Abstract
The beneficial effects of therapy combining an antibiotic and dexamethasone have been reported in human studies on meningitis and in experimental studies on septic arthritis, nephritis, and endophthalmitis. Since most patients with staphylococcal endocarditis need a combination of medical and surgical treatment, the purpose of this study was to determine whether the addition of dexamethasone to vancomycin has any beneficial effect regarding the degree of valve tissue damage or the course of experimental aortic valve endocarditis caused by a methicillin-resistant strain of Staphylococcus aureus. Rabbits with catheter-induced aortic valve vegetations were randomly assigned to a control group and to groups receiving dexamethasone (0.5 mg/kg of body weight, intravenously [i.v.], twice a day [b.i.d]), vancomycin (30 mg/kg, i.v., b.i.d), or dexamethasone plus vancomycin, for a total of 10 doses (two doses per day for 5 days). The severity of valve tissue damage was significantly less in groups receiving vancomycin plus dexamethasone compared with that of the group receiving vancomycin alone (P < 0.001). The severity of tissue damage was inversely correlated with the mean polymorphonuclear leukocyte number in valve tissue. No statistically significant differences were observed between the vancomycin-treated group and the vancomycin-plus-dexamethasone-treated group in survival, blood culture sterilization rate, or reduction of the microbial burden (in CFU per gram) in valvular tissue. In conclusion, treatment with a combination of vancomycin and dexamethasone for 5 days reduces the severity of valve tissue damage in experimental staphylococcal aortic valve endocarditis. These findings could have significant implications in the treatment of staphylococcal endocarditis and deserve further confirmation in clinical trials.
Staphylococcus aureus is the second most common cause of infective endocarditis (IE), affecting both native (25 to 35%) and prosthetic (33) valves, and is characterized by valve destruction and significantly higher in-hospital mortality. In some medical centers, staphylococcal endocarditis may even predominate (23). S. aureus IE may occur in patients of any age who are apparently healthy and in intravascular device users, who are usually young. Patients with community-acquired staphylococcal bacteremia are at high risk for IE. However, the recent increases in the frequencies of intravascular-device-associated and nosocomial staphylococcal bacteremias and even of staphylococcal bacteremia complicating outpatient parenteral antimicrobial therapy are some possible explanations for the increase in the number of patients at risk for IE (6). During the course of staphylococcal endocarditis, most patients will eventually need valve replacement (32). Early combined medical and surgical intervention is more beneficial than medical therapy alone in both native-and prosthetic-valve IE; thus, a more aggressive surgical approach has been recommended during the last 10 years (12, 21, 32).
Dexamethasone has been successfully used together with an antibiotic in the treatment of experimental staphylococcal endophthalmitis (24, 35), septic arthritis (22), septic nephritis (30), and experimental Haemophilus influenzae type b (26) and pneumococcal (19) meningitis. In the case of H. influenzae type b meningitis, clinical investigations with promising results led to the conclusion that the simultaneous use of dexamethasone and antibiotics has beneficial effects (14, 28). However, in the case of pneumococcal meningitis, there are clinical studies that show no beneficial effect from the addition of dexamethasone (1).
The purpose of our research was to evaluate, in a rabbit model of aortic valve endocarditis caused by a methicillin-resistant strain of Staphylococcus aureus (MRSA), the effect of the addition of dexamethasone to the therapeutic regimen. To our knowledge, no similar study has yet been published.
This research was presented previously (P. Siaperas et al., Abstr. 38th Annu. Meet. Infect. Dis. Soc. Am., abstr. 34, 2000).
MATERIALS AND METHODS
Bacterial strain.
The endocarditis-inducing strain of S. aureus (SA-443) used in the present study was a clinical isolate obtained from a patient with sepsis. The bacteria were stored at −80°C in skim milk and were subcultured on blood agar plates 3 days before each experiment.
Susceptibility testing and time-kill curves.
The MICs and minimal bactericidal concentrations (MBCs) of oxacillin (plus 2% NaCl) and vancomycin were determined by a microdilution technique in Mueller-Hinton broth (BBL Microbiology Systems) with the concentrations of the antibiotics tested ranging from 0.25 to 512 μg/ml. The MICs of the antibiotics were determined with inocula of ∼5 × 105 and ∼5 × 107 CFU/ml because the latter number of bacteria simulates more closely the pretreatment bacterial load in the vegetations. The MBC was determined by subculturing 0.1 ml from each clear well onto antibiotic-free blood agar plates and was defined as the lowest concentration that reduced the number of organisms of the initial inoculum by ≥99.9%.
The in vitro bactericidal effect of vancomycin was assessed by the time-kill curve method. An overnight culture in Mueller-Hinton broth was used to prepare inocula of ∼5 × 105 and ∼5 × 107 CFU/ml. The final antibiotic concentration tested was equivalent to the MBC, as determined with an inoculum of ∼5 × 105 CFU/ml. An additional killing-curve study with an inoculum of ∼5 × 107 CFU/ml and a final antibiotic concentration equivalent to the MBC, as determined with an inoculum of ∼5 × 107 CFU/ml, was also performed.
Induction of IE.
Nonbacterial thrombotic endocarditis was induced in female white rabbits (weighing 2.15 to 3.60 kg) according to the method of Perlman and Freedman (18). Twenty-four hours after catheterization, bacterial endocarditis was induced by intravascular inoculation via the marginal ear vein of ∼107 CFU of S. aureus strain SA-443 suspended in 1 ml of saline. Confirmation of IE was based on macroscopic findings (correct placement of the catheter across the aortic valve and the presence of vegetations) and either bacteriological data obtained from culture of the specimens or histopathological data obtained from observation of the aortic valves under light microscopy (presence of bacteria and evidence of inflammation).
In a pilot study, we examined the effect of a regimen consisting of a high dose of dexamethasone (1 mg/kg of body weight every 12 h, intravenously) plus vancomycin (30 mg/kg every 12 h, intravenously) on the sterilization (absence of bacteria) rate and bacterial burden in vegetations. After 5 days of treatment, two out of a total of nine (22%) rabbits had sterile vegetations, while the mean bacterial burden in vegetations was 6.46 ± 3.25 log10 CFU/g (mean ± standard deviation [SD]). Because vancomycin alone, at a lower dose (15 mg/kg every 12 h for 6 days, intravenously), exhibited better results (vegetation sterilization rate, 60%; mean bacterial burden, 5.63 ± 1.61 log10 CFU/g) in a previous study (17), we speculated that the addition of dexamethasone in high doses in the pilot study could be responsible for the lower efficacy of vancomycin compared to that in the previous study (17). Thus, we decided to use a lower dexamethasone dose in the main study. Rabbits were randomly assigned to the following four groups: group a (control group) received no treatment, group b received dexamethasone (0.5 mg/kg every 12 h intravenously) (supplied by Vianex A. E., Athens, Greece), group c received vancomycin (30 mg/kg every 12 h intravenously) (supplied by Eli Lilly Co., Indianapolis, Ind.), and group d received vancomycin (30 mg/kg) plus dexamethasone (0.5 mg/kg) every 12 h intravenously. Treatment was started 24 h after bacterial challenge and was continued for 5 days (total of 10 doses). Animals surviving for the duration of the experiment were sacrificed by rapid intravenous injection of sodium phenobarbital (30 mg/kg) at least 15 h after the administration of the last dose of vancomycin in order to avoid a carryover effect. Untreated controls, regardless of the time of death, and all treated animals that were sacrificed or that died after the initiation of therapy were included in the calculations of survival rates. However, rabbits that had received vancomycin or vancomycin plus dexamethasone were included in the analysis of efficacy (mean bacterial burden [log10 CFU per gram of tissue] and sterilization rate of valve tissue) if they had received three or more doses of the study drugs.
Bacteriological evaluations.
At the time of sacrifice, aortic valvular and left ventricular vegetations as well as a small portion of the vegetation adjacent to the valvular tissue were removed aseptically and were then bisected with a sterilized surgical scalpel. One-half of each specimen was placed immediately in 40% buffered formaldehyde for further histopathological preparation and evaluation, and the other half of each specimen was weighed, homogenized in 1 ml of saline, and quantitatively cultured in duplicate on blood agar plates after seven dilutions, with a 1-log inoculum difference between succeeding dilutions. After incubation for 24 h at 35°C, the colonies of S. aureus growing on the agar plates were counted and the results were expressed as log10 CFU per gram of tissue. The procedure described above was performed each time a rabbit from any group was found dead.
Quantitative peripheral blood samples.
Blood samples (∼1 ml) for quantitative culture were obtained from the ear artery 1 day after inoculation of the bacterial strain, just before the administration of the first dose of antibiotic (day 2), and on days 4 and 6 (day of sacrifice). On day 6, blood was taken from the left ventricle after a small dose of heparin was injected into the sacrificed animal.
Histopathological studies.
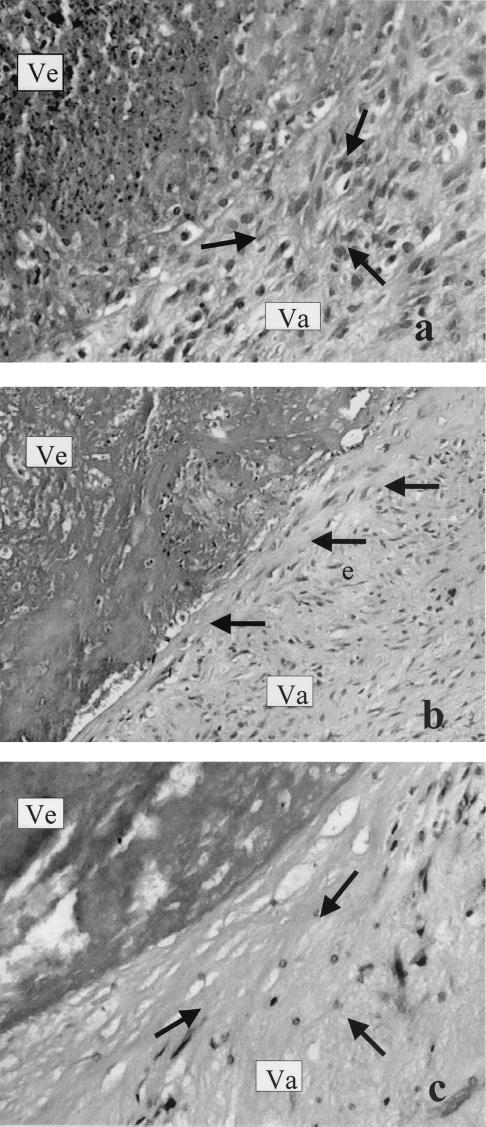
Tissue specimens were fixed immediately in 10% neutral buffered formalin for 24 h and embedded in paraffin. Five-micrometer-thick sections were cut and stained with hematoxylin and eosin and Giemsa stain for bacterial colonies and Masson-trichrome stain for delineation of vegetations. The histological findings were read blindly by the pathologist (A. Kyroudi-Voulgari), without any information regarding either treatments or the results of the valve cultures. The absolute number of neutrophils (polymorphonuclear leukocytes [PMNs]) was evaluated in 10 random optical fields of the periphery of the vegetation on the side of the valve under high-power magnification. PMNs were counted by use of a 100-point double square grid incorporated in the eye lens at a final magnification of ×400. The histopathological findings for the valvular tissue damage were classified into three categories, mild, moderate, and severe. This classification was based on the presence of fibrosis as well as the hyalinization of collagen, which produced injury of the valve bulk. The cases in which the necrotic tissue was replaced by young granulation tissue were characterized as mild (see Fig. 3a). The cases with fibrosis and indications of hyalinization were characterized as moderate (see Fig. 3b). In the cases with severe damage, the valve tissue was converted into dense hyaline material almost completely devoid of cellular structure (see Fig. 3c).
FIG. 3.
The three degrees of valvular tissue (Va) damage in an area proximal to vegetation (Ve) are shown. (a) Mild damage, young granulation tissue (arrows). (b) Moderate damage, hyalinization in a narrow zone in proximity to the vegetation (arrows). (c) Severe damage, conversion of valvular tissue into densely hyaline (almost acellular) material (arrows). Samples were stained with hematoxylin and eosin.
Vancomycin concentration in serum.
The peak and trough concentrations of vancomycin were determined on day 3 by the fluorescence polarization immunoassay (TDx system; Abbott Laboratories, Abbott Park, Ill.) (13). The lower limit of detection of this assay is 0.6 μg/ml. Samples were obtained approximately 15 to 20 min before the administration of vancomycin (trough levels) or 45 to 50 min after the end of the administration of vancomycin (peak levels) in a limited number of animals from both groups c and d.
Statistical methods.
To compare the differences between sterile and nonsterile blood cultures and between sterile and nonsterile tissue cultures, the Fisher exact test was used. To compare the differences between histopathological categories of severity of valvular tissue destruction, the chi-square test was used. For comparisons of mean bacterial burdens either in blood cultures or in tissue cultures, the F-max index showed that it was safe to proceed with parametric analysis and the analysis of variance model was used to assess statistical significance. The F-max index showed that the data were not suitable for parametric analysis of comparisons of the number of PMNs in the valvular tissue. Therefore, we proceeded with Kruskal-Wallis analyses of variance by ranks. The overall differences between the groups were statistically significant; thus, we continued to assess the statistical significance of the differences between the groups with the Mann-Whitney U test. Since the overall differences were statistically significant (Kruskal-Wallis analysis of variance), there was no need for Bonferroni's correction in the Mann-Whitney significance levels. Finally, the log rank test and Kaplan-Mayer curves were used to compare the survival rates among the study groups.
RESULTS
In vitro susceptibility studies.
The MICs and MBCs of the studied antibiotics for inocula of the MRSA strain of ∼5 × 105 CFU/ml were 64 and 128 μg/ml, respectively, for oxacillin and 1 and 1 μg/ml, respectively, for vancomycin, while the MICs and MBCs for inocula of ∼5 × 107 CFU/ml were 128 and 256 μg/ml, respectively, for oxacillin and 1 and 1 μg/ml, respectively for vancomycin. Time-kill studies done with 1 μg of vancomycin per ml demonstrated a reduction of the initial inoculum of ∼5 × 105 CFU/ml to inocula at 6 and 24 h of incubation that were equivalent to 2.5 and 2.3 log10 CFU/ml, respectively. With an initial inoculum of ∼5 × 107 CFU/ml, a reduction equivalent to 2.5 log10 CFU/ml was observed at 6 h of incubation, while after 24 h of incubation, an increase equivalent to 2.0 log10 CFU/ml above the initial inoculum size was observed.
Survival.
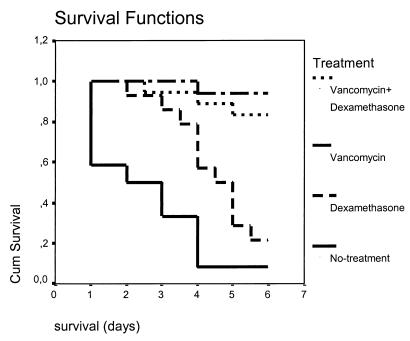
Of the 70 rabbits used in the main study, three died within less than 24 h after placement of the catheter and seven were excluded from further analysis because they did not meet the inclusion criteria. Overall, 60 rabbits were evaluated for heart valve tissue sterilization and reduction of bacterial counts. The mean survival rates of all study groups are presented in Table 1, while the Kaplan-Mayer survival curves are presented in Fig. 1. No statistically significant difference was observed in the survival rates between the group treated with vancomycin and the group treated with vancomycin plus dexamethasone. It is of interest that animals treated with dexamethasone alone exhibited a mean survival rate that was 2 days longer than that of the control animals. However, only three animals (21.5%) treated with dexamethasone were alive until the day of sacrifice.
TABLE 1.
Results of treatment of experimental aortic valve endocarditis due to MRSA with dexamethasone, vancomycin, or vancomycin plus dexamethasonea
| Group (no. of animals) | Treatment (dosage regimen) | Survival (days) (mean ± SD)b | Blood culture results
|
Heart valve tissue culture results
|
No. of specimens with indicated heart valve tissue pathology
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment (day 2)
|
Day 4 (no. sterile/ total no.) | Day 6 (no. sterile/ total no.) | |||||||||
| No. sterile/ total no. | Log10 CFU/ml (mean ± SD) | No. sterile/ total no.c | Log10 CFU/g (mean ± SD)d | No. of PMNs (mean ± SD)e | Bacteria in vegetations (present/absent)f | Severity of tissue damage (mild/ moderate/severe)g | |||||
| a (12) | Control | 2.58 ± 1.7 | 0/8 | 2.74 ± 1.04 | 1/4 | 1/1 | 1/12 | 8.83 ± 3.12 | 9.0 ± 4.2 | 8/0 (4) | 1/0/8 (3) |
| b (14) | Dexamethasone (0.5 mg/kg × 2, i.v.) | 4.57 ± 1.2 | 7/14 | 2.71 ± 1.28 | 2/9 | 2/3 | 0/14 | 9.86 ± 1.86 | 38.3 ± 22.3 | 13/0 (1) | 0/1/10 (3) |
| c (16) | Vancomycin (30 mg/kg × 2, i.v.) | 5.88 ± 0.5 | 3/13 | 2.61 ± 0.78 | 13/15 | 12/12 | 7/16 | 5.23 ± 3.35 | 7.3 ± 4.1 | 12/3 (1) | 1/4/10 (1) |
| d (18) | Vancomycin plus dexamethasone | 5.64 ± 0.9 | 5/18 | 2.67 ± 1.08 | 13/16 | 12/15 | 12/18 | 4.32 ± 3.66 | 24.6 ± 13.9 | 14/3 (1) | 11/3/0 (4) |
For those data consisting of statistical comparisons, only statistically significant differences are given; statistical comparisons regarding blood cultures are presented in the text. For technical reasons, blood samples were not drawn from a limited number of animals.
For the survival data, differences in values were statistically significant as follows: treatment group a versus b, P < 0.015; a versus c or d, P < 0.001; b versus c, P < 0.001, b versus d, P = 0.029.
For the sterile versus nonsterile heart valve tissue data, differences in values were statistically significant as follows: treatment group a versus d, P = 0.002; b versus c, P = 0.007; b versus d, P < 0.001.
For the log10 CFU/g of heart valve tissue data, differences in values were statistically significant as follows: treatment group a versus c, P = 0.037; a versus d, P = 0.003; b versus c, P = 0.002, b versus d, P < 0.001.
For the PMN data, differences in values were statistically significant as follows: treatment group a versus b or d, P < 0.001; b versus c, P < 0.001; c versus d, P < 0.001.
Values in parentheses indicate numbers of specimens not appropriate for histopathological evaluation.
For the severity of heart valve tissue damage data, differences in values were statistically significant as follows: treatment group a versus d, P < 0.001; b versus d, P < 0.001; c versus d, P < 0.001. Values in parentheses indicate numbers of specimens not appropriate for histopathological evaluation.
FIG. 1.
Kaplan-Mayer survival curves. Cum, cumulative.
Bacteriological studies in animals. (i) Blood cultures.
For the pretreatment blood cultures, all study groups yielded comparable mean bacterial burdens (range, 2.61 ± 0.78 to 2.74 ± 1.04 log10 CFU/ml [mean ± SD]) (Table 1). During treatment with vancomycin or vancomycin plus dexamethasone, the few positive blood cultures found yielded mean bacterial burdens similar to those in the pretreatment cultures. In contrast, in the dexamethasone group, the mean bacterial burden on day 3 of treatment was statistically significantly higher than that of the pretreatment group (4.10 ± 0.96 log10 CFU/ml [n = 7] versus 2.71 ± 1.28 log10 CFU/ml [n = 7], respectively; P = 0.046). The numbers of sterile blood cultures on day 3 and day 5 of treatment in the group receiving vancomycin were statistically significantly higher than those of the pretreatment group (P = 0.002 and P < 0.001, respectively). The same was true for the group receiving vancomycin plus dexamethasone (P = 0.001 and P = 0.005, respectively). The number of sterile blood cultures on day 3 of treatment in the group receiving vancomycin was statistically significantly higher than that of the control group (P = 0.037) and that of the group receiving dexamethasone (P = 0.003). The respective P values for the results for the group receiving vancomycin plus dexamethasone were 0.061 (versus results for controls) and 0.009 (versus results for the dexamethasone group).
(ii) Heart valve tissue cultures.
For the sterilization rate of heart valve tissue, the combination of vancomycin plus dexamethasone was more effective than no treatment (P = 0.002), while no statistically significant differences were found between no treatment and treatment with vancomycin (P = 0.088) (Table 1). It is of interest to mention the fact that for one untreated animal in the control group, bacterial colonies were present in the histopathological results but the heart valve culture was sterile. This unexpected finding could represent the spontaneous sterilization of the heart valve tissue (a very unusual fact in staphylococcal endocarditis of the left side of the heart), or it may be the case that the bacterial burden was less than the lower limit of detection of the culture technique. Nevertheless, this particular animal was the only one in the control group who survived for 6 days.
With respect to the reductions of the bacterial burdens measured in heart valve tissues (in mean log10 per gram of tissue), the greater effectiveness of vancomycin treatment compared to that of either no treatment or treatment with dexamethasone was statistically significant (Table 1). There was no statistically significant difference in the reductions of the bacterial counts in heart valve tissue between the vancomycin group and the vancomycin-plus-dexamethasone group (P = 0.86).
In the two main groups of interest, namely, the group treated with vancomycin and the group treated with vancomycin plus dexamethasone, four animals (one in the first group and three in the second group) died before the scheduled day of sacrifice; because of this, we did a separate analysis excluding those four animals in order to eliminate any possible effects of the premature deaths in the results of the study. Again, no statistically significant differences were observed between the two groups regarding the sterilization rate of heart valve tissue (P = 0.264) and the mean bacterial burden (mean log10 CFU per gram of heart valve tissue) (P = 0.184).
Pathology results.
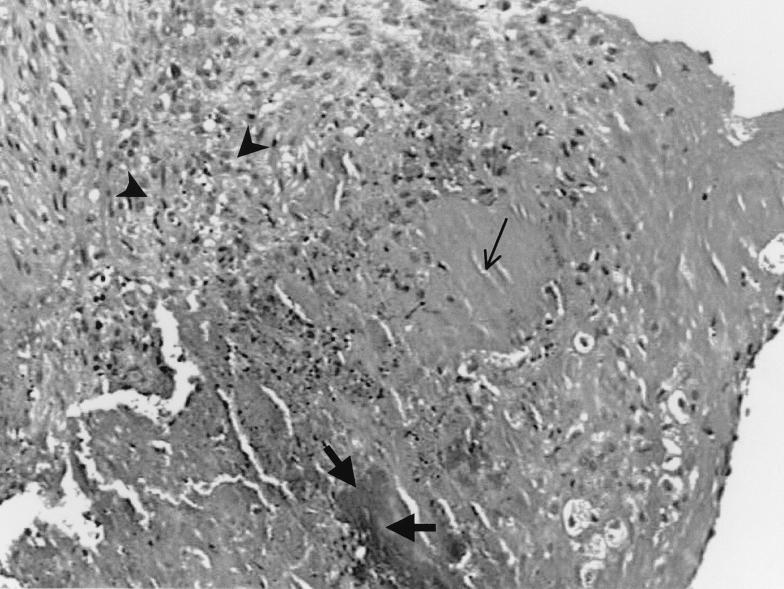
Histological examination of aortic valves revealed ulceration of endothelial surfaces and formation of thrombotic vegetations. The vegetations (Fig. 2) of all animals showed similar pictures, each consisting of acellular fibrin, platelets, and a matrix colonized by bacteria, as previously described (4, 5). Bacterial colonies of various densities were seen only in vegetations. Inflammatory infiltration was observed at the peripheries of the vegetations, particularly in the marginal area of each valve, and not in proximity to bacterial colonies (Fig. 2). The absolute numbers of PMNs in the different groups are given in Table 1. There were statistically significant differences observed in the results for the vancomycin and vancomycin-plus-dexamethasone groups (P < 0.001), with higher numbers of PMNs observed in the latter group. The same was true for comparisons between the control group and the dexamethasone group (P < 0.001). Finally, concerning the severity of valvular tissue damage (Fig. 3), it should be mentioned that a favorable effect was observed only in animals treated with vancomycin plus dexamethasone. Indeed, the majority of animals in the control group and in the dexamethasone- or vancomycin-treated groups had severe tissue damage. In contrast, the majority of animals treated with vancomycin plus dexamethasone had mild or moderate tissue damage, a finding that was also evident in the pilot study, where higher doses of dexamethasone were administered. A statistically significant difference was observed between the animals receiving only vancomycin and the animals receiving the combination of vancomycin plus dexamethasone in the severity of tissue damage of the valve, which was lesser in the latter group (P < 0.001). This favorable result was also evident after the four animals who had died before the scheduled day of sacrifice were excluded from analysis (P = 0.001). Statistically significant differences were also observed between the control group or the dexamethasone group and the vancomycin-plus-dexamethasone group; again, the differences favored the last group (Table 1).
FIG. 2.
Vegetation consists of acellular fibrin (thin arrow), platelets, and matrix colonized by bacteria (thick arrows). Inflammatory infiltrations are observed at the periphery of the vegetation (arrowheads), not in proximity to the bacterial colonies. Samples were stained with hematoxylin and eosin.
Vancomycin concentration in serum.
The mean peak concentration of vancomycin in serum was 72.7 μg/ml (n = 8), while the trough serum vancomycin concentrations were undetectable (n = 8). These serum drug concentrations differ from those usually observed in humans. The concentrations of vancomycin in serum from the vancomycin- and vancomycin-plus-dexamethasone-treated animals were similar.
DISCUSSION
From the results of the present study, it is obvious that with respect to the survival rate, the vegetation sterilization rate, and the mean bacterial burden in heart valve tissue, there are no differences between animals treated with vancomycin and those treated with vancomycin plus dexamethasone (0.5 mg/kg, twice a day). However, we found a beneficial effect in animals treated with dexamethasone plus vancomycin with regard to the tissue damage of the aortic valves. More specifically, tissue damage was more pronounced in the vancomycin-treated animals than in the animals treated with vancomycin plus dexamethasone, and this difference was statistically significant.
The mechanism responsible for this result is unclear. The most likely explanation is that the beneficial effect of dexamethasone is related to its effect on mediators of inflammation. Recent studies showed that downregulation of the responses of lymphocytes and macrophages by corticosteroids alleviates the outcome of sepsis caused by S. aureus (27). Sodequist et al. (25) showed that E-selectin, vascular cell adhesion molecule-1, and tumor necrosis factor alpha concentrations in serum increase in patients with staphylococcal endocarditis. Dexamethasone, due to its anti-inflammatory action, reduces tumor necrosis factor alpha levels (7, 36), and this action, by reducing the tissue damage, may be responsible for the protection of the endothelium of the aortic valve. In the experimental model used in the present study, it has been shown that dexamethasone increases peripheral blood granulocyte counts by approximately 50% during the first days of infection (2). Dexamethasone also increases granulocyte colony-stimulating-factor (G-CSF) levels (11), and this may be an additional mechanism that protects the aortic valve from extensive damage. However, G-CSF did not increase the clearance of methicillin-susceptible S. aureus from aortic valve vegetations in an experimental study (10), despite the fact that it stimulated leukocytosis in infected animals.
Leukocytes are the primary host defense against S. aureus infection (31). In the first study of neutrophil functions in patients with IE, Repine et al. (20) showed that the bactericidal activities of PMNs in untreated patients were significantly depressed but that they returned rapidly to normal during treatment. Bayer et al. (2, 3) showed that intravegetation granulocyte influx plays a significant role in modulating the spontaneous clearance of bacteria in experimental tricuspid valve endocarditis due to Pseudomonas aeruginosa, while this trend toward spontaneous bacterial clearance is not observed in aortic valve vegetations, possibly due to the fact that the granulocytes were distributed on the periphery of the vegetation and not in proximity to the bacterial colonies. However, studies by Meddens et al. (15) have suggested that PMNs play a modulating role by preventing Streptococcus sanguis bacteria from undergoing unbridled growth, even in aortic valve endocarditis. Dexamethasone failed to prevent the spontaneous intravegetation clearance of P. aeruginosa in a study by Bayer et al. (2), a finding in contradiction to those of a study of streptococcal tricuspid endocarditis (8). These disparate results possibly reflect differences in host defenses against gram-positive cocci and gram-negative bacilli (2). Other studies have confirmed the beneficial role of both monocytes and PMNs (16, 29) in the course of staphylococcal aortic valve endocarditis and septicemia (31). Moreover, Frank and Roth (9) have shown that the anti-inflammatory effects of corticosteroids may be partially mediated by factors released by monocytes. The findings of the above-mentioned studies are in accordance with the results of the present study, since the increased numbers of PMNs found in the animals treated with vancomycin plus dexamethasone compared with those found in animals treated with vancomycin only were associated with milder damage of the aortic valves. Nevertheless, PMNs alone are not able to reduce the intravegetation bacterial burden or the severity of tissue damage, since for the animals treated with dexamethasone alone, despite the high numbers of PMNs observed, the severity of tissue damage and the mean bacterial burden were comparable to those of the control animals. It is of interest that dexamethasone-treated animals, which showed a very high mean bacterial burden, exhibited a higher mean survival rate than did untreated controls. However, despite the fact that the mean survival rate of dexamethasone-treated animals was 2 days longer than the mean survival rate of the untreated animals, the vast majority of the former died before the day of sacrifice. This finding suggests that the beneficial effect of dexamethasone lasts for the first 4 days of treatment. In a study by Francioli and Freedman (8), mortality was not affected by dexamethasone in animals with streptococcal aortic valve endocarditis.
Regarding the findings of the histological examination, it is of interest that bacterial colonies were seen in the vast majority of vegetations (Table 1), even in those that did not yield bacteria in tissue culture. This is in disagreement with the findings of the study of Francioli and Freedman (8), where bacterial colonies of S. sanguis were seen when the vegetations contained more than 105 CFU/g. In the present study, inflammatory infiltration was more commonly seen in the dexamethasone-treated groups and was observed at the periphery of vegetations, particularly in the marginal area of each valve, and not in proximity to bacterial colonies; this finding is in accordance with those of the previously mentioned study (8).
In conclusion, the addition of dexamethasone to vancomycin for 5 days of treatment has a beneficial effect, i.e., a reduction in the severity of valve tissue destruction, in the treatment of experimental aortic valve endocarditis due to MRSA while it has no effect on either survival or blood or vegetation sterilization rates. However, the possibility that the steroids might delay valve destruction but not prevent it could not be excluded. In order to elucidate better this possibility, future studies—especially if data regarding heart function obtained by echocardiography (34) are available—with protocols that include treating animals to the point of cure and then observing them without treatment for some period before sacrifice are needed. The clinical relevance of the results of the present experimental study is the possibility that dexamethasone could reduce the severity of valve damage and possibly the need for surgical operation and valve replacement.
ACKNOWLEDGMENTS
We thank A. Mandaraka for technical assistance and Nikolaos Baibas for his help in part of the statistical analysis.
REFERENCES
- 1.Arditi M, Mason E O, Jr, Bradley J S, Tan T Q, Barson W J, Schutze G E, Wald E R, Ginver L B, Kim K S, Yogev R, Kaplan S L. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102:1087–1097. doi: 10.1542/peds.102.5.1087. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A, Yih J, Chiu C Y, Nast C C. Pathogenic effects of monocytopenia, granulocytopenia, and dexamethasone on the course of experimental Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1989;35:278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- 3.Bayer A, Norman D. Valve site-specific pathogenic differences between right-sided and left-sided bacterial endocarditis. Chest. 1990;98:200–205. doi: 10.1378/chest.98.1.200. [DOI] [PubMed] [Google Scholar]
- 4.Buchbinder N A, Roberts W C. Left-sided valvular infective endocarditis. A study of 45 necropsy patients. Am J Med. 1972;53:20–35. doi: 10.1016/0002-9343(72)90112-x. [DOI] [PubMed] [Google Scholar]
- 5.Durack D T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975;115:81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- 6.Fowler V G, Jr, Sanders L L, Kong L K, McClelland R S, Gottlieb G S, Li J, Ryan T, Sexton D J, Roussakis G, Harrell L J, Corey G R. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin Infect Dis. 1999;28:106–114. doi: 10.1086/515076. [DOI] [PubMed] [Google Scholar]
- 7.Franchimont D, Louis E, Dewe W, Martens H, Vrindts-Gevaert Y, De Groote D, Belaiche J, Geenen V. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul Pept. 1998;73:59–65. doi: 10.1016/s0167-0115(97)01063-x. [DOI] [PubMed] [Google Scholar]
- 8.Francioli P B, Freedman L R. Streptococcal infection of endocardial and other intravascular vegetations in rabbits: natural history and effect of dexamethasone. Infect Immun. 1979;24:483–491. doi: 10.1128/iai.24.2.483-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank D E, Roth J A. Factors secreted by untreated and hydrocortisone-treated monocytes that modulate neutrophil function. J Leukoc Biol. 1986;40:693–707. doi: 10.1002/jlb.40.6.693. [DOI] [PubMed] [Google Scholar]
- 10.Frank U, Chambers H F. Treatment of Staphylococcus aureus catheter-related infection and infective endocarditis with granulocyte colony-stimulating factor in the experimental rabbit model. Antimicrob Agents Chemother. 1996;40:1308–1310. doi: 10.1128/aac.40.5.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jilma B, Stohlawetz P, Pernerstorfer T, Eichler H G, Mullner C, Kapiotis S. Glucocorticoids dose-dependently increase plasma levels of granulocyte colony-stimulating factor. J Clin Endocrinol Metab. 1998;83:1037–1040. doi: 10.1210/jcem.83.3.4802. [DOI] [PubMed] [Google Scholar]
- 12.John M D, Hibberd P L, Karchmer A W, Sleeper L A, Calderwood S B. Staphylococcus aureus prosthetic valve endocarditis: optimal management and risk factors for death. Clin Infect Dis. 1998;26:1302–1309. doi: 10.1086/516378. [DOI] [PubMed] [Google Scholar]
- 13.Jolley M E. Fluorescence polarization immunoassay for the determination of therapeutic levels in human plasma. J Anal Toxicol. 1981;5:236–240. doi: 10.1093/jat/5.5.236. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre P B, Berkey C S, King S M, Schaad U B, Kilpi T, Kanra G Y, Perez C M. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:925–931. doi: 10.1001/jama.278.11.925. [DOI] [PubMed] [Google Scholar]
- 15.Meddens M J M, Thompson J, Eulderink F, Bauer W C, Mattie H, Van Furth R. Role of granulocytes in experimental Streptococcus sanguis endocarditis. Infect Immun. 1982;36:325–332. doi: 10.1128/iai.36.1.325-332.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meddens M J M, Thompson J, Bauer W C, Hermans J, van Furth R. Role of granulocytes and monocytes in experimental Staphylococcus epidermidis endocarditis. Infect Immun. 1983;41:145–153. doi: 10.1128/iai.41.1.145-153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perdikaris G, Giamarellou H, Pefanis A, Donta I, Karayiannakos P. Vancomycin or vancomycin plus netilmicin for methicillin- and gentamicin-resistant Staphylococcus aureus aortic valve experimental endocarditis. Antimicrob Agents Chemother. 1995;39:2289–2294. doi: 10.1128/aac.39.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlman B B, Freedman R L. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;44:206–213. [PMC free article] [PubMed] [Google Scholar]
- 19.Rappaport J M, Bhatt S M, Burkard R F, Merchant S N, Nadol J B., Jr Prevention of hearing loss in experimental pneumococcal meningitis by administration of dexamethasone and ketorolac. J Infect Dis. 1999;179:264–268. doi: 10.1086/314531. [DOI] [PubMed] [Google Scholar]
- 20.Repine J E, Clawson C C, Burchell H B, White J G. Reversible neutrophil defect in patients with bacterial endocarditis. J Lab Clin Med. 1976;88:780–787. [PubMed] [Google Scholar]
- 21.Roder B L, Wandall D A, Espersen F, Fridmodt-Moller N, Skinhoj P, Rosdahl V T. A study of 47 bacteremic Staphylococcus aureus endocarditis cases: 23 with native valves treated surgically and 24 with prosthetic valves. Scand Cardiovasc J. 1997;31:305–309. doi: 10.3109/14017439709069552. [DOI] [PubMed] [Google Scholar]
- 22.Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:1596–1605. doi: 10.1002/art.1780390921. [DOI] [PubMed] [Google Scholar]
- 23.Sanabria T J, Alpert J S, Goldberg R, Pape L A, Cheeseman S H. Increased frequency of staphylococcal infective endocarditis: experience at a university hospital, 1981 through 1988. Arch Intern Med. 1990;150:1305–1309. [PubMed] [Google Scholar]
- 24.Smith M A, Sorenson J A, D'Aversa G, Mandelbaum S, Udell I, Harrison W. Treatment of experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis with intravitreal vancomycin and intravitreal dexamethasone. J Infect Dis. 1997;175:462–466. doi: 10.1093/infdis/175.2.462. [DOI] [PubMed] [Google Scholar]
- 25.Sodequist B, Sundqvist K G, Vikerfors T. Adhesion molecules (E-selectin, intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)) in sera from patients with Staphylococcus aureus bacteraemia with or without endocarditis. Clin Exp Immunol. 1999;118:408–411. doi: 10.1046/j.1365-2249.1999.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syrogiannopoulos G A, Olsen K D, Reisch J S, McCracken G H., Jr Dexamethasone in the treatment of Haemophilus influenzae type b meningitis. J Infect Dis. 1987;155:213–219. doi: 10.1093/infdis/155.2.213. . (Erratum, 155:1359). [DOI] [PubMed] [Google Scholar]
- 27.Tarkowsky A, Wagner H. Arthritis and sepsis caused by Staphylococcus aureus: can the tissue injury be reduced by modulating the host's immune system? Mol Med Today. 1998;4:15–18. doi: 10.1016/S1357-4310(97)80540-0. [DOI] [PubMed] [Google Scholar]
- 28.Tunkel A R, Scheld W M. Issues in the management of bacterial meningitis. Am Fam Physician. 1997;56:1355–1362. [PubMed] [Google Scholar]
- 29.Veltrop M H A M, Bancsi M J L M F, Bertina R M, Thompson J. Role of monocytes in experimental Staphylococcus aureus endocarditis. Infect Immun. 2000;68:4818–4821. doi: 10.1128/iai.68.8.4818-4821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verba V, Sakiniene E, Tarkowski A. Beneficial effect of glucocorticoids on the course of haematogenously acquired Staphylococcus aureus nephritis. Scand J Immunol. 1997;45:282–286. doi: 10.1046/j.1365-3083.1997.d01-400.x. [DOI] [PubMed] [Google Scholar]
- 31.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlessis A A, Hovaguimian H, Jaggers J, Ahmad A, Starr A. Infective endocarditis: ten-year review of medical and surgical therapy. Ann Thorac Surg. 1996;61:1217–1222. doi: 10.1016/0003-4975(96)00029-x. [DOI] [PubMed] [Google Scholar]
- 33.Watanakunakorn C, Burkert T. Infective endocarditis at a large community hospital, 1980–1990: a review of 210 episodes. Medicine. 1993;72:90–102. doi: 10.1097/00005792-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y-Q, Kupferwasser L I, Zack P M, Bayer A S. Comparative efficacies of liposomal amikacin (MiKasome) plus oxacillin versus conventional amikacin plus oxacillin in experimental endocarditis induced by Staphylococcus aureus: microbiological and echocardiographic analyses. Antimicrob Agents Chemother. 1999;43:1737–1742. doi: 10.1128/aac.43.7.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshizumi O M, Lee G C, Equi R A, Kim I T, Pitchekian-Halabi H, Adamu S A, Mondino B J. Timing of dexamethasone treatment in experimental Staphylococcus aureus endophthalmitis. Retina. 1998;18:130–135. doi: 10.1097/00006982-199818020-00006. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman S H, Shellhaas J, Butler L D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989;19:301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]