Abstract
In a randomized crossover study, 16 volunteers (8 men, 8 women) received single oral doses of 320 mg of gemifloxacin and 400 mg of ofloxacin on two separate occasions in the fasting state to assess the urinary excretion and urinary bactericidal titers (UBTs) at intervals for up to 144 h. Ofloxacin showed higher concentrations in urine compared with those of gemifloxacin. The median (range) cumulative excretion of gemifloxacin was 29.7% (8.4 to 48.7%) of the parent drug administered, and median (range) cumulative excretion of ofloxacin was 84.3% (46.5 to 95.2%) of the parent drug administered. The UBTs, i.e., the highest twofold dilutions (with antibiotic-free urine as the diluent) of urine that were still bactericidal, were determined for a reference strain and nine uropathogens for which the MICs of gemifloxacin and ofloxacin were as follows: Escherichia coli ATCC 25922, 0.016 and 0.06 μg/ml, respectively; Klebsiella pneumoniae, 0.03 and 0.06 μg/ml, respectively; Proteus mirabilis, 0.125 and 0.125 μg/ml, respectively; Escherichia coli, 0.06 and 0.5 μg/ml, respectively; Pseudomonas aeruginosa, 1 and 4 μg/ml, respectively; Staphylococcus aureus, 0.008 and 0.25 μg/ml, respectively; Enterococcus faecalis, 0.06 and 2 μg/ml, respectively; Staphylococcus aureus, 0.25 and 4 μg/ml, respectively; Enterococcus faecalis, 0.5 and 32 μg/ml, respectively; and Staphylococcus aureus, 2 and 32 μg/ml, respectively. Generally, the UBTs for gram-positive uropathogens were higher for gemifloxacin than for ofloxacin and the UBTs for gram-negative uropathogens were higher for ofloxacin than for gemifloxacin. According to the UBTs, ofloxacin-resistant uropathogens (MICs, ≥4 mg/liter) should also be considered gemifloxacin resistant. Although clinical trials have shown that gemifloxacin is effective for the treatment of uncomplicated urinary tract infections, whether an oral dosage of 320 mg of gemifloxacin once daily is also adequate for the treatment of complicated urinary tract infections has yet to be confirmed.
The rationale for the use of an antimicrobial agent in the management of infectious diseases is its antibacterial activity at the site of the inflammatory process. In the case of urinary tract infection (UTI), the antibacterial spectrum of the antimicrobial agent should include not only Escherichia coli and other species of the family Enterobacteriaceae but also enterococci, staphylococci, and nonfermenting bacteria such as Pseudomonas and Acinetobacter spp. The fluoroquinolones, such as ciprofloxacin and ofloxacin or levofloxacin, which have broad-spectrum antibacterial activities against most gram-negative uropathogens, and the more recent members of this class, which are active against gram-positive uropathogens, are recommended as first-line agents for the treatment of complicated and nosocomial UTIs.
Gemifloxacin, a new fluoroquinolone, has a broad spectrum of activity in vitro against gram-negative bacteria such as E. coli, Proteus mirabilis, some strains of Pseudomonas aeruginosa, and Klebsiella pneumoniae, and against gram-positive bacteria such as streptococci and staphylococci (1). Gemifloxacin has a long half-life in plasma of 8 to 10 h, and approximately 20 to 30% is excreted unchanged into urine (2), with the concentrations in urine thereby reaching those that should provide sufficient antibacterial activity against most bacteria potentially involved in the pathogenesis of UTIs.
The potential antibacterial activity at the site of infection is usually judged by the drug concentrations measured in vivo and the minimal bactericidal concentration (MBC) and MIC determined in vitro. As the antibacterial activities of fluoroquinolones may be inhibited in urine in relation to its pH and contents (8), the antibacterial activities of these agents should be assessed directly in urine. To combine pharmacokinetic and pharmacodynamic properties, the concentrations in urine and urinary bactericidal titers (UBTs) for isolates of the most common uropathogenic bacterial species were determined to approximate more closely their antibacterial activities at the site of infection (7, 10, 13). In the phase I comparative study with a crossover design described here, these parameters were determined after administration of a single oral dose of gemifloxacin (320 mg) and a single oral dose of ofloxacin (400 mg) to healthy volunteers.
MATERIALS AND METHODS
Study design and subject population.
The study was approved by the ethics committee of the Landesärztekammer Bayern. A total of 16 volunteers (8 men, 8 women) were enrolled in this open, randomized, crossover clinical trial. The mean age was 31.5 years (median, 31.5 years; range, 18 to 40 years), the mean body weight was 69.0 kg (median, 66.5 kg; range, 53.3 to 96.7 kg), and the mean height was 171.0 cm (median, 173.5 cm; range, 160 to 179 cm). The individuals were considered healthy on the basis of assessment of medical history, physical examination, hematology parameters (hemoglobin concentration and erythrocyte, leukocyte, and platelet counts), serum chemistry parameters (creatinine, uric acid, γ-glutamyl transferase, alkaline phosphatase, and total bilirubin levels), and urinalysis (glucose and protein levels, white and red blood cell counts, and lack of antibacterial activity, i.e., inhibition of Bacillus subtilis).
After giving written informed consent to participate in the study, the individuals successively received 320 mg of gemifloxacin or 400 mg of ofloxacin in a crossover design at an interval of 14 days according to the randomization schedule. Study drugs were administered with 150 ml of water after a 12-h fasting period and 2 h before a standardized breakfast. A standard lunch and supper were consumed 6 and 10 h after drug intake. The consumption of xanthine derivatives (e.g., caffeine), grapefruit juice, and alcohol was not permitted for up to 48 h after drug administration. The volunteers were asked to drink sufficient and comparable amounts of water to ensure sufficient urine production through both collection periods. Before and after each study phase a physical examination, including electrocardiography, and laboratory tests were performed. Adverse events were recorded continuously throughout the trial period. The volunteers were monitored and urine collection was controlled in the study center for the first 48 h and thereafter at the end of each sampling period for up to 144 h.
Sample collection.
All urine voided was collected over a 12-h interval prior to drug administration and at the following time intervals thereafter: 0 to 6, 6 to 12, 12 to 24, 24 to 48, 48 to 72, 72 to 96, 96 to 120, and 120 to 144 h. All samples were stored at −20°C until assayed.
Sample preparation. (i) Gemifloxacin.
All sample handling was done under protection from daylight. Urine samples were thawed, vigorously mixed, and then diluted with a mobile phase (1:8). After thorough mixing, 10 μl of the diluted samples was analyzed.
(ii) Ofloxacin.
All sample handling was done under protection from daylight. Urine samples were thawed and vigorously mixed, and then 20 μl of each urine sample for ofloxacin assay was diluted with buffer (1:100). After thorough mixing, 10 μl of the diluted samples was analyzed.
Assay conditions. (i) Gemifloxacin.
Chromatographic separation was performed with a reversed-phase column (50 by 4.6 mm) with elution with an isocratic solvent system consisting of 0.01 M ammonium acetate buffer (pH 2.5) and acetonitrile (96/4; vol/vol) and was monitored by liquid chromatography-mass spectroscopy and mass spectroscopy (gemifloxacin, m/z 390 → m/z 313; internal standard, m/z 394 → m/z 313). Under these conditions, gemifloxacin and the internal standard (deuterated gemifloxacin) eluted after approximately 1.1 min. The data acquisition was done with RAD (version 2.6; PE Sciex, Thornhill, Ontario, Canada), and the data processing was done with MacQuan software (version 1.5; 1991 to 1997; Perkin-Elmer, Toronto, Ontario, Canada). The details of the method have been reported previously (6).
(ii) Ofloxacin.
Chromatographic separation was performed with a reversed-phase column, and the effluent was monitored by fluorescence detection (excitation, 293 nm; emission, 490 nm). The method of measuring fluoroquinolones in biological material has been described previously (9). Turbochrom 3 software (version 3.2; 1991; PE Nelson, Cupertino, Calif.) was used for evaluation of chromatograms.
Calibration row and spiked quality controls. (i) Gemifloxacin.
The concentrations in urine samples were measured against a urine calibration row. Calibration standards were prepared by adding the appropriate volumes of a standard solution of gemifloxacin to drug-free human urine. Concentrations were obtained down to a concentration of 0.010 μg/ml of urine.
Spiked quality controls (SQCs) were prepared for determination of interassay variation by the addition of the appropriate volumes of a standard solution of gemifloxacin to a drug-free human urine sample. No interference was observed for gemifloxacin or the internal standard. Weighted linear regression (1/peak area ratio2) was performed for calibration. The linearity of the calibration could be proven for concentrations between 0.010 and 5.00 μg/ml. Quantification levels were identical to the lowest calibration levels. The interassay precisions of the SQCs were 4.7% (4.00 μg/ml), 3.0% (1.00 μg/ml), and 4.6% (0.04 μg/ml); and the accuracies of the standards ranged from 95.8 to 108.2%. The intra-assay precisions of the SQCs ranged from 1.3 to 6.5%, and the accuracies of the standards ranged from 99.2 to 107.4%.
(ii) Ofloxacin.
The concentrations in urine samples were analyzed against a urine calibration row, which was prepared essentially as described above, except for the highest calibration level, which was prepared by dissolving 0.020 g of ofloxacin in 20 ml of tested drug-free urine.
SQCs were prepared for determination of interassay variation by the addition of defined amounts of the stock solution or the spiked control of higher concentration to defined amounts of tested drug-free urine. No interference was observed for ofloxacin or the internal standard. Weighted linear regression (1/concentration) was performed for calibration. The linearity of the calibration could be proven in urine for concentrations between 0.208 and 1,008 μg/ml. Quantification levels were identical to the lowest calibration levels. The interassay precisions of the SQCs were 1.4% (808.5 μg/ml), 1.7% (62.61 μg/ml), 2.2% (4.896 μg/ml), and 6.4% (0.4864 μg/ml); and the accuracies of the standards ranged from 99.1 to 102.5%. The intra-assay precisions of the SQCs ranged from 1.1 to 6.9%, and the accuracies of the standards ranged from 101.1 to 106.9%.
Determination of MICs and MBCs.
The MICs and MBCs were determined by a microdilution method with Mueller-Hinton broth (CM 405; Unipath, Wesel, Germany) with an inoculum of 1.3 × 105 to 9.4 × 105 CFU/ml. The lowest concentration that inhibited visible growth after incubation at 37°C for 18 h was defined as the MIC, while the MBC was defined in a second step by counting the numbers of CFU on antimicrobial-free Columbia agar (Merck, Darmstadt, Germany) supplemented with 5% blood, after additional incubation at 37°C for 18 h. Bactericidal activity was defined as a >99.9% (>3-log) reduction in the numbers of CFU.
Determination of UBTs.
A logarithmic serial dilution (dilution range, 1:2 to 1:1,024) was prepared by taking a 1:1 mixture of the urine sample and the individual's antimicrobial-free urine collected prior to drug administration (7, 10, 13). UBTs were then determined by microdilution, with each well of the microplates containing 100 μl of the prepared dilution. The final inoculum was 105 CFU/ml, and the bactericidal activity was determined according to the recommended guidelines of NCCLS (11). A UBT of 0 was defined as no bactericidal activity, and a UBT of 1:1 was used when only undiluted urine displayed bactericidal activity.
MUBCs.
The division of the antimicrobial concentrations in the urine samples by the corresponding UBTs yielded the respective minimal urinary bactericidal concentrations (MUBCs). For this calculation, UBTs of 0 and 1:≥1,024 were not appropriate.
Test organisms.
UBTs were measured for the reference strain, E. coli ATCC 25922, which is susceptible to nalidixic acid, and nine clinical isolates which were obtained from patients with complicated UTIs: one strain each of E. coli (resistant to nalidixic acid), K. pneumoniae, P. mirabilis, P. aeruginosa, three strains of Staphylococcus aureus, and two strains of Enterococcus faecalis.
Statistical analyses.
UBTs were transformed into ordinal data by using a scale from 1 for UBTs of 0 to 12 for UBTs of 1:≥1,024. The area under the UBT-versus-time curve (AUBT) was calculated by the trapezoidal rule by using the UBT steps (ordinal data) for each test organism and for each drug phase. Summary AUBT data were compared by the paired t test. An α value equal to 0.05 was chosen to determine statistical significance.
RESULTS
Safety and laboratory test results.
Both study drugs were well tolerated. No deaths or serious adverse experiences were considered to be related to a study medication. Nine subjects reported a total of 20 treatment-emergent adverse experiences (AEs) after dosing with ofloxacin, and the same number of subjects reported 19 AEs after dosing with gemifloxacin. The most frequent AEs (reported by more than one subject) after ofloxacin were headache (n = 6), fatigue (n = 4), vertigo (n = 2), and nausea (n = 2), while for gemifloxacin the most frequent AEs were headache (n = 3), fatigue (n = 2), and increased sweating (n = 2). The majority of the AEs were mild or moderate in severity, with the exception of one severe headache (gemifloxacin). The physical examination, including electrocardiography, and the laboratory parameters displayed no clinically relevant changes.
Urinary pHs, volumes, and drug concentrations
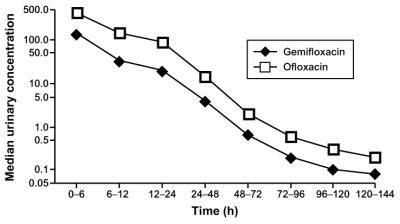
The median (range) values for urinary pHs, volumes, drug concentrations, and cumulative renal excretion obtained in the two study phases are given in Table 1. The data for the corresponding collection periods of the respective study phases showed no marked differences in urinary pHs or volumes. In both study phases, however, the median pH for the collection period from 6 to 12 h (afternoon) was higher than those for the other collection periods. Ofloxacin showed the highest concentrations in urine at 0 to 6 h, with a median of 427 mg/liter, followed by 145 mg/liter at 6 to 12 h and 93 mg/liter at 12 to 24 h. The corresponding concentrations of gemifloxacin in urine were lower, with medians of 144, 33, and 20 mg/liter, respectively (Fig. 1). All urine samples collected prior to drug administration had no detectable drug. The median renal excretions up to 144 h were 84.3% of the dose for ofloxacin and 29.7% of the dose for gemifloxacin.
TABLE 1.
Median (range) urinary pH, volume, parent drug concentration, and cumulative excretion in volunteersa
| Drug and collection period (h) | Urinary pH | Vol (ml) | Conc (mg/liter) | Cumulative excretion (%) |
|---|---|---|---|---|
| Gemifloxacin | ||||
| 0–6 | 5.9 (5.2–8.3) | 293 (72–995) | 144 (59–282) | 16.5 (2.4–23.9) |
| 6–12 | 8.2 (5.9–8.5) | 529 (28–1,195) | 33 (11–70) | 22.5 (4.3–40.9) |
| 12–24 | 6.2 (5.2–7.0) | 713 (309–1,229) | 20 (6–40) | 27.3 (7.2–45.6) |
| 24–48 | 6.2 (5.7–8.2) | 1,368 (671–3,064) | 4 (1–13) | 29.1 (8.1–47.9) |
| 48–72 | 6.6 (5.2–8.0) | 1,534 (842–3,896) | 0.6 (0.1–2) | 29.4 (8.3–48.3) |
| 72–96 | 6.3 (5.6–7.8) | 1,240 (566–2,845) | 0.2 (0.1–0.6) | 29.5 (8.3–48.5) |
| 96–120 | 6.5 (5.3–8.1) | 1,304 (708–2,882) | 0.1 (0.06–0.4) | 29.6 (8.4–48.6) |
| 120–144 | 6.5 (5.4–7.8) | 1,442 (643–3,481) | 0.08 (0.02–0.1) | 29.7 (8.4–48.7) |
| Ofloxacin | ||||
| 0–6 | 5.8 (5.2–8.4) | 417 (157–784) | 427 (227–1107) | 41.8 (18.9–52.5) |
| 6–12 | 7.8 (5.8–8.9) | 623 (64–1450) | 145 (67–327) | 60.4 (31.1–75.9) |
| 12–24 | 6.1 (5.3–7.5) | 664 (316–1690) | 93 (25–197) | 75.5 (42.1–90.4) |
| 24–48 | 6.3 (5.6–8.0) | 1,495 (599–3,235) | 15 (2–30) | 82.9 (44.6–94.4) |
| 48–72 | 6.2 (5.3–7.8) | 1,353 (523–2,461) | 2 (0.8–4) | 83.9 (46.1–94.9) |
| 72–96 | 6.1 (5.4–7.8) | 1,263 (621–2,972) | 0.6 (0.2–1) | 84.1 (46.4–95.0) |
| 96–120 | 6.0 (5.3–7.6) | 1,465 (564–2,276) | 0.3 (LLQb–0.7) | 84.2 (46.5–95.1) |
| 120–144 | 6.6 (5.3–8.2) | 1,311 (691–2,210) | 0.2 (LLQ–0.3) | 84.3 (46.5–95.2) |
A total of 16 volunteers were tested.
LLQ, lower level of quantification.
FIG. 1.
Median (n = 16) concentrations of gemifloxacin (320 mg) and ofloxacin (400 mg) in urine (see Table 1 for concentration ranges).
MICs and MBCs.
The MICs of gemifloxacin and ofloxacin for each of the test organisms, which ranged from 0.008 to 2 mg/liter for gemifloxacin and 0.06 to 32 mg/liter for ofloxacin, are given in Table 2. The corresponding MBCs were identical for most strains or were only 1 dilution step higher (for two strains and for ofloxacin only), except for the enterococcal strain, strain 55, for which the MBCs were fourfold the MICs of both substances.
TABLE 2.
MICs and MBCs of gemifloxacin and ofloxacin for an E. coli test strain (ATCC 25922) and nine uropathogens cultured from the urine of patients with complicated UTIs
| Test strain | Laboratory strain no. | Inoculum (105 CFU/ml) | MIC/MBC (mg/liter)
|
|
|---|---|---|---|---|
| Gemifloxacin | Ofloxacin | |||
| E. coli | ATCC 25922 | 1.3 | 0.016/0.016 | 0.06/0.06 |
| K. pneumoniae | 595 | 3.7 | 0.03/0.03 | 0.06/0.06 |
| P. mirabilis | 414 | 2.5 | 0.125/0.250 | 0.125/0.125 |
| E. coli | 523 | 3.4 | 0.06/0.06 | 0.5/0.05 |
| P. aeruginosa | 568 | 5.6 | 1/2 | 4/4 |
| S. aureus | 83 | 7.1 | 0.008/0.008 | 0.25/0.25 |
| E. faecalis | 60 | 7.0 | 0.06/0.06 | 2/2 |
| S. aureus | 161 | 9.4 | 0.25/0.25 | 4/4 |
| S. aureus | 636 | 5.6 | 2/2 | 32/32 |
| E. faecalis | 55 | 6.0 | 0.5/2 | 32/128 |
UBTs and AUBTs.
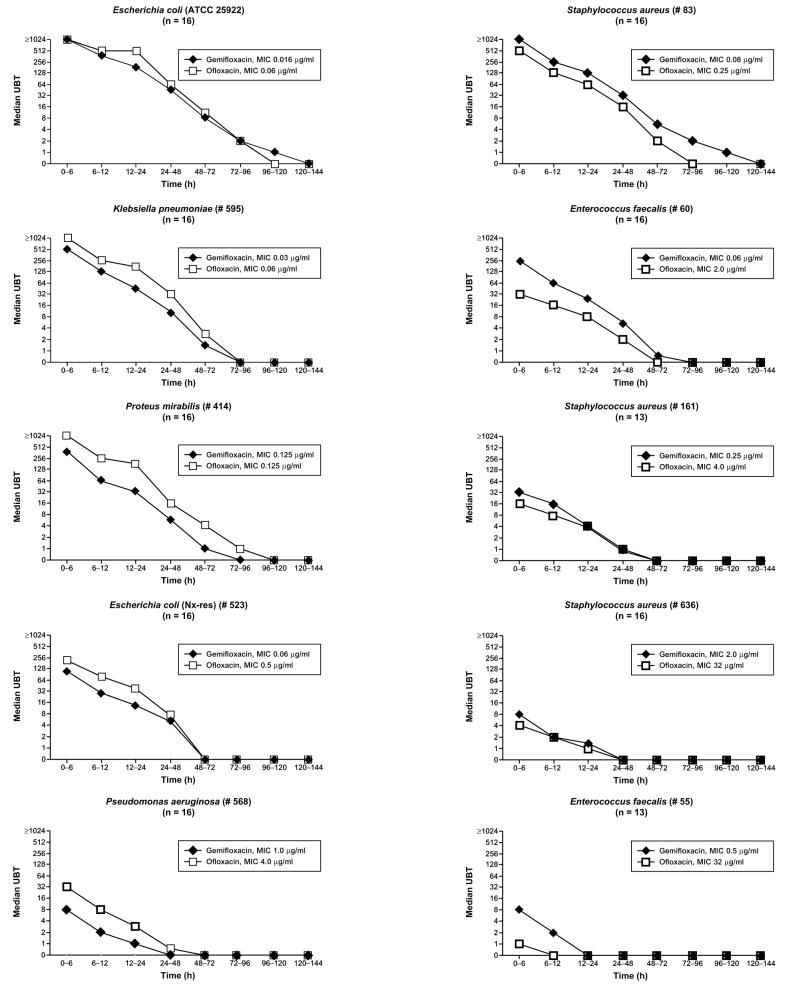
The UBTs and AUBTs for both study drugs for the test organisms are given in Table 3. Before drug intake none of the urine samples showed detectable antibacterial activity against the test strains. For gram-negative uropathogens UBTs and AUBTs were, in general, higher for ofloxacin than for gemifloxacin, and for gram-positive uropathogens they were higher for gemifloxacin than for ofloxacin. In particular, the median UBTs of gemifloxacin were present for up to 5 days for reference strain E. coli ATCC 25922 and decreased from 1:≥1,024 to 1:1. For ofloxacin, they were present for 4 days and decreased from 1:≥1,024 to 1:2 (Fig. 2). The AUBTs of ofloxacin and gemifloxacin were comparable for the reference strain. For the other test organisms, the UBTs were higher for ofloxacin for gram-negative organisms and were higher throughout the study period for gemifloxacin for gram-positive test strains; these data were also reflected by the corresponding AUBTs. Table 4 shows the number of volunteers for whom no urinary bactericidal activity was found in one of the first three collection periods (first 24 h). It can be seen that this was the case for gemifloxacin and ofloxacin for the four strains for which the ofloxacin MIC was ≥4 mg/liter and for gemifloxacin only in the third collection period (12 to 24 h) against the E. coli strain, which was resistant to nalidixic acid.
TABLE 3.
Median (range) reciprocal UBTs for gemifloxacin and ofloxacin
| Drug and strain (no. of volunteers tested) | UBT for the following collection period (h):
|
AUBT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–6 | 6–12 | 12–24 | 24–48 | 48–72 | 72–96 | 96–120 | 120–144 | ||
| Gemifloxacin | |||||||||
| E. coli ATCC 25922 (16) | ≥1,024 (128–≥1,024) | 384 (32–≥1,024) | 192 (4–≥1,024) | 48 (2–128) | 8 (0–32) | 2 (0–16) | 1 (0–4) | 0 (0–2) | 546 (174–816) |
| K. pneumoniae 595 (16) | 512 (16–≥1,024) | 128 (8–256) | 48 (1–256) | 12 (0–64) | 1.5 (0–8) | 0 (0–4) | 0 (0–1) | 0 | 321a (72–564) |
| P. mirabilis 414 (16) | 384 (32–≥1,204) | 64 (16–512) | 32 (1–512) | 6 (1–64) | 1 (0–4) | 0 (0–2) | 0 | 0 | 261a (120–510) |
| E. coli (Nx-res) 523 (16) | 128 (8–512) | 32 (4–128) | 16 (0–64) | 6 (0–16) | 0 (0–2) | 0 (0–1) | 0 | 0 | 237a (48–384) |
| P. aeruginosa 568 (16) | 8 (1–32) | 2 (0–8) | 1 (0–4) | 0 (0–1) | 0 | 0 | 0 | 0 | 48a (12–300) |
| S. aureus 83 (16) | ≥1,024 (128–≥1,024) | 256 (64–≥1,024) | 128 (8–512) | 32 (2–128) | 6 (1–32) | 2 (0–16) | 1 (0–4) | 0a (0–4) | 540a (246–834) |
| E. faecalis 60 (16) | 256 (32–512) | 64 (16–128) | 24 (2–128) | 6 (0–64) | 0.5 (0–4) | 0 (0–4) | 0 | 0 | 246a (96–516) |
| S. aureus 161 (13) | 32 (4–256) | 16 (2–128) | 4 (0–128) | 1 (0–16) | 0 (0–8) | 0 | 0 | 0 | 108 (0–408) |
| S. aureus 636 (16) | 8 (0–32) | 2 (0–8) | 1.5 (0–4) | 0 (0–2) | 0 (0–1) | 0 | 0 | 0 | 60a (0–144) |
| E. faecalis 55 (13) | 8 (1–32) | 2 (1–8) | 0 (0–2) | 0 (0–1) | 0 | 0 | 0 | 0 | 39a (0–102) |
| Ofloxacin | |||||||||
| E. coli ATCC 25922 (16) | ≥1,024 (256–≥1,024) | 512 (32–≥1,024) | 512 (8–≥1,024) | 64 (4–512) | 12 (0–64) | 2 (0–16) | 0 (0–4) | 0 (0–2) | 537 (258–888) |
| K. pneumoniae 595 (16) | ≥1,024 (64–≥1,024) | 256 (16–≥1,024) | 192 (16–≥1,024) | 32 (2–128) | 3 (0–16) | 0 (0–4) | 0 (0–1) | 0 (0–1) | 414a (180–636) |
| P. mirabilis 414 (16) | ≥1,024 (512–≥1,024) | 256 (64–≥1,024) | 192 (16–512) | 16 (2–128) | 4 (0–16) | 1 (0–8) | 0 (0–1) | 0 | 438a (276–654) |
| E. coli (Nx-res) 523 (16) | 256 (16–≥1,024) | 96 (8–512) | 48 (4–256) | 8 (0–64) | 0 (0–4) | 0 (0–1) | 0 | 0 | 276a (90–516) |
| P. aeruginosa 568 (16) | 32 (4–128) | 8 (2–32) | 3 (0–8) | 0.5 (0–4) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 99a (42–258) |
| S. aureus 83 (16) | 512 (128–≥1024) | 128 (4–512) | 64 (2–256) | 16 (2–64) | 2 (0–8) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 387a (162–570) |
| E. faecalis 60 (16) | 32 (8–256) | 16 (8–64) | 8 (4–16) | 2 (0–4) | 0 (0–1) | 0 | 0 | 0 | 135a (90–204) |
| S. aureus 161 (13) | 16 (8–256) | 8 (0–64) | 4 (0–32) | 1 (0–2) | 0 | 0 | 0 | 0 | 105 (0–279) |
| S. aureus 636 (16) | 4 (0–32) | 2 (0–8) | 1 (0–2) | 0 (0–1) | 0 | 0 | 0 | 0 | 39a (0–78) |
| E. faecalis 55 (13) | 1 (0–8) | 0 (0–2) | 0 (0–2) | 0 | 0 | 0 | 0 | 0 | 0a (0–54) |
Significantly different (paired t test; P < 0.05) for gemifloxacin versus ofloxacin.
FIG. 2.
Median reciprocal UBTs of gemifloxacin (320 mg) and ofloxacin (400 mg) for E. coli ATCC 25922 and nine clinical uropathogens (see Table 3 for ranges).
TABLE 4.
Number of volunteers showing no urinary bactericidal activity during the first three collection periods (24 h)
| Strain (no. of volunteers) | No. of volunteers with no urinary bactericidal activity/total no. tested
|
|||||
|---|---|---|---|---|---|---|
| Gemifloxacin
|
Ofloxacin
|
|||||
| 0–6 | 6–12 | 12–24 | 0–6 | 6–12 | 12–24 | |
| E. coli ATCC 25922 (16) | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| K. pneumoniae 595 (16) | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| P. mirabilis 414 (16) | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| E. coli (Nx-res) 523 (16) | 0/16 | 0/16 | 1/16 | 0/16 | 0/16 | 0/16 |
| P. aeruginosa 568 (16) | 0/16 | 1/16 | 6/16 | 0/16 | 0/16 | 1/16 |
| S. aureus 83 (16) | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| E. faecalis 60 (16) | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| S. aureus 161 (13) | 0/13 | 0/13 | 2/13 | 0/13 | 1/13 | 1/13 |
| S. aureus 636 (16) | 2/16 | 3/16 | 5/16 | 2/16 | 3/16 | 7/16 |
| E. faecalis 55 (13) | 0/13 | 0/13 | 7/13 | 6/13 | 9/13 | 12/13 |
MUBCs.
The median (range) MUBCs of both study drugs for the test organisms are given in Table 5. They showed wide inter- and intraindividual ranges and were between 1.1 and 28.2 times higher than the corresponding MICs and MBCs determined in Mueller-Hinton broth (Table 2).
TABLE 5.
Median (range) MUBCs
| Organism (strain) | No. of volunteers for gemifloxacin (n)a | MUBCb for gemifloxacin (μg/ml) | No. of volunteers for ofloxacin (n) | MUBCb for ofloxacin (μg/ml) |
|---|---|---|---|---|
| E. coli (ATCC 25922) | 16 (16) | 0.09 (0.03–1.84) | 16 (16) | 0.27 (0.02–2.51) |
| K. pneumoniae (595) | 16 (32) | 0.42 (0.06–7.27) | 16 (16) | 0.42 (0.08–6.62) |
| P. mirabilis (414) | 16 (48) | 0.63 (0.04–11.0) | 16 (32) | 0.51 (0.17–5.69) |
| E. coli (523) | 16 (32) | 1.02 (0.19–10.4) | 16 (32) | 2.27 (0.26–20.4) |
| P. aeruginosa (568) | 16 (16) | 18.2 (5.29–83.1) | 16 (32) | 15.0 (5.78–109.2) |
| S. aureus (83) | 16 (48) | 0.14 (0.02–1.15) | 16 (16) | 0.27 (0.20–2.51) |
| E. faecalis (60) | 16 (48) | 1.10 (0.01–11.1) | 16 (48) | 11.7 (4.09–38.6) |
| S. aureus (161) | 13 (26) | 3.34 (0.16–20.8) | 13 (13) | 17.0 (2.64–54.6) |
| E. faecalis (55) | 13 (26) | 14.1 (3.70–83.1) |
Values in parentheses are numbers of datum points. b For calculation of the MUBC, only pairs of data with urinary concentration and UBT (different from 0 or ≥1,024) were used if available for all volunteers of the group.
DISCUSSION
Gemifloxacin is a new fluoroquinolone which has a broad antibacterial spectrum in vitro, including various gram-negative and gram-positive strains. Due to its long half-life in plasma and the observed concentrations in urine, it can be considered suitable for the treatment of UTIs, which are among the most frequent bacterial infections in humans. In the present study, we investigated a single oral dose of gemifloxacin (320 mg) and, as a control, a single oral dose of ofloxacin (400 mg), which has been in clinical use for the treatment of complicated and uncomplicated UTIs for several years.
Concentrations in urine and the cumulative renal excretion rate were, in general, higher for ofloxacin than for gemifloxacin, whereas the reported in vitro activity against the test strains was generally higher for gemifloxacin (except against P. mirabilis). However, the bactericidal and inhibitory activities of antimicrobial agents derived from the comparison of data derived from cultures in nutrient broth and individual concentrations in urine sometimes hardly reflect the variability of individual responses to the treatment of UTIs in clinical situations. The estimation of UBTs by use of dilutions with the individual's antimicrobial-free urine may be considered more appropriate for approximation of the expected in vivo activity of an antimicrobial agent (7, 8, 10, 13). Contrary to the results of MIC testing in vitro, the estimation of UBTs yielded higher values for gemifloxacin only for gram-positive strains, whereas for gram-negative uropathogens, UBTs were higher for ofloxacin.
Similar to previous reports, the interindividual variability in the present study is reflected by the wide ranges in the UBTs and MUBCs, although the coefficient of variation of the laboratory method was proven to be rather low (13). As it is the aim of antibacterial treatment to reach efficacy in all treated individuals, the lower range of the UBT results may be regarded as the relevant parameter for clinical dosage recommendations.
Experimental animal and clinical studies have suggested that the effectiveness of fluoroquinolones in the treatment of infectious diseases is more dependent on the area under the concentration-time curve (AUC)/MIC and peak/MIC ratios than on the time above the MIC (5). An AUC/MIC ratio of ≥100 in animal models and an AUC/MIC ratio of ≥125 in clinical studies in seriously ill patients were associated with favorable outcomes (no mortality). To prevent the emergence of resistant mutants during therapy with fluoroquinolones, even higher ratios are necessary (4, 12). These data, however, correspond to levels of total and unbound drug combined in serum and use MICs obtained under standard conditions in a test tube. Therefore, the results from the present study cannot directly be translated into those from that system.
Since the UBTs desirable for the treatment of UTIs have not yet been assessed in clinical trials, only comparative studies with an established antibacterial substance with the same mode of pharmacodynamic action can provide meaningful conclusions. In this context, ofloxacin can be considered a suitable comparator, as it is a fluoroquinolone agent which has been widely used for the treatment of various kinds of UTIs. The advantage of measuring UBTs for comparison is obvious. The possible differences in the intrinsic antibacterial activity of the bladder against the test strains in this environment (urine) and the differences in pharmacokinetic properties, such as bioavailability and renal excretion, need not be considered in addition, and the UBTs obtained with the two study drugs can be compared directly. Since it can be assumed that the pharmacodynamic modes of action of these two fluoroquinolones are comparatively similar in broth, serum, and urine, it can be concluded that higher UBTs are also more favorable in terms of clinical efficacy and the prevention of the emergence of resistant mutants. However, as the UBTs were determined ex vivo, other defense mechanisms which might be relevant in vivo could not be considered in the present study.
Since multiday treatment is required for the treatment of complicated UTIs, the UBTs up to 24 h after administration of the first dose are of special interest, in particular, for gemifloxacin, for which the recommended dosing is once daily. With ofloxacin, the MIC of which was ≥4 mg/liter for three of the four strains, at least one of the volunteers (see lower range) exhibited no urinary bactericidal activity from either the first (0 to 6 h) or the second (6 to 12 h) collection period, while for the pseudomonal strain, there was no bactericidal activity from the third period (12 to 24 h) onwards. These strains are classified resistant according to DIN 58940-4 (3). Although the MICs of gemifloxacin for these four ofloxacin-resistant strains are 2 to 6 dilution steps lower, after administration of the first 320-mg dose of gemifloxacin, at least one of the volunteers also exhibited no urinary bactericidal activity against the pseudomonal strain (MIC, 1 mg/liter) and one of the gram-positive strains (MIC, 2 mg/liter) either from the first (0 to 6 h) or the second (6 to 12 h) collection period onwards or against staphylococcal strain 161 (MIC, 0.25 mg/liter) and E. faecalis strain 55 (MIC, 0.5 mg/liter) from the third collection period (12 to 24 h) onwards. From these findings it can be concluded that uropathogens resistant to ofloxacin should also be regarded as gemifloxacin resistant, despite the lower MICs. This means that the breakpoint for gemifloxacin-resistant uropathogens should be lowered accordingly.
The reason for that might be explained by the lower level of renal excretion of gemifloxacin and the fairly wide range of rates of renal excretion of gemifloxacin. If the lower ranges of the renal excretion rates up to 24 h are compared, a ratio of about 1:6 in favor of ofloxacin is found. Considering that the dose of gemifloxacin (320 mg) was, in addition, lower than that of ofloxacin (400 mg), the corresponding MIC breakpoint for resistance to gemifloxacin would be about 3 dilution steps lower than that for resistance to ofloxacin only in terms of pharmacokinetic considerations.
In particular, the following conclusions may be drawn from the present study. (i) In general, gemifloxacin had higher in vitro activity against the test strains (except for P. mirabilis). (ii) UBTs were higher for gemifloxacin for gram-positive strains, whereas UBTs were higher for ofloxacin for gram-negative strains. (iii) According to UBTs, ofloxacin-resistant uropathogens should also be considered gemifloxacin resistant. (iv) Whether an oral dosage of 320 mg of gemifloxacin once daily is adequate for the treatment of complicated UTIs in addition to uncomplicated UTIs must be confirmed in a clinical trial.
REFERENCES
- 1.Allen A, Bygate E, Teillol-Foo M, Oliver S, Johnson M R, Ward C. Multiple dose pharmacokinetics and tolerability of gemifloxacin following oral doses to healthy volunteers. J Antimicrob Chemother. 1999;44(Suppl. A):133. [Google Scholar]
- 2.Allen A, Bygate E, Teillol-Foo M, Oliver S, Johnson M R, Ward C. Pharmacokinetics and tolerability of gemifloxacin after administration of single oral doses to healthy volunteers. J Antimicrob Chemother. 1999;44(Suppl. A):137. doi: 10.1128/aac.44.6.1604-1608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuth Verlag. Normenausschuβ Medizin (NAMed) im DIN (Deutsches Institut für Normung e.V.). Methoden zur Empfindlichkeitsprüfung von bakteriellen Krankheitserregern (auβer Mykobakterien) gegen Chemotherapeutika. Teil 4. Bewertungsstufen der minimalen Hemmkonzentrationen. DIN 58940-4: September 1995. Berlin, Germany: Beuth Verlag; 1995. [Google Scholar]
- 4.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antimicrobial peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1997;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 6.Doyle, E., S. E. Fowles, D. F. McDonnell, R. McCarthy, and S. A. White. Rapid determination of gemifloxacin in human plasma by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B, in press. [DOI] [PubMed]
- 7.Hofbauer H, Naber K G, Kinzig-Schippers M, Sörgel F, Rustige-Wiedemann C, Wiedemann B, Reiz A, Kresken M. Urine bactericidal activity of pefloxacin versus norfloxacin in healthy female volunteers after a single 800 mg oral dose. Infection. 1977;25:121–126. doi: 10.1007/BF02113592. [DOI] [PubMed] [Google Scholar]
- 8.Naber K G. Antibacterial activity of antibacterial agents in urine. In: Bergan T, editor. Urinary tract infections. Infectiology. Basel, Switzerland: Karger; 1977. pp. 74–83. [Google Scholar]
- 9.Naber K G, Sörgel F, Kinzig M, Weigel D M. Penetration of ciprofloxacin into prostatic fluid, ejaculate and seminal fluid in volunteers after an oral dose of 750 mg. J Urol. 1993;150:1718–1721. doi: 10.1016/s0022-5347(17)35877-9. [DOI] [PubMed] [Google Scholar]
- 10.Naber K G, Theuretzbacher U, Kinzig M, Savov O, Sörgel F. Urinary excretion and bacterial activities of a single oral dose of 400 mg of fleroxacin versus a single oral dose of 800 mg of pefloxacin in healthy volunteers. Antimicrob Agents Chemother. 1998;42:1659–1665. doi: 10.1128/aac.42.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for determining antibacterial activity of antimicrobial agents, vol. 12, no. 19. September. Tentative guideline M26-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 12.Paladino J A, Sperry H E, Backes J M, Gelber J A, Serrianne D J, Cumbo T J, Schentag J J. Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics. Am J Med. 1991;91:462–470. doi: 10.1016/0002-9343(91)90181-v. [DOI] [PubMed] [Google Scholar]
- 13.Well M, Naber K G, Kinzig-Schippers M, Sörgel F. Urinary bactericidal activity and pharmacokinetics of enoxacin versus norfloxacin and ciprofloxacin in healthy volunteers after a single oral dose. Int J Antimicrob Agents. 1998;10:31–38. doi: 10.1016/s0924-8579(98)00014-4. [DOI] [PubMed] [Google Scholar]