Abstract
Integration of acoustic information over time is essential for processing complex stimuli, such as speech, due to its continuous variability along the time domain. In both humans and animals, perception of acoustic stimuli is a function of both stimulus intensity and duration. For brief acoustic stimuli, as duration increases, thresholds decrease by approximately 3 dB for every doubling in duration until stimulus duration reaches 500 ms, a phenomenon known as temporal integration. Although hearing loss and damage to outer hair cells (OHC) have been shown to alter temporal integration in some studies, the role of cochlear inner hair cells (IHC) on temporal integration is unknown. Because IHC transmit nearly all acoustic information to the central auditory system and are believed to code both intensity and timing information, these sensory cells likely play a critical role in temporal integration. To test the hypothesis that selective IHC loss degrades the temporal integration function, behaviorally trained chinchillas were treated with carboplatin, a drug known to selectively destroy IHC with little to no effect on OHC in this species. Pure-tone thresholds were assessed across frequencies (1, 2, 4, 8, 12 kHz) as a function of signal duration (500, 100, 50, 10, and 5 ms). Baseline testing showed a significant effect of duration on thresholds. Threshold decreased as a function of increasing duration, as expected. Carboplatin treatment (75 mg/kg) produced a moderate to severe loss of IHC (45–85%) with little-to-no loss of OHC. Contrary to our hypothesis, post-carboplatin temporal integration thresholds showed no significant differences from baseline regardless of stimulus duration or frequency. These data suggest that few IHC are necessary for temporal integration of simple stimuli. Temporal integration may be sensitive to loss of OHC and loss of cochlear non-linearities but does not appear to be sensitive to selective IHC loss.
Keywords: Inner hair cell loss, Carboplatin, Chinchilla, Synaptopathy, Temporal integration, Pure tone audiometry
INTRODUCTION
In mammals, hearing depends on the function of two distinct sensory cell types in the cochlea. Inner hair cells (IHC) are the primary conduits of acoustic information to the central auditory nervous system via extensive connections with afferent auditory nerve fibers (ANF) (> 90%) whereas outer hair cells (OHC), which are innervated by the remaining 10% ANF, do not appear to transmit acoustic information (Spoendlin 1975). OHC, however, are essential for normal hearing sensitivity as these provide active non-linear cochlear amplification via electromotility (Allen 1980; Johnstone et al. 1986; Preyer and Gummer 1996) and are believed to play an essential role in frequency selectivity (Fettiplace and Fuchs 1999). When OHC are damaged, thresholds increase and frequency selectivity decreases (Cody and Russell 1985; Borg 1987; Davis et al. 1989, 2005; Hamernik et al. 1989; Patuzzi et al. 1989; McFadden et al. 2002).
In addition to elevated thresholds and poorer frequency selectivity, individuals with cochlear hearing loss may also experience other perceptual deficits including poorer auditory temporal processing. For example, hearing-impaired individuals generally show poorer performance in temporal integration tasks and poorer performance on some gap detection tasks (Reed et al. 2009). Poorer temporal processing can be further exacerbated with aging of the auditory system particularly for tasks using time-compressed speech (Gordon-Salant and Fitzgibbons 2001; Gordon-Salant et al. 2007; Manheim et al. 2018).
In humans, cochlear hearing loss is generally correlated with increased difficulty in understanding speech, particularly in the presence of competing background noise. However, the degree of poorer speech understanding does not always correlate with the degree of poorer hearing sensitivity, and individuals with similar audiometric thresholds can demonstrate vastly different levels of speech understanding in noise (Middelweerd et al. 1990; Thibodeau 1996; Badri et al. 2011). Whereas OHC play a major role in frequency tuning and cochlear non-linear gain, IHC provide the first stage of coding of temporal information, as IHC transmit the majority of acoustic information to the central auditory nervous system. Thus, it is possible that individuals with higher levels of degraded speech understanding could have more IHC loss or dysfunction, and that this degradation stems from poorer sensitivity to changes in the temporal domain. In previous reports, sensitivity to temporal changes were assessed by measuring gap detection threshold differences between normal hearing (NH) listeners and listeners with cochlear hearing loss (CL) (i.e., hearing loss that stems from the cochlea and is not conductive or retrocochlear in nature) (see review by Reed et al. 2009). These studies typically controlled for differences in hearing sensitivity between NH listeners and listeners with CL by presenting stimuli at sensation level, or suprathreshold intensity levels to overcome the loss of audibility in participants with CL, or by adding a competing noise stimuli to elevate thresholds in NH listeners (Reed et al. 2009). Though these efforts were made to match the hearing sensitivity across CL and NH groups and control for audibility differences, the studies yielded mixed results. Multiple studies found that CL listeners performed more poorly than aged-matched NH peers on gap detection tasks (Fitzgibbons and Wightman 1982; Florentine and Buus 1984; Grose et al. 1989); whereas other studies found no differences in temporal resolution among CL listeners and age-matched, noise-masked NH listeners (DeFilippo and Snell, 1986; Moore et al. 1992).
The auditory system’s ability to synthesize acoustic information over time is a critical component of psychoacoustic processing. For example, auditory temporal integration results in improved performance on complex auditory tasks with longer stimulus duration such as vowel discrimination (Chiu et al. 2019), frequency modulation detection (Palandrani et al. 2021), and signal detection in noise (Moore et al. 1999). Temporal integration has been operationally defined as the relationship between threshold and the duration of an acoustic stimulus. This relationship has been described in experiments dating as far back as 1876 (Stephens 1973). Over the last century, studies have shown that thresholds depend on constant energy as opposed to constant intensity (Dallos and Johnson 1966; Watson and Gengel 1969; Gengel and Watson 1971; Gerken et al. 1990). For short-duration stimuli at a fixed frequency, thresholds decrease by approximately 3 dB for every doubling of duration until the stimuli duration reaches 500 ms, after which thresholds remain constant (Pedersen and Salomon 1977). Studies in both humans (Gorga et al. 1984; Florentine et al. 1988; Buus et al. 1999) and animals (Henderson 1969; Gleich et al. 2007) have shown that CL from noise or aging increases thresholds and reduces the slope of the temporal integration function. A flatter temporal integration function is correlated with increasing hearing loss. The precise physiological mechanisms underlying this phenomenon, however, are not well understood. It has been hypothesized that a long-term temporal integrator resides in the central auditory nervous system. Studies evaluating temporal integration in humans and the effects of temporal integration on noise-exposed cats have shown few differences in temporal integration between single and multiple burst stimuli (Gerken et al. 1990; Solecki and Gerken 1990). These results suggest that the onset/offset cues in the stimuli that would more vigorously drive the periphery do not play a major role in temporal integration. This lack of difference has been interpreted as a support for a central temporal integrator (Gerken et al. 1990).
However, several studies have challenged the hypothesis of a central generator of temporal integration. Other studies in cats have shown that differences in temporal integration following hearing loss can be explained by changes in hearing sensitivity associated with the loss of OHC and an elevation in the baseline at which sound pressure is effective in driving the auditory system. When adjusted for these changes, temporal integration does not appear to be altered following noise-induced hearing loss, suggesting a peripheral integrator (Neubauer and Heil, 2004). A study using chickens demonstrated concurrent recovery of hearing thresholds and temporal integration after undergoing high-intensity sound exposure (Saunders et al. 1995). Despite the sound exposure initially flattening the animal temporal integration functions, the chicken’s ability to regenerate hair cells following noise-induced structural damage also restored its temporal integration ability. Another study compared the temporal integration function of a breed of canary with congenital hearing loss (Belgian Waterslager Canary) characterized by abnormal, damaged, and missing hair cells to canaries without hearing loss (Lauer et al. 2007). The canaries with hearing loss had shallower temporal integration functions than the normal hearing birds. These studies align with the hypothesis that temporal integration is primarily a peripheral mechanism. Data in humans also suggest a peripheral short-term integrator and the temporal integration functions may be explained by the shift to a linear basilar membrane input–output function associated with cochlear hearing loss. This is most evident at medium sound presentation levels (50–70 dB SPL) where basilar membrane compression is the greatest (Oxenham et al. 1997). Given these results, the short-term temporal integrator is believed to be a function of IHC and auditory nerve activation in the periphery.
Other data in support of a peripheral short-term integrator suggest a repeated sampling “multiple looks” mechanism (Viemeister and Wakefield 1991). The “multiple looks” model posits that when detecting a tone, listeners actively utilize multiple samples from an observed interval. As the observation interval increases, as with longer-duration stimuli, more samples can be obtained and overall listener performance improves, in this case, in the form of lower thresholds. Although the underlying mechanisms are not well understood, individuals with hearing loss typically have more difficulty perceiving short-duration, low-intensity speech components such as consonants (Walden et al. 1981; Turner and Robb 1987). This can be compounded by high-level competing background noise, whereby the signal to noise ratio is reduced and there are fewer opportunities in the time domain to perceive short-duration speech stimuli. Taken together, these experiments suggest that IHC could play a significant role in temporal integration. Consequently, severe loss of IHC may alter temporal integration particularly for short-duration stimuli.
Our previous studies using a chinchilla animal model have focused on the relationship between the loss of IHC and its effects on auditory perception. There are a number of advantages to using the chinchilla for these types of studies. These include similarities with human auditory physiology, anatomy, and hearing sensitivity across a similar frequency range (Trevino et al. 2019). However, one of the most significant advantages of using the chinchilla is that chinchillas are currently the only known species where IHCs can be selectively destroyed pharmacologically without damaging OHC, providing a unique opportunity to study the effects of selective IHC loss on hearing (Takeno et al. 1994a, b; Wake et al. 1994). This unique lesion is possible with the systemic or local administration of the anticancer drug carboplatin. When carboplatin is administered to the chinchilla, IHC can be selectively destroyed in a dose-dependent manner with little to no effect on OHC or cochlear non-linearities (Trautwein et al. 1996; Lobarinas et al. 2013). In a recent publication, we evaluated the effect of IHC loss on gap detection thresholds, an auditory task that requires animals to detect both intensity and temporal changes (Lobarinas et al. 2020). The results of this study showed that gap detection thresholds increased significantly following selective IHC loss (Lobarinas et al. 2020). To further investigate the potential role of IHC on auditory tasks that depend upon detection of changes in the temporal domain, we evaluated the relationship between selective loss of IHC and temporal integration. Temporal integration has been previously measured in chinchillas, but these studies did not assess temporal integration after selective loss of IHC (Henderson 1969; Giraudi et al. 1980; Trevino et al. 2019).
In a previous study, we showed that thresholds to tones with a relatively long duration (500 ms) showed minimal changes following severe IHC loss (Lobarinas et al. 2013, 2016). In contrast, selective loss of IHC significantly elevated gap detection thresholds (Lobarinas et al. 2020). One possible explanation for these results is that selective IHC loss diminishes auditory sensitivity to temporal changes. In order to begin to test this possibility, a behavioral temporal integration task was devised for variable-duration pure-tone stimuli. Given the recent suggestions and results from IHC synaptopathic studies, detailing auditory deficits as a result of IHC synapse loss (Kujawa and Liberman 2009; Song et al. 2016), we hypothesized that thresholds for shorter-duration stimuli would increase following carboplatin-induced moderate to severe IHC loss, and that thresholds for longer-duration stimuli would not change. This differs from experiments with conventional CL, where previous research has shown that the effect of CL creates a flatter temporal integration function. Here, we proposed that the main effect of selective IHC loss would be threshold elevation for short-duration stimuli with a more rapid improvement as a function of increasing duration. As suggested by the “multiple looks” model, it is possible that significant IHC loss would provide fewer opportunities to code acoustic stimuli in the time domain. Specifically, the reduction in the number of available IHC would disproportionally affect thresholds for short-duration stimuli and the slope of the temporal integration function would be steeper.
MATERIALS AND METHODS
Subjects
Eight healthy adult (400–600 g), 1–2-year-old male (n = 4) and female (n = 4) chinchillas, (Chinchilla lanigera, Ryerson Chinchilla Ranch, Plymouth, OH, USA) were used in this study. One animal (male) died of health complications unrelated to the study prior to the completion of post-carboplatin assessment and was excluded from all data analysis (final n = 7).
Temporal integration thresholds in quiet were assessed before and after administration of a single dose of 75 mg/kg carboplatin (intraperitoneal, [i.p.]), a dose reliably shown to produce moderate to severe IHC loss in this species (Hofstetter et al. 1997a, b; Lobarinas et al. 2013, 2016).
All animals were housed in individual cages in a temperature-controlled room with a 12-h light/dark cycle and free access to food and water. Animals were enrolled in an environmental enrichment program at The University of Texas Southwestern Medical Center (UTSW). All procedures were approved by The University of Texas at Dallas (UTD) and UTSW Institutional Animal Care and Use Committees. The UTSW veterinary staff provided oversight and assistance in the care of this USDA-regulated species.
Equipment
Subjects were tested in acoustically transparent (stainless steel bar), operant chambers with dimensions 31.75 (W) × 34.29 (H) × 25.4 (D) cm, (Med Associates, 007-VPX), housed within a sound-attenuating cubicle (Med Associates, ENV-018 V) lined with acoustic foam. The sound-attenuating cubicles resided in a single-walled sound-attenuating booth (WhisperRoom, MDL 4870S). Within each chamber, a photobeam (Med-Associates, ENV-253SD) was placed and centered 19.54 cm above a stainless-steel bar grid floor wired for scrambled foot shock delivery (Med-Associates, ENV-005A-QD and ENV-414S, 2–5 mA scrambled foot shock). The output of the photobeam was fed to an adjustable single-channel IR controller (Med-Associates, ENV-253B) and then to a real-time processor (Tucker Davis Technologies, RP2.1) connected to a personal computer running custom software (Behavior Lab, Daniel Stolzberg). A calibrated speaker (Fostex, FE127E) placed at the top of the operant chamber (26 cm height) was used to deliver all acoustic stimuli. A piezo buzzer (Radio Shack, 273–059) and house lights (Med-Associates, ENV-215 M) provided feedback to the animal during testing.
Study Design
The overall aim of the study was to evaluate the effect of selective IHC loss on temporal integration. A within-subject design was implemented for this study. Temporal integration thresholds were assessed at baseline (pre-carboplatin treatment) and 21 days after carboplatin treatment. The 21-day period post carboplatin administration allowed animals to recover from carboplatin treatment and stabilize the IHC lesion. Animal training and baseline assessment pre-carboplatin lasted approximately 7 weeks; post-carboplatin treatment testing following recovery was performed over the course of 4 weeks. Following post-carboplatin assessment, animals were euthanized, and cochlear tissue was harvested for histological assessment.
Psychophysical Methods
Hearing sensitivity and temporal integration thresholds were obtained using a shock avoidance–conditioning procedure described in detail in previous publications (Lobarinas et al. 2013, 2016), and similar to that described in earlier reports (Blakeslee et al. 1978; Salvi et al. 1978; Giraudi et al. 1980; Giraudi-Perry et al. 1982; Salvi and Arehole, 1985). This procedure has been shown to shorten training times, reduce overall testing times, and does not require food or water deprivation prior to testing (Trevino et al. 2019).
Audiometric Pure-Tone Thresholds
Thresholds were assessed by presenting tone-burst stimuli (500 ms duration, 5 ms rise/fall time) at 1, 2, 4, 8, and 12 kHz. Tone-burst stimuli were generated with a custom MATLAB script (Behavior Lab, Daniel Stolzberg) in conjunction with RPvdsEx software running a real-time processor, RP2.1 (Tucker Davis Technologies). Loudspeaker output was calibrated using a 0.5-in microphone (Larson Davis 2559) and a sound level meter (Larson Davis 800B).
Experimental sessions occurred 3–5 times per week. Each experimental session contained approximately 120 trials. During each trial, six tone bursts (500 ms on/500 ms off, 5 ms rise/fall time) were presented. If the subject reared and broke the plane of the photobeam within the first four of the six tone bursts, the tone-burst signal was terminated, the house light remained on, and the rearing response was recorded as correct (hit). However, if the subject failed to respond within the first four tone bursts, a scrambled foot shock and a buzzer were turned on, the house lights were turned off, and the trial was recorded as an incorrect response (miss).
During a missed trial, the combination of the shock stimulus and buzzer could immediately elicit a response that turned off the shock (escape). Maximum shock time was limited to 2 s and shock intensity was individually adjusted to the minimal level that triggered a rearing response. When tone-burst intensity levels fell below 20 dB SPL, the shock was removed and only the buzzer and light-off condition signaled a miss.
Each trial within a session was initiated using a random intertrial interval that ranged from 18 to 25 s. Initial test sessions presented the stimulus at a high level (> 60 dB SPL) for training purposes. Following training, sessions presented multiple trials at 3–5 different stimulus levels per test frequency (e.g., 45–50-55 dB SPL at 1000 Hz). Subsequent sessions gradually lowered the presentation level until session threshold was achieved based on correct response rates. Session threshold at each frequency was operationally defined as the intensity at which two out of three correct responses (66%) occurred. False alarm rates were monitored, and sessions were considered valid if the false alarm rate was less than 10%. Across conditions, the time needed to assess thresholds in a session was 45 to 60 min. Session thresholds at each frequency were averaged across three sessions to ensure stable baseline pure-tone thresholds at each frequency. Pure-tone thresholds were obtained at baseline and after the 21-day post-carboplatin recovery period.
Temporal Integration Thresholds
Temporal integration thresholds were assessed by varying the duration of pure-tone stimuli presented at frequencies 1, 2, 4, 8, and 12 kHz. Testing was completed using the same equipment and paradigm design as described for pure-tone threshold assessment, with the exception of modifying the duration of the stimuli. Temporal integration thresholds were assessed at durations of 100, 50, 10, and 5 ms with 5 ms rise/fall time for each duration condition. Experimental test sessions were conducted 3–5 times per week and were comprised of approximately 120 trials, during which 6 short-duration tones were presented. Interstimulus intervals were fixed at 500 ms for all duration conditions (e.g., 50 ms on/500 ms off). Temporal integration thresholds were defined as previously stated; the intensity level at which the animals achieved 66% correct responses at each frequency and each tested duration. Temporal integration thresholds were evaluated at baseline and at least 3 weeks post-carboplatin treatment.
Histological Assessment
Histological analysis was conducted in order to confirm IHC and OHC status. Chinchillas were deeply anesthetized with subcutaneous ketamine and xylazine (60 mg/kg and 12 mg/kg, respectively) and decapitated for tissue harvest following the final psychophysical data collection. Both right and left bullae from each animal were harvested. Round and oval windows were opened, and the cochleae were flushed with room temperature fixative (2.5% glutaraldehyde [Electron Microscopy Sciences, Hatfield, PA] or 4% paraformaldehyde [Sigma-Aldrich, St. Louis, MO] in 0.1 M phosphate buffered saline (PBS), pH 7.4, (Sigma-Aldrich, St. Louis, MO) via the opened round window. The tissue was immersed in fixative for at least 24 h at 4 °C, then rinsed in PBS, and stored at 4 °C for future analysis (King et al. 2014). Cochleae were stained with 1% osmium tetroxide (Electron Microscopy Sciences), trimmed and decalcified in ethylenediaminetetraacetic acid tetrasodium salt dehydrate (EDTA; Sigma-Aldrich) for microdissection and brightfield imaged with a compound microscope in a manner similar to that used in earlier studies (Pawlowski et al. 2013). Data were collected on hair cell status along the length of the cochlea from apex to base. A total of 1/3 octave frequency regions were determined using a physiological frequency-position map for chinchillas and presented as cochleograms displaying the mean inner and outer hair cell loss by relative frequency region (Muller et al. 2010).
Data Analysis
All animals were evaluated for audiometric pure-tone thresholds and temporal integration thresholds. Pre- and post-carboplatin threshold averages were compared using a two-way repeated measures analysis of variance (ANOVA) to determine the effect of carboplatin-induced selective IHC loss and the effect of stimulus duration. Sigmaplot 12.5 software was used for all statistical analysis. All statistical comparisons used an alpha level of 0.05; errors bars in relevant figures represent standard deviation and individual animal data are represented with consistent unique symbols per animal across all figures.
RESULTS
IHC Loss
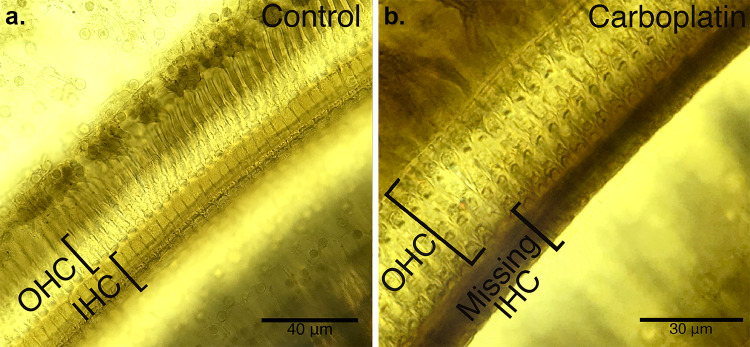
No significant differences were found between left and right ears within individual animals; therefore, histological data are presented from one ear per animal. Images from a control animal cochlea and an experimental animal cochlea can be seen in Fig. 1a, b, respectively. Cochleograms presenting hair cell loss as a function of distance from the apex showed minimal OHC loss (0–5% loss) and moderate (45–70%) IHC loss across anatomical correlates of the experimental frequency regions (1, 2, 4, 8, and 12 kHz) as shown in Fig. 2.
Fig. 1.
Organ of corti stained with osmium tetroxide from a control animal (a) and an animal treated with carboplatin (b). Rows of OHC cell bodies are visible in both images; IHC are missing in the carboplatin image
Fig. 2.
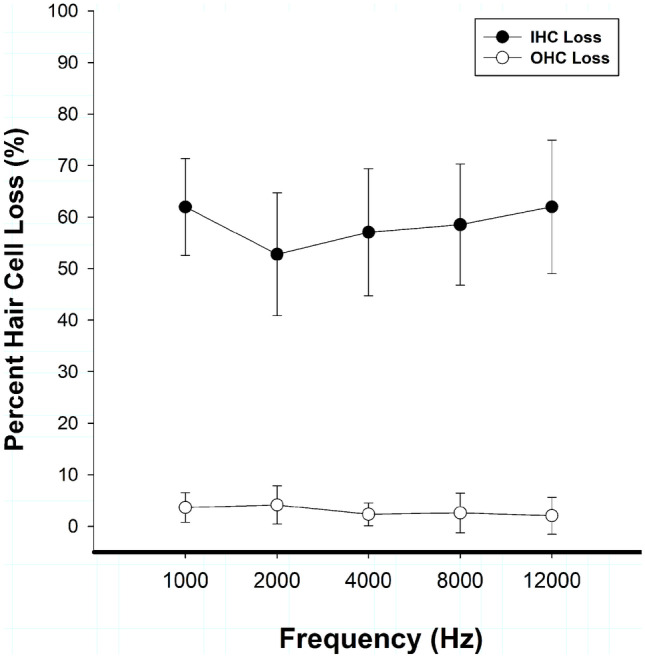
Mean inner (IHC) and outer (OHC) hair cell loss (n = 7) is shown as a function of test frequency. Carboplatin treatment (75 mg/kg) produced moderate (45–70%) IHC loss. In contrast, there was minimal to no evidence (0–5%) of OHC loss. Data are plotted as mean ± standard deviation
Pure-Tone Thresholds
Pre- and post-carboplatin pure-tone thresholds (stimulus duration = 500 ms) are shown in Fig. 3 as a function of frequency. Across frequencies, thresholds remained the same or increased by 2 to 5 dB; however, these changes did not reach statistical significance when analyzed via two-way repeated measures ANOVA [F(1, 69) = 4.725; p = 0.073]. Pure-tone thresholds obtained by the methods described and using the chinchilla carboplatin model are consistent with previous studies (Lobarinas et al. 2013, 2016).
Fig. 3.
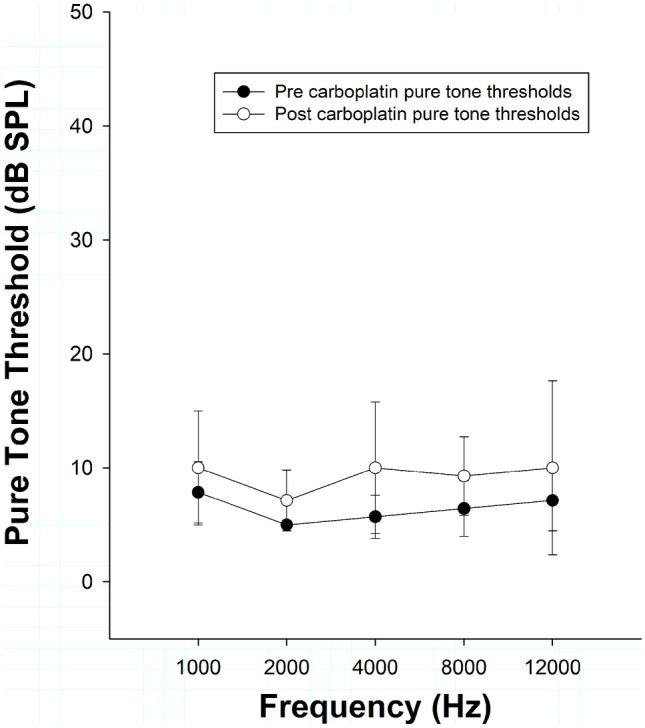
Mean pure-tone thresholds (500 ms; n = 7) are shown as a function of test frequency before and after carboplatin treatment. Thresholds were operationally defined using the 66% correct criteria. Average thresholds at 500 ms showed minimal elevation post carboplatin (2–5 dB)
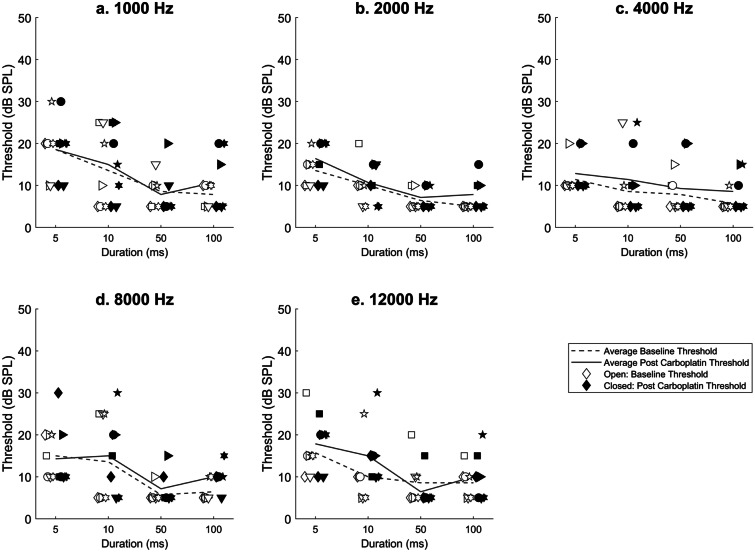
Temporal Integration Thresholds
Temporal integration thresholds (500, 100, 50, 10, 5 ms conditions) for individual animals are shown in Fig. 4a–e. On average, temporal integration thresholds showed little to no change (0–5 dB SPL) across conditions following carboplatin treatment. For all animals, thresholds decreased as stimuli duration increased, in both pre- and post-carboplatin treatment conditions. Thresholds at baseline ranged from 5–10 dB SPL when tested at 100 ms to 15–30 dB SPL in the 5 ms condition. The variables of interest were the treatment condition and stimulus duration at each frequency. A two-way repeated measures ANOVA did not find a statistically significant effect of treatment on temporal integration thresholds at 1000, 2000, 4000, 8000, or 12,000 Hz. F values, p-values, and degrees of freedom [DF] for this analysis are summarized in Table 1. There was main effect of stimulus duration on temporal integration thresholds in all test frequencies. Thresholds increased statistically significantly as a function of stimulus duration at 1000 Hz [F(4,69) = 5.571, p = 0.003]; 2000 Hz [F(4,69) = 15.391, p < 0.001]; 4000 Hz [F(4,69) = 4.494, p = 0.007]; 8000 Hz [F(4,69) = 7.529, p < 0.001]; and 12,000 Hz [F(4,69) = 4.609, p = 0.007]. Of the tested conditions, data within 4000 and 8000 Hz failed the test of equal variance and these data were transformed using a Box-Cox linear transformation performed by Develve v4.7 statistical software. F values, p-values, and degrees of freedom [DF] for this analysis are summarized in Table 2. Although there was no main effect for treatment, post-hoc analysis revealed a small but significant interaction effect between treatment and stimulus duration for one frequency [12000 Hz; F(4,69) = 3.931, p = 0.014].
Fig. 4.
Temporal integration thresholds (n = 7) are shown as a function of duration by test frequency a 1000 Hz, b 2000 Hz, c 4000 Hz, d 8000 Hz, and e 12,000 Hz before and after carboplatin treatment. Temporal integration thresholds were operationally defined at the 66% correct criteria. Post-carboplatin temporal integration thresholds were not statistically different from thresholds obtained before treatment. Individual animal data are plotted pre carboplatin (open symbols) and post carboplatin (closed symbols). Animal symbols are consistent throughout all figures. Mean threshold lines for pre carboplatin (grey, dashed) and post carboplatin (grey, solid) are plotted
Table 1.
F-values and p-values from two-way repeated measures ANOVA for treatment condition (pre vs post carboplatin) are presented for each test frequency (DF = 1.69)
| RM-ANOVA RESULTS | ||
|---|---|---|
| CONDITION (HZ) | F-value | p-value |
| 1000 | 0.234 | 0.646 (n.s.) |
| 2000 | 5.828 | 0.052 (n.s.) |
| 4000 | 3.342 | 0.117 (n.s.)a |
| 8000 | 2.895 | 0.140 (n.s.)a |
| 12,000 | 2.641 | 0.155 (n.s.) |
aDenotes analysis that was conducted using a Box-Cox transform
Table 2.
F-values and p-values from two-way repeated measures ANOVA for stimulus duration (500, 100, 50, 10, and 5 ms) are presented for each test frequency (DF = 4.69)
| RM-ANOVA RESULTS | ||
|---|---|---|
| CONDITION (HZ) | F-value | p-value |
| 1000 | 5.571 | 0.003b |
| 2000 | 15.391 | < 0.001b |
| 4000 | 4.494 | 0.007a,b |
| 8000 | 7.529 | < 0.001 ab |
| 12,000 | 4.609 | 0.007b |
aDenotes analysis that was conducted using a Box-Cox transform
bIndicates statistical significance using alpha level p < .05
Relationship Between IHC Loss and Temporal Integration Thresholds
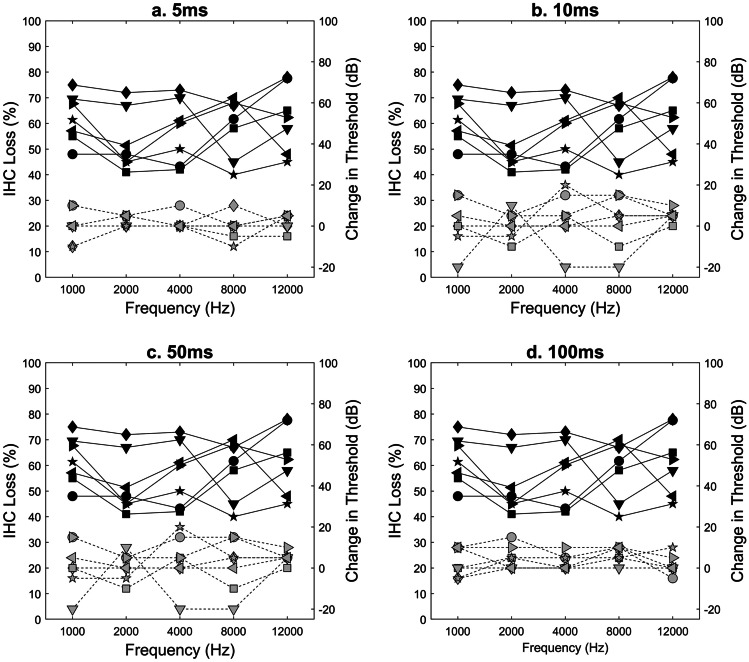
The potential relationship between degree of IHC loss and degree of change in temporal integration thresholds at each duration level (5 ms, 10 ms, 50 ms, and 100 ms) was tested using Spearman’s rank correlation. IHC loss did not significantly correlate with change in threshold at 5 ms, 10 ms, 50 ms, or 100 ms (p > 0.05). Individual animal IHC loss data and change in temporal integration threshold data as a function of frequency at each duration condition are represented in Fig. 5a–d. The change in temporal integration threshold data was calculated by subtracting pre carboplatin thresholds from post carboplatin thresholds at each test frequency for each duration condition.
Fig. 5.
Individual animal IHC loss (percent loss, left y-axis; black symbols and solid lines) and individual animal change in temporal integration threshold (dB, right y-axis, grey symbols, and dashed lines) as a function of frequency at 5, 10, 50, and 100 ms (a–d). Animal symbols are consistent throughout all figures. No significant correlation was found between percent IHC loss and change in temporal integration threshold
Summary of Results
Consistent with our previous studies, carboplatin treatment produced minimal OHC loss and selectively destroyed IHC. There were also no statistically significant differences in pure-tone thresholds (500 ms duration) following carboplatin treatment. As demonstrated in the literature previously, stimulus duration had a significant effect on temporal integration thresholds. Contrary to our hypothesis, however, temporal integration thresholds were not affected by selective IHC loss, suggesting that short-duration threshold measurement is insensitive to this type of cochlear lesion.
DISCUSSION
IHC Loss
Although the animals in this study received the same dose of carboplatin (75 mg/kg, i.p.) used in previous studies, total IHC loss was less than in previous reports (45–70% vs 60–80%). One possibility for this difference was the administration of daily Lactated Ringer’s solution during the recovery period for fluids instead of saline. This change was based on recommendations by the UTSW veterinarians to manage loss of fluid more effectively due to the higher chloride content in saline that can produce vasoconstriction and affect blood flow to the kidney. Because carboplatin is also nephrotoxic, treatment with Lactated Ringer’s solution may have helped clear the carboplatin and overall reduced the ototoxic effects.
Audiometric Pure-tone Thresholds
The insensitivity of the audiogram to IHC loss has been demonstrated previously in our publications (Lobarinas et al. 2013, 2016, 2020). The 500 ms pure-tone threshold results of this study were in agreement with previous reports and showed minimal shifts following carboplatin. These results were expected given the degree of selective IHC loss observed in this experiment.
Temporal Integration Thresholds
Overall, animals showed minimal (1–5 dB increase) or no changes in temporal integration thresholds following carboplatin across duration or frequency conditions. Independent of treatment condition, as tone duration decreased, thresholds predictably increased. The pre-carboplatin findings were in agreement with previous studies that evaluated chinchilla temporal integration and found thresholds increased with shorter-duration stimuli (Henderson 1969; Eddins et al. 1998). Despite IHC loss throughout the cochlea, post-carboplatin temporal integration thresholds remained unchanged from pre-carboplatin thresholds. Furthermore, no relationship between degree of IHC loss and change in temporal integration threshold was found, indicating that the severity of IHC loss in a given animal did not impact delta threshold for that animal. These results suggest that in the absence of OHC loss, relatively few IHC are needed to maintain the temporal integration function.
Classic psychoacoustic studies suggest that individuals with CL have poorer temporal integration than NH individuals (Elliott 1975; Chung 1981; Hall and Fernandes 1983; Carlyon et al. 1990). One possible explanation for degraded performance is that IHC loss or damage could reduce rapid ANF sequential firing needed for acute temporal coding. The volley principle suggests that multiple IHC synapses are recruited and fired asynchronously in order to code small acoustic changes across time (first introduced by Wever and Bray 1930). Based on this model, the lack of available units as a result of damaged IHC would disrupt temporal coding and diminish overall temporal integration. This also fits the “multiple looks” model that proposes listeners obtain multiple samples from a given stimuli, and that longer-duration stimuli affords listeners more samples for improved performance. Based on these models, selective loss of IHC would degrade a listener’s ability to sample effectively due to fewer available sensory cells. However, it is important to note that in human studies of temporal integration, outcomes are characterized by the additional mechanisms associated with age-related hearing loss, noise-induced hearing loss, and genetic differences, including significant loss of OHC and changes in central auditory function. Therefore, it is important to note the inherent challenge in making direct comparisons from the present data to corresponding human studies.
The aforementioned studies in avian species showed a relationship between hair cell loss and reduced temporal integration, suggesting that temporal integration may be mediated by a peripheral mechanism (Saunders et al. 1995; Lauer et al. 2007). In these studies, when chicken hair cells regenerated following sound exposure, the temporal integration function was restored to baseline levels (Saunders et al. 1995). Canaries with damaged and missing hair cells also exhibited poorer temporal integration in comparison to normal control animals (Lauer et al. 2007). In the present study, we induced selective IHC loss but did not alter cochlear nonlinearities associated with OHC loss. In contrast, the disruption of hair cells in these studies was not limited to one type of sensory cell. Of note, avian cochlear anatomy differs from mammalian anatomy in a number of ways including sensory hair cell makeup, i.e., avian cochleae lack inner and outer hair cells and instead are made up of “short” and “tall” sensory hair cells (Janesick et al. 2021). Given that OHC loss alone and selective IHC loss does not appear to impact temporal integration function, it is possible that a presently unknown peripheral mechanism contributes to temporal integration. It is also possible that temporal integration is indeed a central phenomenon, as suggested by the power function model (Gerken et al. 1990). Differences among animal models notwithstanding, we conclude that selective IHC loss does not appear to significantly disrupt temporal integration in the chinchilla.
Across our studies, we have shown that selective IHC loss in the chinchilla model results in functional listening deficits that are not detected by pure-tone thresholds with durations of 500 ms. However, deficits from IHC loss are revealed when assessing suprathreshold measures such as tone-in-noise and gap detection thresholds relative to thresholds assessment in quiet (Lobarinas et al. 2016, 2020). The current study was designed to isolate the effects of selective IHC loss on a single psychoacoustic property by manipulating stimulus duration. Given that we did not find threshold shifts across short-duration conditions in the presence of moderate IHC loss, it is possible that the deficits observed in gap detection and tone-in-noise detection are not the direct result of poorer temporal resolution, although more studies are needed to confirm this. An alternative possibility for our previous gap detection and tone-in-noise results is that IHC loss impairs intensity coding, another key feature of resolving complex auditory stimuli such as speech. Relative intensity changes are also inherent in gap detection tasks and could explain the deficits seen in the lower intensity carrier noise level conditions in our previous experiments (Lobarinas et al. 2020). Gap detection thresholds following selective IHC loss worsened only when the carrier narrowband noise was at the lowest presentation level, and therefore the most difficult condition (40 dB SPL). In that study, both the tested carrier noise levels, 80 dB SPL and 40 dB SPL, were suprathreshold. Thus, if temporal integration was responsible for significant changes, both carrier noise level conditions would have been affected by IHC loss; however, the condition most impacted was the 40 dB SPL carrier, which has a smaller intensity delta. These results suggest that intensity may play a significant role in the observed increased gap detection thresholds, and subsequent research is needed to determine whether selective IHC loss critically impacts intensity discrimination in the chinchilla model.
The overall aim of this study was to assess the effects of selective IHC loss on one metric of sensitivity to changes in the temporal domain by assessing temporal integration. Based on the “multiple looks” model, we hypothesized that a reduction in available IHC would significantly affect the temporal integration function. However, the experimental results failed to support this hypothesis. Additional studies are underway to determine if multiple short stimuli separated by short increments (3–10 ms) or the use of masked stimuli in the temporal integration function is more sensitive to selective loss of IHC.
Abbreviations
- ANOVA
Analysis of variance
- ANF,
Auditory nerve fibers
- CL
Cochlear hearing loss
- dB
Decibel
- IHC
Inner hair cell
- NH
Normal hearing
- OHC
Outer hair cell
- SPL
Sound pressure level
Author Contribution
All authors contributed to data collection and writing of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen JB. Cochlear micromechanics–a physical model of transduction. The Journal of the Acoustical Society of America. 1980;68:1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- Badri R, Siegel JH, Wright BA. Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. The Journal of the Acoustical Society of America. 2011;129:852–863. doi: 10.1121/1.3523476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee EA, Hynson K, Hamernik RP, Henderson D. Asymptotic threshold shift in chinchillas exposed to impulse noise. The Journal of the Acoustical Society of America. 1978;63:876–882. doi: 10.1121/1.381767. [DOI] [PubMed] [Google Scholar]
- Borg E. Loss of hair cells and threshold sensitivity during prolonged noise exposure in normotensive albino rats. Hear Res. 1987;30:119–126. doi: 10.1016/0378-5955(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Buus S, Florentine M, Poulsen T. Temporal integration of loudness in listeners with hearing losses of primarily cochlear origin. The Journal of the Acoustical Society of America. 1999;105:3464–3480. doi: 10.1121/1.424673. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Buus S, Florentine M. Temporal integration of trains of tone pulses by normal and by cochlearly impaired listeners. The Journal of the Acoustical Society of America. 1990;87:260–268. doi: 10.1121/1.399293. [DOI] [PubMed] [Google Scholar]
- Chiu F, Rakusen LL, Mattys SL. Cognitive load elevates discrimination thresholds of duration, intensity, and f0 for a synthesized vowel. The Journal of the Acoustical Society of America. 2019;146:1077. doi: 10.1121/1.5120404. [DOI] [PubMed] [Google Scholar]
- Chung DY. Tone-on-tone masking in subjects with normal hearing and with sensorineural hearing loss. J Speech Hear Res. 1981;24:506–513. doi: 10.1044/jshr.2404.506. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature. 1985;315:662–665. doi: 10.1038/315662a0. [DOI] [PubMed] [Google Scholar]
- Dallos PJ, Johnson KR. Influence of rise-fall time upon short-tone threshold. The Journal of the Acoustical Society of America. 1966;40:1160–1163. doi: 10.1121/1.1910201. [DOI] [PubMed] [Google Scholar]
- Davis B, Qiu W, Hamernik RP. Sensitivity of distortion product otoacoustic emissions in noise-exposed chinchillas. J Am Acad Audiol. 2005;16:69–78. doi: 10.3766/jaaa.16.2.2. [DOI] [PubMed] [Google Scholar]
- Davis RI, Ahroon WA, Hamernik RP. The relation among hearing loss, sensory cell loss and tuning characteristics in the chinchilla. Hear Res. 1989;41:1–14. doi: 10.1016/0378-5955(89)90173-1. [DOI] [PubMed] [Google Scholar]
- DeFilippo CL, Snell KB. Detection of a temporal gap in low-frequency narrow-band signals by normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1986;80:1354–1358. doi: 10.1121/1.394488. [DOI] [PubMed] [Google Scholar]
- Eddins AC, Salvi RJ, Wang J, Powers NL. Threshold-duration functions of chinchilla auditory nerve fibers. Hear Res. 1998;124:190. doi: 10.1016/S0378-5955(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Elliott LL (1975) Temporal and masking phenomena in persons with sensorineural hearing loss. Audiology: official organ of the Int Soc Audio14:336–353 [DOI] [PubMed]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Wightman FL. Gap detection in normal and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1982;72:761–765. doi: 10.1121/1.388256. [DOI] [PubMed] [Google Scholar]
- Florentine M, Buus S. Temporal gap detection in sensorineural and simulated hearing impairments. J Speech Hear Res. 1984;27:449–455. doi: 10.1044/jshr.2703.449. [DOI] [PubMed] [Google Scholar]
- Florentine M, Fastl H, Buus S. Temporal integration in normal hearing, cochlear impairment, and impairment simulated by masking. The Journal of the Acoustical Society of America. 1988;84:195–203. doi: 10.1121/1.396964. [DOI] [PubMed] [Google Scholar]
- Gengel RW, Watson CS. Temporal integration. I. Clinical implications of a laboratory study. II. Additional data from hearing-impaired subjects. J Speech Hear Disord. 1971;36:213–224. doi: 10.1044/jshd.3602.213. [DOI] [PubMed] [Google Scholar]
- Gerken GM, Bhat VK, Hutchison-Clutter M. Auditory temporal integration and the power function model. The Journal of the Acoustical Society of America. 1990;88:767–778. doi: 10.1121/1.399726. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. The Journal of the Acoustical Society of America. 1982;72:1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Giraudi D, Salvi R, Henderson D, Hamernik R. Gap detection by the chinchilla. The Journal of the Acoustical Society of America. 1980;68:802–806. doi: 10.1121/1.384818. [DOI] [PubMed] [Google Scholar]
- Gleich O, Kittel MC, Klump GM, Strutz J. Temporal integration in the gerbil: the effects of age, hearing loss and temporally unmodulated and modulated speech-like masker noises. Hear Res. 2007;224:101–114. doi: 10.1016/j.heares.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Sources of age-related recognition difficulty for time-compressed speech. Journal of Speech, Language, and Hearing Research : JSLHR. 2001;44:709–719. doi: 10.1044/1092-4388(2001/056). [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA. Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. Journal of Speech, Language, and Hearing Research : JSLHR. 2007;50:1181–1193. doi: 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Gorga MP, Beauchaine KA, Reiland JK, Worthington DW, Javel E. The effects of stimulus duration on ABR and behavioral thresholds. The Journal of the Acoustical Society of America. 1984;76:616–619. doi: 10.1121/1.391158. [DOI] [PubMed] [Google Scholar]
- Grose JH, Eddins DA, Hall JW., 3rd Gap detection as a function of stimulus bandwidth with fixed high-frequency cutoff in normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1989;86:1747–1755. doi: 10.1121/1.398606. [DOI] [PubMed] [Google Scholar]
- Hall JW, Fernandes MA. Temporal integration, frequency resolution, and off-frequency listening in normal-hearing and cochlear-impaired listeners. The Journal of the Acoustical Society of America. 1983;74:1172–1177. doi: 10.1121/1.390040. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hear Res. 1989;38:199–211. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Henderson D. Temporal summation of acoustic signals by the chinchilla. The Journal of the Acoustical Society of America. 1969;46:474–475. doi: 10.1121/1.1911714. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36:301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/S0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Janesick A, Scheibinger M, Benkafadar N, Kirti S, Ellwanger DC, Heller S (2021) Cell-type identity of the avian cochlea. Cell Rep 34:108900 [DOI] [PubMed]
- Johnstone BM, Patuzzi R, Yates GK. Basilar membrane measurements and the travelling wave. Hear Res. 1986;22:147–153. doi: 10.1016/0378-5955(86)90090-0. [DOI] [PubMed] [Google Scholar]
- King KA, Gordon-Salant S, Pawlowski KS, Taylor AM, Griffith AJ, Houser A, Kurima K, Wassif CA, Wright CG, Porter FD, Repa JJ, Brewer CC. Hearing loss is an early consequence of Npc1 gene deletion in the mouse model of Niemann-Pick disease, type C. Journal of the Association for Research in Otolaryngology : JARO. 2014;15:529–541. doi: 10.1007/s10162-014-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after "temporary" noiseinduced hearing loss. The Journal of neuroscience: the official journal of the Society for Neuroscience 29:14077–14085 [DOI] [PMC free article] [PubMed]
- Lauer AM, Dooling RJ, Leek MR, Poling K. Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. The Journal of the Acoustical Society of America. 2007;122:3615–3627. doi: 10.1121/1.2799482. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–120. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. Journal of the Association for Research in Otolaryngology : JARO. 2016;17:89–101. doi: 10.1007/s10162-015-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2020) Gap detection deficits in chinchillas with selective carboplatin-induced inner hair cell loss. J Assoc Res Otolaryngol: JARO 21(6):475–483 [DOI] [PMC free article] [PubMed]
- Manheim M, Lavie L, Banai K. Age, hearing, and the perceptual learning of rapid speech. Trends in Hearing. 2018;22:2331216518778651. doi: 10.1177/2331216518778651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ. Chinchilla models of selective cochlear hair cell loss. Hear Res. 2002;174:230–238. doi: 10.1016/S0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Middelweerd MJ, Festen JM, Plomp R (1990) Difficulties with speech intelligibility in noise in spite of a normal pure-tone audiogram. Audiology: official organ of the Int Soc Audio 29:1–7 [DOI] [PubMed]
- Moore BC, Peters RW, Glasberg BR. Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss. The Journal of the Acoustical Society of America. 1992;92:1923–1932. doi: 10.1121/1.405240. [DOI] [PubMed] [Google Scholar]
- Moore BC, Peters RW, Glasberg BR. Effects of frequency and duration on psychometric functions for detection of increments and decrements in sinusoids in noise. The Journal of the Acoustical Society of America. 1999;106:3539–3552. doi: 10.1121/1.428207. [DOI] [PubMed] [Google Scholar]
- Muller M, Hoidis S, Smolders JW. A physiological frequency-position map of the chinchilla cochlea. Hear Res. 2010;268:184–193. doi: 10.1016/j.heares.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Heil P. Towards a unifying basis of auditory thresholds: the effects of hearing loss on temporal integration reconsidered. Journal of the Association for Research in Otolaryngology : JARO. 2004;5:436–458. doi: 10.1007/s10162-004-5031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ, Moore BC, Vickers DA (1997) Short-term temporal integration: evidence for the influence of peripheral compression. J Acoust Soc Am 101:3676–3687 [DOI] [PubMed]
- Palandrani KN, Hoover EC, Stavropoulos T, Seitz AR, Isarangura S, Gallun FJ, Eddins DA. Temporal integration of monaural and dichotic frequency modulation. The Journal of the Acoustical Society of America. 2021;150:745. doi: 10.1121/10.0005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patuzzi RB, Yates GK, Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hear Res. 1989;42:47–72. doi: 10.1016/0378-5955(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Pawlowski K, Koulich E, Wright CG, Roland P (2013) Ototopic applications of povidone iodine/dexamethasone in the rat. Otology & neurotology : official publication of the American Otological Society, Am Neurotol Soc Eur Academy Otol Neurotol 34:167–174 [DOI] [PubMed]
- Pedersen CB, Salomon G. Temporal integration of acoustic energy. Acta Otolaryngol. 1977;83:417–423. doi: 10.3109/00016487709128866. [DOI] [PubMed] [Google Scholar]
- Preyer S, Gummer AW. Nonlinearity of mechanoelectrical transduction of outer hair cells as the source of nonlinear basilar-membrane motion and loudness recruitment. Audiol Neurootol. 1996;1:3–11. doi: 10.1159/000259185. [DOI] [PubMed] [Google Scholar]
- Reed CM, Braida LD, Zurek PM. Review article: review of the literature on temporal resolution in listeners with cochlear hearing impairment: a critical assessment of the role of suprathreshold deficits. Trends Amplif. 2009;13:4–43. doi: 10.1177/1084713808325412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. The Journal of the Acoustical Society of America. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Exp Brain Res. 1978;32:301–320. doi: 10.1007/BF00238704. [DOI] [PubMed] [Google Scholar]
- Saunders SS, Salvi RJ, Miller KM. Recovery of thresholds and temporal integration in adult chickens after high-level 525-Hz pure-tone exposure. The Journal of the Acoustical Society of America. 1995;97:1150–1164. doi: 10.1121/1.412228. [DOI] [PubMed] [Google Scholar]
- Solecki JM, Gerken GM. Auditory temporal integration in the normal-hearing and hearing-impaired cat. The Journal of the Acoustical Society of America. 1990;88:779–785. doi: 10.1121/1.399727. [DOI] [PubMed] [Google Scholar]
- Song Q, Shen P, Li X, Shi L, Liu L, Wang J, Yu Z, Stephen K, Aiken S, Yin S, Wang J (2016) Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Scientific reports 6:25200 [DOI] [PMC free article] [PubMed]
- Spoendlin H. Neuroanatomical basis of cochlear coding mechanisms. Audiology. 1975;14:383–407. doi: 10.3109/00206097509071752. [DOI] [PubMed] [Google Scholar]
- Stephens SD. Auditory temporal integration as a function of intensity. J Sound Vib. 1973;30:109–126. doi: 10.1016/S0022-460X(73)80054-9. [DOI] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y. Induction of selective inner hair cell damage by carboplatin. Scanning Microsc. 1994;8:97–106. [PubMed] [Google Scholar]
- Thibodeau LM. Evaluation of auditory enhancement and auditory suppression in listeners with normal hearing and reduced speech recognition in noise. J Speech Hear Res. 1996;39:947–956. doi: 10.1044/jshr.3905.947. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Trevino M, Lobarinas E, Maulden AC, Heinz MG. The chinchilla animal model for hearing science and noise-induced hearing loss. The Journal of the Acoustical Society of America. 2019;146:3710. doi: 10.1121/1.5132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CW, Robb MP. Audibility and recognition of stop consonants in normal and hearing-impaired subjects. The Journal of the Acoustical Society of America. 1987;81:1566–1573. doi: 10.1121/1.394509. [DOI] [PubMed] [Google Scholar]
- Viemeister NF, Wakefield GH. Temporal integration and multiple looks. The Journal of the Acoustical Society of America. 1991;90:858–865. doi: 10.1121/1.401953. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope. 1994;104:488–493. doi: 10.1288/00005537-199404000-00016. [DOI] [PubMed] [Google Scholar]
- Walden BE, Schwartz DM, Montgomery AA, Prosek RA. A comparison of the effects of hearing impairment and acoustic filtering on consonant recognition. J Speech Hear Res. 1981;24:32–43. doi: 10.1044/jshr.2401.32. [DOI] [PubMed] [Google Scholar]
- Watson CS, Gengel RW. Signal duration and signal frequency in relation to auditory sesitivity. The Journal of the Acoustical Society of America. 1969;46:989–997. doi: 10.1121/1.1911819. [DOI] [PubMed] [Google Scholar]
- Wever EG, Bray CW. Auditory nerve impulses. Science. 1930;71:215. doi: 10.1126/science.71.1834.215.a. [DOI] [PubMed] [Google Scholar]