Abstract
Background
The efficacy of transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT) is limited. There are insufficient data on TACE-lenvatinib sequential therapy for HCC with PVTT. We aimed to assess the efficacy and safety of TACE-lenvatinib sequential therapy for the treatment of HCC and PVTT.
Methods
We retrospectively reviewed 12 consecutive patients with HCC and PVTT who underwent TACE-lenvatinib sequential therapy between July 2018 and May 2021. Lenvatinib treatment was started 1 week after TACE at a dose of 8 or 12 mg daily depending on the patient weight. Follow-up examinations were performed at 4 week and then every 8 weeks after the first TACE procedure. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) and adverse events (AEs) were calculated. Survival curves of PFS and OS were estimated using the Kaplan-Meier method.
Results
The median OS and PFS were 16.9 and 6.15 months, respectively. The ORR and DCR were 75% and 91.7%, respectively. The most common lenvatinib-related AE was hypertension (33.3%), and the most common TACE-related AE was elevated liver enzymes (100%). No treatment-related deaths or grade 4 events were observed.
Conclusions
TACE-lenvatinib sequential therapy may be safe and well tolerated, and may improve OS and PFS for HCC patients with PVTT. Further randomized controlled trials with larger cohorts are needed to confirm its efficacy and safety.
Keywords: Hepatocellular carcinoma (HCC), lenvatinib, portal vein tumor thrombus (PVTT), sequential therapy, transarterial chemoembolization (TACE)
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related death worldwide (1). More than 60% of patients are diagnosed at an intermediate or advanced stage (2), and the presence of macroscopic vascular invasion is considered to be a poor prognostic factor for overall survival (OS) (3). The median OS of patients who are untreated for HCC and portal vein tumor thrombus (PVTT) is 2.7–4.0 months (2). PVTT can lead to series of complications, such as portal hypertension, liver function damage and tumor dissemination (4,5). The treatment for HCC with PVTT includes systemic therapy, hepatectomy, radiotherapy, transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy, portal vein revascularization by endovascular stenting and radioembolization with yttrium-90 microspheres (6). The efficacy of monotherapy remains limited. Combination treatment has been used in patients with HCC and PVTT. In previous studies, TACE-sorafenib treatment improved progression-free survival (PFS) and OS compared with TACE monotherapy (7,8). The mechanism is that sorafenib inhibits neoangiogenesis after TACE (9,10). In the REFLECT trial, lenvatinib was shown to be non-inferior to sorafenib in OS and showed better improvement in PFS and the objective response rate (ORR) (11). As a novel oral multikinase inhibitor, lenvatinib suppresses vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor receptors (FGFRs) 1–4, platelet-derived growth factor receptor ɑ, KIT, and RET. In 2018 lenvatinib was approved by the US Food and Drug Administration and the European Medicines Agency as a first-line drug for advanced HCC (12,13). In a real-world study from Korea, lenvatinib was demonstrated to be more effective and showed an ORR superior to sorafenib as a salvage therapy for TACE in unresectable HCC (14). A price reduction has made lenvatinib more accessible for Chinese patients.
The survival benefit of TACE-lenvatinib sequential therapy for patients with HCC and PVTT needs to be further explored, so we conducted a retrospective study of 12 patients with HCC and PVTT to evaluate the efficacy and safety of sequential therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-239/rc).
Methods
Patients
Between July 2018 and May 2021, 12 consecutive patients who were diagnosed with unresectable HCC and PVTT and underwent TACE-lenvatinib sequential therapy in the Department of Interventional Radiology (The First Medical Center of Chinese PLA General Hospital, Beijing, China) were retrospectively enrolled in this study based on the eligibility criteria. HCC was diagnosed according to the American Association for the Study of Liver Diseases (AASLD) (15). The presence of PVTT was confirmed by demonstration of an intraluminal filling defect with generalized enhancement in the main portal vein or portal vein branches on contrast-enhanced magnetic resonance imaging (MRI) (5,16). PVTT was classified into three types according to previous studies (8,17). Type A: PVTT in the main portal vein; type B: PVTT in the first-order portal vein branch (the right or left portal vein); and type C: PVTT in the second- or lower-order portal vein branches (segmental branches of portal vein or higher). The eligibility criteria were: (I) patient age between 18 and 75 years; (II) HCC invading the portal vein; (III) Child-Pugh class A or B; (IV) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. The exclusion criteria were: (I) ECOG performance status of 3 or 4; (II) main portal vein completely obstructed; (III) severe vital organ dysfunction. All patients were treated with TACE-lenvatinib sequential therapy during the study period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by the Ethics committee of The First Medical Center of Chinese PLA General Hospital. The informed consent was not required because of the retrospective nature of this study.
TACE
Hepatic artery and superior mesenteric artery angiography was performed with a 4-F catheter (RH, Terumo Corporation, Japan) to assess the arterial blood supply of the tumor, and portal vein patency. A 2.7-F miniature catheter (Progreat, Terumo Corporation, Japan) was selected for the feeding artery, and an emulsion of 4–20 mL of lipiodol (Laboratorie Guerbet, Aulnay-sous-Bois, France), and 2–5 chemotherapy drugs from among epirubicin, oxaliplatin, mitomycin, 5-fluorouracil and calcium folinate was infused into the feeding artery of the tumor in a 1:1 ratio depending on the tumor size and liver function until small peripheral portal branches around the tumor were visualized. Finally, embolization with polyvinyl alcohol particles and gelatin sponge particles was performed to block the blood supply of the tumor.
Lenvatinib therapy
Lenvatinib treatment was started 1 week after TACE if the patient’s liver function was Child-Pugh class A or B. If the patient’s weight was <60 kg, a 12 mg dose of lenvatinib was given once daily; If the patient’s weight was >60 kg, the dose of lenvatinib was 8 mg once daily. Doses were modified depending on side effects. If grade 3 or 4 adverse events (AEs) occurred, a dose reduction to 8 mg once daily or a temporary drug interruption was required until the AEs were alleviated or disappeared.
Follow-up
To evaluate the effect of the sequential therapy, follow-up examinations were performed at 4 week and then every 8 weeks after the first TACE procedure until the date of disease progression, death, or last follow-up. The examination included routine blood tests, liver function tests, serum hepatitis B virus DNA, alpha-fetoprotein (AFP) and abdominal contrast-enhanced MRI. If residual viable tumor or recurrent lesion was found on MRI, TACE was repeated.
Efficacy assessment
The patients’ responses to treatment were assessed based on the best response according to the modified Response Evaluation Criteria in Solid Tumors criterion (18): complete response (CR) = disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR) = sum of the diameters of viable lesions decreased at least 30%; progressive disease (PD) = sum of the diameters of viable lesions increased at least 20%; stable disease (SD) = any case that does not meet the criteria for PD or PR. The ORR was the proportion of patients of patients who achieved CR and PR, and the DCR was the percentage of patients with a tumor response of CR, PR, or SD. PFS was defined as the time from the start of treatment to disease progression or death. OS was defined as the time from the start of treatment to death or the last follow-up.
Safety assessment
AEs were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (19).
Statistical analysis
All statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and Prism 8 (GraphPad Software, San Diego, CA, USA). Survival curves of PFS and OS were estimated using the Kaplan-Meier method.
Results
Baseline characteristics
The median follow-up time was 15.2 months. Table 1 summarizes the baseline clinical characteristics and Table 2 shows the laboratory data.
Table 1. Baseline characteristics.
| Characteristics | Number |
|---|---|
| Sex, n (%) | |
| Male | 10 (83.3) |
| Female | 2 (16.7) |
| Mean age (years) | 55.9±8.4 |
| Etiology, n (%) | |
| Hepatitis B virus | 12 (100.0) |
| Hepatitis C virus | 0 |
| Unknown | 0 |
| BCLC stage, n (%) | |
| A | 0 |
| B | 0 |
| C | 12 (100.0) |
| PVTT grade, n (%) | |
| A | 3 (25.0) |
| B | 9 (75.0) |
| C | 0 |
| Extrahepatic metastasis, n (%) | 7 (58.3) |
| Lung | 3 (25.0) |
| Lymph node | 4 (33.3) |
| Bone | 2 (16.7) |
| Paranephros | 1 (8.3) |
| Pleura | 1 (8.3) |
| Initial dose of lenvatinib, n (%) | |
| 12 mg | 4 (33.3) |
| 8 mg | 8 (66.7) |
| Reduced initial dose of lenvatinib | 1 (8.3) |
BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombus.
Table 2. Laboratory data of patients.
| Variables | Number |
|---|---|
| AST (U/L) | 36.7±15.6 |
| ALT (U/L) | 24.7±13.1 |
| PLT (×109) | 126.3±65.5 |
| TB (µmol/L) | 16.7±6.2 |
| ALB (g/L) | 39.2±3.9 |
| PT (s) | 16.5±0.85 |
| AFP (µg/L) | 2,976.3±4,132.3 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelets; TB, total bilirubin; ALB, albumin; PT, prothrombin time; AFP, alpha-fetoprotein.
Response and survival outcomes
CR occurred in 2 patients (16.7%), PR in 7 patients (58.3%), SD in 2 patients (16.7%), and PR in 1 patient (8.3%). The ORR was 75.0% (9/12) and the DCR was 91.7% (11/12). Median OS was 16.9 months (Figure 1), and median PFS was 6.15 months (Figure 2).
Figure 1.
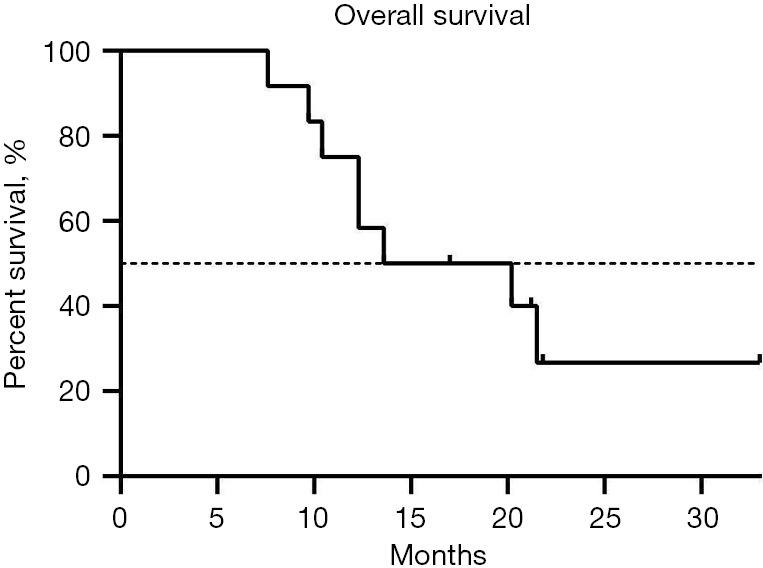
Overall survival of the 12 patients with hepatocellular carcinoma and portal vein tumor thrombus.
Figure 2.
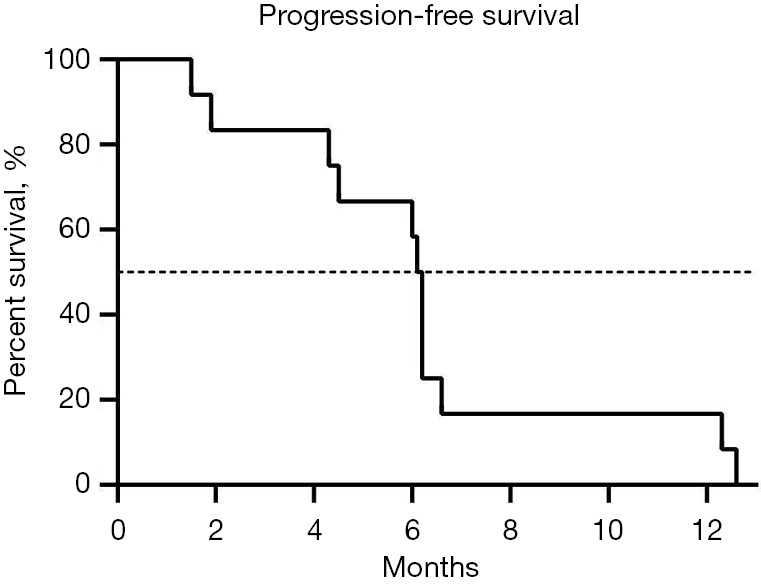
Progression-free survival of the 12 patients with hepatocellular carcinoma and portal vein tumor thrombus.
Safety outcomes
Lenvatinib-related AEs included hypertension (33.3%), fatigue (8.3%), myalgia (8.3%), hand-foot skin reaction (8.3%), rash (8.3%), decreased appetite (8.3%), decreased platelets (8.3%), diarrhea (8.3%), and edema (8.3%) (Table 3). TACE-related AEs included elevated aspartate aminotransferase (AST: 100.0%), elevated alanine aminotransferase (ALT: 100.0%), abdominal pain (91.7%), fever (75.0%), elevated bilirubin (50%), vomiting (25.0%), abdominal distension (16.7%), and constipation (8.3%) (Table 4).
Table 3. Lenvatinib-related adverse events.
| Adverse event | All grades, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Hypertension | 4 (33.3) | 1 (8.3) | 0 |
| Fatigue | 1 (8.3) | 0 | 0 |
| Myalgia | 1 (8.3) | 0 | 0 |
| Hand-foot skin reaction | 1 (8.3) | 0 | 0 |
| Rush | 1 (8.3) | 0 | 0 |
| Decreased appetite | 1 (8.3) | 0 | 0 |
| Decreased platelet | 1 (8.3) | 0 | 0 |
| Diarrhea | 1 (8.3) | 0 | 0 |
| Edema | 1 (8.3) | 0 | 0 |
Table 4. TACE-related adverse events.
| Adverse event | All grades, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Elevated AST | 12 (100.0) | 4 (33.3) | 0 |
| Elevated ALT | 12 (100.0) | 3 (25.0) | 0 |
| Abdominal pain | 11 (91.7) | 0 | 0 |
| Fever | 9 (75.0) | 0 | 0 |
| Elevated bilirubin | 6 (50.0) | 1 (8.3) | 0 |
| Vomiting | 3 (25.0) | 0 | 0 |
| Abdominal distension | 2 (16.7) | 0 | 0 |
| Constipation | 1 (8.3) | 0 | 0 |
TACE, transarterial chemoembolization; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
In 2007 sorafenib was the first molecular targeted drug to be approved for first-line treatment for advanced HCC (20). Lenvatinib is also recommended by international guidelines as a first-line treatment for advanced HCC (21-24). In the REFLECT trial, lenvatinib showed a meaningful improvement in PFS and ORR compared with sorafenib (11), but the majority of patients who receive lenvatinib experience disease progression at a relatively early stage (25). The efficacy of TACE for HCC with PVTT is also limited. Therefore, it is necessary to find a more effective therapy. Previous studies have explored the TACE-lenvatinib combination. Fu et al. compared the efficacy and safety of combined treatment with lenvatinib and TACE versus TACE in patients with unresectable HCC. Their results showed that the ORR was better with TACE plus lenvatinib treatment (ORR: 68.3% vs. 31.7%) and DCR numerically increased in the TACE-plus treatment (93.3% vs. 86.7%) (26). Kawamura et al. revealed that using lenvatinib-TACE sequential therapy after progression during lenvatinib treatment was associated with better progression-free survival (PFS) (25). However, there are insufficient data on TACE-lenvatinib sequential therapy for HCC with PVTT, so we conducted the present study to evaluate its efficacy and safety. Our results demonstrated that TACE-lenvatinib was effective in patients with advanced HCC and PVTT, with median PFS and OS of 6.15 and 16.9 months, respectively. Previous study showed that the median OS of HCC patients with segmental PVTT after TACE was 15.5 months (27), which was shorter than in present study. And repeated TACE leads to deterioration of liver function. Ding et al. claimed that the TACE plus lenvatinib had favorable efficacy for HCC with PVTT compared with TACE plus sorafenib, and presented a median TTP (time from randomization to disease progression) of 4.7 months compared with 3.1 months in the patients receiving TACE plus sorafenib treatment (28). In our study, we observed a better median PFS of 6.15 months. The OS was also longer than those reported for TACE plus sorafenib. Zhu et al. reported a median OS for TACE plus sorafenib of 11.0 months for the treatment of HCC with PVTT (8), and Pan et al. reported a median OS of 13 months (29). Our results suggested that TACE-lenvatinib sequential therapy is a possible useful option for patients with HCC and PVTT. The encouraging survival benefits of TACE-Lenvatinib sequential therapy may be attributed to the following potential mechanism. TACE creates a hypoxic environment within the tumor, and increases the secretion of angiogenic factors such as VEGF and FGF, which lead to tumor angiogenesis and recurrence (9). Lenvatinib is a novel anti-angiogenic, multikinase inhibitor that targets VEGF receptors 1–3 and FGF receptors 1–4 (12,13), which inhibits angiogenesis and tumor recurrence after TACE. In addition, TACE induces tumor necrosis by directly killing tumor cells and blocking the supply vessels of the tumor. As a systemic therapy, lenvatinib targets local tumor, PVTT and extrahepatic metastases to improve tumor control.
The present study also revealed that the most common lenvatinib-related AE was hypertension, and the most common TACE-related AE was elevated AST and ALT. Treatment-related deaths and grade 4 AEs were not observed. All of the AEs were well tolerated. Although liver dysfunction occurred in all patients after TACE, it was transient and reversible with proper management. The other TACE-related AE was postembolization syndrome, which was relieved with appropriate measures. Lenvatinib-related AEs were hypertension, fatigue, myalgia, hand-foot skin reaction, rash, decreased appetite, decreased platelets, diarrhea and edema. These AEs were manageable with dose adjustment or interruption, or symptomatic treatment.
The present study had some limitations. First, it was a retrospective and single-center study. Second, it lacked a control group. Third, the number of patients enrolled in the study was small.
In conclusion, TACE-lenvatinib sequential therapy may be safe and well tolerated, and may improve OS and PFS for HCC patients with PVTT. Further randomized controlled trials with larger cohorts are needed to confirm its efficacy and safety.
Acknowledgments
Funding: This study was supported by National Key R&D Program of China (2020YFC2008900)and National Nature Science Foundation of China (81671790, 82104404).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by the Ethics committee of The First Medical Center of Chinese PLA General Hospital. The informed consent was not required because of the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-239/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-239/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-239/coif). The authors have no conflicts of interest to declare.
(English Language Editor: K. Brown)
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. 10.3322/CA.2007.0010 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol 2017;67:999-1008. 10.1016/j.jhep.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 4.Katamura Y, Aikata H, Takaki S, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol 2009;44:492-502. 10.1007/s00535-009-0033-y [DOI] [PubMed] [Google Scholar]
- 5.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 2012;82:2004-11. 10.1016/j.ijrobp.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721-30. 10.1016/S2468-1253(19)30178-5 [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu K, Chen J, Lai L, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology 2014;272:284-93. 10.1148/radiol.14131946 [DOI] [PubMed] [Google Scholar]
- 9.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21. 10.1111/j.1572-0241.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 10.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008;99:2037-44. 10.1111/j.1349-7006.2008.00909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014;6:18. 10.1186/2045-824X-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878-82. 10.3748/wjg.v10.i19.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Sung PS, Yang H, et al. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J Clin Med 2020;9:4121. 10.3390/jcm9124121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 16.Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95:588-95. 10.1002/cncr.10694 [DOI] [PubMed] [Google Scholar]
- 17.Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol 2015;26:320-9.e6. 10.1016/j.jvir.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Division of Cancer Treatment and Diagnosis. Cancer therapy evaluation program. Adverse events/CTCAE [accessed 2018 Dec 23]. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
- 20.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129-40. 10.1158/1535-7163.MCT-08-0013 [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. [Internet] NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx [DOI] [PMC free article] [PubMed]
- 23.Vogel A, Cervantes A, Chau I, et al. Correction to: "Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann Oncol 2019;30:871-3. 10.1093/annonc/mdy510 [DOI] [PubMed] [Google Scholar]
- 24.Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. 10.1016/j.annonc.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y, Kobayashi M, Shindoh J, et al. Lenvatinib-Transarterial Chemoembolization Sequential Therapy as an Effective Treatment at Progression during Lenvatinib Therapy for Advanced Hepatocellular Carcinoma. Liver Cancer 2020;9:756-70. 10.1159/000510299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663-75. 10.1007/s12072-021-10184-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Kim HC, Lee JH, et al. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol 2017;27:1448-58. 10.1007/s00330-016-4511-3 [DOI] [PubMed] [Google Scholar]
- 28.Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer 2021;127:3782-93. 10.1002/cncr.33677 [DOI] [PubMed] [Google Scholar]
- 29.Pan T, Li XS, Xie QK, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol 2014;69:e553-61. 10.1016/j.crad.2014.09.007 [DOI] [PubMed] [Google Scholar]


