates have been noted in California (32 and 12% to erythromycin and clindamycin, respectively), with lower rates in Florida (9 and 2%, respectively) (16). In Canada, a study of invasive isolates collected from several provinces in 1996 revealed that 7% were resistant to erythromycin and 4% were resistant to clindamycin (30).
The most frequently encountered macrolide resistance mechanisms in streptococci are ribosomal modification by a methylase encoded by an erm gene (33) and drug efflux by a membrane-bound protein encoded by a mef gene (17). Presence of the Erm methylase confers resistance to erythromycin and inducible or constitutive resistance to lincosamides and streptogramin B (macrolide-lincosamide-streptogramin B [MLS] phenotype), whereas presence of the Mef pump confers resistance only to 14- and 15-membered macrolides (M phenotype). A second efflux mechanism, encoded by the mreA gene, has been described for GBS (7). However, susceptible GBS strains have also been shown to possess the mreA gene, and it might function as a housekeeping gene (G. Clarebout and R. Leclercq, 39th Intersci. Conf. Antimicrob. Agents Chemother. abstr. 840, p. 115, 1999). Although several previous studies have documented the prevalence of macrolide resistance in GBS (2, 10, 16, 19, 22, 30), few have identified the mechanisms of resistance or have compared the prevalence of resistance in early- and late-onset neonatal sepsis or contemporaneous neonatal sepsis and maternal vaginal isolates. The relative contribution of the presence of the erm and mef genes in contributing to a resistance phenotype may have important implications for therapy, since there are differences in drug susceptibility depending on the mechanism of resistance. In this study we report the prevalence and mechanisms of macrolide-lincosamide resistance in neonatal sepsis GBS isolates collected from 1995 to 1998 and vaginal isolates from pregnant women collected in 1999.
MATERIALS AND METHODS
Strains.
A total of 279 GBS strains were screened for antimicrobial susceptibility. Strains were obtained from cases of neonatal sepsis (n = 179) as well as from pregnant women (n = 101). Neonatal sepsis isolates were collected from 1995 to 1998 through the Toronto Invasive Bacterial Diseases Network, a population-based surveillance system for invasive bacterial diseases, including GBS, in the metropolitan Toronto and Peel region (population, 3.5 million in 1996). All hospitals and the three largest private laboratories serving residents of the population area reported sterile site isolates of GBS to the central study office. Clinical data were obtained from attending physicians and infection control practitioners. Lab audits were conducted semiannually to ensure complete reporting. Vaginal isolates were collected from screening vaginal or combined vaginal and rectal swabs in pregnant women in March 1999 by the largest private laboratory serving an estimated 60% of physician offices in metropolitan Toronto and the surrounding area. All isolates were confirmed to be GBS by standard methodology.
Susceptibility testing.
Strains were tested against penicillin, amoxicillin, cefuroxime, cefotaxime, ceftriaxone, erythromycin, clindamycin, tetracycline, gentamicin, and chloramphenicol by broth microdilution following NCCLS guidelines (21). In order to distinguish between macrolide (M) and MLS or macrolide-lincosamide (ML) phenotypes, clindamycin discs were placed on plates at 12 and 22 mm from a central erythromycin (15-μg) disk and incubated overnight at 37°C in 5% CO2. Blunting of the growth between clindamycin and erythromycin discs and no inhibition around the clindamycin disk indicated inducible MLS resistance (MLS I). Constitutive resistance (MLS C) was defined as growth inhibition zones of ≤15 mm around both the clindamycin and erythromycin disks. Heteroresistance to clindamycin was defined as inhibition zones of ≥15 mm around the clindamycin disk but with resistant colonies growing within this zone. The M phenotype was characterized by resistance to erythromycin and susceptiblity to clindamycin.
Detection of resistance genes.
PCR was used to detect erm and mef genes in the erythromycin- and clindamycin-resistant isolates as described previously (9). Primers for detection of the mreA gene were based on GenBank accession no. U92073 (8) as follows: MREA (bp 291 to 314), 5′-3′ AGA CAC CTC GTC TAA CCT TCG CTC; and MREB (bp 706 to 684), 5′-3′ TCC ATG TAC TAC CAT GCC ACA GG. Cycling was carried out using 5 μl of template (9) in a 25-μl reaction mixture in a 9600 Perkin-Elmer Thermocycler as follows: initial denaturation at 94°C for 4 min followed by denaturation at 93°C for 30 s, annealing at 50°C for 30 s, and elongation at 72°C for 1 min for a total of 30 cycles. A final extension step was carried out at 72°C for 5 min. Primers and PCR conditions for detection of the linB gene were as described previously (5). PCR products were resolved on 1% agarose gels.
Macrolide consumption.
Data on macrolide usage in the province of Ontario were obtained through IMS HEALTH, Montreal, Canada.
RESULTS
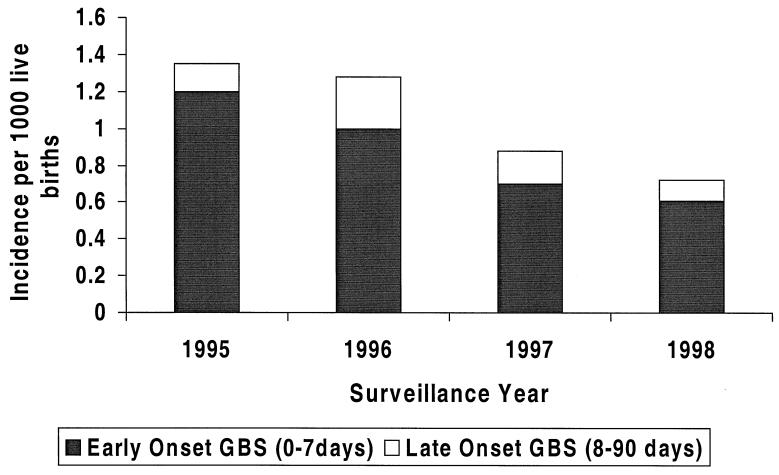
The GBS neonatal sepsis cases identified from 1995 to 1998 comprised 163 cases of early-onset disease (illness during the first week of life) and 33 cases of late-onset disease (illness from days 7 to 90). A statistically significant decrease in incidence of invasive disease (P = 0.001; chi-square test for trend) was noted over the period of the study (Fig. 1). Isolates were available for 178 cases, of which 153 were early-onset disease and 25 were late-onset disease.
FIG. 1.
Prevalence of early-onset and late-onset GBS disease among neonates from 1995 to 1998.
Susceptibility testing revealed that none of the isolates were resistant to β-lactam drugs or vancomycin, although one neonatal sepsis isolate showed intermediate susceptibility to penicillin (MIC, 0.25 μg/ml). This isolate, although susceptible to ceftriaxone, was less so than other strains (MIC, 0.5 μg/ml for this isolate versus ≤0.125 μg/ml for other strains). The majority of neonatal sepsis strains (87%) showed resistance to tetracycline, and a few (2.8%) were resistant to chloramphenicol. Erythromycin resistance was present in 8% of strains, with 4.5% of these also resistant to clindamycin. A single isolate was susceptible to erythromycin but resistant to clindamycin (MIC, 4 μg/ml). Although the incidence of invasive disease decreased over the period of the study, the rate of erythromycin resistance increased from 5% in 1995–96 to 13% in 1998–99 (P = 0.06; chi-square test). Macrolide usage in Ontario increased from 11.8 to 14.3 prescriptions/100 persons between 1992 and 1997. There was no difference in clinical severity or outcome in cases associated with macrolide resistance, and these cases were not clustered geographically within the surveillance area.
Of the 101 consecutive vaginal and rectal and vaginal isolates collected in 1999, 18% were resistant to erythromycin and 8% were constitutively or inducibly resistant to clindamycin. Isolates were susceptible to penicillin and chloramphenicol, but as noted with the neonatal sepsis isolates, resistance to tetracycline was high (82% resistant). In total, 11.5% of erythromycin-resistant strains were identified among neonatal sepsis and vaginal isolates combined.
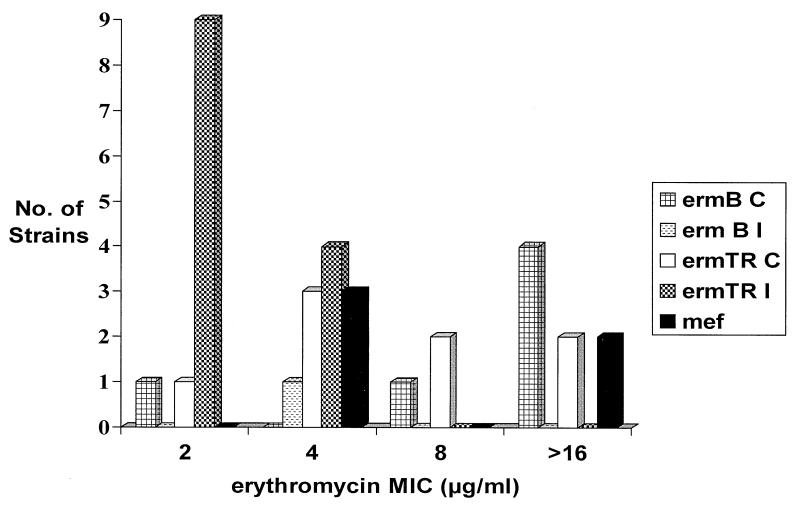
The association of resistance mechanisms with erythromycin and clindamycin MICs is shown in Table 1. For only one M phenotype strain (mef+) was the erythromycin MIC high (≥16 μg/ml); for the other three, the MIC was 4 μg/ml. The majority of erythromycin-resistant strains with constitutive or inducible clindamycin resistance possessed the ermTR gene, an allele of ermA (24) (Table 1 and Fig. 2). All of the ermB strains were constitutively resistant, with one strain showing heteroresistance to clindamycin. By comparison, only 38% of strains with ermTR/ermA showed constitutive resistance and one of these also possessed a mef gene (erythromycin and clindamycin MICs were each ≥16 μg/ml). Clindamycin had a MIC of ≥16 mg/liter for all the ermTR/ermA constitutively resistant strains but only for three of seven ermB strains. Inducibly resistant strains appeared to have a susceptible clindamycin MIC by broth microdilution and single-disk diffusion.
TABLE 1.
Association of phenotype, genotype, and MICs of erythromycin and clindamycin
| Phenotype | Genotype | No. of strains | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|---|---|
| Erythromycin
|
Clindamycin
|
|||||||
| 50% | 90% | Range | 50% | 90% | Range | |||
| MLS Cb | ermB | 7 | ≥16 | ≥16 | 2–≥16 | 4 | ≥16 | 2–≥16 |
| ermTR | 8 | 4 | ≥16 | 2–≥16 | ≥16 | ≥16 | ≥16 | |
| MLS I | ermB | 0 | ||||||
| ermTR | 13 | 2 | 4 | 2–4 | 0.25 | 0.25 | 0.25 | |
| M | mef | 4 | 4 | 16 | 4–≥16 | 0.25 | 0.25 | 0.25 |
| L | linB | 1 | NAc | NA | 4 | 0.12 | 0.12 | 0.12 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
One ermB strain showed heteroresistance; one MLS phenotype strain harbored both an ermTR/ermA and a mef gene.
NA, not applicable.
FIG. 2.
Distribution of erm and mef genes according to erythromycin MIC.
A single isolate which was susceptible to erythromycin but resistant to clindamycin was found to have the linB gene. The mreA gene was present in all the erythromycin-resistant strains as well as in 10 randomly selected erythromycin-susceptible strains from patients with neonatal sepsis.
DISCUSSION
In this study, a marked increase in prevalence of erythromycin resistance in invasive isolates occurred between 1995 and 1997 and this increase was reflected in the vaginal and rectal isolates collected in 1999, of which 18% were erythromycin resistant. There was a temporal increase in macrolide usage in the province of Ontario between 1994 and subsequent years, which could account for the increase in resistance.
High rates of tetracycline resistance were noted, as has been described in other studies (3, 4, 11, 31), and this is likely due to the presence of the tetM gene (27). tetM can be readily transferred via conjugative transposons (29), which may account for its spread under selective pressure. However, the reason for its high prevalence in GBS is unclear since tetracycline is not used prophylactically or therapeutically in pregnant women or in the pediatric population.
Most studies on antimicrobial susceptibility of GBS strains have found uniform susceptibility to penicillin (1, 2, 10, 16, 19, 22, 30, 31), although increased resistance to ampicillin, in particular as a result of ante- and intrapartum treatment with this agent, has been described (18). However, Vermillion et al. (32), in a study of 574 GBS isolates collected from an obstetric population in the United States in 1998, reported 1.8% of strains with nonsusceptibility to penicillin; MIC levels were not reported. In our study one neonatal sepsis strain showed intermediate penicillin resistance (MIC, 0.25 μg/ml). Emergence of resistance to penicillin should be monitored since this is the first-line agent for prophylaxis.
Erythromycin resistance was found to be predominantly due to the presence of an Erm methylase and the ermTR/ermA gene was most prevalent (75% of MLS strains). An ermTR/ermA gene was identified in a strain collected as far back as 1995. The ermTR/ermA gene has been identified in group A streptococcus (GAS) strains from different geographical locations (9, 11, 13, 14), in Group C and G streptococci (15, 28), and in Peptostreptococcus strains (23). In these studies, the ermTR/ermA strains rarely, if ever, possessed a constitutive phenotype. Our previous study of GAS strains identified only 1 of 19 ermTR/ermA strains with constitutive resistance. The number of constitutively resistant ermTR/ermA strains identified in this study suggests that this phenotype is more highly associated with GBS than with GAS. In addition, a high clindamycin MIC (≥16 mg/liter) was associated with all of the constitutively resistant ermTR/ermA strains but with only 42% of ermB strains with this phenotype. Interestingly, inducibly resistant ermTR/ermA strains showed a susceptible clindamycin MIC by broth microdilution as well as by single disk diffusion and based on these tests alone, they would be phenotypically indistinguishable from mef-positive strains. In a routine clinical diagnostic laboratory, it would be important to carry out a double-disk diffusion test as described here to verify clindamycin resistance.
Bozdogan et al. in 1999 described a novel lincosamide-inactivating nucleotidyltransferase encoded by the linB gene in Enterococcus faecium (5). Our studies demonstrate its presence in GBS. It will be interesting to see whether, or more likely how soon, this gene spreads to other GBS strains or indeed to other streptococcal species.
The low prevalence of the mef gene (16% of erythromycin-resistant strains) is in contrast to erythromycin-resistant GAS strains isolated in Ontario, where 70% of strains possessed this gene (9). Among pneumococcal strains collected from across Canada, 55% were mef positive (12). The mef gene has been shown to be mobile in a variety of gram-positive bacteria (17), and its low incidence in GBS is surprising, although the potential for spread is presumably high. A second efflux mechanism, encoded by the mreA gene, has been reported to be associated with macrolide resistance in GBS (8). However, all strains tested (both resistant and susceptible) possessed this gene, which adds weight to its questionable role as a cause of macrolide resistance (Clarebout and Leclercq, 39th ICAAC).
Our study shows that although the incidence of invasive neonatal GBS disease is decreasing in association with increasing use of intrapartum antimicrobial prophylaxis, macrolide resistance in GBS strains appears to be increasing. The clinical relevance of macrolide-resistant GBS in women treated with macrolides for intrapartum prophylaxis needs to be assessed.
ACKNOWLEDGMENT
This work was supported by a grant from the Canadian Bacterial Diseases Network.
REFERENCES
- 1.Adhikari M, Coovadia Y M, Singh D. A 4-year study of neonatal meningitis: clinical and microbiological findings. J Trop Pediatr. 1995;41:81–85. doi: 10.1093/tropej/41.2.81. [DOI] [PubMed] [Google Scholar]
- 2.Andrews J I, Diekema D J, Hunter S K, Rhomberg P R, Pfaller M A, Jones R N, Doern G V. Group B streptococci causing neonatal bloodstream infection: antimicrobial susceptibility and serotyping results from SENTRY centers in the Western hemisphere. Am J Obstet Gynecol. 2000;183:859–862. doi: 10.1067/mob.2000.108839. [DOI] [PubMed] [Google Scholar]
- 3.Anthony B F, Concepcion N F. Group B streptococcus in a general hospital. J Infect Dis. 1975;132:561–567. doi: 10.1093/infdis/132.5.561. [DOI] [PubMed] [Google Scholar]
- 4.Baker C J, Webb B J, Barrett F F. Antimicrobial susceptibility of group B streptococci isolated from a variety of clinical sources. Antimicrob Agents Chemother. 1976;10:128–131. doi: 10.1128/aac.10.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozdogan B, Berrezouga L, Kuo M S, Yurek D A, Farley K A, Stockman B J, Leclercq R. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob Agents Chemother. 1999;43:925–929. doi: 10.1128/aac.43.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Early-onset group B streptococcal disease–United States, 1998–1999. Morb Mortal Wkly Rep. 2000;49:793–796. [Google Scholar]
- 7.Clancy J, Dib-Hajj F, Petitpas J W, Yuan W. Cloning and characterization of a novel macrolide efflux gene, mreA, from Streptococcus agalactiae. Antimicrob Agents Chemother. 1997;41:2719–2723. doi: 10.1128/aac.41.12.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 9.De Azavedo J C, Yeung R H, Bast D J, Duncan C L, Borgia S B, Low D E. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob Agents Chemother. 1999;43:2144–2147. doi: 10.1128/aac.43.9.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez M, Hickman M E, Baker C J. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrob Agents Chemother. 1998;42:1517–1519. doi: 10.1128/aac.42.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovanetti E, Montanari M P, Mingoia M, Varaldo P E. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains in Italy and heterogeneity of inducibly resistant strains. Antimicrob Agents Chemother. 1999;43:1935–1940. doi: 10.1128/aac.43.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston N J, De Azavedo J C, Kellner J D, Low D E. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2425–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataja J, Huovinen P, Seppala H. Erythromycin resistance genes in group A streptococci of different geographical origins. J Antimicrob Chemother. 2000;46:789–792. doi: 10.1093/jac/46.5.789. [DOI] [PubMed] [Google Scholar]
- 14.Kataja J, Huovinen P, Skurnik M, Seppala H the Finnish Study Group for Antimicrobial Resistance. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataja J, Seppala H, Skurnik M, Sarkkinen H, Huovinen P. Different erythromycin resistance mechanisms in group C and group G streptococci. Antimicrob Agents Chemother. 1998;42:1493–1494. doi: 10.1128/aac.42.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F Y, Azimi P H, Weisman L E, Philips III J B, Regan J, Clark P, Rhoads G G, Clemens J, Troendle J, Pratt E, Brenner R A, Gill V. Antibiotic susceptibility profiles for group B streptococci isolated from neonates, 1995–1998. Clin Infect Dis. 2000;31:76–79. doi: 10.1086/313936. [DOI] [PubMed] [Google Scholar]
- 17.Luna V A, Coates P, Eady E A, Cove J H, Nguyen T T, Roberts M C. A variety of gram-positive bacteria carry mobile mef genes. J Antimicrob Chemother. 1999;44:19–25. doi: 10.1093/jac/44.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Mercer B M, Carr T L, Beazley D D, Crouse D T, Sibai B M. Antibiotic use in pregnancy and drug-resistant infant sepsis. Am J Obstet Gynecol. 1999;181:816–821. doi: 10.1016/s0002-9378(99)70307-8. [DOI] [PubMed] [Google Scholar]
- 19.Morales W J, Dickey S S, Bornick P, Lim D V. Change in antibiotic resistance of group B streptococcus: impact on intrapartum management. Am J Obstet Gynecol. 1999;181:310–314. doi: 10.1016/s0002-9378(99)70553-3. [DOI] [PubMed] [Google Scholar]
- 20.Munoz P, Llancaqueo A, Rodriguez-Creixems M, Pelaez T, Martin L, Bouza E. Group B streptococcus bacteremia in nonpregnant adults. Arch Intern Med. 1997;157:213–216. doi: 10.1001/archinte.1997.00440230087011. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7–A5. NCCLS document M100–S10/M7. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 22.Pearlman M D, Pierson C L, Faix R G. Frequent resistance of clinical group B streptococci isolates to clindamycin and erythromycin. Obstet Gynecol. 1998;92:258–261. doi: 10.1016/s0029-7844(98)00155-0. [DOI] [PubMed] [Google Scholar]
- 23.Reig M, Galan J C, Baquero F, Perez-Diaz J C. Macrolide resistance in Peptostreptococcus spp. mediated by ermTR: possible source of macrolide-lincosamide-streptogramin B resistance in Streptococcus pyogenes. Antimicrob Agents Chemother. 2001;45:630–632. doi: 10.1128/AAC.45.2.630-632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag S J, Zywicki S, Farley M M, Reingold A L, Harrison L H, Lefkowitz L B, Hadler J L, Danila R, Cieslak P R, Schuchat A. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 26.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz S, Wibawan I W, Lammler C. Distribution of genes conferring combined resistance to tetracycline and minocycline among group B streptococcal isolates from humans and various animals. Zentbl Bakteriol. 1994;281:526–533. doi: 10.1016/s0934-8840(11)80341-5. [DOI] [PubMed] [Google Scholar]
- 28.Seral C, Gonzalez V, Castillo J, Garcia C, Rubio M C, Gomez-Lus R. Study of macrolide-resistant genes in group C and G streptococci. Rev Esp Quimioter. 2000;13:171–175. [PubMed] [Google Scholar]
- 29.Speer B S, Shoemaker N B, Salyers A A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992;5:387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyrrell G J, Senzilet L D, Spika J S, Kertesz D A, Alagaratnam M, Lovgren M, Talbot J A. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study–1996. Sentinel Health Unit Surveillance System Site Coordinators. J Infect Dis. 2000;182:168–173. doi: 10.1086/315699. [DOI] [PubMed] [Google Scholar]
- 31.Uh Y, Jang I H, Yoon K J, Lee C H, Kwon J Y, Kim M C. Colonization rates and serotypes of group B streptococci isolated from pregnant women in a Korean tertiary hospital. Eur J Clin Microbiol Infect Dis. 1997;16:753–756. doi: 10.1007/BF01709259. [DOI] [PubMed] [Google Scholar]
- 32.Vermillion S T, Bland M L, Soper D E, Austin M. Antibiotic resistance patterns of group B streptococcus in late-third-trimester rectovaginal cultures. Obstet Gynecol. 2000;95:S79–S80. doi: 10.1067/mob.2001.115478. [DOI] [PubMed] [Google Scholar]
- 33.Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression–a review. J Antimicrob Chemother. 1985;16(Suppl. A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]