Abstract
Genitourinary syndrome of menopause significantly affects the quality of life in postmenopausal women with few evidence-based alternatives to vaginal estrogen for women with contraindications. This systematic review evaluates the evidence for vaginal vitamin E efficacy in reducing patient-reported genitourinary symptoms in healthy postmenopausal women compared to placebo or vaginal control therapy. This systematic review evaluated randomized controlled trials before October 2020 that assessed the efficacy of vitamin E vaginal suppositories in reducing genitourinary symptoms in postmenopausal women compared with a control group of healthy postmenopausal women. Outcomes included patient-perceived genitourinary symptoms. Of the 31 studies, four met the inclusion criteria. One 8-week trial (n = 42) found a significant reduction in vaginal symptoms in the 1 mg vitamin E group than the placebo group (difference in means, 5.3; 95% confidence interval [CI], 4.4 to 6.2). Another 8-week trial (n = 40) found 5 mg vaginal hyaluronic acid superior to 1 mg vitamin E (difference in means -0.50, 95% CI, -0.95 to -0.05). Two 12-week trials (n = 52 in each) found no difference between 0.5 g vaginal estrogen and 100 IU vaginal vitamin E in healthy postmenopausal women (difference in means: -0.19, 95% CI, -4.4 to 4.0, and -3.47, 95% CI, -13.8 to 6.8). Evidence from small, limited studies suggests that vaginal vitamin E may be effective in alleviating symptoms of genitourinary syndrome of menopause; however, additional high-quality studies are needed to determine efficacy, ideal dosing, and long-term safety.
Keywords: Atrophic vaginitis, Dyspareunia, Menopause, Urinary tract infections, Vitamin E
Graphical Abstract
INTRODUCTION
Genitourinary syndrome of menopause (GSM) describes a constellation of urinary, sexual, genital, and somatic symptoms associated with the onset of menopause and its associated hypoestrogenic state [1,2,3]. Notable genital symptoms of GSM include vaginal dryness, burning, pruritus, and pain. Urinary symptoms include urinary urgency, dysuria, and recurrent urinary tract infections. Sexual symptoms include lack of lubrication, discomfort or pain, decreased libido, and impairment of arousal and orgasm [4,5]. While estimates vary as to the prevalence of GSM symptoms in postmenopausal women, roughly half of postmenopausal women in Western countries report symptoms of GSM, with approximately half reporting moderate to severe symptoms [6,7,8]. Unlike vasomotor symptoms, GSM tends to be both chronic and progressive [9], with one study finding 84% of postmenopausal women to exhibit signs of GSM by six years after menopause [10].
Symptoms of GSM can markedly influence quality of life [4,6,8,11]. A survey of 300 postmenopausal women compared mean scores for the Menopause Specific Quality of Life Scale (MSQLS), for women with and without GSM, and found that quality of life was significantly lower in postmenopausal women with GSM [12]. A review of GSM found that self-esteem and intimacy appear to be negatively affected by GSM symptoms, decreasing the quality of life of women [3]. For women with moderate or severe genital symptoms, the negative impact on quality of life can be comparable to the impact of chronic diseases such as arthritis and chronic obstructive pulmonary disease [6]. A survey of nearly 4,000 postmenopausal women found that up to 45% complained of vaginal symptoms, with a majority reporting negative impact of these symptoms on their lives [13].
Given the high prevalence of GSM in postmenopausal women and the significant influence it has on quality of life, adequate medical attention and treatment of these symptoms is critical [7]. Treatment goals of GSM are focused on alleviating symptoms [4]. Mild symptoms are often treated with nonhormonal lubricants during intercourse, or regular use of vaginal moisturizers. Most of these are available over the counter, with few clinical studies to support efficacy [4].
For moderate to severe symptoms, prescription therapies include vaginal DHEA, oral ospemifene, and vaginal estrogen therapy [4]. Vaginal estrogen products, the mainstay of treatment for moderate to severe GSM, can be administered as creams, tablets, or a slow-release intravaginal ring, all of which have shown efficacy as compared to placebo [4,14,15,16,17]. Vaginal estrogen therapy is thought to have a favorable safety profile compared to oral estrogen: the low doses used for vaginal therapy have not been shown to elevate serum estradiol levels above the normal postmenopausal range, and observational studies and systematic reviews have failed to show increased risk for venous thromboembolism (VTE) or cardiovascular disease [4,18]. However, long-term prospective safety data on estrogen-dependent cancer and VTE risk are lacking due to lack of randomized controlled trials (RCTs) lasting longer than 52 weeks [4]. Undiagnosed vaginal bleeding is an absolute contraindication to vaginal estrogen use, with additional relative contraindications for estrogen-dependent cancers and increased VTE risk. Product packaging for vaginal estrogen therapy includes the same labeling as systemic doses, including warnings about stroke, heart disease, dementia, VTE, and breast/endometrial cancer, which can lead to patient concern about use of these products [18]. In addition, vaginal estrogen formulations can be expensive, and use can be limited by cost and insurance coverage. In women with breast cancer and genitourinary symptoms, treatment options are limited due to the use of estrogen-antagonist adjuvant treatments, the limited clinical trials in this population, and the lack of consensus in the healthcare community on appropriate therapy.
Given these limitations of vaginal estrogen and the paucity of evidence for commonly used nonhormonal topical therapies for GSM, review of existing evidence for promising nonhormonal therapies is important both in guiding therapy as well in illuminating directions for additional research. One such therapy meriting review is vaginal vitamin E. Vitamin E is a potent chain-breaking antioxidant that inhibits the production of reactive oxygen species molecules during fat oxidation and the propagation of free radical reactions, thus decreasing oxidation and cell damage [19]. Vitamin E also protects cell membranes against free radicals by inhibiting lipid peroxidation, potentially preventing or delaying disease processes associated with reactive oxygen species molecules, i.e., aging cells. In addition, vitamin E increases the stability of the cell by creating a tighter order in the membrane lipid packaging and helps repair the cell membrane by preventing the oxidation of phospholipids [20]. All of these qualities contribute to the potential of vitamin E to repair the vaginal epithelium, increasing the efficacy of the mucus producing cells in the vagina and lowering local inflammation. These in turn may decrease the level of atrophy and dryness in the vaginal mucosa that leads to the primary genitourinary symptoms.
Several studies have explored vitamin E’s potential to repair vaginal epithelium. In 150 postmenopausal women using suppositories containing vitamin E, vitamin A and hyaluronic acid, vaginal dryness, as measured by a four-point visual analogue scale, improved after 4 weeks of treatment [21]. Parnan Emamverdikhan et al. [22] compared vaginal maturation value (VMV) in 26 women treated with 100 IU vitamin E suppositories compared with 26 women using 0.5 g of conjugated estrogen cream for 12 weeks. While the estrogen group’s VMV was significantly better at 4 weeks, the VMV was not significantly different between the two groups using vitamin E and vaginal estrogen at 8 or 12 weeks of treatment [22]. An eight-week study comparing VMV and pH in 20 women using 1 mg vitamin E suppositories to 22 women using placebo suppositories found decreased vaginal pH and increased VMV in the vitamin E group compared to the placebo group [23].
This systematic review focuses on RCTs to explore whether vaginally administered vitamin E provides an effective alternative treatment for patients who are unable or unwilling to use estrogen products for GSM. The objective is to determine whether vaginal vitamin E alleviates patient-reported symptoms of GSM in healthy postmenopausal women.
MATERIALS AND METHODS
This systematic review was conducted using the guidelines of “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” [24]. Detailed search strategy and study protocol are registered with the International Prospective Register of Systematic Reviews (PROSPERO): CRD42020214246. Electronic database searches were conducted without language or date restrictions in MEDLINE (Ovid interface), CINAHL, Cochrane Central Register of Controlled Trials (CENTER), Cochrane Database of Systematic Reviews, and Scopus. Searches included articles spanning back to each databases’ initiation through the date of the last search on October 14th, 2020. In addition, the references of relevant articles were scanned for additional studies. The exemplary search strategy for MEDLINE (Ovid interface) can be found at the following link: https://www.crd.york.ac.uk/PROSPEROFILES/214246_STRATEGY_20201019.pdf
Inclusion criteria for this review included RCTs comparing vaginally administered vitamin E with a control group (placebo or another vaginally administered treatment). Participants were limited to postmenopausal women with genitourinary or sexual symptoms. In addition, selected studies had to report on the primary outcome of patient-perceived genitourinary and sexual symptoms on a standardized questionnaire or scale as compared to a control group or placebo. To limit heterogeneity of studies and focus on the highest quality trials with outcomes relevant to patient care, exclusion criteria included: studies that used oral therapy, lacked control groups or randomization, or focused on subject groups with chemically-induced menopause. In addition, studies were excluded if they lacked a patient-centered outcome. Because of unclear correlations between VMV and patient symptoms [25], studies were excluded that evaluated only VMV, without assessment of patient symptoms.
Study selection
Search results were reviewed for relevance by abstract and title by the authors, with at least two reviewers per abstract (L.P., N.W., Z.S.D., F.S., and A.S.). Records were excluded if the study did not meet inclusion criteria. The full texts of selected articles were each further assessed by the authors for eligibility, and each was coded by two separate reviewers (L.P., N.W., Z.S.D., F.S., and A.S.), with disagreements resolved by discussion and consensus. Data extracted from each clinical trial included: 1) trial structure (blinding, control group, number of centers); 2) characteristics of participants (including age, health status) and the trial’s exclusion/inclusion criteria; 3) the type of intervention (including dose, administration, frequency, duration, and attrition); 4) the type of placebo or control group (including type, dose, frequency, and duration, and attrition); 5) the type of outcome measure (validated method of scoring symptoms and/or related quality of life); and 6) results (the size in difference between groups). The principal summary measure was difference between the mean scores of patient-reported symptoms between intervention and placebo group after completion of treatment period. The risk of bias in each individual study was assessed by two reviewers using the Oxford Centre for Evidence-Based Medicine Risk of Bias tool [26]. Disagreements were resolved by discussion, consensus, and by a third reviewer (L.P.).
RESULTS
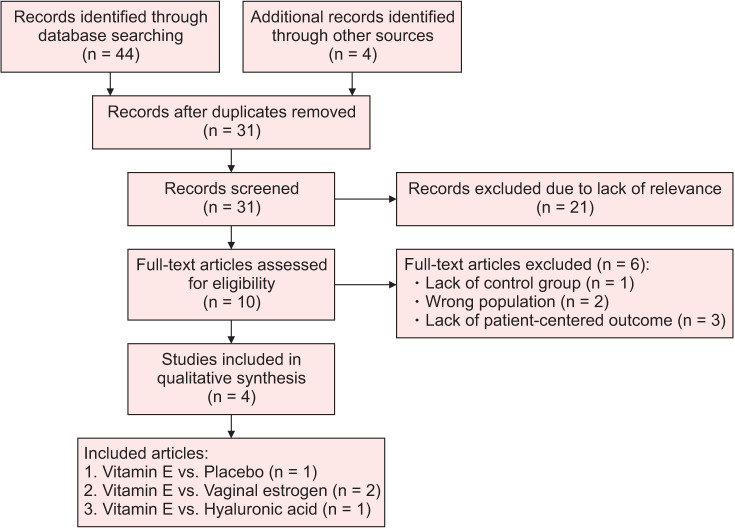
The search yielded 44 records with an additional four studies identified through scanning references. After elimination of duplicates, the abstracts and titles of 31 articles were screened for relevance and 21 were excluded due to lack of randomization, control group, or due to lack of an intervention that included vaginal vitamin E. After full-text review of the remaining 10 articles, an additional six articles were excluded due to inappropriate population (post-operative patients), lack of a control group, or due to lack of a patient-centered outcome. Four remaining RCTs were included in the systematic review (Fig. 1) [24].
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart of inclusion and exclusion process.
All four studies described adequate processes for randomization, similarity at baseline between control and intervention groups, and equal treatment of groups (Table 1). All subjects in the included studies were accounted for. In the Ziagham et al.’s studies [23,27], double-blinding was used, while Golmakani et al. [28] described single-blinding (of investigators), and Parnan Emamverdikhan et al. [29] did not comment on whether investigators were blinded to the subjects’ allocation group.
Table 1. Methodologic features of studies.
The four RCTs included in the review were all conducted in Iran through university-based health centers between 2010 and 2014 (see study characteristics, Table 2). Two eight-week trials reported by Ziagham et al. [23,27] recruited women from 2010 to 2011, and two 12 weeks trials were reported by Parnan Emamverdikhan et al. [29] and Golmakani et al. [28], recruiting from 2013 to 2014. In all studies, the intervention was vaginal vitamin E suppositories in healthy postmenopausal women with genitourinary symptoms. Ziagham et al. [23] tested the efficacy of suppositories containing 1 mg (2.22 IU) of vitamin E compared to placebo for eight weeks and Ziagham et al. [27] compared suppositories containing 1 mg (2.22 IU) of vitamin E to hyaluronic acid. Parnan Emamverdikhan et al. [29] and Golmakani et al. [28] each compared 100 IU of vaginal vitamin E to 0.5 vaginal estrogen for 12 weeks. Meta-analysis was not appropriate for this systematic review because three different instruments were used for patient self-rating of symptoms.
Table 2. Characteristics of included studies.
| Study | n | Patient-centered outcome | Intervention (dose) (n) [mean age, y] | Control group (dose) (n) [mean age, y] | Length of study, treatment regimen |
|---|---|---|---|---|---|
| Ziagham et al. [27] (2012) | 40 | CSVS Max = 12, higher scores reflect more severe symptoms |
Vaginal vitamin E (1 mg/2.22 IU) (n = 20) [54.9 ± 4.38] | Vaginal hyaluronic acid (5 mg) (n = 20) [54 ± 5.16] | 8 weeks Weeks 1–2: daily Weeks 3–8: every other day |
| Ziagham et al. [23] (2013) | 42 | CSVS Max score = 12, higher score reflect more severe symptoms |
Vaginal vitamin E (1 mg/2.22 IU) (n = 20) [54.9 ± 5.16] | Placebo: semi-synthetic fatty acid TG (n = 22) [53.77 ± 5.3] | 8 weeks Weeks 1–2: daily Weeks 3–8: every other day |
| Golmakani et al. [28] (2019) | 52 | ASFQ Max score = 36, higher scores reflect better sexual function |
Vaginal vitamin E (100 IU) (n = 26) [unavailable] | Conjugated vaginal estrogen cream (0.5 g) (n = 26) [unavailable] | 12 weeks Weeks 1–2: daily Weeks 3–12: twice weekly |
| Parnan Emamverdikhan et al. [29] (2014) | 52 | MEQOL Higher scores reflect lower quality of life |
Vaginal vitamin E (100 IU) (n = 26) [52.11 ± 4.70] | Conjugated vaginal estrogen cream (0.5 g) (n = 26) [52.88 ± 6.30] | 12 weeks Weeks 1–2: daily Weeks 3–12: twice weekly |
CSVS: Composite Score of Vaginal Symptoms, ASFQ: Abbreviated Sexual Function Questionnaire, MEQOL: Menopause-Specific Quality of Life.
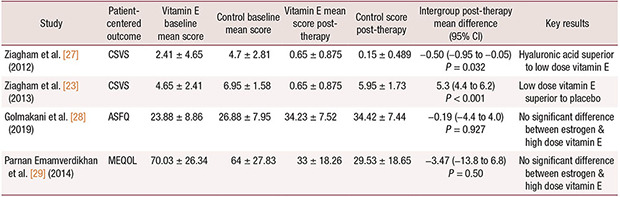
Ziagham et al. [23] found low dose vitamin E far outperformed a placebo in reducing vaginal symptoms (P < 0.001, Table 3). They compared participants in a vitamin E group (n = 20) with a placebo group (n = 22). Vitamin E suppositories contained a semi-synthetic fatty acid triglyceride along with 1 mg (2.22 IU) of vitamin E and placebo suppositories contained semisynthetic fatty acid triglycerides. Suppositories were administered daily for the first two weeks, then continued every other day for an additional six weeks, for a total of 8 weeks. Symptoms were rated on the Composite Score of Vaginal Symptoms (CSVS; range, 0–12), assessing symptoms of irritation, itching, vaginal dryness, and dyspareunia on a four-point scale (0–3), with higher scores indicating more severe symptoms [23]. At eight weeks, the vitamin E group (n = 20) had significantly fewer symptoms (mean, 0.65 ± 0.875) compared to the placebo group (n = 22; mean, 5.95 ± 1.73) and had improved from baseline of 4.65 ± 2.41. Difference in means was 5.3 (95% CI, 4.4 to 6.2; P < 0.001). No adverse effects were reported.
Table 3. Results of included studies.
| Study | Patient-centered outcome | Vitamin E baseline mean score | Control baseline mean score | Vitamin E mean score post-therapy | Control score post-therapy | Intergroup post-therapy mean difference (95% CI) | Key results |
|---|---|---|---|---|---|---|---|
| Ziagham et al. [27] (2012) | CSVS | 2.41 ± 4.65 | 4.7 ± 2.81 | 0.65 ± 0.875 | 0.15 ± 0.489 | –0.50 (–0.95 to –0.05) P = 0.032 |
Hyaluronic acid superior to low dose vitamin E |
| Ziagham et al. [23] (2013) | CSVS | 4.65 ± 2.41 | 6.95 ± 1.58 | 0.65 ± 0.875 | 5.95 ± 1.73 | 5.3 (4.4 to 6.2) P < 0.001 |
Low dose vitamin E superior to placebo |
| Golmakani et al. [28] (2019) | ASFQ | 23.88 ± 8.86 | 26.88 ± 7.95 | 34.23 ± 7.52 | 34.42 ± 7.44 | –0.19 (–4.4 to 4.0) P = 0.927 |
No significant difference between estrogen & high dose vitamin E |
| Parnan Emamverdikhan et al. [29] (2014) | MEQOL | 70.03 ± 26.34 | 64 ± 27.83 | 33 ± 18.26 | 29.53 ± 18.65 | –3.47 (–13.8 to 6.8) P = 0.50 |
No significant difference between estrogen & high dose vitamin E |
CSVS: Composite Score of Vaginal Symptoms, ASFQ: Abbreviated Sexual Function Questionnaire, MEQOL: Menopause-Specific Quality of Life, CI: confidence interval.
Using the same design and outcome measure, Ziagham et al. [27] found hyaluronic acid superior to low dose vitamin E (Table 3). Ziagham et al. [27] compared 20 participants in a vitamin E group with a hyaluronic acid group containing 20 participants. Vitamin E suppositories contained a semi-synthetic fatty acid triglyceride along with 1 mg (2.22 IU) of vitamin E and hyaluronic acid suppositories contained 5 mg of hyaluronic acid. At eight weeks, patient-rated CSVS scores were significantly less severe in the hyaluronic acid group (mean, 0.15 ± 0.489) compared to the vitamin E group (mean, 0.65 ± 0.875), with both groups improving from baseline scores of 2.41 ± 4.65 in the vitamin E group and 4.7 ± 2.81 in the hyaluronic acid group. Difference in means was –0.50 (95% CI, –0.95 to –0.05; P = 0.032). No side effects were reported for either intervention.
Parnan Emamverdikhan et al. [29] found no difference in quality life between vaginal estrogen and high-dose vitamin E. Parnan Emamverdikhan et al. [29] reported scores from the Menopause-Specific Quality of Life (MEQOL) scale with 29 questions (6-point rating scales) for vasomotor, psycho-social, physical, and sexual symptoms and lower scores indicating better quality of life. At 12 weeks, the vitamin E group score (mean, 33 ± 18.26) showed no significant difference from the estrogen group (mean, 29.53 ±18.65). Difference in means after treatment was –3.47 (95% CI, –13.8 to 6.8; P = 0.50), with both groups improving from baseline scores of 70.03 ± 26.34 in the vitamin E group and 64 ± 27.83 in the estrogen group. Side effects were not reported other than “sensitivity to medications,” which was listed as a reason for study discontinuation.
Using the same design and treatment protocol, Golmakani et al. [28] found no difference in sexual symptoms scores between vaginal estrogen and high-dose vitamin E. Golmakani et al. [28] reported on the Abbreviated Sexual Function Questionnaire (ASFQ) with 15 questions assessing symptoms of sexual desire, sensation, lubrication, and orgasm, with higher scores indicating better sexual function. At 12 weeks, the vitamin E group score (mean, 34.23 ± 7.52) was not significantly different from the estrogen group (mean, 34.42 ± 7.44). The difference in means after treatment was –0.19 (95% CI, –4.4 to 4.0; P = 0.927), with scores in both groups improving from baselines of 23.88 ± 8.86 in the vitamin E group and 26.88 ± 7.95 in the estrogen group. Side effects reported in the vitamin E group were vaginal burning, discharge and bleeding (13%) while vaginal discharge, hypersensitivity and breast enlargement were reported in the estrogen group (13%).
DISCUSSION
This review found evidence that vaginal vitamin E may be an effective short-term treatment for GSM, although additional high-quality placebo-controlled studies are needed to clarify efficacy, ideal dosing, and safety. Self-rated patient scores for those treated with vitamin E improved from baseline in all studies, and showed superiority compared to placebo and no significantly difference compared with vaginal estrogen. Low dose vitamin E suppositories were, however, less effective compared with hyaluronic acid.
Although the four trials were generally of good to fair quality and suggest that vitamin E may reduce genitourinary (GU) symptoms, this review has some limitations. All included trials were conducted in Iran, and thus, a lack of ethnic diversity in the subject groups may limit generalizability of the study findings. In addition, the two studies comparing high dose vitamin E with estrogen were limited by lack of patient blinding. All studies include outcomes that measure self-reported patient symptoms. While this has the advantage of a focus on outcomes that are meaningful for patients, the subjectivity of symptoms makes them more prone to influence by the placebo effect, though this is likely mitigated by blinding in the Ziagham studies [23,27]. In addition, as in any systematic review, results have the potential to be skewed by publication bias, with studies without significant findings or with negative results being less likely to be published, and thus, eligible for inclusion in this review [30].
A further possible limitation relates to the question of how vitamin E subject groups are represented by the four RCTs. In the Parnan Emamverdikhan et al. [29] and Golmakani et al. [28]’s trials, the similarities in the recruitment period and baseline characteristics of the groups, as well as the identical number of participants in each group raise the question of whether these two studies may explore two different outcomes for the same group of subjects. Likewise, in the Ziagham et al.’s publications [23,27], the similarity in recruitment period, baseline characteristics, and the identical number of participants and final CSVS scores for the vitamin E groups in both publications raise the question of whether the same vitamin E subject group may have been used in both studies in comparison to two different controls (placebo and hyaluronic acid). There is a discrepancy in the reported pre-treatment CSVS scores for the vitamin E groups in the Ziagham et al.’s publications [23,27] (4.65 ± 2.41 vs 2.41 ± 4.65); however, this may be a typographical error. The authors of the studies were unavailable for clarification of this question when contact was attempted. Nevertheless, the subgroup comparisons provide novel evidence about effectiveness of vitamin E to reduce GU symptoms.
Statistical power is another important consideration for evaluating quality of included trials. Although Ziagham et al. [23] reported considering statistical power in determining their sample size, Ziagham et al. [27] explicitly stated that their sample size with 22 subjects in each group was to detect a difference of 0.63 standard deviations difference between groups on their outcome measure (alpha = 0.05, beta = 0.20). Their estimation was based on Ekin et al. [31] who actually found a larger difference between groups (1.19 standard deviations). Notably, the Ziagham et al.’s studies [23,27] detected a large difference between groups and did not lack statistical power. These studies also may have gained further statistical power by controlling for baseline scores.
Parnan Emamverdikhan et al. [29] and Golmakani et al. [28] also sought to detect a large difference between groups, powering their sample size to detect approximately a standard deviation difference between group means. If we apply the same criteria for a meaningful difference across all four studies, results can be interpreted as superiority of low dose vitamin E when compared to placebo and noninferiority between high dose vitamin E and vaginal estrogen. To achieve statistical power of 0.80 (with alpha of 0.05), a difference of 0.90 standard deviations or larger can be detected with at least 20 people per group in a comparison of postintervention scores [32]. Parnan Emamverdikhan et al. [29] and Golmakani et al. [28]’s studies observed very small differences (0.19 and 0.03 standard deviations, respectively) between groups. Thus, these studies did not observe a meaningful difference between groups and also were not significantly different.
Of note, official societies such as the North American Menopause Society, or the European Menopause and Andropause Society do not currently recommend use of vaginal vitamin E as part of the guidelines for management of GSM [4,33]. The findings of this systematic review suggest that additional trials of high-quality are needed before such recommendations would be appropriate.
In conclusion, while high-quality studies are needed to further explore potential, these limited RCTs suggest vaginal vitamin E may have potential meriting further exploration as an alternative treatment for GSM. A low dose vitamin E suppository appears to perform significantly better than placebo and may offer postmenopausal women a more effective non-hormonal treatment for GSM symptoms. This review sheds light on the need for additional high-quality RCTs to further explore both efficacy and safety of vaginal vitamin E for this condition. Future RCTs should evaluate the effect of different doses of vitamin E in terms of both tolerability and benefit. If short-term treatment appears effective, long-term safety and efficacy should be studied.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Mastroianni J, Thompson JA, Shifren JL, Zuckerman AL, Pereira K. Improving the identification of genitourinary syndrome of menopause through the utilization of the Day-to-Day Impact of Vaginal Aging questionnaire. Menopause. 2020;27:1295–1301. doi: 10.1097/GME.0000000000001668. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi J, Chen A, Dagur G, Suh Y, Smith N, Cali B, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;215:704–711. doi: 10.1016/j.ajog.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 3.Kim HK, Kang SY, Chung YJ, Kim JH, Kim MR. The recent review of the genitourinary syndrome of menopause. J Menopausal Med. 2015;21:65–71. doi: 10.6118/jmm.2015.21.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976–992. doi: 10.1097/GME.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 5.Gabes M, Knüttel H, Stute P, Apfelbacher CJ. Measurement properties of patient-reported outcome measures (PROMs) for women with genitourinary syndrome of menopause: a systematic review. Menopause. 2019;26:1342–1353. doi: 10.1097/GME.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 6.DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and Western Europe. J Womens Health (Larchmt) 2015;24:713–722. doi: 10.1089/jwh.2014.5177. [DOI] [PubMed] [Google Scholar]
- 7.Nappi RE, Kokot-Kierepa M. Women's voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67:233–238. doi: 10.1016/j.maturitas.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Parish SJ, Nappi RE, Krychman ML, Kellogg-Spadt S, Simon JA, Goldstein JA, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447. doi: 10.2147/IJWH.S44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturdee DW, Panay N International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13:509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- 10.Palma F, Volpe A, Villa P, Cagnacci A Writing group of AGATA study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44. doi: 10.1016/j.maturitas.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Shifren JL, Zincavage R, Cho EL, Magnavita A, Portman DJ, Krychman ML, et al. Women's experience of vulvovaginal symptoms associated with menopause. Menopause. 2019;26:341–349. doi: 10.1097/GME.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 12.Karakoç H, Uçtu AK, Özerdoğan N. Genitourinary syndrome of menopause: effects on related factors, quality of life, and self-care power. Prz Menopauzalny. 2019;18:15–22. doi: 10.5114/pm.2019.84152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantine GD, Graham S, Clerinx C, Bernick BA, Krassan M, Mirkin S, et al. Behaviours and attitudes influencing treatment decisions for menopausal symptoms in five European countries. Post Reprod Health. 2016;22:112–122. doi: 10.1177/2053369116632439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachtigall LE. Clinical trial of the estradiol vaginal ring in the U.S. Maturitas. 1995;22 Suppl:S43–S47. doi: 10.1016/0378-5122(95)00963-9. [DOI] [PubMed] [Google Scholar]
- 15.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259–263. doi: 10.1016/0378-5122(95)00955-8. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen PS, Rasmussen H. Low-dose 17 beta-estradiol vaginal tablets in the treatment of atrophic vaginitis: a double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol. 1992;44:137–144. doi: 10.1016/0028-2243(92)90059-8. [DOI] [PubMed] [Google Scholar]
- 17.Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;2016:CD001500. doi: 10.1002/14651858.CD001500.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Goldstein SR, Kagan R, Kaunitz AM, Liu JH, Pinkerton JV, et al. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21:911–916. doi: 10.1097/GME.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 19.Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 20.Szczeklik A, Gryglewski RJ, Domagala B, Dworski R, Basista M. Dietary supplementation with vitamin E in hyperlipoproteinemias: effects on plasma lipid peroxides, antioxidant activity, prostacyclin generation and platelet aggregability. Thromb Haemost. 1985;54:425–430. [PubMed] [Google Scholar]
- 21.Costantino D, Guaraldi C. Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: an open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 2008;12:411–416. [PubMed] [Google Scholar]
- 22.Parnan Emamverdikhan A, Golmakani N, Tabassi SA, Hassanzadeh M, Sharifi N, Shakeri MT. A survey of the therapeutic effects of Vitamin E suppositories on vaginal atrophy in postmenopausal women. Iran J Nurs Midwifery Res. 2016;21:475–481. doi: 10.4103/1735-9066.193393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziagham S, Abbaspoor Z, Safyari S, Rad P. Effect of vitamin E vaginal suppository on atrophic vaginitis among postmenopausal women. Jundishapur J Chronic Dis Care. 2013;2:11–19. [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila GW, Singh A, Karapanagiotou I, Woodhouse S, Huber K, Zimberg S, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;188:382–388. doi: 10.1067/mob.2003.23. [DOI] [PubMed] [Google Scholar]
- 26.University of Oxford. Centre for Evidence Based Medicine. Randomised Controlled Trials (RCT) critical appraisal sheet. Oxford: University of Oxford; 2021. [cited 2021 Feb 20]. Available from: https://www.cebm.ox.ac.uk/files/ebm-tools/rct.pdf . [Google Scholar]
- 27.Ziagham S, Abbaspoor Z, Abbaspour M. Effect of hyaluronic acid and vitamin E vaginal tablets on atrophic vaginitis: a randomized controlled trial. Afr J Pharm Pharmacol. 2012;6:3124–3129. [Google Scholar]
- 28.Golmakani N, Parnan Emamverdikhan A, Zarifian A, Sajadi Tabassi SA, Hassanzadeh M. Vitamin E as alternative local treatment in genitourinary syndrome of menopause: a randomized controlled trial. Int Urogynecol J. 2019;30:831–837. doi: 10.1007/s00192-018-3698-z. [DOI] [PubMed] [Google Scholar]
- 29.Parnan Emamverdikhan A, Golmakani N, SharifiSistani N, Shakeri MT, Hasanzade MM, Sajadi Tabassi A. Comparing two treatment methods of vitamin E suppository and conjugated estrogen vaginal cream on the quality of life in menopausal women with vaginal atrophy. J Midwifery Reprod Health. 2014;2:253–261. [Google Scholar]
- 30.Dalton JE, Bolen SD, Mascha EJ. Publication bias: the elephant in the review. Anesth Analg. 2016;123:812–813. doi: 10.1213/ANE.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekin M, Yaşar L, Savan K, Temur M, Uhri M, Gencer I, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283:539–543. doi: 10.1007/s00404-010-1382-8. [DOI] [PubMed] [Google Scholar]
- 32.Weller SC. Sample size estimation: the easy way. Field Methods. 2015;27:333–347. [Google Scholar]
- 33.Hirschberg AL, Bitzer J, Cano A, Ceausu I, Chedraui P, Durmusoglu F, et al. Topical estrogens and non-hormonal preparations for postmenopausal vulvovaginal atrophy: an EMAS clinical guide. Maturitas. 2021;148:55–61. doi: 10.1016/j.maturitas.2021.04.005. [DOI] [PubMed] [Google Scholar]