Abstract
Background
Single-drug albumin-bound paclitaxel is one of the standard second-line treatments for advanced gastric cancer. Some clinical studies suggest that albumin-bound paclitaxel combined with S-1 can be used in the first-line treatment of gastric cancer. Both the two regimens have been commonly used in the past few years. Which is more effective? What’s the safety?
Methods
From 2016 to 2021, a total of 70 untreated patients with advanced gastric cancer were included in our study. They all received at least two cycles of chemotherapy. Among them, 37 cases received standard S-1 and oxaliplatin (SOX) regimen, and 33 cases received albumin-bound paclitaxel combined with S-1 (aTS) regimen. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and adverse events (AEs) were analyzed. The OS and PFS curves were estimated using the Kaplan-Meier method.
Results
The PFS of the aTS group was higher than that of the SOX group (9.27 vs. 7.03 months; P=0.046), but there was no significant difference in the OS between the two groups (19.2 vs. 12.5 months; P=0.131). The ORR of the aTS group was higher than that of the SOX group, and the side effects were tolerable.
Conclusions
Both regimens can be applied to advanced gastric cancer patients. Albumin-bound paclitaxel showed a higher ORR and could effectively prolong PFS.
Keywords: Albumin-bound paclitaxel, S-1, advanced gastric cancer, first-line therapy
Introduction
In 2020, the incidence of gastric cancer ranked sixth in the world, and it was the third leading cause of cancer-related death globally (1). In the past decade, compared with other malignancies, the progress in the treatment of gastric cancer has been relatively slow. Despite advances in the field of molecular targeted drug therapies, so far, the chemotherapy of gastric cancer has not made a breakthrough and the survival time of such patients has not been markedly improved. While the advent of immunotherapy paved the way for some remarkable progress, at present, chemotherapy is still the cornerstone in the treatment of gastric cancer, and mainly consists of dual-drug therapy, such as oxaliplatin combined with the S-1 regimen. However, chemotherapy, as one of the main treatments, has not made any significant progress in recent years.
The guidelines from both the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) recommend oxaliplatin combined with fluorouracil as first-line therapy and albumin-bound paclitaxel as second-line therapy in advanced gastric cancer. Efficacy of albumin-bound paclitaxel combined with S-1 in first-line treatment has recently been confirmed (2). In that study, The scheme achieves a high response rate. However, to date, there have been no studies comparing the efficacy and safety of albumin-bound paclitaxel combined with S-1 (aTS) regimen and the standard S-1 and oxaliplatin (SOX) regimen.
In recent years, our center has achieved good results when using aTS as the first-line treatment in patients with advanced gastric. This retrospective study was performed to compare the aTS regimen with standard treatment over the same period. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-279/rc).
Methods
Patients
Patients diagnosed with advanced gastric cancer and who were treated with the SOX regimen or the aTS regimen in our hospital between 2016 and 2021 were retrospectively enrolled in this study. The following inclusion criteria were applied: (I) patients >18 years old; (II) the Eastern Cooperative Oncology Group (ECOG) score was ≤2; (III) patients had pathologically confirmed locally advanced unresectable or metastatic gastric cancer; (IV) the tumors were human epidermal growth factor receptor 2 (HER-2) negative; (V) patients did not receive any previous radiotherapy or chemotherapy (prior adjuvant/neoadjuvant therapy was allowed if at least 6 months had elapsed between completion of adjuvant/neoadjuvant therapy); (VI) the expected survival time was >3 months; (VII) the lesions were measurable; (VIII) the functions of the liver, kidney, and bone marrow hematopoiesis were good, as indicated by absolute neutrophil count ≥1,000/mm3, platelet count ≥7.5×104/mm3, total bilirubin ≤1.5 mg/dL, aspartate aminotransferase ≤100 IU/L, and alanine aminotransferase ≤100 IU/L (for patients with liver metastasis, total bilirubin ≤2.0 mg/dL, aspartate aminotransferase ≤200 IU/L, and alanine aminotransferase ≤200 IU/L); and (IX) patients received at least 2 cycles of aTS or SOX treatment in our institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Changzhou Cancer Hospital (No. 2017-SY-012). Written informed consent was obtained from the patients for publication of this study.
Treatment
Patients in the SOX group were administered oxaliplatin 130 mg/m2 and S-1 40 mg/m2 (b.i.d d1–14, q3w). Patients in the aTS group were given albumin-bound paclitaxel 120 mg/m2 (d1, 8) and S-1 40 mg/m2 (b.i.d d1–14, q3w). Up to 6 cycles of the treatments were administered, and if the disease remained stable after 6 cycles, oral maintenance therapy was continued with S-1. If there was disease progression or intolerable side effects, or the patients withdrew for personal reasons, the treatments were terminated.
Patients were not randomly assigned SOX or aTS treatment. A decision on which treatment regimen would be administered was made via consultation between the doctor and patient, and the following factors were considered: the patient’s physical condition, economic status, treatment purpose, and the requirements for efficacy and side effects. This was deemed a better reflection of the medical situation in the real world.
Assessments
The study was conducted to compare the efficacy and safety of the aTS regimen and the SOX regimen, including objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). The ORR was evaluated with enhanced computed tomography (CT) every 2 cycles, including chest and abdomen CT, according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (3).
Safety was assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
The t-tests and chi-square tests were used to compare the baseline parameters of the patients (a two-sided 5% was considered statistically significant). The OS and PFS curves were estimated using the Kaplan-Meier method, using an unstratified log-rank test with a two-sided 5% significance level. The hazard ratio (HR) was estimated using the Cox proportional hazards model. Univariate and multivariable analyses were also performed using the Cox proportional hazards model. Univariate analysis was performed to explore prognostic factors. Statistical analyses were conducted using the SPSS 26.0 software (IBM Corp., Armonk, NY, USA).
Results
Basic patient characteristics
A total of 70 patients were enrolled in the study, including 37 patients in the SOX group and 33 patients in the aTS group. There were no significant differences in the baseline characteristics of the patients in the two groups (Table 1). The longest follow-up period was 2 years.
Table 1. Basic patient characteristics.
| Characteristics | aTS (n=33) | SOX (n=37) | P value |
|---|---|---|---|
| Age (years) | 0.280 | ||
| Median [range] | 65.45 [46–78] | 62.97 [36–75] | |
| Sex, n (%) | 0.820 | ||
| Male | 24 (72.7) | 26 (70.3) | |
| Female | 9 (27.3) | 11 (29.7) | |
| Disease site, n (%) | 0.469 | ||
| Cardia | 18 (54.5) | 19 (51.4) | |
| Gastric fundus | 1 (3.0) | 0 (0.0) | |
| Gastric body | 5 (15.2) | 10 (27.0) | |
| Gastric antrum | 9 (27.3) | 8 (21.6) | |
| Histology, n (%) | 0.906 | ||
| Well differentiated | 0 (0.0) | 0 (0.0) | |
| Moderately differentiated | 2 (6.1) | 2 (5.4) | |
| Poorly differentiated | 31 (93.9) | 35 (94.6) | |
| Site of metastases, n (%) | |||
| Liver | 12 (36.4) | 19 (51.4) | 0.208 |
| Lung | 2 (6.1) | 3 (8.1) | 0.740 |
| Retroperitoneal lymph nodes | 19 (57.6) | 15 (40.5) | 0.155 |
| Others | 13 (39.4) | 20 (54.1) | 0.220 |
| Cycles of first-line chemotherapy | 0.186 | ||
| Mean ± standard deviation | 4.81±1.42 | 4. 49±1.75 | |
| Cycles of posterior line chemotherapy | 0.544 | ||
| Mean ± standard deviation | 2.1±2.19 | 2.04±3.15 | |
aTS, albumin-bound paclitaxel combined with S-1; SOX, standard S-1 and oxaliplatin.
Efficacy and safety
Of the 37 patients in the SOX group, 1 (2.7%) had complete response (CR), 10 (27.0%) showed partial response (PR), 22 (59.5%) had stable disease (SD), and 4 (10.8%) progressed. Of the 33 patients in the aTS group, none (0.0%) experienced CR, 18 (54.5%) showed PR, 15 (45.5%) had SD, and none (0.0%) progressed. There was a significant difference between these two groups (P=0.038) (Table 2).
Table 2. Efficacy of aTS and SOX treatment.
| Efficacy | aTS (n=33) | SOX (n=37) | P value |
|---|---|---|---|
| CR, n (%) | 0 (0.0) | 1 (2.7) | 0.038 |
| PR, n (%) | 18 (54.5) | 10 (27.0) | – |
| SD, n (%) | 15 (45.5) | 22 (59.5) | – |
| PD, n (%) | 0 (0.0) | 4 (10.8) | – |
| ORR (%) | 54.5 | 29.7 | – |
aTS, albumin-bound paclitaxel combined with S-1; SOX, standard S-1 and oxaliplatin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate.
The median PFS was 7.03 months in the SOX group [95% confidence interval (CI): 4.77 to 9.29 months] and 9.27 months in the aTS group (95% CI: 5.61 to 12.91 months) (P=0.046; Figure 1). The median OS was 12.5 months in the SOX group (95% CI: 6.83 to 18.17 months) and 19.2 months in the aTS group (95% CI: 9.48 to 28.92 months) (P=0.131; Figure 2). Univariate analysis showed that PFS was related to chemotherapy regimen and whether there were more than 2 cycles of chemotherapy. Multivariate analysis of variance suggested that PFS was related to chemotherapy regimen (aTS vs. SOX: HR =0.605; P=0.047; 95% CI: 0.369 to 0.992), and whether there were more than 2 cycles of chemotherapy (HR =0.228; P=0.000; 95% CI: 0.116 to 0.452) (Table 3).
Figure 1.
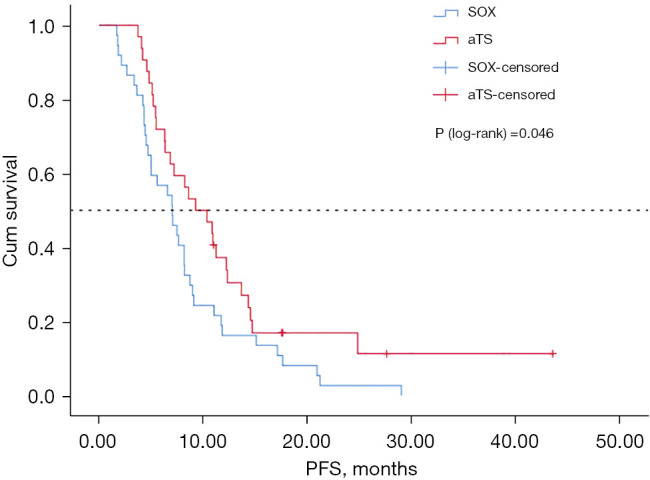
Kaplan-Meier PFS for aTS vs. SOX. SOX, standard S-1 and oxaliplatin; aTS, albumin-bound paclitaxel combined with S-1; PFS, progression-free survival.
Figure 2.
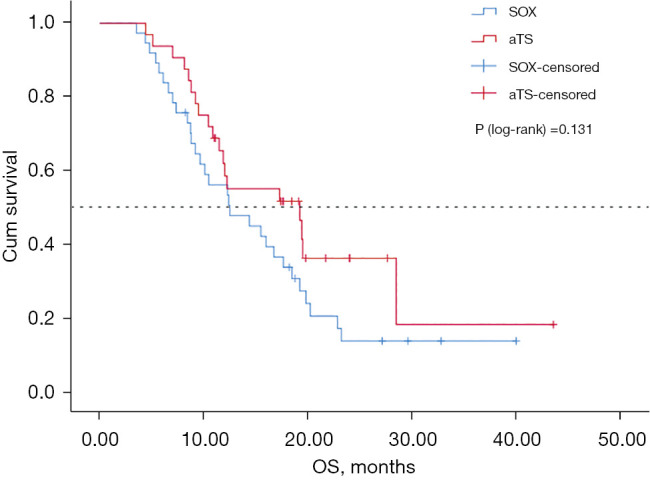
Kaplan-Meier OS for aTS vs. SOX. SOX, standard S-1 and oxaliplatin; aTS, albumin-bound paclitaxel combined with S-1; OS, overall survival.
Table 3. Side effects associated with aTS and SOX treatment.
| Side effects | aTS (n=33) | SOX (n=37) | |||
|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | ||
| Diarrhea, n (%) | 2 (6.1) | 0 (0.0) | 2 (5.4) | 0 (0.0) | |
| Nausea/vomiting, n (%) | 15 (45.5) | 2 (6.1) | 17 (45.9) | 2 (5.4) | |
| Mucositis, n (%) | 1 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hand & foot syndrome, n (%) | 3 (9.1) | 0 (0.0) | 3 (8.1) | 1 (2.7) | |
| Asthenia, n (%) | 4 (12.1) | 0 (0.0) | 5 (13.5) | 0 (0.0) | |
| Live toxicity, n (%) | 1 (3.0) | 0 (0.0) | 1 (2.7) | 0 (0.0) | |
| Renal toxicity, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Peripheral sensory neuropathy, n (%) | 17 (45.5) | 1 (3.0) | 27 (73.0) | 1 (2.7) | |
| Neutropenia, n (%) | 19 (57.6) | 3 (9.1) | 19 (51.3) | 1 (2.7) | |
| Thrombocytopenia, n (%) | 4 (12.1) | 1 (3.0) | 10 (27.0) | 3 (8.1) | |
| Febrile neutropenia, n (%) | 1 (3.0) | 2 (6.1) | 1 (2.7) | 2 (5.4) | |
| Anemia, n (%) | 4 (12.1) | 0 (0.0) | 6 (16.2) | 0 (0.0) | |
| Alopecia, n (%) | 22 (66.7) | 4 (12.1) | 1 (2.7) | 0 (0.0) | |
aTS, albumin-bound paclitaxel and S-1; SOX, standard S-1 and oxaliplatin.
The adverse events (AEs) of all participants are summarized in Table 4. The two schemes were well-tolerated, with most side effects classified as grade 1–2 (Table 4). Neutropenia and gastrointestinal reactions were common. The incidence of peripheral neurotoxicity was high in both groups. The incidences of neutropenia and alopecia were higher in the aTS group compared to the SOX group. The incidence of thrombocytopenia in the SOX group was higher than in the aTS group, and this may be related to oxaliplatin. No obvious cardiotoxicity was observed in either group.
Table 4. Cox regression analysis (PFS).
| Variables | Univariate Cox regression model | Multivariate Cox regression model | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Sex (male vs. female) | 0.923 | 0.531–1.606 | 0.778 | ||||
| Age (years, <65 vs. ≥65) | 1.106 | 0.679–1.803 | 0.685 | ||||
| Disease site | |||||||
| Cardia | 1.0 | ||||||
| Gastric fundus | – | – | – | ||||
| Gastric body | 1.411 | 0.762–2.613 | 0.274 | ||||
| Gastric antrum | 1.068 | 0.584–1.953 | 0.831 | ||||
| Histology | |||||||
| Differentiation (1= moderately; 2= poorly) | 1.274 | 0.461–3.520 | 0.641 | ||||
| Site of metastases | |||||||
| Liver | 0.974 | 0.592–1.603 | 0.918 | ||||
| Lung | 0.975 | 0.389–2.422 | 0.957 | ||||
| Retroperitoneal lymph node metastasis | 1.163 | 0.710–1.907 | 0.549 | ||||
| Others | 1.575 | 0.962–2.580 | 0.071 | ||||
| Therapy (aTS vs. SOX) | 0.610 | 0.372–0.999 | 0.050 | 0.605 | 0.369–0.992 | 0.047 | |
| Cycles of first line chemotherapy (>2 vs. 2) | 0.230 | 0.117–0.455 | 0.000 | 0.228 | 0.116–0.452 | 0.000 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; aTS, albumin-bound paclitaxel and S-1; SOX, standard S-1 and oxaliplatin.
Discussion
For advanced gastric cancer, fluorouracil combined with platinum is the most commonly used first-line treatment (4,5). The choice of platinum can be cisplatin or oxaliplatin, while fluorouracil can be either S-1 or capecitabine. Since S-1 is oral fluorouracil, it is simple and convenient to administer and is thus widely used in Asian countries, including China (6,7). The combination of docetaxel, fluorouracil, and cisplatin is the standard first-line and three-drug regimen (8), especially in Europe and the United States, and is superior to fluorouracil plus cisplatin in terms of efficiency. However, there is no statistical difference in OS compared to the conventional two-drug regimens. On the contrary, the higher incidence of toxic reactions limits its routine use. A meta-analysis found that there was no statistical difference in ORR or OS when platinum was used as a first-line treatment compared with the new generation of non-platinum drugs (paclitaxel and irinotecan). Furthermore, platinum drugs are significantly more toxic in terms of hematotoxicity, nausea, vomiting, and neurotoxicity. In the mouse model, paclitaxel and S-1 were found to have a synergistic effect (9). Several phase II or III clinical trials reported that paclitaxel or docetaxel combined with S-1 was effective and well-tolerated (10,11).
In addition, effective platinum-free first-line chemotherapy is needed, especially when patients are unable to accept platinum drugs due to renal dysfunction or other side effects. Paclitaxel is a better choice for platinum-tolerant patients. It is therefore imperative to explore paclitaxel non-platinum chemotherapy with equivalent low toxicity as a first-line treatment.
Compared with ordinary paclitaxel, albumin-bound paclitaxel has a higher concentration in tumor tissues. Due to the change of solvent, the incidence of allergic reactions is also significantly reduced. Prophylactic antiallergic therapy is no longer needed before infusion. Presently, it is widely used in malignancies such as breast cancer, pancreatic cancer, and non-small cell lung cancer (12-14). However, in some cancer types, a study has confirmed that albumin-bound paclitaxel does not improve OS compared to common paclitaxel drugs (15). In terms of gastric cancer, current guidelines (NCCN guidelines and CSCO guidelines) recommend that albumin-bound paclitaxel can be used alone for second-line treatment of advanced gastric cancer.
A phase II trial in Japan (16) demonstrated that albumin-bound paclitaxel (NAB-PTX; 260 mg/m2, d1, q3w) as second-line chemotherapy in advanced gastric cancer had good activity and tolerance. A phase III clinical trial (17) showed that NAB-PTX (100 mg/m2, d1, 8, and 15) once every 3–4 weeks, was as safe as solvent-based paclitaxel in terms of OS, and had fewer allergic reactions.
Recently, in the phase II clinical trial organized by Professor Xu Ruihua of Sun Yat-sen University, the efficacy and safety of albumin-bound paclitaxel combined with S-1 in the treatment of advanced gastric cancer were evaluated (2). Previously untreated patients with metastatic gastric adenocarcinoma were administered 40 mg [body surface area (BSA) <1.25 m2], 50 mg (1.25≤ BSA <1.50 m2), or 60 mg (BSA ≥1.50 m2) twice a day on days 1–14, combined with albumin-bound paclitaxel (120 mg/m2, d1 and 8) every 21 days. A total of 73 patients were enrolled. The median PFS was 9.63 months, and the OS rate was 14.6 months. There were 4 cases of CR and 39 cases of PR, with an ORR of 58.9% and a disease control rate (DCR) of 87.7%. Most of the toxic reactions were mild and there were no treatment-related deaths. Grade 3–4 adverse reactions occurred in 22 cases (30.1%), including leukopenia (13.7%), neutropenia (12.3%), anemia (5.5%), thrombocytopenia (1.4%), diarrhea (6.8%), etc. The study concluded that S-1 combined with albumin-bound paclitaxel is an effective and safe first-line treatment for advanced gastric cancer.
Another phase III multicenter, open-label, randomized, controlled, clinical trial protocol (18) is underway to explore the application of albumin-bound paclitaxel combined with S-1 in adjuvant chemotherapy after D2 radical resection of gastric cancer. The primary endpoint is the 3-year DFS defined as the time from randomization to the time of recurrence of the original gastric cancer, development of a new gastric cancer, or death from any cause. The secondary endpoints are OS (defined as the time from the date of randomization to the date of death from any cause) and safety (any AE).
This current retrospective study analyzed patients who were treated with SOX or aTS. The results demonstrated that the PFS of the aTS group was longer than that of the SOX group (9.27 vs. 7.03 months; P=0.046), and the ORR was significantly increased (54.5% vs. 29.7%). Therefore, the aTS regimen may be more suitable for patients who require a reduction in tumor size over a short period of time, to relieve symptoms or to reach the maximum reducing staging to facilitate surgical options.
There was no significant difference in OS between the two groups (P=0.131), which may be related to the second-line application of albumin-bound paclitaxel or immunotherapy in some patients. However, the number of samples involved was small and the distribution of cases did not strictly follow the principle of randomization. Future large-scale clinical studies are warranted to verify these findings.
In summary, compared with the SOX regimen, the aTS regimen can improve the ORR and prolong the PFS, but the extension of PFS does not translate into increased OS.
Acknowledgments
The authors would like to thank the staff of the Department of Oncology of Changzhou Cancer Hospital.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Changzhou Cancer Hospital (No. 2017-SY-012). Written informed consent was obtained from the patients for publication of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-279/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-279/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-279/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Teoh)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.He MM, Wang F, Jin Y, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci 2018;109:3575-82. 10.1111/cas.13813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer 2013;49:3616-24. 10.1016/j.ejca.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435-42. 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547-53. 10.1200/JCO.2009.25.4706 [DOI] [PubMed] [Google Scholar]
- 7.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. 10.1016/S1470-2045(09)70259-1 [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 9.Nukatsuka M, Fujioka A, Nakagawa F, et al. Antimetastatic and anticancer activity of S-1, a new oral dihydropyrimidine-dehydrogenase-inhibiting fluoropyrimidine, alone and in combination with paclitaxel in an orthotopically implanted human breast cancer model. Int J Oncol 2004;25:1531-6. 10.3892/ijo.25.6.1531 [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, Yamagishi H, Ichikawa D, et al. Multicenter phase II study of weekly paclitaxel plus S-1 combination chemotherapy in patients with advanced gastric cancer. Gastric Cancer 2010;13:149-54. 10.1007/s10120-010-0548-1 [DOI] [PubMed] [Google Scholar]
- 11.Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 2014;140:319-28. 10.1007/s00432-013-1563-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794-803. 10.1200/JCO.2005.04.937 [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. 10.1200/JCO.2011.39.5848 [DOI] [PubMed] [Google Scholar]
- 15.Yoneshima Y, Morita S, Ando M, et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J Thorac Oncol 2021;16:1523-32. 10.1016/j.jtho.2021.03.027 [DOI] [PubMed] [Google Scholar]
- 16.Sasaki Y, Nishina T, Yasui H, et al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 2014;105:812-7. 10.1111/cas.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:277-87. 10.1016/S2468-1253(16)30219-9 [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Wu D, Xu N, et al. Adjuvant albumin-bound paclitaxel combined with S-1 vs. oxaliplatin combined with capecitabine after D2 gastrectomy in patients with stage III gastric adenocarcinoma: a phase III multicenter, open-label, randomized controlled clinical trial protocol. BMC Cancer 2021;21:56. 10.1186/s12885-020-07772-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


