Abstract
Background
More and more evidence has confirmed the efficacy and safety of immunotherapy drugs, such as camrelizumab and pembrolizumab. There are several phase-I/II studies showing that toripalimab has an acceptable safety profile and promising clinical activity in patients with advanced solid tumors. To further confirm its efficacy and safety, the aim of the study was to evaluate toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESCC).
Methods
This study was an investigator-initiated, open-label, non-randomized, single-arm, single-center phase II trial (registration number: ChiCTR2100052784). The patients eligible for inclusion criteria at Fujian Medical University Union Hospital from October 2019 to October 2020 were included in this study. Patients who were suitable for surgery underwent minimally invasive esophagectomy (MIE) within 4–6 weeks after neoadjuvant therapy. Pathological complete response (pCR) and adverse events (AEs) were the primary end points. Secondary endpoints included R0 resection rate, major pathological response (MPR), interval to surgery, and 30-day complications.
Results
A total of 20 patients were enrolled from October 2019 to October 2020. All patients successfully completed 2 cycles of neoadjuvant therapy. Treatment-related AEs were common during neoadjuvant therapy, with leucopenia the most frequently occurring AE (4/20, 25%). With respect to immune-related AEs, immune dermatitis occurred in 2 patients, including 1 patient with grade I and 1 patient with grade III. Based on radiologic evaluation, the objective response rate (ORR) was 70% (14/20). Twelve patients underwent McKeown MIE. The pCR rate of the primary tumor was 16.7% (2/12), and the MPR rate of the primary tumor was 5/12 (41.7%). The mean interval to surgery was 33.2 days, and no patients experienced delayed surgery due to treatment-related AEs. Pneumonia was the most common 30-day postoperative complication (3/12, 25%). Anastomotic leakage (AL) only occurred in 1 patient during the hospital stay. There were no treatment- or surgery-related deaths.
Conclusions
Based on our results, toripalimab combined with docetaxel and cisplatin as a novel neoadjuvant therapy was safe and effective in locally advanced ESCC.
Keywords: Clinical trial, toripalimab, neoadjuvant therapy, minimally invasive surgery, esophageal squamous cell carcinoma (ESCC)
Introduction
With more than 600,000 new cases annually, esophageal carcinoma (EC) was one of the top 10 cancers for cases and deaths worldwide in 2020 (1). There are two main types of esophageal cancer, squamous cell carcinoma (ESCC) and adenocarcinoma (AC). The incidence of ESCC was highest in South-East and Central Asia, where 79% of all ESCC cases occurred (2). As a standard treatment for patients diagnosed with locally advanced ESCC, surgery after preoperative chemotherapy has significantly better overall survival than surgery alone (3). However, 31–39% of patients still relapse within 3–5 years after surgery (4,5).
PD-1/PD-L1 signaling can be blocked by Inhibitors of programmed cell-death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1). Therefore, PD-1/PD-L1 reverses T cell suppression and enhances antitumor immune response (6). In multiple solid tumors including EC, a high frequency of mutations was correlated with clinical benefits from PD-1 or PD-L1 checkpoint inhibitors (7). Immunotherapy has been proven to significantly improve the 5-year survival rate of advanced ESCC (8). More and more evidence has confirmed the efficacy and safety of immunotherapy drugs, such as camrelizumab and pembrolizumab (9,10). Toripalimab is a humanized immunoglobulin G (IgG)4κ monoclonal antibody (mAb) with hinge mutation (S228P) specific for human PD-1 receptor and blocks PD-1/PD-L1 interactions. In 2018, toripalimab was approved in China for second-line treatment of metastatic melanoma. There are several phase-I/II studies showing that toripalimab has an acceptable safety profile and promising clinical activity in patients with advanced solid tumors (11,12). More and more clinical evidence indicates that immunotherapy combined with radiotherapy can improve the efficacy of anti-tumor therapy (13-15). And the role of PD-1 or PD-L1 inhibitors combined with chemoradiotherapy has been evaluated (10). Furthermore, chemotherapy with pembrolizumab or nivolumab shows the better response rates than chemotherapy alone in observational studies of neoadjuvant therapy (16,17).
In this clinical trial, we evaluated the efficacy and safety of surgery after neoadjuvant treatment with toripalimab plus docetaxel and cisplatin in patients diagnosed with locally advanced ESCC. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-131/rc).
Methods
Study design
This was a single-center, single-arm, prospective clinical study of preoperative toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal ESCC (registration number: ChiCTR2100052784). The primary outcome was R0 resection rate. The secondary endpoints included objective response rate (ORR), adverse events (AEs) during neoadjuvant immunochemotherapy, and intraoperative and postoperative conditions. The patients were closely monitored for toxic effects of chemotherapy based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Fujian Medical University (No. 2019YF033-01) and informed consent was taken from all the patients.
Patients
Patients with previously untreated, locally advanced ESCC at Fujian Medical University Union Hospital from October 2019 to October 2020 were included in this study.
Patients were enrolled in the study if the following inclusion criteria were satisfied: (I) aged between 18 and 75; (II) resectable ESCC proven by pathology (including histology or cytology); (III) esophageal cancer stage ≥ cT3 or ≥ N+, confirmed by computed tomography (CT)/magnetic resonance imaging (MRI) or color ultrasound, positron emission tomography (PET)-CT, or ultrasonic gastroscopy before surgery; (IV) newly diagnosed patients without prior surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy, or other treatments; (V) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score 0–3; (VI) no serious heart, lung, or liver dysfunction, and no acute infection; (VII) main organs functioned normally and met the following standards: (I) routine blood examination criteria (no blood transfusion within 14 days): hemoglobin (HB) 100 g/L or higher, white blood cells (WBC) 3.5×109/L, neutrophil count (NC) 1.5×109/L, and platelets (PLT) 100×109/L or higher; (II) biochemical tests met the following standards: bilirubin (BIL) <1.5× upper limit of normal (ULN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) <2.5× ULN, glutamate pyruvate transaminase (GPT) ≤1.5× ULN, serum creatinine (Cr) ≤1 ULN, and endogenous creatinine clearance >60 mL/min (Cockcroft-Gault formula); (VIII) not participating in other clinical studies within 3 months before treatment; and (IX) subjects voluntarily joined the study and signed informed consent with good compliance and follow-up.
The following exclusion criteria were applied: (I) previous surgical history of thoracic malignant tumor; (II) prior systemic antitumor therapy, including radiotherapy, cytotoxic drugs, targeted drugs, monoclonal antibodies, or experimental therapy, and patients who had taken antitumor Chinese herbal medicine within 7 days prior to enrollment; (III) patients with small cell carcinoma, distant metastasis, or tumor invasion of cervical esophagus or upper thoracic segment requiring laryngectomy; (IV) patients with serious heart, liver, kidney neurological, or mental diseases, or with severe infection; (V) pregnant or lactating women; (VI) patients with hypertension who could not be reduced to the normal range by antihypertensive medication (systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg); (VII) patients with grade I or above coronary heart disease, arrhythmia [including prolonged corrected QT (QTc) interval >450 ms in males and >470 ms in females], or cardiac insufficiency; (VIII) patients with high risk of bleeding or perforation due to obvious tumor invasion of adjacent organs (major arteries or trachea) or esophageal lesions, or patients who had developed fistulas; (IX) patients with interstitial lung disease requiring steroid therapy, active pulmonary tuberculosis, a known history of primary immunodeficiency, or an active autoimmune disease requiring symptomatic treatment or a history of the disease within 2 years; (X) a history of severe allergy, anaphylaxis, or other hypersensitivity reactions to chimeric or humanized antibodies or fusion proteins; (XI) patients who had previously received allogeneic stem cell or solid organ transplantation; and (XII) patients who were considered unsuitable for inclusion by researchers.
Neoadjuvant therapy
The chemotherapy regimen included docetaxel (75 mg/m2) and cisplatin (60 mg/m2), which were administrated intravenously on days 1 and 22. Toripalimab was given on days 1 and 22 of the neoadjuvant therapy intravenously at a dose of 240 mg. There were 2 cycles in all, and the interval between each cycle was 3 weeks. After the completion of neoadjuvant therapy, physical examination, routine laboratory tests, contrast-enhanced chest CT, pulmonary function test and echocardiography were performed for disease reassessment and to exclude cases with contraindication to surgery.
Surgery
Patients who met the surgical criteria underwent surgery within 4–6 weeks after completion of neoadjuvant therapy. For tumors located in the upper esophagus or patients with cervical lymph node involvement, McKeown esophagectomy with 3-field or 2-field lymph node dissection was performed. For tumors located in the middle and lower esophagus, Ivor-Lewis esophagectomy with 2-field lymph node dissection was performed. Gastric-tube reconstruction with a cervical anastomosis was performed for restoring the continuity of the digestive tract. Mechanical cervical anastomoses and all thoracic anastomoses were performed with end-to-side stapled techniques, and the thoracic drainage tube was inserted into the posterior mediastinum.
Pathological evaluation
Reports on pathological examination represented the tumor type and extension, lymph nodes, and resection margins. In defect of macroscopical tumor, any tissue appearing abnormal was paraffin-embedded so as to create an adequate assessment for the presence of residual tumor and the effects of therapy. The histomorphologic regression of residual esophageal tumors was divided into 4 categories: grade I [no evidence of vital residual tumor cells, pathologic complete response (pCR)]; grade II (less than 10% vital residual tumor cells); grade III (11–50%); and grade IV (more than 50%) (18,19).
Statistical analysis
The data in this study are descriptive. Data are presented as mean value ± standard deviation for continuous variables and percentages for categorical variables. All statistical analyses were performed using SPSS 21 software.
Results
Clinical characteristics
Between October 2019 and October 2020, 20 ESCC patients agreed to receive neoadjuvant toripalimab combined with docetaxel and cisplatin. The patient selection process is summarized in Figure 1. Most patients were male (17/20, 85%) and 3 were female. The median age was 58.3 years (range, 49–69 years). Three patients had hypertension and 2 had diabetes. The median American Society of Anesthesiologists (ASA) status score was II. All patients completed 2 cycles of preoperative treatment, which comprised a flat dose of toripalimab plus docetaxel and cisplatin on days 1 and 22. The clinical characteristics of the included patients are summarized in Table 1.
Figure 1.
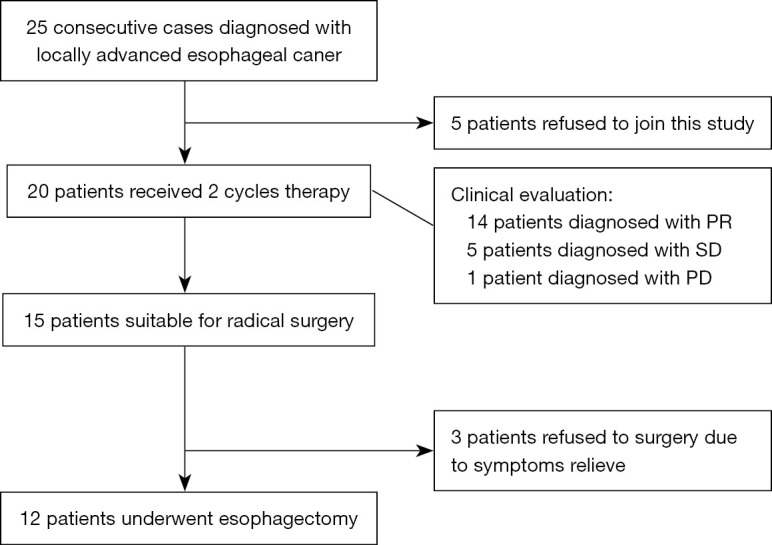
Flowchart of patient selection. PR, partial response; SD, stable disease; PD, progressive disease.
Table 1. Baseline demographic and clinical characteristics.
| Characteristic | Value |
|---|---|
| Mean age [range], years | 58.3 [49–69] |
| Gender, n (%) | |
| Male | 17 (85.0) |
| Female | 3 (15.0) |
| BMI (mean) | 22.20 |
| Range | 18.49–26.30 |
| ≤25, n (%) | 18 (90.0) |
| >25, n (%) | 2 (10.0) |
| Preoperative complications, n (%) | |
| Hypertension | 3 (15.0) |
| Diabetes | 1 (5.0) |
| Coronary heart disease | 0 (0.0) |
| ASA status | 2 [2–2] |
| Smoking history, n (%) | 13 (65.0) |
| Drinking history, n (%) | 4 (20.0) |
| Tumor location, n (%) | |
| Upper | 2 (10.0) |
| Middle | 13 (65.0) |
| Lower | 5 (25.0) |
| Effect after immunotherapy, n (%) | |
| cCR | 0 (0.0) |
| cPR | 14 (70.0) |
| cSD | 5 (25.0) |
| cPD | 1 (5.0) |
| Objective response rate | 14 (70.0) |
BMI, body mass index; ASA status, The American Society of Anesthesiologists' Physical Status; cCR, clinical complete response; cPR, clinical partial response; cSD, clinical stable disease; cPD, clinical progressive disease.
Treatment-related AEs
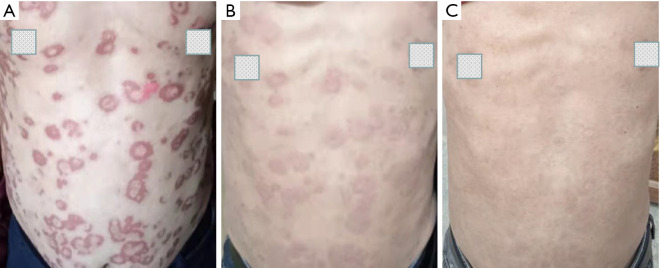
Treatment-related AEs of any grade during neoadjuvant treatment period are shown in Table 2. Treatment-related AEs were common, and most were grade I–II. One patient with grade III leucopenia, 1 patient with grade III anemia, and 1 patient with increased transaminase were observed. With respect to immunotherapy-related AEs, there were 2 patients with immune dermatitis, which included 1 patient diagnosed with grade I and 1 patient diagnosed with grade III (Figure 2). Other toxicities of immunotherapy were not observed, including pneumonia, myocarditis, hepatitis, nephritis, thyroiditis, and hypophysitis.
Table 2. Treatment-related adverse events.
| Adverse events | 1–2 grade, n (%) | ≥≥3 grade, n (%) |
|---|---|---|
| Leucopenia | 3 (15.0) | 1 (5.0) |
| Neutropenia | 3 (15.0) | 0 |
| Lymphopenia | 2 (10.0) | 0 |
| Anemia | 1 (5.0) | 1 (5.0) |
| Increased transaminase | 2 (10.0) | 1 (5.0) |
| Alopecia | 4 (20.0) | 0 |
| Constipation | 2 (10.0) | 0 |
| Diarrhea | 1 (5.0) | 0 |
| Fatigue | 3 (15.0) | 0 |
| Nausea | 4 (20.0) | 0 |
| Vomiting | 3 (15.0) | 0 |
| Immune dermatitis | 1 (5.0) | 1 (5.0) |
| Immune pneumonitis | 0 (0.0) | 0 (0.0) |
Figure 2.
Appearance of immune dermatitis. (A) Immune dermatitis occurred after 2 cycles neoadjuvant therapy; (B) immune dermatitis relieved after 1 week of oral prednisone; (C) immune dermatitis disappeared after 2 weeks of oral prednisone.
All patients successfully received 2 cycles of neoadjuvant immunotherapy combined with chemotherapy. After 2 cycles of neoadjuvant therapy, clinical evaluation and multidisciplinary discussion were conducted to determine further treatment. The ORR was 70% (14/20), and 1 patient achieved clinical partial response (cPR). A total of 15 patients were suitable for radical surgery after 2 cycles of neoadjuvant therapy, and 3 patients refused surgery after symptoms were relieved. The median interval between the completion of preoperative treatment and surgery was 33.2±8.5 days. No treatment-related surgical delays were observed, and surgery was conducted in all patients between 4–6 weeks after neoadjuvant therapy.
Preoperative outcomes and pathological response
Twelve patients underwent minimal invasive esophagectomy (MIE), and no patients required conversion to thoracotomy. Further, all patients achieved standard R0 resection. The mean operation time was 321.75±50.74 minutes, and the mean blood loss was 131.66±64.22 mL. Pneumonia occurred in 3 patients (3/12, 25%), 2 patients experienced cardiac events (2/12, 16.7%), chylothorax occurred in 1 patient (1/12, 8.3%), and 1 patient had anastomotic leakage (AL) (1/12, 8.3%). There was no 30-day mortality or 30-day readmission in this cohort. Preoperative outcomes and postoperative complications are shown in Table 3. Five patients (41.7%) were stage I–II, and 7 patients (50.0%) were stage IIIA–IVA. There were 2 (16.7%) patients who had pCR, and 5 (41.7%) patients achieved at least major pathological response (MPR). The conditions of nerve or vascular invasion and lymphatic metastasis are shown in Table 4.
Table 3. Preoperative outcomes and postoperative complications.
| Content | Value |
|---|---|
| Number receiving surgery | 12 |
| Interval to surgery (d) | 33.2±8.5 |
| Conversion into open surgery | 0 |
| Operation time (min) | 321.75±50.74 |
| Blood loss (mL) | 131.66±64.22 |
| ICU admission (d) | 2±3.69 |
| Postoperative hospital stay (d) | 12.41±5.55 |
| Chest tube duration (d) | 5.5±4.46 |
| Postoperative complications, n (%) | |
| Pneumonia | 3 (25.0) |
| Thrombogenesis | 0 |
| Chylothorax | 1 (8.3) |
| Cardiac events | 2 (16.7) |
| Anastomotic leakage | 1 (8.3) |
| Intestinal obstruction | 0 |
| Postoperative bleeding | 0 |
| Secondary operation | 0 |
| 30-day mortality | 0 |
| 30-day readmission | 0 |
ICU, intensive care unit.
Table 4. Pathological results in patients receiving neoadjuvant immunotherapy and chemotherapy plus surgery.
| Content | Number (%) |
|---|---|
| Pathology response in tumor | |
| 1 | 2 (16.7) |
| 2 | 3 (25.0) |
| 3 | 6 (50.0) |
| 4 | 1 (8.3) |
| Pathological stage | |
| I | 4 (33.3) |
| II | 1 (8.3) |
| IIIA | 1 (8.3) |
| IIIB | 6 (50.0) |
| IVA | 0 |
| Nerve invasion | 3 (25.0) |
| Vascular invasion | 4 (33.3) |
| Lymphatic metastasis | 7 (58.3) |
| Thoracic lymphatic metastasis | 6 (50.0) |
| Abdominal lymphatic metastasis | 2 (16.7) |
Discussion
Several recent studies, including KEYNOTE-181 and ATTRACTION-3 (8,20), have shown that immunotherapy inhibitors of PD-1/PD-L1 for advanced esophageal cancer offer a significant improvement as second-line therapy. Compared with surgery or adjuvant therapy alone, neoadjuvant therapy before surgery can improve the survival rate of patients with advanced ESCC (3,21). Toripalimab is a humanized IgG4κ mAb with hinge mutation (S228P) specific for human PD-1 receptor and blocks PD-1/PD-L1interactions. It is used as second-line treatment for metastatic melanoma, and toripalimab plus chemotherapy has shown promising antitumor activity with acceptable safety profiles as a second-line treatment for patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) (22). However, there are few studies reporting on toripalimab for locally advanced esophageal cancer.
Our study showed that neoadjuvant treatment of locally advanced ESCC patients with toripalimab plus docetaxel (75 mg/m2) and cisplatin (60 mg/m2) was well tolerated, causing a small number of grade ≥3 AEs, no treatment-related surgical delays, and no severe perioperative complications. Two patients experienced immune dermatitis, including 1 patient diagnosed with grade I and 1 patient with grade III. The pattern of AE grades in this cohort was similar to neoadjuvant chemoradiotherapy (23), which validated the manageable safety and feasibility of our therapeutic regimen in patients with locally advanced ESCC.
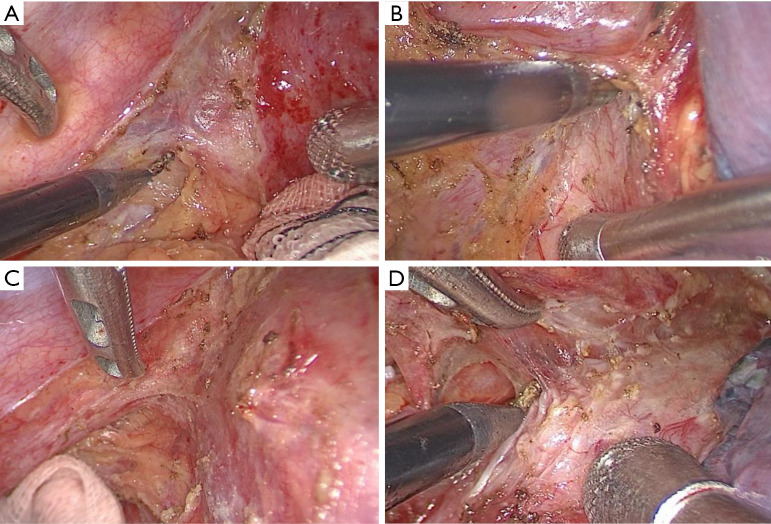
A concern for our surgeons was whether additional immunotherapy would increase the surgical difficulty and technical challenge. All patients in our study successfully underwent MIE without conversion to thoracotomy, and for experienced surgeons, MIE was applicable for patients receiving neoadjuvant toripalimab plus docetaxel and cisplatin. In contrast, the previous study have shown that surgical resection of NSCLC after neoadjuvant immunotherapy was more difficult and challenging due to dense fibrosis, especially in mediastinal and hilar dissection (24). In addition, unexpected thoracotomy may occur in patients after immunotherapy (25,26). To the best of our knowledge, there are no similar reports in patients receiving additional immunotherapy. During the surgical procedure, we noticed fibrosis in the esophageal mesentery; however, the fibrosis was not dense and did not significantly increase the difficulty in distinguishing the tumor from surrounding organs or tissues (Figure 3). When the primary tumor is in the upper third or middle third thoracic region, or when the primary tumor is large, the surgeon needs to carefully locate the boundary between the tumor and normal tissue to avoid injury to surrounding organs, such as the thoracic duct, aorta, and trachea.
Figure 3.
Intraoperative tissue fibrosis. (A) Fibrosis in esophageal mesentery near primary tumor; (B) fibrosis in esophageal mesentery near pericardium; (C) fibrosis in esophageal mesentery near thoracic aorta; (D) fibrosis in esophageal mesentery near trachea.
We observed that the use of 2 cycles of neoadjuvant therapy in patients with esophageal cancer resulted in 16.7% of tumors being eligible for pCR. Samson et al. reported a large cohort from the National Cancer Database, including 916 patients receiving neoadjuvant chemotherapy and 6,422 receiving neoadjuvant chemoradiation. The pCR rate was 17.2% and 6.4% in the neoadjuvant chemoradiation group and neoadjuvant chemotherapy group, respectively (27). The pCR rate in our cohort was superior to that for neoadjuvant chemotherapy and inferior compared to neoadjuvant chemoradiotherapy. One study recently presented at European Society for Medical Oncology (ESMO) Virtual Congress 2020 employed a regimen including nab-paclitaxel/S1 plus toripalimab for neoadjuvant treatment with a pCR rate of 16.67% (28), which was similar to our findings. Shen et al. reported a retrospective cohort received neoadjuvant PD-1 (including nivolumab, pembrolizumab, and camrelizumab) plus chemotherapy with a pCR rate of 33% (29). Yang et al. reported a retrospective cohort received treatment with camrelizumab plus nab-paclitaxel/S1 with a pCR rate of 33.3% (4/12) (30). The above two regimens seemed to have a higher pCR rate than that in this cohort, indicating that homogenous PD-1 inhibitor and the number of neoadjuvant cycles may have different effects on pathological response. Considering the limited size of the present report, further studies are necessary to explore more suitable regimens for patients with locally advanced ESCC in China.
The postoperative complications in this cohort were manageable. Only 1 patient (1/12, 8.3%) had AL. According to previous research, the incidence of AL ranges between 11.4–21.2% (31). Neoadjuvant chemoradiotherapy has undergone decades of development and is recommended as first-line treatment for locally advanced ESCC. However, the evidence regarding the extent and dose of neoadjuvant chemoradiotherapy affecting AL remains controversial, particularly regarding the “safe” dose of chemoradiotherapy in the fundus (anastomotic area used in gastric tube reconstruction). Thus, we hypothesized that different regimens, cycles, and surgical intervals may have different effects on the occurrence of AL. Whether the use of additional neoadjuvant therapy would increase the risk of AL requires further investigation.
To the best of our knowledge, this is the first report of neoadjuvant immunotherapy and chemotherapy (nICT) using toripalimab for locally advanced ESCC. The combination of immunotherapy and chemotherapy can modulate the tumor microenvironment to increase cytotoxic drug sensitivity and achieve synergistic antitumor effect. However, this study had several limitations. First, the sample size was small and came from a single center. The patients were recruited through a highly selective process and all were diagnosed with ESCC. Second, this was a single-arm study that required a control group to definitively assess the role of toripalimab in nICT. Thus, our conclusions may not be applicable to patients treated with other surgical procedures, other histological types, or other immune checkpoint inhibitors. Third, an investigation of overall survival and disease-free survival is necessary to evaluate function of pCR in nICT for locally advanced ESCC.
Conclusions
Based on our results, toripalimab combined with docetaxel and cisplatin as a novel neoadjuvant therapy was safe and effective in locally advanced ESCC.
Acknowledgments
Funding: This study was sponsored by Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University, National Natural Science Foundation of China (Grant No. 82070499), National Natural Science Foundation of China (Grant No. 81773129), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant No. 2020Y9073), Natural Science Foundation, Fujian Province (Grant No. 2020J011036), and Program for Innovative Research Team in Science and technology in Fujian Province University (Grant No. 2018B053).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Fujian Medical University (No. 2019YF033-01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-131/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-131/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-131/coif). The authors have no conflicts of interest to declare.
(English Language Editor: A. Muylwyk)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. 10.1136/gutjnl-2014-308124 [DOI] [PubMed] [Google Scholar]
- 3.Boonstra JJ, Kok TC, Wijnhoven BP, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer 2011;11:181. 10.1186/1471-2407-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi M, Yang Y, Zhang L, et al. Multi-institutional Analysis of Recurrence and Survival After Neoadjuvant Chemoradiotherapy of Esophageal Cancer: Impact of Histology on Recurrence Patterns and Outcomes. Ann Surg 2019;269:663-70. 10.1097/SLA.0000000000002670 [DOI] [PubMed] [Google Scholar]
- 5.Kong M, Shen J, Zhou C, et al. Prognostic factors for survival in esophageal squamous cell carcinoma (ESCC) patients with a complete regression of the primary tumor (ypT0) after neoadjuvant chemoradiotherapy (NCRT) followed by surgery. Ann Transl Med 2020;8:1129. 10.21037/atm-20-4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtulus S, Madi A, Escobar G, et al. Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1-CD8+ Tumor-Infiltrating T Cells. Immunity 2019;50:181-194.e6. 10.1016/j.immuni.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarchoan M, Johnson BA, 3rd, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:209-22. 10.1038/nrc.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Zheng Q, Chen H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis 2021;13:3518-28. 10.21037/jtd-21-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021;144:232-41. 10.1016/j.ejca.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Ying J, Xu J, et al. Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. JAMA Netw Open 2020;3:e2013770. 10.1001/jamanetworkopen.2020.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang FH, Wei XL, Feng J, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol 2021;39:704-12. 10.1200/JCO.20.02712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-e509. 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 14.Gong J, Le TQ, Massarelli E, et al. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer 2018;6:46. 10.1186/s40425-018-0361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. 10.1016/S1470-2045(17)30181-X [DOI] [PubMed] [Google Scholar]
- 17.Van Schil PE. Reply to "Pulmonary metastasectomy: where is the evidence?": absence of evidence is not evidence of absence!. J Thorac Oncol 2015;10:e14-e15. 10.1097/JTO.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 18.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. 10.1002/cncr.20916 [DOI] [PubMed] [Google Scholar]
- 19.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 21.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. 10.1245/s10434-011-2049-9 [DOI] [PubMed] [Google Scholar]
- 22.Jiang T, Wang P, Zhang J, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther 2021;6:355. 10.1038/s41392-021-00751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bott MJ, Cools-Lartigue J, Tan KS, et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann Thorac Surg 2018;106:178-83. 10.1016/j.athoracsur.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaft JE, Hellmann MD, Velez MJ, et al. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg 2017;104:e217-8. 10.1016/j.athoracsur.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samson P, Robinson C, Bradley J, et al. Neoadjuvant Chemotherapy versus Chemoradiation Prior to Esophagectomy: Impact on Rate of Complete Pathologic Response and Survival in Esophageal Cancer Patients. J Thorac Oncol 2016;11:2227-37. 10.1016/j.jtho.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang G, Hu Y, Yang B, et al. A single-centre, prospective, open-label, single-arm trial of toripalimab with nab-paclitaxel and S-1 as a neoadjuvant therapy for esophageal squamous cell carcinoma (ESCC). Ann Oncol 2020;31:S645-S671. 10.1016/j.annonc.2020.08.1178 [DOI] [Google Scholar]
- 29.Shen D, Chen Q, Wu J, et al. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol 2021;12:1-10. 10.21037/jgo-20-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G, Su X, Yang H, et al. Neoadjuvant programmed death-1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med 2021;9:1254. 10.21037/atm-21-3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabbi M, Hagens ERC, van Berge Henegouwen MI, et al. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 2021;34:doaa039. [DOI] [PMC free article] [PubMed]