Abstract
We established a human immunodeficiency virus type 1 (HIV-1) envelope (Env)-mediated membrane fusion assay and examined the small-molecule CCR5 antagonist TAK-779 and its derivatives for their inhibitory effects on HIV-1 Env-mediated membrane fusion and viral replication. The membrane fusion assay is based on HIV-1 long terminal repeat-directed β-d-galactosidase reporter gene expression in CD4- and CCR5-expressed HeLa (MAGI-CCR5) cells after cocultivation with effector 293T cells expressing HIV-1 Env. Inhibition of HIV-1 replication was also determined in MAGI-CCR5 cells infected with the corresponding cell-free HIV-1. TAK-779 effectively suppressed R5 HIV-1 (strain JR-FL) Env-mediated membrane fusion as well as viral replication. Its 50% inhibitory concentrations (IC50s) for membrane fusion and viral replication were 0.87 ± 0.11 and 1.4 ± 0.1 nM, respectively. These values corresponded well to the IC50 for 125I-RANTES (regulated on activation, T cell expressed, and secreted) binding to CCR5 (1.4 nM). The inhibitory effects of 18 TAK-779 derivatives on membrane fusion differed from one compound to another. However, there was a close correlation among their inhibitory effects on membrane fusion, viral replication, and RANTES binding. The correlation coefficient between their IC50s for membrane fusion and viral replication was 0.881. Furthermore, since this assay depends on Env expressed in the effector cells, it is also applicable to the evaluation of CXCR4 antagonists. These results indicate that the HIV-1 Env-mediated membrane fusion assay is a useful tool for the evaluation of entry inhibitors.
The advent of highly active antiretroviral therapy with reverse transcriptase and protease inhibitors has achieved high-level suppression of viral load in human immunodeficiency virus type 1 (HIV-1)-infected individuals (8). However, a recent report suggests that the chemotherapy presently available is not sufficient for virus eradication (17). In addition, there are few alternative chemotherapy options in cases of treatment failure with existing antiretrovirals, which target only two different events in the HIV-1 replication cycle. Therefore, it is mandatory to discover novel anti-HIV-1 agents with a different mechanism of action. HIV-1 entry is one of the promising targets, since T20, an inhibitor of gp41-mediated HIV-1 entry, has shown efficacy in a recent phase I/II clinical trial (19). The chemokine receptors CCR5 and CXCR4 act as major coreceptors for the entry of macrophage-tropic (CCR5-using or R5) and T cell line-tropic (CXCR4-using or X4) HIV-1 into host cells, respectively (2, 10, 12–14, 16). Natural ligands for CCR5 (regulated on activation, normal T cell expressed, and secreted [RANTES] and macrophage inflammatory proteins 1α and 1β) and for CXCR4 (stromal cell-derived factors 1α and 1β) are known to block R5 and X4 HIV-1 infections, respectively (7, 11, 23). Therefore, chemokine receptor antagonists functioning as HIV-1 entry inhibitors may be promising candidates for the treatment of HIV-1 infection.
Cell-to-cell membrane fusion assays have been employed widely to study HIV-1 entry mechanisms because they are easy to operate and do not need an infectious virus. The assays may also be a useful tool for the screening of HIV-1 entry inhibitors. However, it has not been demonstrated whether the inhibitory effects of entry inhibitors on envelope (Env)-mediated membrane fusions exactly reflect those on viral entry. In particular, small-molecule inhibitors do not seem to cover completely the HIV-1 Env-binding regions of chemokine receptors. There are several methods to detect the cell-to-cell membrane fusion. For instance, fluorescent dye transfer and morphological change (syncytium formation) can be detected by microscopy (6, 18). This technique provides only semiquantitative evaluation for membrane fusion. Assays with either β-d-galactosidase, luciferase, or chloramphenicol acetyltransferase as a reporter gene are commonly used for quantitative detection (22, 24). However, these methods require preparation of cell lysate for measurement of reporter activities, which is laborious and not suitable for high-throughput screening. Direct detection of reporter activities without the requirement for preparation of cell lysate is desirable for this purpose.
TAK-779 is a small-molecule CCR5 antagonist with highly potent and selective antiviral activity against R5 HIV-1 (4). TAK-779 derivatives also proved inhibitory to RANTES binding in CCR5-expressing cells (26), yet their activities against HIV-1 replication and Env-mediated membrane fusion have not been determined. In this study, we constructed an HIV-1 Env-mediated membrane fusion assay and evaluated various TAK-779 derivatives for their inhibitory effects on membrane fusion. We also examined their inhibitory effects on HIV-1 replication and found that there was a close correlation between inhibition of membrane fusion and viral replication.
MATERIALS AND METHODS
Cells and virus.
MAGI-CCR5, a HeLa-CD4 cell line that expresses CCR5 and that has an integrated copy of the HIV-1 long terminal repeat (LTR)-driven β-d-galactosidase reporter gene (9), were maintained in Dulbecco's modified Eagle's medium (Nikken BioMedical Laboratory, Kyoto, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Gaithersburg, Md.), 100 U of penicillin per ml and 100 μg of streptomycin per ml (Life Technologies), 0.2 mg of G418 (Life Technologies) per ml, 0.2 mg of hygromycin B (Boehringer Mannheim, Mannheim, Germany) per ml, and 1 μg of puromycin (Sigma, St. Louis, Mo.) per ml. 293T cells were maintained using Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. The R5 HIV-1 strain JR-FL was used in this study. The JR-FL strain was propagated in MOLT-4/CCR5 cells, which are highly permissive for the replication of R5 HIV-1 (3). The virus stocks were determined for their p24 antigen levels with a sandwich enzyme-linked immunosorbent assay kit (ZeptoMetrix Corporation, Buffalo, N.Y.) and stored at −80°C until use.
Compounds.
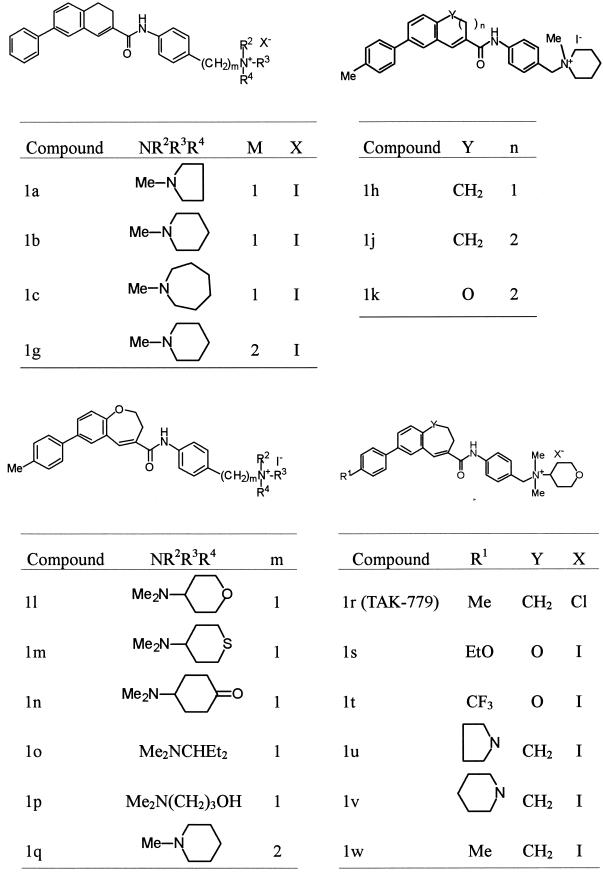
TAK-779 and 18 derivatives were used in this study. These compounds were synthesized by Takeda Chemical Industries (Osaka, Japan). All compounds were dissolved in dimethyl sulfoxide at 20 mM and stored at −20°C until use. Their chemical structures are shown in Fig. 1.
FIG. 1.
Chemical structures of TAK-779 derivatives.
HIV-1 replication assay.
The inhibitory effects of the test compounds on HIV-1 replication are due to the inhibition of virus-induced infectious focus formation in MAGI-CCR5 cells (20). Briefly, MAGI-CCR5 cells were seeded in a 96-well plate at 1.5 × 104 cells per well. The culture supernatants were removed on the next day, and fresh culture medium containing the virus (approximately 300 focus-forming units per well) and various concentrations of the test compounds were added to each well. On day 2 after viral infection, the culture supernatants were removed and fixing solution (1% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline [PBS]) was added to each well. The cells were fixed at room temperature for 5 min and washed twice with PBS. X-Gal staining solution (4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 2 mM magnesium chloride, and 0.4 mg of 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside per ml in PBS) was added to each well, and the cells were stained at 37°C for 45 min. The number of infected (blue) cells was counted microscopically.
Env-mediated membrane fusion assay.
The inhibitory effects of the test compounds on HIV-1 Env-mediated membrane fusion were determined by a β-d-galactosidase reporter gene system. For preparation of the effector cells, 293T cells were seeded in a six-well plate at 106 cells per well. The culture supernatants were removed on the next day, and the cells were transfected with 0.6 μg of Env expression vector, 0.2 μg of p-rev encoding HIV-1 Rev, and 1.0 μg of pSV2tat encoding HIV-1 Tat with Lipofectamine (Life Technologies). After a 6-h incubation, the mixtures were removed and the cells were incubated with fresh culture medium for 2 days. For preparation of the target cells, MAGI-CCR5 cells were seeded in a 96-well plate at 104 cells per well. Culture supernatants were removed on the next day, and fresh culture medium containing transfected 293T cells (104 cells per well) and various concentrations of the test compounds were added to each well. The target and effector cell suspensions were incubated at 37°C. After an overnight incubation, Gal-Screen (Tropix, Foster City, Calif.) was added to each well and the mixtures were incubated at 30°C for 45 min. The β-d-galactosidase activity in each well was measured with a luminometer (Microlumat LB96P; Berthold, Wildbad, Germany).
Data analysis.
Fifty percent inhibitory concentrations (IC50s) of the test compounds for membrane fusion and HIV-1 replication were determined by least-squares linear regression analysis of the ascending linear portions of the dose-response curves.
RESULTS
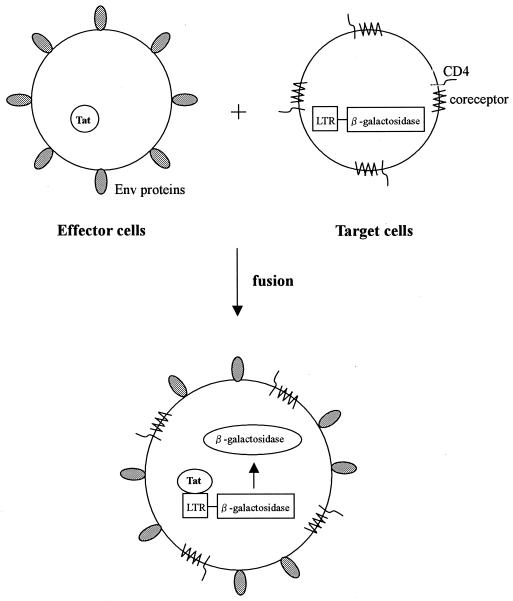
To detect the Env-mediated membrane fusion, we established a cell-to-cell membrane fusion assay using an HIV-1 Tat- and LTR-driven-β-d-galactosidase reporter system. A schematic presentation of this assay system is shown in Fig. 2. 293T cells transiently expressing HIV-1 Tat and Env were used as the effector cells, and MAGI-CCR5 cells were used as the target cells. When cell-to-cell membrane fusion occurs between effector and target cells, the reporter gene is activated by Tat under the control of HIV-1 LTR. Reporter activity was detected by chemiluminescence without preparation of cell lysate. The signal-to-noise ratios for JR-FL Env-mediated membrane fusion after 24- and 48-h reactions were about 10 and greater than 20, respectively (data not shown). As a signal-to-noise ratio greater than 10 is sufficient to obtain reliable results, a 24-h reaction time was chosen for the following experiments. Env from another HIV-1 strain is also applicable in this system. In fact, we could obtain similar results with expression vectors of Env derived from HXB2 (X4) and 89.6 (R5X4) and from other R5 HIV-1 strains (data not shown).
FIG. 2.
Schematic presentation of the HIV-1 Env-mediated membrane fusion assay. 293T cells transiently expressing HIV-1 Tat and Env were used as the effector cells, and MAGI-CCR5 cells were used as the target cells. When cell-to-cell membrane fusion occurs between the effector and target cells, the reporter gene is activated by Tat under the control of HIV-1 LTR. Reporter activity was detected by chemiluminescence.
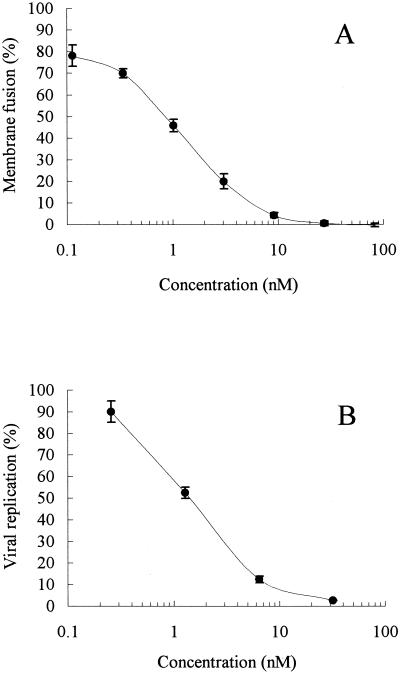
TAK-779 was previously reported to inhibit R5 but not X4 HIV-1 replication in MAGI-CCR5 cells through the blocking of viral entry (4). In this study, we examined whether TAK-779 interfered specifically with R5 HIV-1 Env-mediated membrane fusion. TAK-779 proved inhibitory to JR-FL Env-mediated membrane fusion in a dose-dependent fashion (Fig. 3A). A similar dose-response curve was also obtained in the replication assay (Fig. 3B). IC50s of TAK-779 for membrane fusion and viral replication were 0.87 and 1.4 nM, respectively. TAK-779 was totally inactive against X4 HIV-1 (HXB2) Env-mediated membrane fusion (data not shown). In addition, the CXCR4 antagonist AMD3100 did not inhibit JR-FL Env-mediated membrane fusion at concentrations up to 10 μM (data not shown). These data suggest that TAK-779 blocks R5 HIV-1 replication at a stage of membrane fusion.
FIG. 3.
Inhibitory effects of TAK-779 on HIV-1 Env-mediated membrane fusion (A) and on virus replication (B). Assay procedures are described in Materials and Methods. All data represent means ± standard errors of the means obtained in three separate experiments.
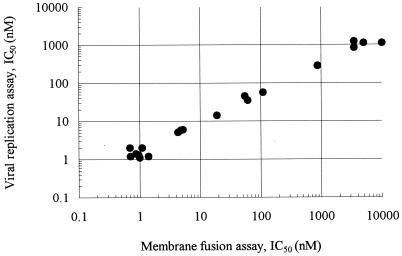
To elucidate the correlation between the inhibition of membrane fusion and HIV-1 replication, TAK-779 and 18 derivatives were examined for their inhibitory effects on membrane fusion and viral replication (Table 1). Their IC50s for 125I-RANTES binding to CCR5 ranged from 1.4 to 1,100 nM, as previously described (26), whereas the IC50s for membrane fusion ranged from 0.69 to 10,000 nM. Ten compounds, 1l, 1m, 1n, 1o, 1r (TAK-779), 1s, 1t, 1u, 1v, and 1w, displayed potent activities against membrane fusion, with IC50s ranging from 0.69 to 5.2 nM. These compounds also showed potent inhibitory effects on viral replication, with IC50s ranging from 1.1 to 6.0 nM. Four compounds (1j, 1k, 1p, and 1q) had moderate activities against membrane fusion (IC50s of 19 to 110 nM) and viral replication (IC50s of 14 to 56 nM) as well as 125I-RANTES binding (IC50s of 6.8 to 110 nM). Five compounds (1a, 1b, 1c, 1g, and 1h) could not achieve 50% inhibition, even at a concentration of 200 nM in all assays. No compound showed any cytotoxicity in MAGI-CCR5 cells at concentrations up to 20 μM (data not shown). The correlation coefficient was calculated using the IC50s for membrane fusion and viral replication. As shown in Fig. 4, there was a close correlation (r = 0.881) between them, indicating that inhibition of the fusion process is a principal mechanism of the inhibition of HIV-1 replication by TAK-779 and its derivatives.
TABLE 1.
Inhibitory effects of TAK-779 and its derivatives on HIV-1 Env-mediated membrane fusion and virus replication
| Compound | IC50 (nM) for:
|
||
|---|---|---|---|
| Membrane fusiona | HIV-1 replicationa | 125I-RANTES bindingb | |
| 1a | 10,000 ± 4,800 | 1,100 ± 100 | 430 |
| 1b | 3,500 ± 1300 | 1,200 ± 100 | 390 |
| 1c | 3,500 ± 1300 | 820 ± 110 | 380 |
| 1g | 5,000 ± 2500 | 1,100 ± 100 | 1,100 |
| 1h | 870 ± 430 | 280 ± 50 | 240 |
| 1j | 61 ± 17 | 35 ± 4 | 25 |
| 1k | 55 ± 22 | 45 ± 9 | 43 |
| 1l | 1.1 ± 0.2 | 2.0 ± 0.2 | 1.4 |
| 1m | 5.2 ± 1.5 | 6.0 ± 0.3 | 3.1 |
| 1n | 4.8 ± 1.1 | 5.8 ± 0.6 | 4.5 |
| 1o | 4.3 ± 1.2 | 5.1 ± 0.4 | 3.3 |
| 1p | 19 ± 4 | 14 ± 1 | 6.8 |
| 1q | 110 ± 20 | 56 ± 3 | 110 |
| 1r (TAK-779) | 0.87 ± 0.11 | 1.4 ± 0.1 | 1.4 |
| 1s | 0.69 ± 0.11 | 1.8 ± 0.2 | 1.8 |
| 1t | 1.4 ± 0.4 | 1.2 ± 0.02 | 1.5 |
| 1u | 1.0 ± 0.1 | 1.1 ± 0.1 | 3.8 |
| 1v | 0.96 ± 0.06 | 1.3 ± 0.2 | 2.2 |
| 1w | 0.71 ± 0.17 | 1.2 ± 0.1 | 1.8 |
Data represent means ± standard errors of the means in three separate experiments.
Data are taken from reference 26.
FIG. 4.
Correlation between the inhibitory effects of TAK-779 derivatives on HIV-1 Env-mediated membrane fusion and viral replication. Each point represents the IC50s for membrane fusion and viral replication (r = 0.881).
DISCUSSION
Since RANTES, a natural ligand for CCR5, is known to inhibit R5 HIV-1 replication (11), assays for RANTES-binding inhibition of CCR5-expressing cells are considered useful tools for finding CCR5 antagonists with anti-HIV-1 activities. In fact, we found TAK-779 by using a screening assay for 125I-RANTES-binding inhibition (4). However, several studies indicate that the binding site of β-chemokines on CCR5 does not overlap completely with that of either recombinant gp120 or virions (27). β-Chemokines bind predominantly to the second extracellular loop of CCR5, whereas R5 HIV-1 gp120 interacts with the N terminus and the second extracellular loop of CCR5 (1, 5, 21, 25). A recent report has also shown that TAK-779 binds within a cavity formed between transmembrane domains of CCR5 and induces its conformational change (15), findings based on the assumption that the inhibition of gp120-coreceptor interaction by TAK-779 is attributable to its allosteric effect on CCR5. Therefore, screening of compounds by ligand-binding inhibition might have some limitations for the discovery of entry inhibitors with potent anti-HIV-1 activities.
In this study, we established a quantitative Env-mediated membrane fusion assay and examined various TAK-779 derivatives for their inhibitory effects on membrane fusion and HIV-1 replication. The IC50s of the compounds for membrane fusion were found to be closely correlated with their IC50s for viral replication, indicating that a membrane fusion assay could replace a viral replication assay using infectious HIV-1. In the cases of TAK-779 and its derivatives, their inhibitory effects on viral replication also have a close correlation with those on 125I-RANTES binding (r = 0.856) because they were screened in a 125I-RANTES-binding inhibition assay (13). However, it is likely that a compound interacting directly with the N terminus of CCR5 would not be detectable in the 125I-RANTES-binding inhibition assay, even though it is a potent inhibitor of HIV-1 entry. From this point of view, the membrane fusion assay seems superior to the 125I-RANTES-binding assay as an efficient tool for the screening of entry inhibitors. However, it should be pointed out that only TAK-779 derivatives were used as entry inhibitors in this study. To gain further insight into the usefulness of the membrane fusion assay, the correlation between inhibition of membrane fusion and HIV-1 replication should be determined for various types of entry inhibitors. Furthermore, in the membrane fusion assay, Tat-induced and HIV-1 LTR-driven transcriptional activation was required for the expression of β-d-galactosidase. This requirement could generate false-positive results when some inhibitors of Tat- or HIV-1 LTR-driven gene expression are examined. Although TAK-779 strongly inhibited membrane fusion mediated by R5 HIV-1 (JR-FL) Env, it was not inhibitory to that by X4 HIV-1 (HXB2), which indicates that TAK-779 did not affect the transcriptional activation of the reporter gene.
In conclusion, the HIV-1 Env-mediated membrane fusion assay is a safe and reliable system for the screening of entry inhibitors. Quantitative measurement of β-d-galactosidase activity can be performed with a luminometer in a 96-well plate. Thus, the membrane fusion assay might also be applicable to high-throughput screening of effective entry inhibitors of HIV-1.
ACKNOWLEDGMENTS
We thank S. Shiki for technical assistance. The JR-FL Env-expressing vector and p-rev were kindly provided by D. Littman and T. Parslow, respectively. MAGI-CCR5 cells and pSV2tat were obtained through the NIH AIDS Research and Reference Reagent Program, NIAID, Bethesda, Md. (contributed by J. Overbaugh and A. Frankel, respectively).
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
REFERENCES
- 1.Alkhatib G, Ahuja S S, Light D, Mummidi S, Berger E A, Ahuja S K. CC chemokine receptor 5-mediated signaling and HIV-1 co-receptor activity share common structural determinants. Critical residues in the third extracellular loop support HIV-1 fusion. J Biol Chem. 1997;272:19771–19776. doi: 10.1074/jbc.272.32.19771. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Baba M, Miyake H, Okamoto M, Iizawa Y, Okonogi K. Establishment of a CCR5-expressing T-lymphoblastoid cell line highly susceptible to R5 HIV Type 1. AIDS Res Hum Retrovir. 2000;16:935–941. doi: 10.1089/08892220050058344. [DOI] [PubMed] [Google Scholar]
- 4.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S G, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter C C J, Cooper D A, Fischl M A, Gatell J M, Gazzard B G, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schechter M, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy in adults: updated recommendations of the international AIDS society-USA panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 13.Dornaz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Trkola A, Thompson D A D, Cormier E G, Kajumo F A, Maxwell E, Lin S W, Ying W, Smith S O, Sakmar T P, Moore J P. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 18.Furuta R A, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 19.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 20.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 24.Rucker J, Doranz B J, Edinger A L, Long D, Berson J F, Doms R W. Cell-cell fusion assay to study role of chemokine receptors in human immunodeficiency virus type I entry. Methods Enzymol. 1997;288:118–133. doi: 10.1016/s0076-6879(97)88011-1. [DOI] [PubMed] [Google Scholar]
- 25.Samson M, LaRosa G, Libert F, Paindavoine P, Detheux M, Vassart G, Parmentier M. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J Biol Chem. 1997;272:24934–24941. doi: 10.1074/jbc.272.40.24934. [DOI] [PubMed] [Google Scholar]
- 26.Shiraishi M, Aramaki Y, Seto M, Imoto H, Nishikawa Y, Kanzaki N, Okamoto M, Sawada H, Nishimura O, Baba M, Fujino M. Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem. 2000;43:2049–2063. doi: 10.1021/jm9906264. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackey C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]